Abstract
GABA is the main inhibitory neurotransmitter in the adult forebrain, where it activates ionotropic type A and metabotropic type B receptors. Early studies have shown that GABAA receptor-mediated inhibition controls neuronal excitability and thus the occurrence of seizures. However, more complex, and at times unexpected, mechanisms of GABAergic signaling have been identified during epileptiform discharges over the last few years. Here, we will review experimental data that point at the paradoxical role played by GABAA receptor-mediated mechanisms in synchronizing neuronal networks, and in particular those of limbic structures such as the hippocampus, the entorhinal and perirhinal cortices, or the amygdala. After having summarized the fundamental characteristics of GABAA receptor-mediated mechanisms, we will analyze their role in the generation of network oscillations and their contribution to epileptiform synchronization. Whether and how GABAA receptors influence the interaction between limbic networks leading to ictogenesis will be also reviewed. Finally, we will consider the role of altered inhibition in the human epileptic brain along with the ability of GABAA receptor-mediated conductances to generate synchronous depolarizing events that may lead to ictogenesis in human epileptic disorders as well.
Keywords: Epileptiform synchronization, GABA, High frequency oscillations, Limbic structures
1. Background
The amino acid GABA, originally identified as factor I by Florey and McLennan (1955) and by Basemore et al. (1956), is the main inhibitory neurotransmitter in the adult forebrain where, once released from interneuron terminals, it activates pre- and postsynaptic GABA receptors (Martin and Olsen, 2000; Farrant and Kaila, 2007). GABA receptors are divided into three types: A, B and C. GABAA receptors are receptor-operated ionotropic channels while signals mediated by GABAB receptors are metabotropic and have slower and longer effects because they activate second messengers; It is also well established that presynaptic GABAB receptors control transmitter release from excitatory and inhibitory terminals whereas a similar function for GABAA receptors remains controversial (Draguhn et al., 2008). GABAC receptors, which were considered confined to the retina in the adult central nervous system (Enz et al., 1995, but see Boue-Grabot et al., 1998), have been later reported to play inhibtory functions in the adult rodent hippocampus where they may be extrasynaptically located and activated via spillover of synaptically released GABA (Alakuijala et al., 2006).
The relation between GABA and seizures was first identified when infants fed with a formula that was accidentally deficient in pyridoxine (also known as vitamin B6) presented with seizures (Molony and Parmelee, 1954; Coursin, 1954). Pyridoxine is the coenzyme for the synthesis of GABA from glutamic acid via the enzyme glutamic acid decarboxylase (GAD). Shortly after its identification, GABA was also found to prevent seizures while drugs interfering with GABA synthesis and signaling were demonstrated to induce convulsions (Hawkins and Sarett, 1957; Hayashi, 1959; Benassi and Bertolotti, 1962). Studies performed in the seventies and early eighties have confirmed that GABAA receptor-mediated currents control neuronal excitability and thus epileptiform activity (see Section 4). According to this view, failure of GABA receptor was assumed to be a conditio sine qua non for seizure appearance (Krnjevic, 1983). In addition, altered GABA receptor signaling has been proposed to be involved in the generation of abnormal activity in other neurological and psychiatric conditions (Llinás et al., 2005; Uhlhaas and Singer, 2010).
Later studies, however, have challenged this assumption, by showing that inhibition per se or some types of inhibitory mechanisms are preserved in animal models of epilepsy (Davenport et al., 1990; Esclapez et al., 1997; Prince & Jacobs, 1998; Cossart et al., 2001, 2005) and in post-surgical human temporal lobe tissue (Isokawa-Akesson et al., 1989; Babb et al., 1989; Avoli and Olivier, 1989; Avoli et al., 1995; Cohen et al., 2002). Moreover, more complex, at times ambiguous, roles for GABAergic signaling in epilepsy were identified. Indeed, evidence is emerging that GABA may assist, support or shape epileptiform synchrony (Avoli et al., 1993, 1996a,b,c, 2002; Mann and Mody, 2008; de Curtis and Gnatkovsky, 2009a,b). In addition, recent findings indicate a role played by GABAA receptor-mediated mechanisms in epileptogenetic processes both in adult and immature brains.
Here, we will review experimental data that point at the role played by GABAA receptor-mediated mechanism in synchronizing neuronal networks, and in particular those of limbic structures such as hippocampus and parahippocampal cortices. After having summarized the fundamental characteristics of GABAA receptor-mediated mechanisms we will discuss (i) their role in the generation of network oscillations and their contribution to epileptiform synchronization; (ii) whether they can influence the interaction between limbic networks leading to ictogenesis; and (iii) how they are altered in the epileptic brain.
2. GABA as a transmitter at central synapses
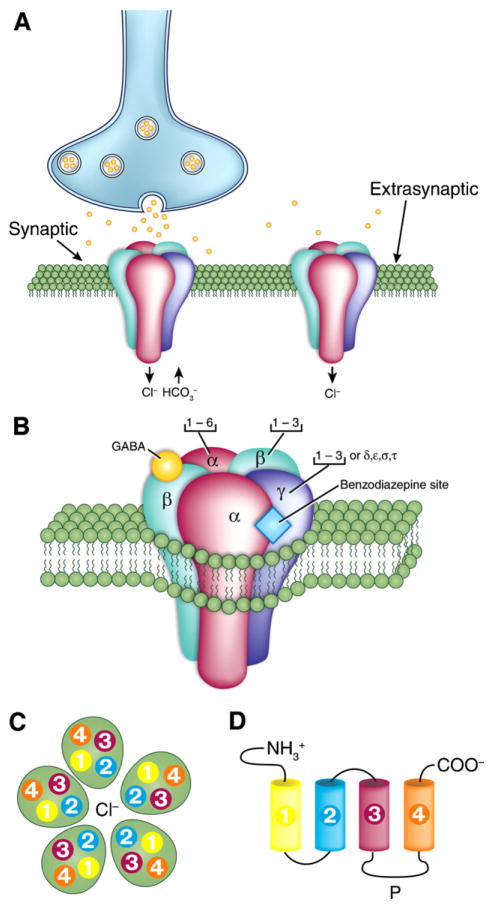
As illustrated in Fig. 1, ionotropic GABAA receptors in mammals are heteropentameric molecules formed from a family of at least 19 related subunits named α (six varieties identified as 1–6), β (1–4), γ (1–3), δ, ε, π and ρ (1–3) plus a few splice variants (Burt and Kamatchi, 1991; Macdonald and Olsen, 1994; Olsen and Macdonald, 2002; Kaila, 1994; Bormann, 2000; Farrant and Kaila, 2007; Jacob et al., 2008; Olsen and Sieghart, 2008). GABAA receptors with different subunit composition have specific functional and pharmacological characteristics and are differentially expressed in various regions of the brain. Moreover, there are both synaptically and extrasynaptically located GABAA receptors (Fig. 1A); these two categories of receptors are believed to mediate phasic and tonic inhibition, respectively.
Fig. 1. Molecular structure of the ionotropic GABAA receptor.
(A) Localization of GABAA receptors in the postsynaptic neuron membrane. Both a synaptic and an extra- or peri-synaptic receptor are shown. (B) Structure of the GABAA receptor and its subunit composition; the heteropentameric, Cl−-permeable channel is made of five subunits that come from seven subunit subfamilies (α, β, γ, δ, ε, θ and π). Note that receptors composed of α (1–3) subunits together with β and γ subunits are presumably synaptically localized, whereas those containing α5, β an γ receptors are located at extrasynaptic sites. Both types of GABAA modulated by benzodiazepine, while extrasynaptically localized receptors composed of α (4 or 6), β and δ are benzodiazepine insensitive. Note also that binding of GABA occurs at the interface between α and β subunits while the benzodiazepine binding occurs at the interface between α (1, 2, 3 or 5) and γ subunits. (C) Top view of the GABAA receptor. Note that each subunit comprises four hydrophobic transmembrane domains (TM1–TM4) with TM2 providing the lining of the Cl− pore. (D) Unfolded view of the transmembrane domains (TM1–TM4). Note that: (i) the extracellular amino terminus is the site of GABA binding, and also contains binding sites for psychoactive drugs, such as benzodiazepines; (ii) the large intracellular loop between TM3 and TM4 (P) is the site for various protein interactions as well as for various post-translational modifications that modulate receptor activity.
Panels in this figure were drawn according to information obtained from Bormann (2000) and Jacob et al. (2008).
Four binding site domains are known to be present in the synaptically located GABAA receptor (Fig. 1B): (i) the GABA site that binds both agonists (such as muscimol or THIP) and antagonists (e.g., bicuculline or gabazine) and it is located at the interface between the α and β subunits (ii) the Cl− channel site that is inhibited non-competitively by the convulsant drug picrotoxin; (iii) the benzodiazepine site that provides for modulation by this category of drugs and is positioned between α and γ subunits; and (iv) a fourth binding site that is postulated to mediate the specific direct interaction with barbiturates and related CNS depressant drugs. GABAA receptors are unique among neurotransmitter receptors in the number of allosteric ligands that modulate their function (Olsen et al., 2004).
GABAA receptor subunits consist of four transmembrane domains termed TM1-4 with TM2 that plays a role in forming the pore of the Cl− channel (Fig. 1C). The large extracellular amino acid terminus is the site for binding GABA, and presumably modulatory drugs such as benzodiazepines while the large intracellular domain between TM3 and TM4 is the site for various protein interactions as well as for various post-translational modifications that modulate receptor activity.
Alhough the membrane topology of GABAC receptors appears very similar to that of GABAA receptors, the formers are composed of ρ subunits, which can assemble into either homo-oligomeric or pseudohomooligomeric (e.g., ρ1–ρ2) receptors. The GABAC receptor also represents a Cl− pore. However, it is selectively activated by cis-4-aminocrotonic acid; moreover it is competitively antagonized by [1,2,5,6-tetrahydropyridine-4-yl(methyl-phosphinic acid)] and noncompetitively by picrotoxinin. Bicuculline, baclofen as well as GABAA receptor modulatory drugs such as benzodiazepine are all inactive at this GABA receptor. Data on the dynamic regulation of GABAA receptor composition, trafficking and movement of these receptors between synaptic and extrasynaptic locations as well as on their regulation by pharmacological agents have been recently reviewed by Jacob et al. (2008) and by Uusi-Oukari and Korpi (2010).
2.1. Interneurons as GABA releasing nerve cells
Histochemical tools have been proved successful in identifying cortical cells that release GABA (thereafter termed interneurons). Besides GAD – which is the synthesizing enzyme of GABA – and GABA itself (Ribak et al., 1978), various other markers (e.g., calretinin, somatostatin, parvalbumin, substance P, neuropeptide Y, each co-expressed in a fraction of 10–20% interneurons) have been used, leading to sub-classifications (Freund and Buzsáki, 1996; Maccaferri and Lacaille, 2003; Klausberger and Somogyi, 2008; Somogyi and Klausberger, 2005). Visual identification of cells has allowed morphological characterization of interneurons into subtypes, which vary according to the localization of their somas as well as to the regional distribution of their dendritic and axonal arbors.
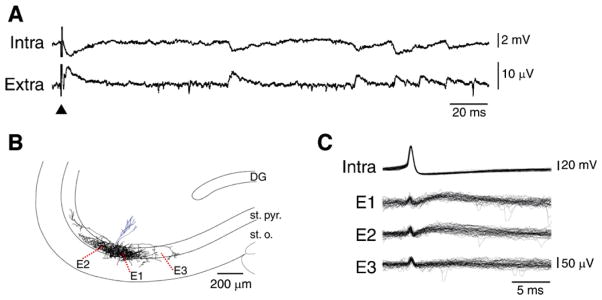
Two main classes of interneurons then appear to emerge (Freund and Buzsáki, 1996; Maccaferri and Lacaille, 2003). The first includes basket or axo-axonic interneurons that form multiple synaptic contacts with the peri-somatic region of principal cells and contribute to the fast GABAA receptor-mediated IPSPs. A recent study by Bazelot et al. (2010) has demonstrated that inhibitory field potentials can be detected over several hundreds of μm from the soma of the initiating interneuron (Fig. 2). It should also be emphasized that perisomatic interneurones establish several thousand terminals at a density of approx. 80 per 100 μm3 (Oláh et al., 2009), a value that is much higher than what reported for principal cells (cf. for CA3 pyramidal cells Wittner and Miles, 2007). Thus inhibitory synaptic events may contribute to the EEG more than those excitatory in nature (cf. also Trevelyan, 2009).
Fig. 2. Unitary inhibitory field potentials recorded from the rat hippocampal CA3 area.
(A) Simultaneous intracellular (intra) and extracellular (extra) recordings obtained from the CA3 area in a brain slice that was superfused with ionotropic glutamatergic receptor antagonists; the extracellular electrode was at ~100 μm from the pyramidal cell. Note that many spontaneously occurring intracellular and extracellular events are correlated as well as that their shapes closely resemble the signals recorded following a weak stimulus (triangle) delivered in the stratum pyramidale at 200 μm from the recorded pyramidal cell. (B) Reconstruction of the dendritic and axonal arbour of a biocytin-filled presumptive interneuron. Axon terminals were largely confined to the CA3 stratum pyramidale (st. pyr.) with some in stratum oriens (st. o.), and were distributed over about 1 mm along CA3 st. pyr. with their density falling with distance. Extracellular recordings shown in C were obtained from sites E1-3. (C) Single action potentials generated by a presumptive interneuron evoke field IPSPs in two of three extracellular recording sites. Thirty responses recorded from electrodes positioned at E1–E3 are shown below. Note that intracellular action potentials elicited extracellular field potentials at two of three recording sites in stratum pyramidale while no extracellular event was detected by a third electrode located at a site which was not innervated by this cell.
Modified from Bazelot et al. (2010) with permission.
The second main class of interneurons comprises a variety of distinct subtypes that make selective contacts with different dendritic domains of principal cells. It has been proposed that these interneurons may be involved in the generation of slow GABAA receptor-mediated potentials (Pearce, 1993; Freund and Buzsáki, 1996; Banks et al., 1998). In line with this view it has been recently reported that in the CA3 subfield inhibitory events generated at dendritic sites have similar amplitudes but occurred less frequently and had slower kinetics than perisomatic events generated in stratum pyramidale (Bazelot et al., 2010). Zarnowska et al. (2009) have proposed that GABAA receptors containing alpha 5 subunits contribute to these slow IPSPs.
GABAergic cells have in most cases distinctive electrophysiological characteristics that include short-lasting Na+ spikes, pronounced afterhyperpolarizations, and thus the ability to generate high frequency, repetitive action potentials (Lacaille and Williams, 1990; Freund and Buzsáki, 1996). Interneuron communication is provided by glutamatergic transmission (Lacaille, 1991) but also by GABAA receptor postsynaptic currents that are frankly excitatory (Michelson and Wong, 1991) along with gap junctions containing specific types of connexin proteins (Gibson et al., 1999, 2004; Traub et al., 2003, 2004; Mancilla et al., 2007).
2.2. GABAA receptor-mediated phasic inhibition
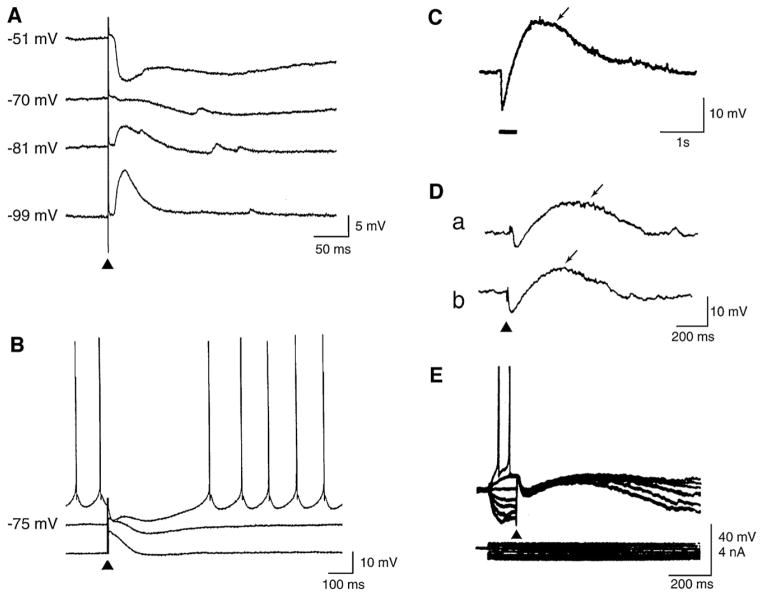
GABAA receptor activation opens channels that are permeable to Cl− and to a lesser extent to HCO3−. With a reversal potential that is negative to or around the resting potential, GABA release induces a post-synaptic potential that is fast and inhibitory (fast IPSP), tending to hyperpolarize post-synaptic cells, thus suppressing action potential firing (Fig. 3A and B). The inhibitory effect is also sustained by a large increase in membrane conductance that is responsible for the IPSP “shunting effect”; as a consequence, inhibition, i.e., a reduction of the likelihood of neurons to discharge action potentials, can be achieved even without prominent hyperpolarization. These are indeed the “classical” IPSPs described in all neurobiology textbooks.
Fig. 3. Cortical post-synaptic GABA receptor-mediated potentials.
(A) Intracellular potentials recorded from a regularly firing neuron in the rodent perirhinal cortex in response to single-shock stimuli delivered in the amygdala in an in vitro slice preparation. Responses were recorded during application of control medium with a K-acetate-filled microelectrode at resting membrane potential (−70 mV), and at depolarizing and hyperpolarizing levels set by intracellular injection of steady current. (B) Intracellular responses generated by a neocortical pyramidal cell in an in vitro slice preparation following single-shock stimuli delivered in medium containing glutamatergic receptor antagonists; these responses were recorded with a K-acetate-filled microelectrode at resting membrane potential (−75 mV) and at depolarized and hyperpolarized potentials. Note in A and B that the hyperpolarizing response comprises an early (GABA type A) and a late (GABA type B) component. (C) Intracellular response recorded from a hippocampal neuron following a train of 40 stimuli delivered at 100 Hz; this recording was performed in medium containing glutamatergic receptor antagonists with a patch microelectrode (from Kaila et al., 1997). (D) Spontaneous (a) and stimulus-induced (b) intracellular potentials recorded from a pyramidal cell in a hippocampal slice that was superfused with medium containing 4AP. Note that both here and in C the intracellular responses consist of an early hyperpolarizing component followed by a long-lasting depolarization. (E) Superimposed intracellular potentials recorded from a cortical neuron in an in vitro slice preparation during 4AP application in response to single-shock stimuli. Responses were recorded with a K-acetate-filled microelectrode at different membrane potentials set by intracellular injection of current pulses as indicated by the current trace (below).
The driving force for the Cl− current that is necessary for hyperpolarizing IPSPs is generated by the K+-Cl− cotransporter KCC2, a neuron-specific cotransporter that extrudes Cl− under physiological conditions in adulthood (Farrant and Kaila, 2007; Blaesse et al., 2009). Accordingly, the change in GABAA receptor-mediated responses from depolarizing to hyperpolarizing seen during early neuronal development is coupled to the induction of the KCC2 expression (Rivera et al., 1999); these data thus support the view that KCC2 is the main Cl− extruder to promote fast hyperpolarizing postsynaptic inhibition in the brain. However, another prerequisite for the generation of GABAA receptor-mediated hyperpolarizing IPSPs is that at resting membrane potential the HCO3− current mediated by GABAA receptor activation (see below, Section 2.4) is not larger than the Cl− current (Kaila, 1994; Rivera et al., 2005).
2.3. GABAA receptor-mediated tonic inhibition
During the last decade, several studies have shown that GABAA receptor activation can also mediate tonic inhibition; these receptors are extrasynaptically and perisynaptically localized and contain a distinct, high affinity subunit composition made of δ and α4, α5 & α6 subunits (Nusser et al., 1998; Yeung et al., 2003; Semyanov et al., 2003, 2004; Cope et al., 2005; Scimemi et al., 2005; Glykys et al., 2008). Hence, GABAA receptors can generate two types of current, depending on their location and subunit composition: the first type is the classic synaptic phasic (or ‘transient’) current that results from the release of GABA from synaptic vesicles in the synaptic cleft; the second type is a tonic (‘always on’) current that is caused by GABAA receptors responding to low levels of ambient GABA (Farrant and Nusser, 2005). Recently, it has been demonstrated that GABA leading to tonic activation of GABAA receptors is released, at least in cerebellum, from glial cells by permeation through bestropin 1 anion channels (Lee et al., 2010). It has also been reported that taurine can activate extrasynaptic GABAA receptors in the mouse ventrobasal thalamus thus reducing the excitability of thalamocortical relay neurons (Jia et al., 2008).
Neurosteroids appear to modulate preferentially tonic rather than phasic inhibition (Mihalek et al., 1999; Lambert et al., 2009). Indeed, neuroactive steroids may play a role in catamenial epilepsy and in temporal lobe epilepsy (TLE) as suggested by their ability to delay the establishment of this chronic condition following pilocarpine-induced status epilepticus in rodents. These findings have been recently reviewed (Biagini et al., 2010) and, therefore, will not be further discussed here.
2.4. GABAA receptor-mediated depolarizing actions
Postsynaptic activation of GABAA receptors can also cause membrane depolarization of both interneurons and principal cells. In the immature brain (Ben-Ari et al., 1989; Cherubini et al., 1991; see for review Ben-Ari et al., 2007) as well as in some adult long-axoned neurons (Gulledge and Stuart, 2003) or interneurons (Michelson and Wong, 1991, 1994) these depolarizing post-synaptic currents may be strong enough to result in excitation. This type of response, which was originally identified in hippocampal pyramidal cells by applying exogenous GABA to the dendrites (Andersen et al., 1980; Alger and Nicoll, 1982), has been the focus of many studies over the last three decades. Several mechanisms may contribute to these GABAA receptor-mediated potentials. First, accumulation of intracellular Cl− ([Cl−]i) – eventually favored by decreased KCC2 activity – can shift the EGABA to values positive to resting membrane potential; indeed, since dendritic receptor activation often produces depolarization, it was proposed that [Cl−]i in cortical cell dendrites could be higher than in the soma, where hyperpolarizations are commonly recorded (Misgeld et al., 1986). Second, [Cl−]i accumulation is caused (at least in immature neurons) by the activation of NKCC1 cotransporter that imports Na+, K+, and Cl− (Achilles et al., 2007; Sipilä et al., 2009). As discussed in Section 9, this mechanism may play an important role in neonatal seizures (Dzhala et al., 2005, 2010; Nardou et al., 2009). Third, GABAA receptor-mediated depolarizations are contributed by HCO3− currents that have quite positive reversal potentials (−10 to −15 mV) (Staley et al., 1995; Lamsa and Kaila, 1997; Kaila et al., 1993, 1997; Rivera et al., 2005). Fourth, as discussed in Section 5.2.2, postsynaptic GABAA receptor activation can produce per se increases in extracellular K+ concentration ([K+]o) (Barolet and Morris, 1991), a phenomenon that is known to depolarize neighboring nerve cells and to cause seizure activity (Zuckermann and Glaser, 1968). Fifth, HCO3− dependent depolarization may lead to the activation of voltage-gated Ca2+ channels (Autere et al., 1999). In line with this view, it has been reported that antiepileptic drugs such as topiramate inhibit the cytosolic carbonic anhydrase (Dodgson et al., 2000) thus decreasing or abolishing GABAA receptor-mediated depolarizing responses (Herrero et al., 2002). In addition, it has been proposed that inhibiting NKCC1 with bumetanide can reduce and even abolish seizures in rodent models of neonatal seizures (Dzhala et al., 2005, 2010; Nardou et al., 2009) and in human neonates (Kahle et al., 2009). NKCC1, which increases intracellular Cl−, is elevated in neonates where GABAA receptor-mediated conductances are mainly excitatory. The action of bumetanide has, however, been questioned in other studies (Vanhatalo et al., 2009; Kilb et al., 2007).
Central to this review is the evidence that massive activation of GABAA receptors, even in cells that generate fast hyperpolarizing IPSPs, can result in a long-lasting GABAergic depolarization. This response, which usually includes a brief hyperpolarizing component followed by a long-lasting depolarization (LLD) can be evoked by high-frequency stimulation of GABAergic interneurons (Smirnov et al., 1999; Kaila et al., 1997) (Fig. 3C) or during application of the K+ channel blocker 4-aminopyridine (4AP) (Avoli and Perreault, 1987; Perreault and Avoli, 1989, 1991, 1992; Staley et al., 1995; Lamsa and Kaila, 1997) (Fig. 3D). It should be emphasized that 4AP enhances the release of both excitatory and inhibitory neurotransmitters (Buckle and Haas, 1982; Perreault and Avoli, 1991) as well as that it does not influence the postsynaptic responses generated by hippocampal pyramidal cells to exogenous GABA (Perreault and Avoli, 1991).
GABAA receptor-mediated depolarizations recorded during 4AP application can, however, retain some inhibitory function, presumably, through shunting of the membrane. Thus, as shown in the experiment illustrated in Fig. 3E, the action potential firing generated by a cortical neuron during intracellular injection of depolarizing current, is abolished throughout the duration of a stimulus-induced GABAergic event that includes an early hyperpolarizing component as well as a subsequent LLD.
3. Role of GABAA receptors in neuronal network oscillations
Since early in the sixties, several investigators have suggested that GABAergic networks contribute to organize neuronal ensemble synchronization during the generation of brain rhythms such as the alpha pattern (around 12 Hz) that is recorded in the EEG (Andersen et al., 1966, 1967). More recently, cortical inhibitory networks have also been proposed to play a critical role in the generation of faster activities that include oscillations in the low (i.e, beta-gamma oscillations at 20–80 Hz) and high frequency range (>80 Hz, so called ripples) (Buzsáki et al., 1992). Interestingly, both beta-gamma rhythms and ripples occurring in cortical areas (Gray et al., 1989; Murthy and Fetz, 1992; Singer and Gray, 1995), including those of the limbic system (Bragin et al., 1995; Chrobak and Buzsáki, 1998; Csicsvari et al., 1999, 2003), have been implicated in higher brain processes such as attention, sensorimotor integration, consciousness, learning and memory (Girardeau et al., 2009; Montgomery and Buzsáki, 2007). Therefore, it has been suggested that these oscillatory rhythms represent the basic neuronal processing state of the brain (Basar et al., 1999).
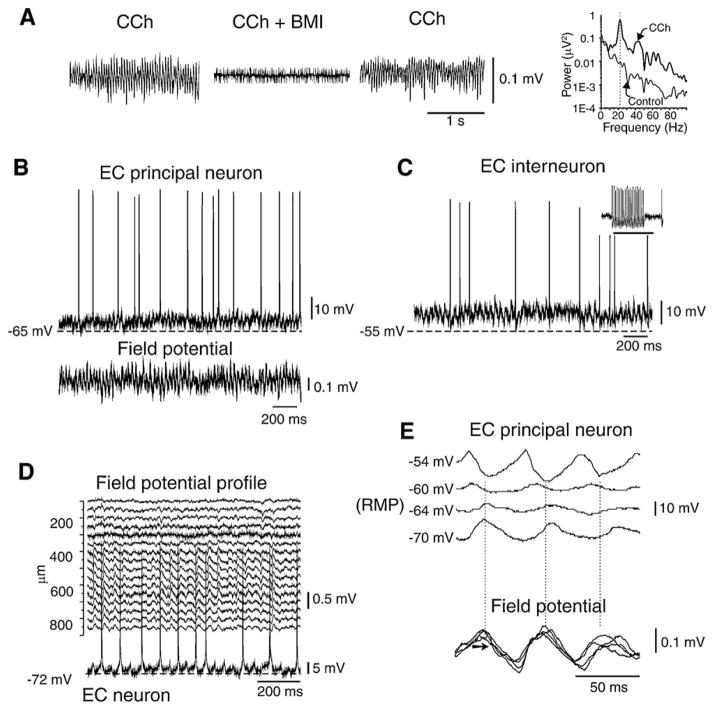
Fast oscillations in limbic and extra-limbic structures can be reproduced in vitro by electrical tetanic stimulation (Whittington et al., 1997; Bracci et al., 1999; Vreugdenhil et al., 2005) as well as by the application of carbachol, high-K+ solutions, kainic acid, or metabotropic agonists (Whittington et al., 1995; Fisahn et al., 1998; van der Linden et al., 1999; Dickson et al., 2000). Several findings support the view that these cortical activities reflect the synchronization of inhibitory GABAergic networks (see Traub et al., 1999; Whittington and Traub, 2003; Mann and Paulsen, 2007), with or without the contribution of excitatory glutamatergic networks (Bartos et al., 2007). For instance, gamma activities induced by the cholinergic agonist carbachol are abolished or greatly diminished by GABAA receptor (Fig. 4A) or AMPA receptor antagonists (Fisahn et al., 1998; Dickson et al., 2000), whereas fast oscillations induced by tetanic stimulation, kainic acid and metabotropic glutamate receptor agonists are resistant to ionotropic glutamatergic antagonists, but sensitive to GABAA receptor blockers (Whittington et al., 1995; Fisahn et al., 1998). Hence, different populations of interconnected interneurons play a key role in gamma synchronization in spite of a feedback excitatory input from principal cells.
Fig. 4. Fast oscillation generated by carbachol (CCh) perfusion in the entorhinal cortex (EC) of the isolated in vitro guinea pig brain preparation.
(A) Extracellular recording of fast activity induced by CCh (100 μM) is blocked by local co-perfusion with bicuculline methiodide (BMI, 50 μM). The peak activity at 22 Hz induced by CCh is shown in the spectrogram on the right (thick line). Frequency content of the signals in control conditions and during CCh is illustrated in the right panel. (B and C) Intracellular recordings performed in a principal neuron (stellate cell in B) and a putative interneuron (in C) during carbachol-induced gamma oscillations. In the inset in panel C, the typical fast firing that characterizes interneurons is shown. The simultaneous field potential recording is shown in B. (D) Extracellular laminar profile of gamma activity recorded with a 16-channel silicon probe and simultaneous recording from a pyramidal EC neuron. (E) Correlation between oscillations of a stellate EC cell (upper traces) and superimposed simultaneous field potential recordings (lower traces). The intracellular signals recorded at different membrane potentials (left of each trace) and synchronized with reference to the simultaneous extracellular recording demonstrate sequences of PSPs with different reversal potentials. A component of the oscillation shows a reversal membrane potential around −60 mV, close to the values that characterize GABAA receptor-mediated reversal potentials.
As shown in Fig. 4, experiments performed in the isolated guinea pig brain preparation have revealed that fast oscillations of the membrane potential can be recorded in both principal cells (Fig. 4B) and interneurons (Fig. 4C) of the entorhinal cortex during bath application of carbachol (Dickson et al., 2000, 2003). These subthreshold oscillations closely correlate to the field potentials recorded with an extracellular microelectrode (Fig. 4D), and are supported by sequences of postsynaptic potentials that are generated by principal neurons (Fig. 4E). Moreover, one component of the oscillation cycle shows a reversal potential close to the GABAA-mediated reversal (around −60 mV). In keeping with this evidence, computer modeling supports the hypothesis that gamma oscillations reflect the interactions within interneuron networks (Wang and Buzsáki, 1996; White et al., 1998; Traub, 2000; Bartos et al., 2001).
Networks of fast-spiking interneurons co-expressing GABA and parvalbumine have been shown to promote synchronization via perisomatic interneuron–interneuron interaction in the hippocampus, in the entorhinal cortex, and in the neocortex (Gulyás et al., 1996; Tamás et al., 1998; Galarreta and Hestrin, 1999; Gibson et al., 1999; Bartos et al., 2002, 2007; Hájos et al., 2004). In addition, non-synaptic interactions through gap junctions may represent an additional mechanism for interneurons synchronization during gamma oscillations (Bartos et al., 2002; Tamás et al., 2000). In vitro studies have also demonstrated that fast oscillations in the gamma range occur spontaneously in isolated cortical networks during up-down states usually associated to both sleep and anesthesia (Sanchez-Vives et al., 2000; Dickson et al., 2003). Also, under these experimental conditions – which more closely represent a physiological synchronization that is independent from pharmacological manipulations – both interneurons and principal cells contribute to fast oscillation generation (Dickson et al., 2003; Gnatkovsky et al., 2007; Compte et al., 2008). As further discussed in Section 6, fast activities at 20–80 Hz can also be observed in association with prolonged periods of epileptiform synchroniations that resemble electrographic seizures, and are induced by experimental procedures that do not block GABAA receptor-mediated signaling (Velazquez and Carlen, 1999; Köhling et al., 2000; Fujiwara-Tsukamoto et al., 2004; Gnatkowsky et al., 2008).
High frequency oscillations at >100 Hz have been recorded in the cortex of humans and animals in conditions of physiological excitability and in focal epilepsy (see for review Engel et al., 2009). Physiological ripples (frequency up to 200 Hz) – which appear to be implicated in the process of memory consolidation – are believed to represent population IPSPs generated by principal neurons entrained by network of synchronously active interneurons (Buzsáki et al., 1992; Ylinen et al., 1995; Chrobak and Buzsáki, 1998). However, ripples may also occur independently on interneuronal networks as proposed by Dzhala and Staley (2004); they have shown that high-frequency oscillations originate from the action potential bursting generated by CA3 pyramidal cells that are synchronized through recurrent excitatory connections.
Ripples have also been reported to occur from epileptic patients during pre-surgical intracranial explorations in cases of drug-resistant TLE (Bragin et al., 1999, 2002; Staba et al., 2002; Urrestarazu et al., 2007; Le Van Quyen et al., 2008). Indeed, in some of these studies high frequency oscillation at 250–600 Hz were also recorded and termed fast ripples. Although, the frequency delimitation of ripples and fast ripples remains a matter of debate, it has been proposed that fast ripples, unlike ripples, are seen exclusively in the epileptic tissue (Bragin et al., 1999; Jirsch et al., 2006). Moreover, experiments performed in both in vitro and in vivo preparations indicate that fast ripples do not depend on inhibitory transmission as they are easily observed during GABAA receptor blockade and, indeed, they appear to represent the synchronous firing of principal (glutamatergic) neurons (D’Antuono et al., 2005; Engel et al., 2009; Bragin et al., 2011). The possibility that fast ripples emerge as the result of loss of synchronization during jittery, out-of-phase burst firing of principal cells in the epileptic hippocampus has been proposed (Foffani et al., 2007; Ibarz et al., 2010).
4. Models of epileptiform synchronization
As discussed in Section 1, blocking GABAA receptor-mediated inhibition induces epileptiform synchronization in cortical regions both in in vivo (Matsumoto and Ajmone-Marsan, 1964; Prince, 1968; Dichter and Spencer, 1969; Ayala et al., 1973) and in vitro animal models (Schwartzkroin and Prince, 1978, 1980; Johnston and Brown, 1981; Gutnick et al., 1982; Wong et al., 1986). Indeed, application of GABAA receptor antagonists (such as bicuculline, picrotoxin or penicillin) to “isolated” hippocampal or neocortical brain slices maintained in vitro has represented the core of basic research in epilepsy for several decades. These studies have firmly established that GABAA receptor function is necessary for limiting neuronal network synchrony as well as for controlling transmission in existing but not functional poly-synaptic pathways (Miles and Wong, 1983, 1987).
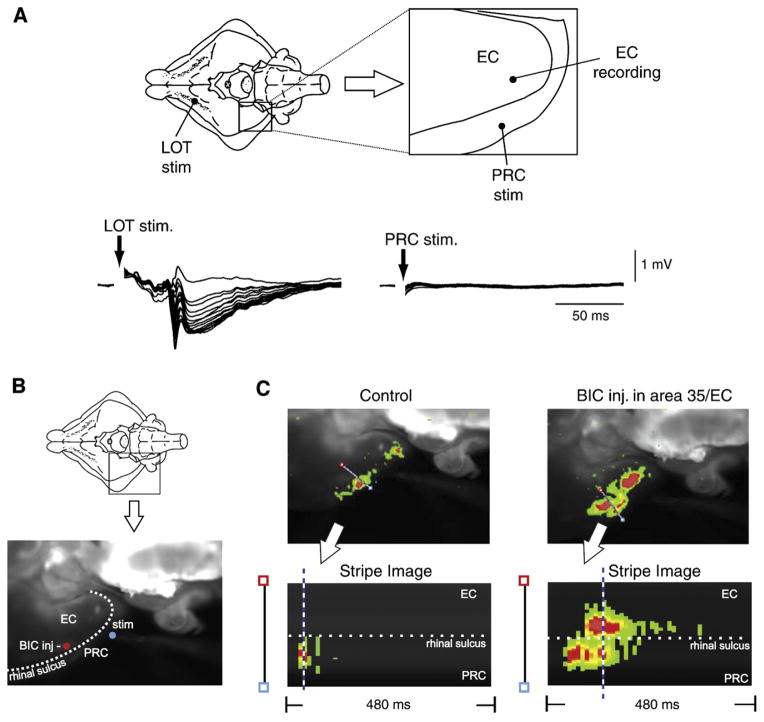
However, the epileptiform activity recorded during full blockade of GABAA receptor-mediated activity (Fig. 5A) usually consists of either short-lasting discharges resembling the interictal spikes or the prolonged afterdischarges observed after robust high-frequency brain stimulation (see for review de Curtis and Avanzini, 2001). Interestingly, local cortical application of drugs that interfere with GABAA receptor-mediated inhibition (such as penicillin) usually induces interictal discharges in in vivo preparations as well; in fact, unless repetitive cortical electrical stimulation was delivered, seizure-like activities have been recorded in only a few cases in these studies (Matsumoto and Ajmone-Marsan, 1964; Prince, 1968; Ayala et al., 1973). In addition, local application of GABAA receptor blockers in the piriform and entorhinal cortices of the isolated guinea pig brain failed to induce seizure-like events, but caused the appearance of interictal spikes with or without afterdischarges (de Curtis et al., 1994; Forti et al., 1997; Librizzi and de Curtis, 2003). Therefore, these findings demonstrate that only interictal spikes and afterdischarges are generated in most of the cases when GABAergic inhibition is blocked by GABAA receptor antagonists and suggest that the generation of seizure-like events requires the participation (and presumably the disinhibition) of larger networks that should include several cortical structures.
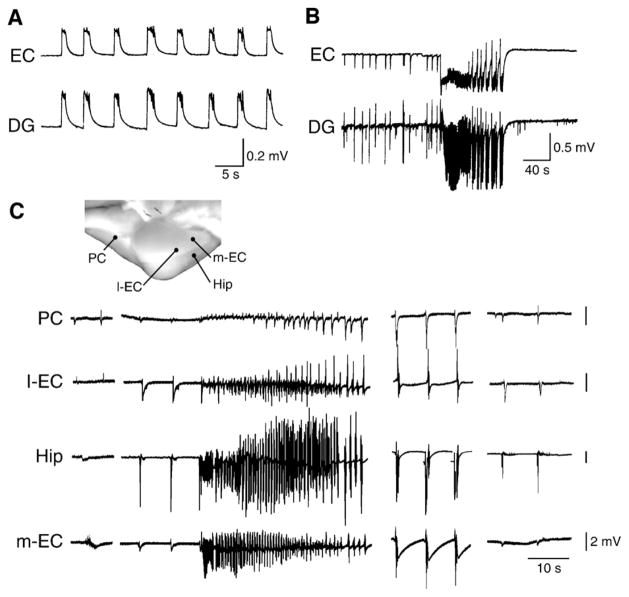
Fig. 5. Interictal- and ictal-like discharges analyzed in two in vitro brain preparations.
A and B panels illustrate the synchronous field discharges recorded in an in vitro brain slice preparation from the entorhinal cortex (EC) and the dentate gyrus (DG) during application of the GABAA receptor antagonist picrotoxin (50 μM) (A) or 4AP (50 μM) (B). Note that only short-lasting interictal discharges occur in the presence of picrotoxin while during 4AP both interictal and ictal events are generated. (C) Epileptiform activity induced in the isolated guinea pig brain preparation by a brief (3 min) arterial perfusion with medium containing the GABAA receptor antagonist bicuculline methiodide (50 μM). Recordings were performed in the piriform cortex (PC), in the lateral and medial entorhinal cortex (l-EC and m-EC) and in the CA1 region of the hippocampus (Hip). Note that seizure-like activity is mainly present in CA1 and medial entorhinal cortex. The late bursting phase of the seizure is shown in the middle and right group of traces.
In line with this view, seizure-like events can be recorded when brain slices that include interconnected areas such as the rodent hippocampal-parahippocampal slice preparation (Walther et al., 1986; Jones and Lambert, 1990a,b; Dreier and Heinemann, 1991), en block preparations such as the immature rat hippocampus (Khalilov et al., 1997; Derchansky et al., 2008) or the adult isolated guinea pig brain (de Curtis et al., 1998; Uva et al., 2005; Gnatkowsky et al., 2008) are employed. Moreover, it appears that prolonged epileptiform discharges are easily induced by experimental procedures that either do not fully block GABAA receptor inhibition or even enhance it (Fig. 5B). These long-lasting epileptiform events – which may represent the equivalent of ictal phenomena and/or of status epilepticus seen in patients and in animal models in vivo – are induced by experimental procedures that include the application of the K+ channel blocker 4AP, the cholinergic agonist pilocarpine (Nagao et al., 1996), trains of high frequency electrical stimuli as well as increased [K+] or removal of Mg2+ in the bathing medium, or (Jefferys, 1990; Avoli, 1990, 1996; Avoli et al., 2002; Fujiwara-Tsukamoto et al., 2004, 2006, 2007; de Curtis and Gnatkovsky, 2009a,b; and Section 6). Seizure-like events that involve different limbic structures following a 3-minute application of bicuculline methiodide (which presumably caused an approx. 40% reduction of GABAA receptor-mediated inhibition) are shown in Fig. 5C (Uva et al., 2005; Gnatkowsky et al., 2008).
5. Contribution of GABAA receptors to interictal spikes
Epileptiform discharges resembling in shape and duration interictal spikes recorded between seizures in the EEG of patients presenting with several types of partial epileptic disorders (Chatrian et al., 1974), are the most common response induced by epileptogenic manipulations. During 4AP application, two types of interictal discharges can be recorded from extended brain slices that include the hippocampus proper and one or more parahippocampal areas, as diagrammatically shown in Fig. 6A. The first type of discharge (identified by multiple arrows in Fig. 6B) is consistently initiated by CA3 networks, lasts 50–300 ms and occurs at frequency of 0.25–1 Hz. The second type (identified by asteriks in Fig. 6B) can be initiated by any limbic areas (including different hippocampal subregions), occurs at a slower pace (the interval of occurrence can vary between 2 and 50 s) and has longer durations (up to 2.5 s). These two types of interictal pattern are typically seen during 4AP application but can also be recorded in the presence of pilocarpine (Nagao et al., 1996) or during application of Mg2+-free medium (Barbarosie and Avoli, 1997). In this section we will focus on the contribution of GABAA receptor-mediated mechanisms to these two types of interictal activity.
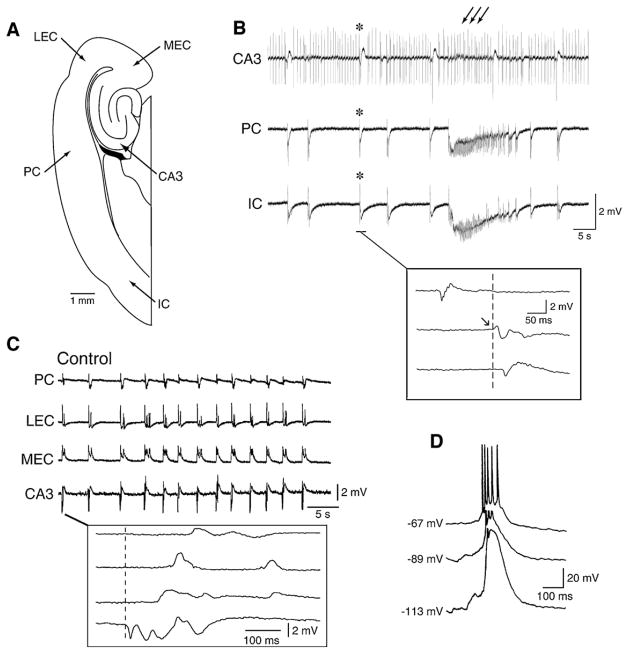
Fig. 6. Characteristics of the fast, CA3-driven interictal discharges.
(A) Diagramatic drawing of an extended brain slice including the hippocampus, the entorhinal and perirhinal cortices as well as the insular cortex. (B) Interictal and ictal discharges are recorded from a brain slice similar to that shown in A during application of 4AP. Note that two type of interictal events can be identified; the first type (multiple arrows) is recorded in this experiment in the CA3 area only, while the second type (asteriks) is seen in all limbic areas. Note also that the second type of interictal discharge occurs at a slower pace than the CA3-driven discharge. (C) CA3-driven interictal activity can occur simultaneously in several limbic areas in the presence of 4AP (note in the expanded sample the different onset latencies). (D) Changes induced by intracellular injections of hyperpolarizing current on the amplitude of the fast interictal events recorded from a CA3 pyramidal cell during 4AP application; note that at resting membrane potential (−67 mV) the interictal discharge is associated with an action potential burst that rides on a depolarization that increases in amplitude when the membrane potential is made more negative; this characteristic suggests that the interictal depolarizations (also termed paroxysmal depolarizing shifts) are largely contributed by synaptic currents
5.1. Fast CA3-driven interictal activity
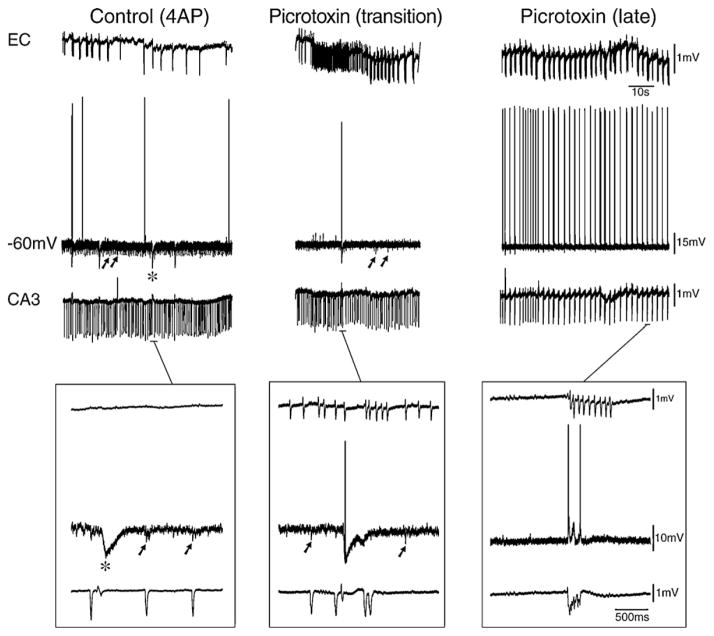
CA3-driven interictal discharges are recorded in isolated hippocampal slices as well as in brain slices that include parhippocampal areas such as the entorhinal and perirhinal cortices, the amygdala or the insular cortex. The evidence for the role played by CA3 networks in initiating these discharges rests on the analysis of propagation performed with simultaneous field potential recordings as well as on the fact that interictal events in CA1 or other distant areas are abolished by cutting the Schaffer collaterals, which represents one of the main outputs of the CA3 area (Barbarosie and Avoli, 1997; de Guzman et al., 2004; Benini et al., 2003). As discussed in Section 7.1, CA3-driven interictal events induced by 4AP application can be restrained to the hippocampus (Fig. 6B) or they can spread to the connected parahippocampal areas to re-enter the hippocampus proper via the perforant path (Fig. 6C). Interestingly, CA3-driven interictal discharges in combined hippocampus-entorhinal cortex slices treated with either pilocarpine (Nagao et al., 1996), 4AP, or Mg2+-free medium (unpublished data from our laboratories) appear earlier than other types of epileptiform activity.
The propensity of the CA3 area to generate such a stereotyped interictal behavior is presumably caused by the presence of recurrent excitatory connections among neighboring CA3 pyramidal cells, along with their ability to generate voltage-gated Ca2+ bursting (Miles and Wong, 1983, 1987; Traub and Wong, 1982; Traub and Jefferys, 1994). When recorded intracellularly from hippocampal pyramidal cells, these 4AP-induced, CA3-driven interictal discharges are associated with action potential bursts riding on a depolarization (Fig. 6D); hence, they resemble the paroxysmal depolarizing shifts described in vivo by several investigators following a variety of experimental procedures, many of which consisted of antagonizing GABAA receptors (Matsumoto and Ajmone-Marsan, 1964; Prince, 1968; Ayala et al., 1973). The presumed excitatory-driven paroxysmal depolarizing shift has been considered for a long time the hallmark of focal epileptiform interictal activity (cf., de Curtis and Avanzini, 2001).
The exact roles played by GABAA receptor-mediated mechanisms in the CA3-driven interictal activity induced by experimental procedures that do not interfere with inhibition remains to be determined. However, the pioneering studies made in Johnston’s laboratory with single-electrode voltage-clamp recordings have demonstrated that the amplitude of the interictal responses recorded during application of medium containing 4AP, tetraethylammonium, or elevated [K+] are characterized by reversal potentials that are more negative than those associated with reversal potentials of paroxysmal depolarization shifts induced by GABAA receptor antagonists (Rutecki et al., 1985, 1987, 1990). Hence, synaptic inhibition is operative in these in vitro models of interictal discharge. Interestingly, enhanced synchronization of GABAergic inputs to CA3 neurons was demonstrated just ahead of seizures in juvenile rat hippocampal slices bathed in low-magnesium medium (Lasztocki et al., 2009). The changes induced by intracellular injections of hyperpolarizing current on the amplitude of the fast interictal events recorded from pyramidal cells during application of 4AP are indicative of the participation of synaptic currents (Fig. 6D).
5.2. Slow interictal discharges
Field potential recordings obtained in vitro from several parahippocampal areas have revealed interictal spikes that are rather heterogeneous in terms of rate of occurrence and shape, even when the same pharmacological treatment (e.g., bath application of 4AP at concentrations ranging between 50 and 100 μM) is used to induce epileptiform synchronization. Interestingly, the original work made with 4AP in the isolated hippocampal slice preparation was the first to show the coexistence of CA3-driven interictal activity and less frequent interictal spikes (Voskuyl and Albus, 1985; Perreault and Avoli, 1991, 1992) (see Fig. 7Ba). It was reported in these studies that the slow interictal discharges did not have a fixed site of initiation in the different hippocampal regions analyzed with simultaneous field potential recordings. This evidence has later been confirmed in hippocampus-entorhinal cortex slices (Avoli et al., 1996a) and in brain slice preparations that included the entorhinal and perirhinal cortices (de Guzman et al., 2004) (Fig. 7A), the amygdala (Benini et al., 2003), the insular cortex (Sudbury and Avoli, 2007), or the cingulate cortex (Panuccio et al., 2009).
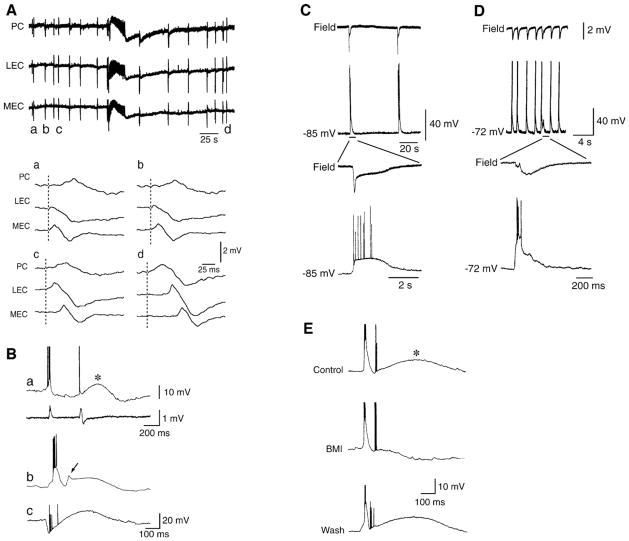
Fig. 7. Slow interictal discharges recorded from the hippocampus and from the entorhinal-perirhinal cortices in the presence of 4AP.
(A) Field potential recordings obtained in vitro from the medial and lateral aspects of the entorhinal cortex (MEC and LEC, respectively) and from the perirhinal cortex (PC) demonstrate the occurrence of interictal and ictal discharges; note in the expanded panels (a–d) that these slow interictal discharges do not have a fixed site of initiation. (B) Intracellular characteristics of the slow interictal discharges recorded from hippocampal pyramidal cells; in a, both field and intracellular signals were recorded. Note in all examples the presence of a long-lasting depolarization that in a follows a single action potential, in b appears to be initiated by an action potential burst, and in c arises from a clear hyperpolarizing event during which ectopic action potentials occur. (C and D) Simultaneous field potential and “sharp” intracellular recordings obtained from the rat entorhinal cortex in the presence of 4AP. Note that both the rate of occurrence and the duration of these interictal discharges are quite diverse in spite of an identical pharmacological procedure and of the similar in vitro brain preparation. (E) Local applications of the GABAA receptor antagonist bicuculline methiodide (BMI) to the CA1 stratum radiatum blocks the long-lasting depolarization and unmasks a long-lasting hyperpolarization. Intracellular recordings in all samples (B–E) were obtained by employing K-acetate-filled “sharp” microelectrodes.
5.2.1. Involvement of GABAA receptor-mediated mechanisms in the slow interictal discharges
Intracellular recordings with K-acetate-filled “sharp” microelectrodes from principal neurons of the hippocampus (Perreault and Avoli, 1991, 1992), entorhinal cortex (Lopantsev and Avoli, 1998a), and amygdala (Benini et al., 2003) have demonstrated that during 4AP application slow interictal events are usually characterized by long-lasting depolarizations that can trigger few action potentials (Fig. 7B and C). However, intracellular “spikes” of variable amplitude, presumably arising from axon terminals (Perreault and Avoli, 1989; Avoli et al., 1998), are often generated during these slow interictal events (Fig. 7Bc and E). Axon terminal hyperexcitability is indeed known to occur in models of epileptogenesis both in vivo (Gutnick and Prince, 1972; Schwartzkroin et al., 1975) and in vitro (Stasheff et al., 1993a,b) and it is presumably caused by by local, transient elevations in extracellular [K+] (Avoli et al., 1998). However, it cannot be excluded that some ectopic action potentials occurring during the long-lasting depolarization are generated in the dendrites (Johnston et al., 1999; Frick and Johnston, 2005; Wong and Prince, 1979; Wong and Stewart, 1992).
Long-lasting depolarizations associated to action potential discharge have been reported to occur during 4AP application in interneurons of the dentate hilus where it has been proposed that depolarizing GABAA receptor-mediated signaling implements excitatory communication among interneurons (Michelson and Wong, 1991, 1994). Moreover, Benardo (1997) has shown that during 4AP application neocortical interneurons generate sustained action potential firing. Finally, long-lasting depolarizations with associated firing have been reported to occur in principal cells of the neocortex (Aram et al., 1991; Avoli et al., 1994) and of the cingulate cortex (Panuccio et al., 2009) during 4AP treatment.
The role played by GABAA receptor-mediated conductances in the generation of these slow interictal discharges has been identified both electophysiologically and pharmacologically. The intracellular depolarization in CA1 pyramidal cells reverses in polarity at values that are close to those of the reversal potential of GABAA-mediated IPSPs (i.e., between −50 and −70 mV, with the initial component displaying more negative values (Perreault and Avoli, 1989) (see also Fig. 3E). In addition, by employing KCl- or KCl/QX314-filled microelectrodes for “sharp” intracellular recordings, it has been reported that the long-lasting depolarizations recorded from entorhinal cortex neurons during 4AP application increase in amplitude over time thus confirming the contribution of GABAA receptor-mediated Cl− conductances (Lopantsev and Avoli, 1998a). Finally, as illustrated in Fig. 7E, by using local applications of GABAA receptor antagonists to stratum radiatum of the CA1 subfield, we found that the long-lasting depolarization is blocked to unmask a prolonged hyperpolarization that is presumably mediated by GABAB receptors (Perreault and Avoli, 1989). These data have suggested that GABAA receptors involved in the generation of the long-lasting depolarization are mainly located in the dendrites of hippocampal pyramidal cells; such a conclusion is in line with the known ability of exogenous GABA to induce depolarizing responses when applied to the apical dendrites of principal neurons (Andersen et al., 1980; Alger and Nicoll, 1982; Grover et al., 1993).
As mentioned above, the slow interictal events generated by parahippocampal networks can vary among experiments both in their interval of occurrence (between 2.5 and 50 s) and in their duration (from a few hundred of ms up to 2.5 s) with these two parameters being directly correlated. For instance, experiments perfomed by the same investigators in rat entorhinal cortex slices during 4AP treatment have shown that either slow, long-lasting (Fig. 7C) or fast, relatively short-lasting (Fig. 7D) interictal events can be recorded (Lopantsev and Avoli, 1998a,b). To date, it is unclear why a similar pharmacological procedure (i.e., 4AP application) performed in the same type of tissue can result in such great variability; however, as shown in Fig. 7C and D, the presence of a GABAergic LLD appears to be a consistent feature of interictal events that occur at a slower pace and with longer durations. Since these data have been obtained from brain slices maintained in vitro, a possible explanation for the large variability in duration and frequency of occurrence of interictal discharge (and the fact that there appears to exist a different expression of GABAA receptor-mediated conductances) may rest on the variable preservation of interneuron networks following slicing as well as on the different expression of interneurons in ventral versus dorsal parts of the limbic system. These hypotheses, however, remain to be tested.
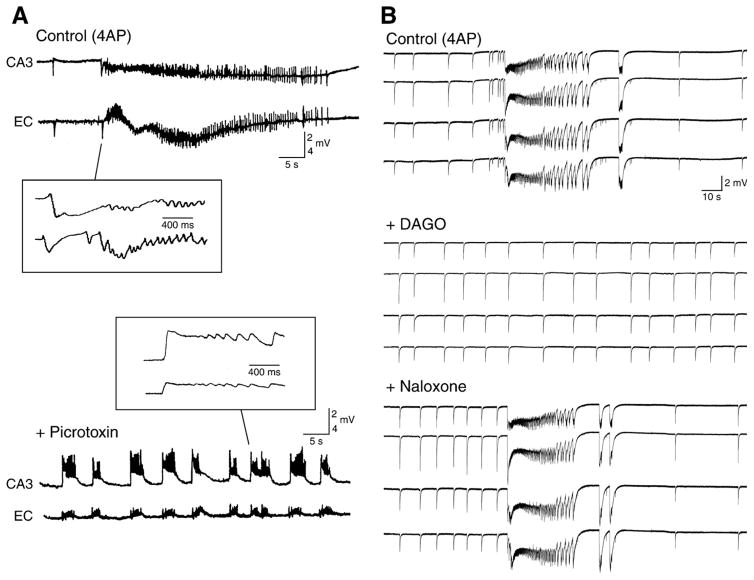
Further evidence for the participation of inhibitory mechanisms to the slow interictal spikes recorded during 4AP application emerges from pharmacological manipulations aimed at blocking ionotropic glutamate receptors. Slow interictal events induced by 4AP are minimally affected by the application of NMDA receptor antagonists, and surprisingly, continue to occur in the presence of NMDA and non-NMDA glutamatergic receptor antagonists. This type of glutamatergic-independent synchronous activity can be recorded from practically any limbic structures maintained in the brain slice (Avoli et al., 1996a, 2002; Benini et al., 2003; Sudbury and Avoli, 2007; Panuccio et al., 2009) as well as in the isolated guinea pig brain (Uva et al., 2009). It has also been reported that the rate of occurrence of these GABAergic spikes are not influenced by modulating metabotropic glutamate receptor activity (Salah and Perkins, 2008).
As illustrated in Fig. 8A, slow interictal discharges recorded during concomitant application of 4AP and ionotropic glutamatergic receptor antagonists continue to be recorded from different areas of a brain slice that comprised the perirhinal and insular cortices, while fast, CA3-driven interictal activity is abolished. It should also be emphasized that these glutamatergic-independent events occurred synchronously in both perirhinal and insular cortices without a preferential site of initiation (Fig. 8B).
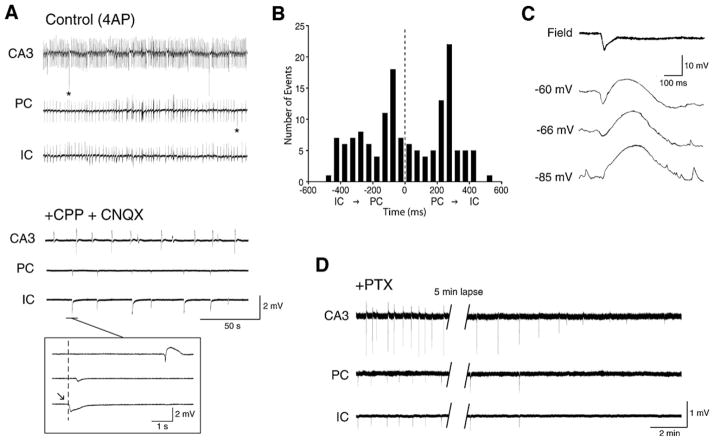
Fig. 8. 4AP-induced, slow interictal discharges continue to occur during blockade of ionotropic glutamatergic receptors.
(A) Effects induced by concomitant application of NMDA (CPP) and non-NMDA (CNQX) glutamatergic receptor antagonists on the 4AP-induced epileptiform discharges recorded from a combined brain slice that included the entorhinal (EC) and perirhinal (PC) cortices as well as the insular cortex (IC) (see Fig. 4A for detail on this preparation); note that during CPP + CNQX application spontaneous field events continue to occur as well as that this glutamatergic-independent synchronous activity is seen in different areas while fast, CA3-driven interictal activity is readily abolished. (B) Histogram of the latencies of the glutamatergic-independent events recorded from perirhinal and insular cortices; note that these field potentials occur without a preferential site of initiation. (C) Intracellular recording obtained with a K-acetate filled microelectrode from a perirhinlal cortex principal cell during application of medium containing 4AP + CPP + CNQX demonstrates a sequence of hyperpolarizing-depolarizing potentials that change in amplitude during injection of steady hyperpolarizing and depolarizing current (RMP = −66 mV); note that the early hyperpolarizing component inverts in polarity between −66 and −85 mV suggesting the contribution of Cl− conductances. (D) Glutamatergic independent interictal spikes – which were recorded from a combined brain slice that comprised the entorhinal and perirhinal cortices as well as the insular cortex (see Fig. 4A) during application of medium containing 4AP + CPP + CNQX – are abolished by the application of the GABAA receptor antagonist picrotoxin.
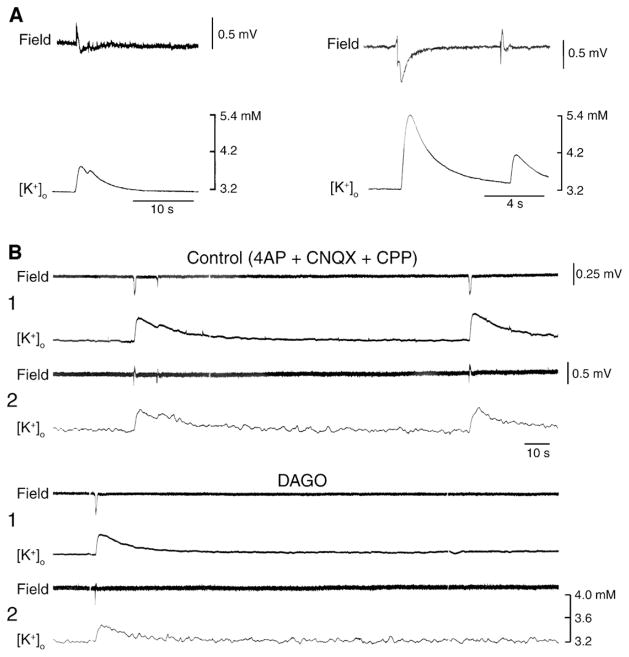
The contribution of GABAA receptors to the 4AP-induced slow, interictal events recorded during blockade of glutamatergic receptors is further supported by intracellular data obtained from principal cells in the neocortex (Aram et al., 1991; Avoli et al., 1994), hippocampus (Perreault and Avoli, 1992) and in other limbic areas such as the amygdala or the entorhinal cortex. In all cases, the counterpart of the slow discharges that continue to occur in the presence of glutamatergic receptor antagonists is a sequence of hyperpolarizing-depolarizing potentials that change in amplitude as expected for a post-synaptic response due to Cl− conductances (Fig. 8C). In addition, these glutamatergic independent interictal events are abolished by GABAA receptor antagonists (Fig. 8D) as well as by the μ-opioid receptor agonist [D-ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAGO) (Avoli et al., 1996a,b; Sudbury and Avoli, 2007; Panuccio et al., 2009) (Fig. 10B). It is well-established that the latter pharmacological procedure blocks the presynaptic release of GABA from interneurons (Madison and Nicoll, 1988; Capogna et al., 1993).
Fig. 10. Field potential and [K+]o features of the interictal discharges induced by 4AP in the rat entorhinal cortex.
(A) Two examples of slow interictal spikes obtained during application of 4AP containing medium, and recorded with simultaneous field (upper trace) and K+ selective microelectrodes. Note that these interictal events are associated with transient increases in [K+]o from a baseline of 3.2 mM up to approx. 5.3 mM, and that, in the left sample, the [K+]o remains slightly elevated during the period of field oscillations that follow the initial negative field transient. Note also in the right sample the close relation between field amplitude and transient increases in [K+]o. (B) Field events and similar concomitant elevations in [K+]o (see Table I) can be recorded during application of medium containing 4AP and glutamatergic receptor antagonists (Control). This pattern of spontaneous activity is depressed by application of the μ-opioid receptor agonist DAGO. In this experiment field potential and [K+]o were recorded simultaneously by two electrodes (indicated as 1 and 2) that were positioned in the deep layers of the entorhinal cortex, approx. 1 mm apart.
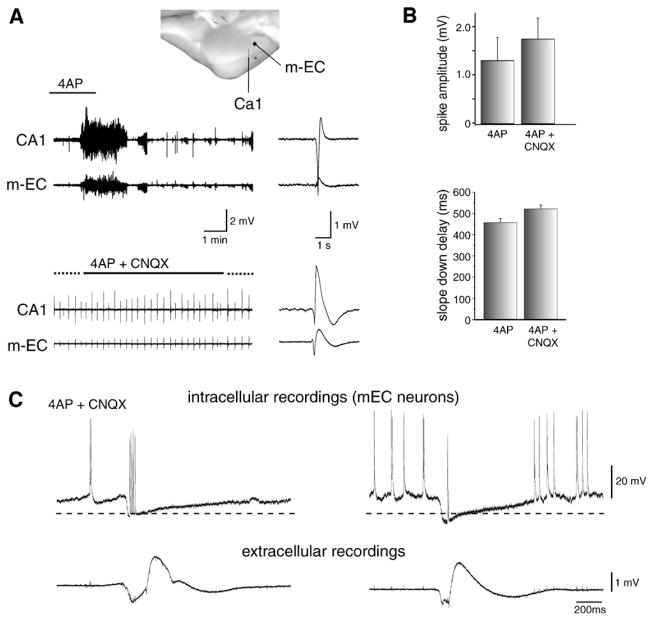
As already mentioned, hypersynchronous GABAergic potentials are also recorded from several areas of the isolated guinea pig brain preparation during 4AP application (Uva et al., 2009; Carriero et al., 2010). As illustrated in Fig. 9A, these GABAergic spikes are present during application of 4AP and continue to occur in isolation during blockade of ionotropic glutamatergic transmission; they can, however, be reduced and eventually abolished by additional application of GABAA receptor antagonist, bicuculline methiodide (Uva et al., 2009). The time course and distribution of these slow GABAergic interictal events are similar to the spikes observed before glutamatergic receptor blockade (Fig. 9B) (Carriero et al., 2010). Moreover, they can be generated in different subfields of the hippocampus/entorhinal cortex and propagate with a velocity of tens of ms within homologous regions of the two hemispheres.
Fig. 9. Seizure-like activity and glutamatergic independent interictal-like events recorded in the in vitro, isolated guinea pig brain during arterial perfusion with 4AP.
(A) Schematic representation of of the recording electrodes that were positioned in the medial entorhinal cortex (mEC) and in the hippocampal CA1 field is shown in the top panel. Seizure-like discharges along with interictal spikes induced by 4AP are illustrated in the upper panel; note that one interictal-like spike is shown at fast speed in the right panel. In the lower part only interictal spikes but not seizure-like events are recorded during concomitant application of 4AP and the non-NMDA glutamatergic receptor antagonist CNQX; also in this case interictal-like spike is illustrated in the right panel at high speed. Note the similar shape of the interictal spike under these two conditions. (B) Amplitude of spikes (upper graph) and duration of the slow component that follows the spike (lower panel) recorded during arterial perfusion of 4AP and during co-perfusion of 4AP + CNQX. (C) Simultaneous intracellular (upper traces) and extracellular (lower traces) recordings obtained from two different experiments from the medial entorhinal cortex during arterial perfusion of 4AP + CNQX. Note in both cases that these glutamatergic independent events are characterized by a pronounced hyperpolarization as well as that action potentials are evident during the hyperpolarizing component of the discharge thus suggesting that they were ectopically generated. Resting membrane potentials (dotted lines) were −65 mV and −60 mV for the neurons on the left and right, respectively, in C.
Intracellular recordings obtained from principal neurons of the entorhinal cortex in the guinea pig isolated brain have demonstrated that, as in the experiments performed in brain slices (see above), these GABAergic spikes correlate with inhibitory (but in this preparation mainly hyperpolarizing) postsynaptic potentials with the reversal of GABAergic IPSPs that carry at the troth small amplitude, presumably ectopic, action potentials (Fig. 9C) (Uva et al., 2009). Although most of the data reported to date on the mechanisms contributing to the slow, GABAergic, interictal spikes have been obtained by employing the 4AP procedure, yet similar characteristics can be identified in the entorhinal cortex with the interictal activity induced by the application of either Mg2+ free-medium (M. D’Antuono and M. Avoli, unpublished data), or bicuculline (Gnatkowsky et al., 2008) or by cholinergic receptor activation following carbachol treatment (Dickson and Alonso, 1997). Glutamatergic independent IPSPs have also been recorded from neurons of the guinea pig dentate hilus by Michelson and Wong (1991) during application of medium containing high [K+].
The role played by GABAA receptor-mediated transmission in epileptiform synchronization is further supported by the experiments performed in rat hippocampal slices by Uusisaari et al. (2002). It was shown in this study that a progressive synchronization of interneuronal activity develops following rather prolonged pharmacological blockade of both GABAB and ionotropic glutamatergic receptors, and that this process can lead in some cases to the occurrence of GABAA receptor-mediated epileptiform activity in the CA1 subfield. Moreover, these epileptiform discharges were shown to be dependent on intracellular carbonic anhydrase and potentiated by maneuvers that increased intracellular pH. Finally, they were blocked by a Cx36-specific gap-junction blocker suggesting that epileptiform activity in the absence of ionotropic glutamatergic transmission results from enhanced GABAergic activity that depends on interneuronal connectivity provided by gap junctions. It should be noted that the contribution of gap junction to synchronize neuronal network during epileptiform activity has also been identified in the in vitro 4AP model, although the well-known ability of gap junction decouplers to influence other mechanisms of neuronal excitability hampers a definitive characterization of their role (Gigout et al., 2006).
5.2.2. [K+]o elevations and slow interictal events
As shown in Fig. 10, simultaneous field potential and K+- selective recordings from both hippocampus and entorhinal cortex in the in vitro slice preparation, have revealed that the slow interictal events induced by 4AP are associated with transient increases in [K+]o from a resting value of 3.25 mM (Morris et al., 1996; Avoli et al., 1996a,b,c; Barbarosie et al., 2000, 2002). Table 1 summarizes the peak values and the durations of the transient elevations in [K+]o that were recorded in these studies during the slow interictal events induced by 4AP.
Table 1.
Peak values and durations of the [K+]o increases recorded in vitro from adult rodent limbic areas during 4AP-induced slow interictal discharges.
| Control conditions (4AP only)
|
+ Glutamatergic receptor blockers
|
|||
|---|---|---|---|---|
| [K+]o peak | Duration | [K+]o peak | Duration | |
| CA1a | 4.7 ± 0.3 mM | 32 ± 5.5 s | Values similar to control conditions | |
| CA3b | – | – | 4.3 ± 0.1 mM | 29 ± 3 s |
| ECc | 4.2 ± 0.1 mM | Approx. 45 s | ||
| ECd | 4.3 ± 0.3 mM | |||
| DGd | 4.6 ± 0.7 mM | – | ||
Reference source:
Abbreviations: CA1 and CA3, Cornus Ammonis subfield 1 and 3; EC, entorhinal cortex; DG, dentate gyrus.
Increases in [K+]o are not unexpected during hypersynchronous activity such as that associated with an interictal discharge that is contributed by glutamatergic conductances leading to sustained action potential firing, and they have been well characterized both in in vivo (Heinemann et al., 1977; Krnjevic et al., 1980) and in vitro preparations (Lux et al., 1986; Avoli et al., 1990). What is surprising in the 4AP model is that similar [K+]o elevations can be observed concomitant to the glutamatergic-independent interictal discharges generated by neuronal networks during application of ionotropic glutamatergic receptor antagonists (Fig. 10B). This evidence suggests, therefore, that the postsynaptic activation of GABAA receptors is sufficient to cause elevations in [K+]o. This conclusion is supported by two sets of experimental findings. First, as shown in Fig. 10B, both the slow interictal events and the associated increases in [K+]o recorded during blockade of excitatory glutamatergic receptors are abolished by pharmacological activation of μ-opioid receptors; as already mentioned, this procedure blocks the release of GABA from interneurons. Second, increases in [K+]o can be induced by application of exogenous GABA or GABAA (but not GABAB) receptor agonists in the presence of tetrodotoxin, thus ruling out a meaningful contribution of action potential firing (Barolet and Morris, 1991); it should be emphasized that in this pioneering study, Barolet and Morris (1991) demonstrated that the [K+]o elevations induced by GABA application were abolished by the GABAA receptor antagonist bicuculline.
As summarized in Table 1, the transient elevations in [K+]o associated with the slow interictal spikes induced by 4AP, attain similar values both under control conditions and following blockade of ionotropic glutamatergic receptors thus suggesting that GABA per se is the main factor for these [K+]o increases. Moreover, similar peak values are attained by [K+]o following trains of electrical stimuli delivered in hippocampal slices during application of medium containing ionotropic glutamatergic antagonists but no 4AP (Smirnov et al., 1999; Kaila et al., 1997). This experimental procedure induces an intracellular response that is almost identical to what seen during the slow interictal spikes induced by 4AP (Fig. 3C).
Several mechanisms play a role in the GABA receptor-dependent elevations in [K+]o. They presumably reflect the depolarization of neurons and even more so, of glial cells caused by the activation of GABAA receptors (Krnjevic and Schwartz, 1967; Gilbert et al., 1984; MacVicar et al., 1989; Fraser et al., 1995) in response to the synchronous release of GABA from interneurons. This depolarization should lead to outward counter/cotransport of K+ with Cl−/HCO3+ anion shift (cf. Kaila, 1994; Kaila et al., 1997). Recently, Viitanen et al. (2010) have proposed that the elevation in [K+]o induced by high-frequency stimulation or GABAA receptor agonist application are generated by the KCC2 and that the transporter-mediated KCl extrusion is critically dependent on the bicarbonate-driven accumulation of [Cl−]i. The [K+]o increases could also be contributed by the firing of action potentials generated by interneurons discharging during the synchronous GABA receptor-mediated potential (Benardo, 1997), although the data obtained by Barolet and Morris (1991) during blockade of voltage-gated Na+ channels suggest that this is not a significant mechanism.
Regardless the precise contribution of these neuronal and/or glial mechanisms to the GABAA receptor-mediated elevations in [K+]o, the established ability of extracellular K+ in modulating neuronal excitability suggests that this transient increases may contribute to the spread of these potentials once glutamateric transmission is blocked (Avoli et al., 2002; Uva et al., 2009). A similar process has also been documented by employing K+-selective electrodes in the human neocortex in an in vitro brain slice preparation during bath application of 4AP (Louvel et al., 2001).
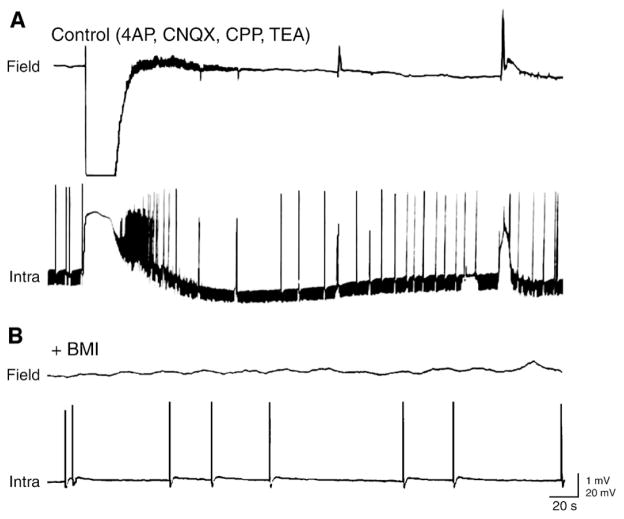
GABAA receptor-mediated [K+]o elevations associated with the glutamatergic-independent spikes can be so intense to trigger spreading depression-like episodes. This phenomenon has been reported in juvenile hippocampal slices during 4AP application (Psarropoulou and Avoli, 1993) and in adult tissue following concomitant application of 4AP and tetraethylammonium which is another K+ channel blocker (Avoli et al., 1996c; Fig. 11A). The role of GABAA receptor-mediated mechanisms in triggering and sustaining these in vitro episodes of spreading depression is supported by the ability of GABAA receptor antagonists to abolish them (Fig. 11B). As discussed in Section 6.1, GABAA receptor-mediated elevations in [K+]o occurring during 4AP application can play a meaningful role in ictal discharge initiation.
Fig. 11. Glutamatergic-independent interictal spikes and spreading depression like-episodes are contributed by GABAA receptor-mediated conductances.
(A) Under control condition (i.e., in the presence of 4AP, CNQX, CPP and TEA) a spreading depression-like event (a), a prolonged field-potential discharge (b) and a negative-going field potential (c) are recorded with simultaneous extracellular (Field) and “sharp” intracellular (Intra) microelectrodes from the CA3 stratum radiatum and a CA3 pyramidal cell, respectively. Note that a large decrease in membrane input resistance, monitored by continuous injection of brief (100 ms; −0.3 nA) hyperpolarizing current pulses, characterizes all three types of synchronous activity (RMP of the CA3 neuron = −75 mV). (B) Effects induced by the GABAA receptor antagonist bicuculline methiodide (BMI) on the pattern spontaneous activity shown in A; note that this pharmacological procedure abolishes all types of synchronous events.
6. Role of GABAA receptors in ictal discharge generation
Pharmacological procedures that cause in vitro epileptiform synchronization by fully antagonizing GABAA receptors elicit robust interictal activity but fail to induce sustained ictal discharges (see Section 4). On the contrary, reduction (but not abolition) or reinforcement of GABAergic inhibition can generate seizure-like activity. Incidentally, a partial decrease of fast GABAA-mediated inhibition has been proposed to be responsible of seizure generation in computer models of temporal lobe seizures (Wendling et al., 2002; Labyt et al., 2006). Hence, these findings – which have been obtained in different in vitro preparations such as brain slices, en block hippocampus or isolated guinea pig brain – suggest that ictogenesis relies on GABAA receptor function, and challenge the notion that focal seizure activity can solely be promoted by a build-up of excitatory neurotransmission and concomitant reduction in the efficacy of synaptic inhibition. In this section we will review the role of GABAA receptor-mediated mechanisms in the initiation and maintainance of prolonged discharges resembling the electrographic seizures recorded in epileptic patients as well as in in vivo and in vitro animal models of epilepsy.
6.1. Initiation
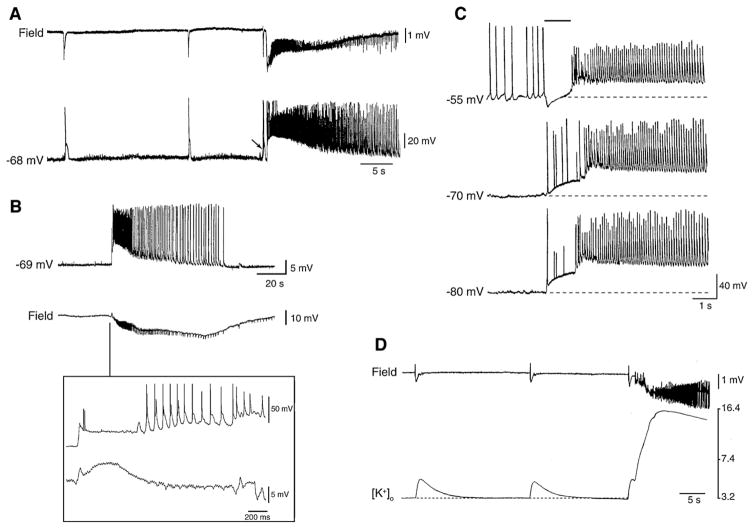
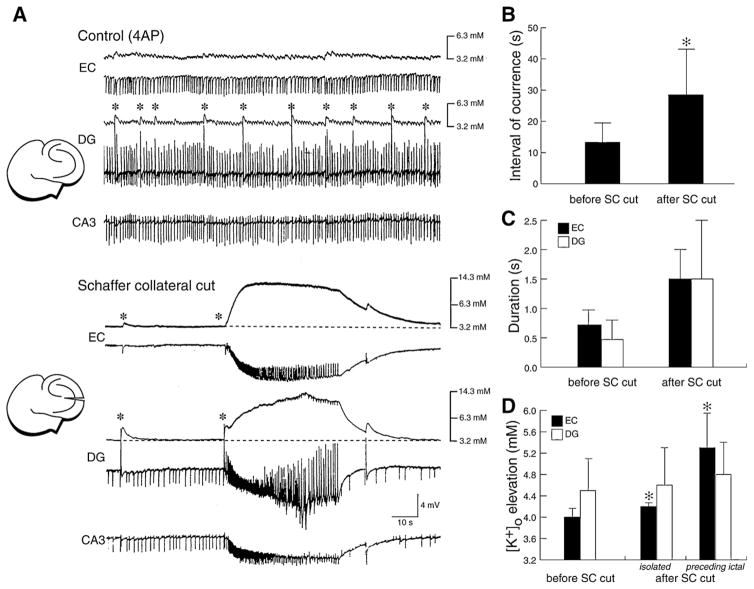
Several electrophysiological data support the view that a transient enhancement of intereuron network activity can be responsible for seizure onset. The first demonstration that a strong inhibitory event can set off an ictal-like discharge was reported by us (Avoli, 1990; Avoli et al., 1993) in the CA3 area of juvenile rat hippocampal slices during 4AP treatment. Later, these findings have been confirmed in the adult rat entorhinal cortex and in the amygdala by employing intracellular sharp electrode recordings (Lopantsev and Avoli, 1998a; Benini et al., 2003) as well as analysis of [K+]o (Avoli et al., 1996a; Barbarosie et al., 2000, 2002). These experiments have demonstrated that the onset of an ictal discharge is characterized by the occurrence of a long-lasting depolarization (resembling those seen in association with the slow interictal spikes) (Fig. 12A and B). Moreover, as illustrated in Fig. 12C, this initial component becomes hyperpolarizing during steady injection of depolarizing current, indicating that under these experimental conditions, ictal discharges appear, paradoxically, to originate from a synchronous event that is presumably contributed by the post-synaptic activation of GABAA receptor. In addition, this initial slow potential is accompanied by elevations in [K+]o that are often larger that those recorded in association with the slow spikes occurring during the interictal period (Fig. 12D). Interestingly, [K+]o measurements performed at different depth of the entorhinal cortex during application of 4AP and glutamatergic receptor antagonists have revealed that the largest increases in [K+]o occur in the deep layers, which are the site where ictal activity is initiated (Avoli et al., 1996a).
Fig. 12. Relationship between slow interictal spikes and ictal discharges recorded from different limbic structures during 4AP application.
(A) Simultaneous extracellular (Field) and “sharp” intracellular (−68 mV) recordings obtained from the rat perirhinal cortex during 4AP application in an in vitro brain slice preparation. Both slow interictal spikes and an ictal discharges are illustrated. Note that the ictal discharge appears to be initiated by an interictal spike (arrow). (B) Ictal discharge recorded with simultaneous “sharp” intracellular (−69 mV) and extracellular (Field) recordings in the lateral amygdala in an in vitro slice preparation during 4AP application. Note in the expanded panel that a long-lasting depolarization, which is associated with only two small amplitude “spikes”, characterizes the ictal discharge onset as well as that the corresponding field recording shows a spike-slow wave pattern. (C) Electrophysiological features of the long-lasting depolarization occurring at ictal discharge onset in an entorhinal cortex neuron recorded with K-acetate-filled microelectrode during 4AP application. RMP of this neuron = −70 mV. Note that when the membrane is hyperpolarized by intracellular injection of steady negative current (−80 mV trace) both long-lasting depolarization and ictal depolarization increase in amplitude as compared with the RMP sample; on the contrary, when the neuronal membrane is depolarized by intracellular injection of steady positive current (−55 mV) the amplitude of the sustained ictal depolarization decreases while the initial long-lasting depolarization becomes hyperpolarizing as compared with the recording obtained at RMP; the time occupied by this initial long-lasting depolarization is indicated by the continuous line on top of the −55 mV trace. (D) Simultaneous field and [K+]o recordings obtained from the rat entorhinal cortex at seizure onset during 4AP application. Note that the slow interictal spikes are associated with transient increases in [K+]o, while a sustained elevation is seen during the tonic phase of the ictal discharge; note that the ictal discharge is initiated by an increase in [K+]o that is larger than what observed during the isolated slow interictal discharges.
As already mentioned, a similar pattern of ictal discharge onset is seen during 4AP treatment in isolated rat hippocampal slices that were obtained from young (11–23 day-old) animals (Avoli, 1990; Avoli et al., 1993, 1996b). It should be noted that in these studies we found that 4AP discloses ictal discharges in the juvenile but not in the adult hippocampal tissue. This “propensity” for ictogenesis may be due to the better ability of the latter to control changes in [K+]o. In line with this view, the elevations in [K+]o associated with the slow interictal events recorded during blockade of glutamatergic transmission in the juvenile hippocampal slices are larger than those seen in the adult hippocampus under similar experimental conditions (Avoli et al., 1996b). It should also be emphasized that both experimental (Swann and Brady, 1984; Ben-Ari et al., 1989; Cherubini et al., 1991; Gloveli et al., 1995; Luhmann et al., 2000) and clinical (Aicardi, 1986) studies have provided evidence for dramatic developmental changes in neurotransmitter signaling and, therefore, brain excitability.
Overall these data indicate that GABAA receptor-mediated synchronization during 4AP treatment serves as a powerful implement for the initiation of ictal discharges. This mechanism presumably relies on the GABAA receptor-dependent increase in [K+]o that can cause positive shifts of both GABAA and GABAB receptor-mediated postsynaptic inhibition, can depolarize neighbour neurons and can disinhibit excitatory postsynaptic interactions. The role of GABAA receptor-mediated inhibition in the onset of electrographic seizure-like discharges has also been obtained by superfusing brain slices with Mg2+-free solutions (Köhling et al., 2000). It was shown in this study that the initiation of ictal discharges generated by CA1 neurons is facilitated by GABAA receptor-mediated oscillations in the gamma range.
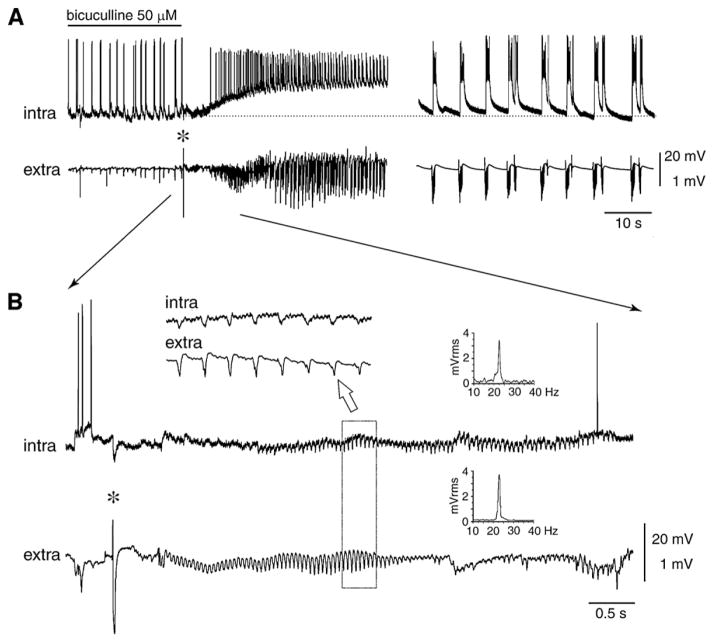
Slow interictal spikes induced by arterial perfusion of 4AP in the guinea pig isolated brain preparation have been reported to occur just ahead of seizures in the hippocampus and the entorhinal cortex (Uva et al., 2009; Carriero et al., 2010). Interestingly, the time course and distribution of these slow interictal spikes were similar to those of the isolated spikes observed during glutamate receptor blockade. Since these pharmacologically isolated spikes are blocked by GABAA receptor antagonism (Uva et al., 2009), it is enticing to suggest that as in the in vitro slice preparation treated with 4AP, recruitment of inhibitory mechanisms is crucial for the initiation of seizure activity in the guinea pig isolated brain preparation. This conclusion is also supported by the findings reported by Gnatkowsky et al. (2008) who have found that a transient period of partial ‘disinhibition’ induced by brief arterial application of bicuculline in this type of in vitro preparation, leads to a peculiar seizure pattern in the entorhinal-hippocampal region.
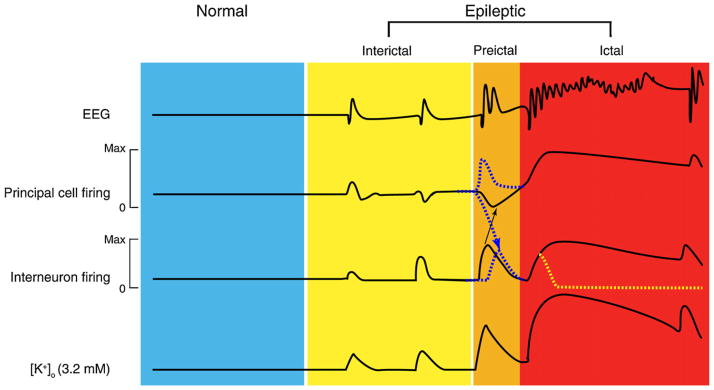
As shown in Fig. 13, fast activity in the beta-gamma range (preceeded by large amplitude interictal spikes) can be observed shortly before the onset of seizures in these experiments. Moreover, intracellular recordings obtained from principal neurons of the deep and superficial layers of the entorhinal cortex have demonstrated that both pre-ictal spikes and fast activity at seizure onset correlate with hyperpolarizing events (Fig. 13B) that have a reversal potential that is typical of GABAA receptor-mediated IPSPs (Gnatkowsky et al., 2008). Thus, also in this model of epileptiform synchronization the onset of a seizure activity is associated with a period of intense activation of inhibitory interneurons along with inhibiton (or silencing) of principal neurons. This phase of robust inhibitory network activation lasts several seconds and gradually shifts into a phase during which the action potential firing generated by principal cells resumes thus progressing into a pattern of recurrent population discharges as those seen during an electrographic seizure (Trombin et al., 2011; Fig. 13A).
Fig. 13. Seizure-like activity in the isolated guinea pig brain induced by arterial perfusion of bicuculline methiodide.
“Sharp” intracellular (intra) and extracellular (extra) recordings, which were obtained from the medial entorhinal cortex, are illustrated at different time scale in A and B. Note in A that the ictal discharge (i) rises from a very slow depolarization that leads to sustained action potential firing during the “tonic phase” and then (ii) progresses to the “clonic phase” that is characterized by rhythmic bursting. Note in B that the very slow depolarization is associated to fast oscillatory activity at 20–30 Hz in both intracellular and field recordings; moreover, the intracellular recording reveals that these fast oscillations consist of IPSPs that are in phase with the extracellular oscillation. The peak frequencies of the oscillations recorded extra- and intracellularly are shown in the FFT plots in the inserts. The asterisks point to pre-ictal spikes that correlate at intracellular level with a hyperpolarizing potential (presumably an IPSP). Resting membrane potentials (dotted lines) were −63 mV.
The active role played by GABAA receptor-mediated mechanisms in the initiation of ictal discharges has, however, been challenged by Derchansky et al. (2008), who employed “low-Mg2+” medium to induce ictal events in the isolated immature mouse hippocampus. By recording from CA1 pyramidal cells and from fast- and non-fast-spiking interneurons in the CA1 stratum oriens, these investigators have shown that the transition from interictal to ictal discharge is characterized by a shift from dominant phasic inhibition to dominant phasic excitation. This evidence is in line with findings obtained in another study in which simultaneous dual and triple whole-cell and extracellular recordings were obtained from pyramidal cells and interneurons in the CA1 area of rat hippocampal slices bathed in 4AP and Mg2+-free medium (Ziburkus et al., 2006). It was shown in this study that interneurons increase their activity during the interictal period that precedes the ictal discharge onset (also termed pre-ictal) but then they undergo long-lasting periods of depolarization block (with suppression of action potential generation) during the ictal discharge, while principal cells produce sustained action potential discharges.
Similar results have recently been published by Huberfeld et al. (2011) who studied the transition from interictal- to ictal-like epileptiform discharges in the subiculum of brain slices that were obtained from TLE patients undergoing surgery. It should be noted that in order to obtain a pattern of ictal-like activity these authors had to employ multiple pharmacological manipulations of the superfusing medium including high K+, low Mg2+ and Ca2+, or external alkalinization, since a “single” manipulation consisiting of bath application of 4AP, high K+, low Mg2+ or bicuculline failed in inducing ictal-like events. In these experiments, Huberfeld et al. (2011) found that distinct pre-ictal discharges, which emerged concurrently with interictal events, depend on glutamatergic mechanisms and are preceded by pyramidal cell firing, whereas interneuron firing precedes interictal events that depend on both glutamatergic and depolarizing GABAergic signaling; moreover, preictal spikes spread faster that interictal-like discharges suggesting a reduced inhibitory restraint.
Therefore, in the experiments performed by Ziburkus et al. (2006) and by Derchansky et al. (2008) in rodent slices as well as in human recordings of Huberfeld et al. (2011), GABAA receptor-mediated mechanisms – which are operative and enhanced during the interictal period – appear to fade away in the preictal phase and even more once the ictal activity occurs. Whether the different results obtained in the 4AP model (in which mostly GABAergic spikes appear to initiate ictal-like activity) are due to differences in drug treatment, brain structure analyzed or maturity of the tissue remains a matter of discussion.
6.2. Mainteinance
Electrophysiological evidence for a role played by GABAA receptor-mediated conductances during ictal synchronization originates from the intracellular analysis of the depolarizations that occur in entorhinal cortex neurons during the early ‘tonic’ phase (characterized by sustained action potential firing) and the late ‘clonic’ phase (associated with action potential bursts) in the 4AP model (Lopantsev and Avoli, 1998a). These experiments have shown that the reversal potentials of these ictal depolarizations had values more negative than what expected for an isolated EPSP or an epileptiform depolarization induced by GABAA receptor antagonists (cf. Johnston and Brown, 1981; Gutnick et al., 1982). In addition, when KCl or KCl/QX314-filled microelectrodes were used for recording intracellularly from entorhinal cortex neurons, these ictal depolarizations had amplitudes that were larger than those recorded with microelectrodes filled with K-acetate. Therefore, GABAA receptor-mediated conductances are operative during ictal depolarizations induced by 4AP in the rat entorhinal cortex. It was also found in these experiments that when recorded with K-acetate/QX314-filled microelectrodes, ictal depolarizations have reversal potentials that become more negative as the epileptiform discharge progresses from the ‘tonic’ to the ‘clonic’ phase. Hence, the progression of ictal discharges induced by 4AP in the entorhinal cortex appears to be accompanied by a progressive enhancement of Cl−-dependent inhibition, suggesting that GABAA receptor activation may also contribute to the termination of ictal synchronization in this in vitro model (Lopantsev and Avoli, 1998a).
As illustrated in Fig. 14, the role of GABAA receptor-mediated inhibition to in vitro ictogenesis is further supported by the ability of drugs that interfere with GABAergic transmission to abolish ictal discharges induced by 4AP in several limbic structures (Avoli et al., 1993, 1996a,b; Lopantsev and Avoli, 1998a; Benini et al., 2003; Sudbury and Avoli, 2007). Similar evidence has been reported in other in vitro models of epileptiform synchronization. Accordingly, ictal-like afterdischarges induced by high-frequency-stimulation are blocked (Higashima et al., 1996; Fujiwara-Tsukamoto et al., 2006) or at least shortened (Perez Velazquez and Carlen, 1999) by GABAA receptor antagonists while ictal events generated by hippocampal CA1 networks in the Mg2+-free model are abolished by GABAA receptor-blockade (Köhling et al., 2000; Fujiwara-Tsukamoto et al., 2006).
Fig. 14. Blockade or reduction of GABAA receptor signaling abolishes ictal discharges in limbic areas.
(A) Effects induced by bath application of picrotoxin in a combined hippocampus-entorhinal cortex slice after Schaffer collateral cut. Note that picrotoxin abolishes both the slow interictal events and the ictal discharges seen under control conditions (i.e., during application of 4AP only), and discloses a robust pattern of intercital discharges. Note also the inversion in polarity of the discharges during picrotoxin application. The expanded panels show the onset of the ictal discharge seen under control conditions (i.e., 4AP) and of an interictal event recorded during additional application of picrotoxin. (B) Epileptiform activity in the anterior cingulate cortex is modulated by mu-opioid receptors. Note that ictal activity induced by 4AP (Control) no longer persists after bath-application of the μ-opioid agonist DAGO; this effect is reversed by the opioid antagonist naloxone. Field signals were obtained from different areas of the cingulate cortex.
These findings indicate that GABAA receptor-mediated receptor activation actively contributes to sustained prolonged period of epileptiform synchronization. In line with this view, Traub et al. (1996) modeled the contribution of GABAA receptor-mediated mechanisms to epileptiform synchronization induced by 4AP, and proposed that depolarizing GABAA receptor-mediated conductances modulate secondary, non-NMDA receptor-dependent bursts in CA3 networks (cf., Avoli et al., 1993). However, both the experimental and the modeling findings obtained from the 4AP model are at variance with those reported by Ziburkus et al. (2006) and Derchansky et al. (2008) since, as already reviewed in Section 6.1, interneurons recorded during ictal activity appear to be silent due to depolarization block. It remains to be clarified whether these different findings are due to removal of Mg2+ from the bathing medium, an experimental procedure that may indeed influence interneuron intrinsic excitability.
7. GABAA receptors and limbic structure interactions
GABAA receptor-mediated inhibition not only plays a role in modulating the occurrence of interictal and ictal discharges in limbic areas. GABAA receptor-mediated mechanisms, in fact, can control the spread of neuronal activity within the limbic system thus restraining the propagation of epileptiform discharges. The following sections are aimed at reviewing this experimental evidence that was obtained by employing electrophysiological recordings in the limbic system in in vitro experiments carried out in the isolated guinea pig brain or in rodent brain slices.
7.1. Control of spread of synchronous activity
Interactions within different parahippocampal regions as well as between these areas and the hippocampus proper are controlled by powerfull inhibitory mechanisms (de Curtis and Paré, 2004). Studies performed in vivo as well as in different types of in vitro preparations have demonstrated that the reciprocal propagation of activity between the temporal neocortex, the perirhinal cortex and the entorinal cortex is restrained by feed-forward inhibitory networks (Iijima et al., 1996; Martina et al., 2001; Biella et al., 2002, 2003; Pelletier et al., 2004). These studies have proposed that the cross-talk between perirhinal and entorhinal cortices is not organized to unrestrictedly transfer information, but to select relevant inputs (de Curtis and Paré, 2004). In line with this view it has been shown that electrical stimuli delivered in the perirhinal cortex do not evoke field potential responses in the entorhinal cortex (Fig. 15A), even though anatomical studies demonstrated that these two structures are strongly reciprocally connected (Amaral and Witter, 1989; Witter et al., 1989; Witter, 1993). Massive propagation of synchronous activity between these two regions can, however, be released when seizure activity is repeatedly generated within the hippocampal-entorhinal region, as demonstrated in the isolated guinea pig brain in vitro preparation (Uva et al., 2005). Recent studied employing voltage-sensitive dyes have confirmed that the blockade of GABAA receptors in area 35 of the perirhinal region facilitates the reciprocal propagation of activity between these rhinal cortices (Fig. 15B) (Biella et al., 2010). Hence, local, possibly feed-forward inhibitory networks in this strip of cortex are crucial to control reciprocal entorhinal-perirhinal cortex interactions and, therefore, to regulate the function of the parahippocampal region (de Curtis and Paré, 2004).
Fig. 15. Inhibitory networks control propagation of activity from perirhinal to entorhinal cortex.
(A) Schematic drawing of the position of the stimulation and recording electrodes in the isolated guinea pig brain preparation are shown in the top part. Laminar profiles of the responses recorded in the entorhinal cortex (EC) with a 16-channel linear slicon probe (100 μM interelectrode spacing) to the lateral olfactory tract (LOT) and to the perirhinal cortex (PRC) are shown in the bottom part. Note that LOT stimulation induced a field response in EC, while PRC stimulation did not evoke a field response in the EC. (B) Ventral view of the guinea pig brain and photomicrograph of the imaging area with the stimulation electrode in PRC (blue dot) and the bicuculline (BIC) injection site (red dot). (C) Optical imaging of the signals generated by voltage-sensitive dye (2-ANEPS) in the entorhinal cortex following perirhinal cortex stimulation under control conditions and during local injection of 1 mM bicuculline (BIC) in area 35. In the right panels, stripe images representing the changes in optical signal over time along a line positioned across the rhinal sulcus between the perirhinal and entorhinal cortices are shown. In control conditions, PRC-evoked responses do not propagate to the entorhinal cortex (left). The same stimulus applied after BIC injection induced both a stronger activation of the PRC and a spread of signal into the entorhinal cortex across the rhinal sulcus.
The function of GABAA receptor-mediated inhibition in delimiting synchronous epileptiform discharges emerges further when experiments are carried out in extended brain slices that include both the hippocampus and other parahippocampal structures. In these experiments, CA3-driven interictal discharges disclosed by bath application of 4AP, are often restrained to the hippocampus proper only (see Section 5.1). However, following application of the GABAA receptor antagonist picrotoxin these epileptiform discharges become more robust and spread to distant areas such as the enthorinal cortex (Benini and Avoli, 2005) or even the insular cortex (Sudbury and Avoli, 2007). As illustrated in Fig. 16, this phenomenon is presumably caused by ability of picrotoxin to block GABAA receptor-mediated inhibition in the subiculum (Benini and Avoli, 2005). The inability of CA3-driven interictal activity to propagate outside the hippocampus proper has also been documented in rat brain slices treated with Mg2+-free medium (Dreier and Heinemann, 1991).
Fig. 16. GABAA receptor-mediated inhibition in subiculum controls the spread of epileptiform discharges from the hippocampus to entorhinal cortex.
Under control conditions (4AP only in the superfusing medium) independent fast and slow interictal discharges are recorded with field microelectrodes from the CA3 and entorhinal cortex (EC), respectively, while hyperpolarizing IPSPs are generated by a subicular cells (−60 mV) analyzed intracellularly with a K-acetate-filled “sharp” microelectrode. During application of picrotoxin, both amplitude and rate of occurrence of the spontaneous IPSPs in the subicular cell decrease in amplitude and frequency of occurrence while an initial ictal discharge occurs in the EC (transition panel). Later, IPSPs disappear in the subiculum and CA3-driven interictal discharge spread to subiculum and EC (late panel). Expanded traces are shown in the lower insterts.
7.2. CA3-driven outputs control the expression of GABAergic synchronization and thus ictogenesis
Evidence supporting the view that GABAA receptor-mediated inhibition can modulate the interaction between limbic structures stems from experiments performed in adult rodent brain slices in which reciprocal connections between the hippocampus proper and para-hippocampal areas remained preserved. It was shown in these studies that (i) ictal discharges – which are consistently initiated by parahippocampal neuronal networks – disappear over 1–2 h of bath application of 4AP or Mg2+-free medium, while (ii) CA3-driven, fast interictal activity continues to occur throughout the experiment (Barbarosie and Avoli, 1997; Barbarosie et al., 2000, 2002; D’Antuono et al., 2002; Benini et al., 2003; Panuccio et al., 2010). In these experiments, Schaffer collateral cut restores ictal activity generation thus suggesting that CA3-driven interictal activity exerts an unexpected “inhibitory” effect on the expression of ictal discharges in structures such as the entorhinal cortex, the amygdala and even the insular cortex. Hence, as originally proposed by Swartzwelder et al., 1987), CA3-driven interictal discharges likely control rather than sustain limbic seizures.
Several mechanisms may contribute to this phenomenon including activity-dependent changes in synaptic and non-synaptic mechanisms that control neuronal excitability. However, Barbarosie et al. (2000, 2002) have proposed that the control of ictal discharges exerted by CA3 outputs is due to their ability to modulate (and actually reduce) the size of the slow interictal GABAergic events along with the associated increases in [K+]o. In line with this view, following the Schaffer collateral cut, slow interictal discharges become less frequent and are accompanied by larger [K+]o elevations than what seen during the pre-cut control condition; in addition, these changes are characterized by the ability of slow interictal discharge to trigger ictal events. These data are illustrated in Fig. 17 where cutting the Schaffer collateral prevents CA3-driven interictal activity to propagate to the entorhinal cortex and uncovers ictal discharges that are usually preceded (and thus presumably initiated) by large transient increases in [K+]o.
Fig. 17. Changes induced by Schaffer collateral cut on the 4AP-induced epileptiform activity.
Synchronous activity was recorded durring application of 4AP with field potential and [K+]o selective electrodes in the entorhinal cortex and dentate gyrus, and with a field electrode in the CA3 area before and after cutting the Schaffer collateral. Note in A that under control conditions intercital discharges are recorded in EC and DG; note also that the slow (presumptive GABAergic) spikes (asterisks) are accompanied by larger increases in [K+]o than CA3 driven interictal events. Cutting the Schaffer collateral prevents CA3-driven interictal activity to propagate to the entorhinal cortex and uncovers ictal discharge. Note that under these conditions the slow interictal event eirther occurs in isolation or shortly precedes the ictal discharge onset. (B) Histogram showing the changes in rate of occurrence of the slow interictal events before and after Schaffer collateral cut (n = 8 experiments; *p < 0.05). (C) Quantitative summary of the changes in duration of the slow interictal events recorded in dentate gyrus and entorhinal cortex before and after Schaffer collateral cut (n = 7 experiments; p < 0.05). (D) Histogram showing the effects of Schaffer collateral cut on the increases in [K+]o associated with the slow interictal discharge and recorded in entorhinal cortex and dentate (*p < 0.05).
The ability of CA3-driven interictal discharges to control the expression of synchronous GABAergic mechanisms that are instrumental for the occurrence of ictal discharge is supported by experiments in which parahippocampal structures such as the entorhinal cortex, the amydala or the insular cortex are stimulated electrically at frequencies that are similar to those of the CA3-driven interictal activity (i.e., at 0.25–1.0 Hz) (Barbarosie and Avoli, 1997; Barbarosie et al., 2002; D’Arcangelo et al., 2005; Sudbury and Avoli, 2007). It has been demonstrated in these studies that ictal discharge generation in brain slices that have undergone a lesion of the Schaffer collateral is depressed throughout the period of stimulation. As discussed below (Section 8.2), this experimental evidence suggests that the functional integrity of hippocampal output neurons may represent a critical control point in TLE pathophysiogenesis and gives to GABAergic synchronization a role in epileptic disordes (see also Avoli et al., 2002).
8. GABAA receptor function in the epileptic brain
Failure of GABAA receptor-mediated inhibition was assumed in the early 1980s to be a conditio sine qua non for the generation of epileptic discharges (see Section 1). This view was soon supported by studies that identified marked decreases in interneurons in some experimental models of epilepsy (Ribak and Reiffenstein, 1982; Ribak et al., 1982). These findings have been, however, challenged by subsequent studies that employed histological and electrophysiological tools to analyze the function of GABAergic inhibition in the epileptic brain in both humans and animal models. In this section we will briefly summarize the evidence regarding the changes in inhibition and in inhibitory cells that occur in TLE patients and in animal models. In addition, we will review experimental data pointing at the role played by GABAA receptor-mediated conductances in synchronizing neuronal networks and thus, presumably, in facilitating ictogenesis in the epileptic brain.
8.1. GABAA receptor-mediated inhibition in temporal lobe epilepsy
TLE is a partial epilepsy disorder in which seizure discharges involve the hippocampus proper, extrahippocampal structures such as the entorhinal and perirhinal cortices and the amygdala, as well as the temporal neocortex. TLE patients present with a pattern of brain damage known as Ammon’s horn sclerosis (or mesial temporal sclerosis) that is typically characterized by neuronal loss in hippocampal CA1/CA3 subfields, dentate hilus, layer III of the medial EC, and amygdala (Gloor, 1991, 1997; Houser, 1999; Du et al., 1993). Similar histopathological changes have been reported to occur in laboratory animals by injecting convulsant drugs such as pilocarpine or kainic acid, or by repetitive electrical stimulation of limbic pathways (Ben-Ari, 1985; Cavalheiro et al., 1991; Curia et al., 2008; Du et al., 1995). These experimental procedures induce an initial status epilepticus and cause 1–2 weeks later a chronic condition of recurrent limbic seizures. Interestingly, a similar, seizure-free, latent period can be observed in TLE patients who can present with an initial insult in early childhood (e.g., birth trauma, complicated febrile convulsions, brain injury or meningitis) and later develop partial seizures in adolescence or early adulthood.
Inhibitory mechanisms are altered in patients presenting with TLE and in animal models mimicking this disorder (Obenaus et al., 1993; Bernard et al., 2000; Houser and Escaplez, 1996; Fritschy et al., 1999; Gorter et al., 2001; Knopp et al., 2008; Maglóczky and Freund, 2005; van Vliet et al., 2004). However, these changes are complex and they cannot be epitomized as a simple, general decrease in the number of GABAergic cells along with inhibition collapse. For instance, robust periods of firing depression (presumably corresponding to IPSPs) have been recorded in electrophysiological studies performed pre-surgically with intracranial electrodes or extracellular unit recordings in TLE patients (Wilson and Engel, 1993; Isokawa-Akesson et al., 1989). Moreover, histochemical studies have demonstrated that some interneuron subtypes are preserved in both human and experimental animal epileptic tissue (Babb et al., 1989; Davenport et al., 1990; Esclapez et al., 1997) even though some other subtypes are clearly decreased in number in specific limbic structures (de Guzman et al., 2006, 2008).
Besides the altered number of interneurons (i.e., GABAergic inhibitory cells) that can characterize the epileptic tissue, several researchers have shown that attenuated network inhibition in epileptic tissue results from a reduced excitatory drive on inhibitory interneurons. Such a mechanism has been reported in the hippocampus and EC layers II/III (Bekenstein and Lothman, 1993; Williams et al., 1993; Sloviter, 1987; Doherty and Dingledine, 2001; Sloviter et al., 2003; Kumar and Buckmaster, 2006). Experiments performed in pilocarpine-treated rats have also demonstrated that pharmacologically isolated, GABAA receptor-mediated IPSPs have more positive reversal potentials in neurons recorded in vitro from the epileptic subiculum, peirhinal cortex and amygdala (de Guzman et al., 2006; Benini and Avoli, 2005; Benini et al., 2011); these data often correlated with reduced levels of mRNA expression and immunoreactivity of the neuron-specific cotransporter KCC2. In line with the view that altered homeostasis of intracellular [Cl−] is associated with epileptogenesis, NKCC1 mRNA and proteins in the mouse CA1 subfiled are up-regulated up to 45 days following pilocarpine-induced status epilepticus, while KCC2 is down-regulated (Li et al., 2008). Laschet et al. (2007) have also demonstrated that GABAergic inhibition is more lable in human epileptogenic tissue than in non-epileptogenic tissue, a functional difference that appears to result from an intrinsic deficiency of GABAA receptor endogenous phosphorylation.
The complex relations between GABAA receptors and epileptic disorders become even more evident when analyzing the changes in inhibition in the kindling model of TLE. At least in the hippocampal dentate gyrus, inhibition appears to be augmented, rather than diminished after kindling (Otis et al., 1994). In addition, Buhl et al. (1996) have reported that in this hippocampal area the excitatory drive onto inhibitory interneurons was increased and was paralleled by a reduction in the presynaptic autoinhibition of GABA release in brain slices obtained from kindled rats,. According to these experiments, the augmented inhibition found in the epileptic tissue is however decreased by the Zn2+ that is released from aberrantly sprouted mossy fiber terminals of granule cells during seizures. Evidence has also been obtained for selective changes in receptor subunit expression in animal models of TLE (Kamphuis et al., 1994, 1995; Rice et al., 1996; Brooks-Kayal et al., 1998).
8.2. GABAA receptor-mediated inhibition may implement epileptiform synchronization in temporal lobe epilepsy and other epileptic disorders
Studies performed on post-surgical human tissue obtained from surgery on patients with pharmacoresistant TLE maintained in vitro have demonstrated interictal discharges that are supported by GABAA receptor-mediated conductances in the neocortex (Köhling et al., 1998) and the subiculum (Cohen et al., 2002; Huberfeld et al., 2007; Wozny et al., 2003, 2005b). This conclusion stems from the ability of GABAA receptor antagonists to suppress synchronous, interictal population discharges in these cortical structures. Experiments performed with intracellular recordings from these subicular neurons have also shown that the reversal potential for GABAA-mediated synaptic events was depolarizing (with respect to resting potential) in a minority of pyramidal cells of the subiculum. Hence, in this cell group GABA provides an excitatory drive which combines with that transmitted via glutamatergic synapses to assure synchrony.
Huberfeld et al. (2007) have later reported that KCC2 immunostaining in those subicular neurons with a less negative IPSP reversal potential is reduced indicating a perturbation in Cl− homeostasis. Invetigators have speculated that the partial denervation of subicular cells in TLE, due to the loss of their pre-synaptic CA1 subfield inputs region, induces a return to an immature pattern of Cl− homeostasis. This view is supported by experimental evidence in which it was shown that following damage to afferents in the spinal cord, BDNF liberated by microglia activates trkB receptors leading to a KCC2 down-regulation and a depolarizing shift in GABAergic reversal potentials associated with pathological pain responses (Price et al., 2005; Lu et al., 2008). It has also been shown that experimentally induced interictal activity in hippocampal slices maintained in vitro, downregulates KCC2 mRNA and protein expression in CA1 pyramidal neurons, which leads to a reduced capacity for neuronal Cl− extrusion (Rivera et al., 2002, 2004; Blaesse et al., 2009). Alterations in the balance of NKCC1 and KCC2 activity have also been identified in the subiculum and hippocampus of epileptic patients by Munoz et al. (2007). These investigators, by employing double immunolabeling of NKCC1 and KCC2, have reported that in the transition zone from the sclerotic CA1 area to the subiculum, approximately 20% of the NKCC1-immunoreactive neurons do not express KCC2. In addition, they found that some neurons were hyperinnervated by parvalbumin-positive hypertrophic basket formations.
Changes in limbic network interactions, which may result from seizure-induced cell damage, characterize epileptic pilocarpine-treated rodent slices. These changes – which may lead to limbic seizures in vivo and perhaps in patients presenting with TLE – consist of a decreased control exerted by hippocampal outputs on entorhinal cortex excitability along with reverberant activity between entorhinal cortex and CA1/subiculum networks (D’Antuono et al., 2002; Wozny et al., 2005a; Panuccio et al., 2010). The functional integrity of hippocampal neurons that contribute to the generation of normal output activity may thus represent a critical control point for limbic seizure generation and recurrence. It has also been hypothesized that removing or decreasing this hippocampal output activity (as the result of CA3 neuronal cell loss, and Schaffer collateral damage) can lead to the functional disclosure of a direct temporoammonic path that excite both CA1 and subicular neurons (Barbarosie and Avoli, 1997; D’Antuono et al., 2002). In line with this view, Ang et al. (2006) have found that the temporoammonic pathway is transformed in pilocarpine-treated epileptic rats from a spatially restricted, highly regulated input to a strong excitatory projection to the CA1 hippocampal area. Since the temporoammonic pathway is critically positioned to modulate the propagation of seizure activity to the hippocampus (van Groen and Lopes da Silva, 1986; van Groen et al., 1986), this process can produce reverberant interactions between hippocampus/subiculum and the entorhinal cortex.
An active, “pro-epileptogenic” role of GABAA receptor-mediated mechanisms has been reported in other epileptic disorders as well. For instance, a synchronous GABAA receptor-mediated event appears to be able to initiate ictal discharges in post-surgical neocortical tissue removed from patients with Taylor type focal cortical dysplasia (Avoli et al., 1999; D’Antuono et al., 2004); it was shown in these experiments that, as reported in rodent limbic structures, this synchronous GABAergic potential is accompanied by a transient increase in [K+]o as well as that the ictal activity is abolished by GABAA receptor antagonists or μ-opioid receptor agonism. Interestingly, it has been reported that the dysplastic tissue resected from these patients presents with decreased number of presumptive interneurons that, however, provide increased GABAergic innervation to principal cells (Spreafico et al., 1998; Tassi et al., 2002). In addition, Alonso-Nanclares et al. (2005) have reported that giant ectopic neurons in the white matter are surrounded by hypertrophic basket formations. Finally, Munakata et al. (2007) have found decreased KCC2 immunohistochemical expression in focal cortical dysplastic specimens as compared to mature nondysplastic cortex, and have proposed that this alteration may affect intracellular Cl− and K+ homeostasis. Hence, hyperinnervation of principal neurons by “surviving” inhibitory cells appears to be the hallmark of the epileptic tissue in patients presenting with either TLE (Munoz et al., 2007) or focal cortical dysplasia. This over-expression of GABAergic axon terminals on principal glutamatergic neurons along with changes in KCC2 and NKCC1 may indeed represent an important tool for implementing GABAA receptor-mediated synchronization.
Enhanced GABAergic inhibition has been shown to promote seizures in a transgenic murine model of frontal lobe epilepsy that is linked to a nicotinic acetylcholine receptor mutation (Klaassen et al., 2006). Neocortical seizures sustained by nicotinic activation are suppressed in these animals by GABAA receptor antagonists, suggesting that seizure onset involves phase resetting of synchronization of principal cells determined by acetylcholine-dependent synchronization of inhibitory cortical networks.
9. Conclusive remarks
In this review we have focused on the role played by GABAA receptor-mediated mechanisms in synchronizing neuronal networks in different limbic areas. Contrary to the common view that has considered reduced efficacy of inhibition as an obligatory step for seizure occurrence both in normal and epileptic brains, the studies reviewed here demonstrate that GABAergic mechanisms can actively contribute to neuronal network oscillations, interictal spiking and seizures in different models of epileptiform synchronization.
This view is summarized in the schematic drawings shown in Fig. 18, where the overall activity (expressed as amount of firing) of principal glutamatergic cell and interneuron networks along with the [K+]o changes are shown under different conditions, from normal (blue area) to ictal (red area) as epitomized in the EEG recording. Resting neuronal firing of both principal and local inhibitory neurons is assumed to increase during interictal spikes – identified as one of the prominent interictal phenomena (yellow area) – although principal neuron firing may also be inhibited by the influence of inhibitory inputs. Two possible sequence of events, both grounded on experimental observations in different seizure models, are active in the preictal state (green area in Fig. 18). According to one view (Avoli et al., 1993, 1996a; Lopantsev and Avoli, 1998a; Fujiwara-Tsukamoto et al., 2004; Gnatkowsky et al., 2008; Lasztocki et al., 2009), interneuronal activity is enhanced and synchronized in the period that precedes a seizure (continuous line). This reduces the firing of principal neurons at seizure onset. However, synchronous interneuronal firing leads to GABA release thus promoting a large increase in [K+]o; this in turn restores neuronal firing in all cells and promotes the transition into the massive, synchronous activity typically observed during the full-blown phase of a seizure (Trombin et al., 2011). An alternative hypothesis (blue dotted line) is based on data obtained in the CA1/subiculum area by Derchansky et al. (2008), Ziburkus et al. (2006) and Huberfeld et al. (2011). According to these findings principal glutamatergic cells are massively recruited in the pre-ictal phase and the consequent synaptic release of glutamate along with the [K+]o increase promote the transition into seizure; hence, in this type of preictal stage interneurons are secondarily activated by principal glutamatergic cells. In addition, according to Ziburkus et al. (2006) during the ictal phase interneurons are transiently hyperactivated and undergo depolarizing block of firing (yellow dotted line), thus further reinforcing the activation of principal neurons. It should be mentioned that according to both hypotheses, the preictal phase can be considered part of the ictal discharge; therefore, its definition as preictal is a matter of semantics.
Fig. 18.
Interplay between principal cells, interneurons and [K+]o during different EEG states. Drawings summarizing the role of GABAA receptor signaling subsequent to GABA released by interneurons during normal and epileptic conditions. The hypothetical EEG, the total amounts of firing generated by principal cell and by interconnected interneuron networks, as well as the [K+]o are shown. In the epileptic condition, interictal, preictal and ictal discharges are considered. Note that an increase in interneuron network activity (and consequent GABA release) occurs in all three cases, while a decrease in principal cell network activity (secondary to activation of GABAA receptors) can result during the interictal discharges as well as during the pre-ictal spike as indicated by the experimental data shown in Fig. 12C and Fig. 13. The preictal mechanism proposed by Ziburkus et al. (2006), Derchansky et al. (2008) and Huberfeld et al. (2011) in which field spikes leading to an ictal discharge are mainly glutamatergic and capable of activating interneurons secondarily is hihglighted by the dotted blue lines. Note that the dotted yellow line indicates the possible decrease in interneuron firing secondary do depolarization block as reported by Ziburkus et al. (2006).
In conclusion, given the complexity of the mechanisms that regulate brain functions, we propose that seizure generation (and perahps epileptogenesis) results from structural and functional alterations in neuronal transmission that involve both excitatory and inhibitory networks. For sure, many of the studies reviewed here indicate that GABAA receptor-mediated mechanisms rather than being silenced or playing the role of innocent by-standers, contribute to synchronizing neuronal networks in several epilepsy-prone brain structures. The challenge for future studies will be to further dissect the molecular, pharmacological and cellular bases of GABAA receptor-mediated signaling in order to identify the most relevant for designing new, more efficacious and safer antiepileptic strategies.
Acknowledgments
The original studies reviewed in this paper were supported by grants from the Canadian Institutes of Health Research, the Savoy Foundation, CURE, the Piergiorgio and Luisa Mariani Foundation and the Italian Health Ministry. We thank Drs. J.-C. Lacaille and S. Vicini for reading an early draft of this paper, and Ms. R. Herrington for reference editing. We also thank Ms. Toula Papadopoulos for continuous assistance.
Abbreviations
- 4AP
4-aminopyridine
- BIC
bicuculline
- CA1 and CA3
Cornus Ammonis subfield 1 and 3
- [Cl−]i
intracellular Cl− concentration
- DG
dentate gyrus
- EC
entorhinal cortex
- GAD
glutamic acid decarboxylase
- [K+]i
intracellular K+ concentration
- LLD
long-lasting depolarization
- PRC
perirhinal cortex
- TLE
temporal lobe epilepsy
References
- Aicardi J. Epilepsy in children. In: Aicardi J, editor. International Review of Child Neurology Series. Raven Press; New York: 1986. p. 413. [Google Scholar]
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Yamada J, Fukuda A, Luhmann HJ, Kilb W. Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons. J Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakuijala A, Alakuijala J, Pasternack M. Evidence for a functional role of GABA-C receptors in the rat mature hippocampus. Eur J Neurosci. 2006;23:514–520. doi: 10.1111/j.1460-9568.2005.04572.x. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Nanclares L, Garbelli R, Sola RG, Pastor J, Tassi L, Sreafico R, De Felipe J. Microanatomy of the dysplastic neocortex from epileptic patients. Brain. 2005;128:158–173. doi: 10.1093/brain/awh331. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of the anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen P, Andersson SA, Lǿmo T. Patterns of spontaneous rhythmic activity within various thalamic nuclei. Nature. 1966;211:888–889. doi: 10.1038/211888b0. [DOI] [PubMed] [Google Scholar]
- Andersen P, Andersson SA, Junge K, Lǿmo T, Sveen OH. Physiological mechanism of the slow 10c/sec cortical rhythmic activity. Electroencephalogr Clin Neurophysiol. 1967;23:394–395. [PubMed] [Google Scholar]
- Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Laursen AM. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aram JA, Michaelson HB, Wong RKS. Synchronized GABAergic IPSPs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J Neurophysiol. 1991;65:1034–1041. doi: 10.1152/jn.1991.65.5.1034. [DOI] [PubMed] [Google Scholar]
- Autere AM, Lamsa K, Kaila K, Taira T. Synaptic activation of GABAA receptors induces neuronal uptake of Ca2+ in adult hippocampal slices. J Neurophysiol. 1999;81:811–815. doi: 10.1152/jn.1999.81.2.811. [DOI] [PubMed] [Google Scholar]
- Avoli M. Epileptiform discharges and a synchronous GABAergic potential induced by 4-aminopyridine in the rat immature hippocampus. Neurosci Lett. 1990;117:93–98. doi: 10.1016/0304-3940(90)90125-s. [DOI] [PubMed] [Google Scholar]
- Avoli M. GABA-mediated synchronous potentials and seizure generation. Epilepsia. 1996;37:1035–1042. doi: 10.1111/j.1528-1157.1996.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, Perreault P. A GABAergic depolarizing potential in the hippocampus disclosed by the convulsant 4-aminopyridine. Brain Res. 1987;400:191–195. doi: 10.1016/0006-8993(87)90671-8. [DOI] [PubMed] [Google Scholar]
- Avoli M, Olivier A. Electrophysiological properties and synaptic responses in the deep layers of the human epileptogenic neocortex in vitro. J Neurophysiol. 1989;61:589–606. doi: 10.1152/jn.1989.61.3.589. [DOI] [PubMed] [Google Scholar]
- Avoli M, Drapeau C, Perreault P, Louvel J, Pumain R. Epileptiform activity induced by low chloride medium in the CA1 subfield of the hippocampal slice. J Neurophysiol. 1990;64:1747–1757. doi: 10.1152/jn.1990.64.6.1747. [DOI] [PubMed] [Google Scholar]
- Avoli M, Mattia D, Siniscalchi A, Perreault P, Tomaiuolo F. Pharmacology and electrophysiology of a synchronous GABA-mediated potential in the human neocortex. Neuroscience. 1994;62:655–666. doi: 10.1016/0306-4522(94)90467-7. [DOI] [PubMed] [Google Scholar]
- Avoli M, Psarropoulou C, Tancredi V, Fueta Y. On the synchronous activity induced by 4-aminopyridine in the CA3 subfield of juvenile rat hippocampus. J Neurophysiol. 1993;70:1018–1029. doi: 10.1152/jn.1993.70.3.1018. [DOI] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Drapeau C, Pumain R, Kurcewicz I. GABAA-mediated inhibition and in vitro epileptogenesis in the human neocortex. J Neurophysiol. 1995;73:468–484. doi: 10.1152/jn.1995.73.2.468. [DOI] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lucke A, Nagao T, Lopantsev V, Kohling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996a;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Kurcewicz I, Pumain R, Barbarosie M. Extracellular free potassium and calcium during synchronous activity induced by 4-aminopyridine in the immature rat hippocampus. J Physiol. 1996b;493:707–717. doi: 10.1113/jphysiol.1996.sp021416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Nagao T, Köhling R, Lücke A, Mattia D. Synchronization of rat hippocampal neurons in the absence of excitatory amino acid-mediated transmission. Brain Res. 1996c;735:188–196. doi: 10.1016/0006-8993(96)00376-9. [DOI] [PubMed] [Google Scholar]
- Avoli M, Methot M, Kawasaki H. GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci. 1998;10:2714–2722. doi: 10.1046/j.1460-9568.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, Bernasconi A, Mattia D, Olivier A, Hwa GG. Epileptiform discharges in the human dysplastic neocortex: in vitro physiology and pharmacology. Ann Neurol. 1999;46:816–826. doi: 10.1002/1531-8249(199912)46:6<816::aid-ana3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system. Progr Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. doi: 10.1016/0006-8993(73)90647-1. [DOI] [PubMed] [Google Scholar]
- Babb TL, Pretorius JK, Kupfer WR, Crandall PH. Glutamate decarboxylase- immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci. 1989;9:2562–2574. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA. The synaptic basis of GABAA, slow. J Neurosci. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustain in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, Kurcewicz I, Avoli M. CA3-Released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol. 2000;83:1115–1124. doi: 10.1152/jn.2000.83.3.1115. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, D’Antuono M, Kurcewicz I, Avoli M. Masking synchronous GABA-mediated potentials controls limbic seizures. Epilepsia. 2002;43:1469–1479. doi: 10.1046/j.1528-1157.2002.17402.x. [DOI] [PubMed] [Google Scholar]
- Barolet AW, Morris ME. Changes in extracellular K+ evoked by GABA, THIP and baclofen in the guinea-pig hippocampal slice. Exp Brain Res. 1991;84:591–598. doi: 10.1007/BF00230971. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Geiger JR, Jonas P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci. 2001;21:2687–2698. doi: 10.1523/JNEUROSCI.21-08-02687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schürmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Eng Med Biol Mag. 1999;18:56–66. doi: 10.1109/51.765190. [DOI] [PubMed] [Google Scholar]
- Basemore A, Elliott KA, Florey E. Factor I and gamma-aminobutyric acid. Nature. 1956;178:1052–1053. doi: 10.1038/1781052a0. [DOI] [PubMed] [Google Scholar]
- Bazelot M, Dinocourt C, Cohen I, Miles R. Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J Physiol. 2010;588:2077–2090. doi: 10.1113/jphysiol.2009.185918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993;259:97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- Benardo LS. Recruitment of GABAergic inhibition and synchronization of inhibitory interneurons in rat neocortex. J Neurophysiol. 1997;77:3134–3144. doi: 10.1152/jn.1997.77.6.3134. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Benassi P, Bertolotti P. Experimental research on the anticonvulsant properties of 1-glutamine, 1-asparagine, gamma-aminobutyric acid and gamma-amino-beta-hydroxybutyric acid. Riv Sper Freniatr Med Leg Alien Ment. 1962;86:342–354. [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol. 2005;566:885–900. doi: 10.1113/jphysiol.2005.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benini R, Longo D, Biagini G, Avoli M. Perirhinal cortex hyperexcitability in pilocarpine-treated epileptic rats. Hippocampus. 2011;21:702–713. doi: 10.1002/hipo.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Cossart R, Hirsch JC, Esclapez M, Ben-Ari Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia. 2000;41(Suppl 6):S90–S95. doi: 10.1111/j.1528-1157.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Biagini G, Panuccio G, Avoli M. Neurosteroids and epilepsy. Curr Opin Neurol. 2010;23:170–176. doi: 10.1097/WCO.0b013e32833735cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biella G, Uva L, Hoffman U, de Curtis M. Associative potentials in the entorhinal cortex of the guinea pig. J Neurophysiol. 2002;88:1159–1165. doi: 10.1152/jn.2002.88.3.1159. [DOI] [PubMed] [Google Scholar]
- Biella G, Gnatkovsky V, de Curtis M. Olfactory projections to the parahippocampal region of the isolated guinea pig brain. Eur J Neurosci. 2003;18:95–101. doi: 10.1046/j.1460-9568.2003.02730.x. [DOI] [PubMed] [Google Scholar]
- Biella G, Toselli M, de Curtis M, Gnatkovsky V. Functional perirhinal-postrhinal-entorhinal connections revealed by voltage sensitive dye imaging in the isolated guinea pig brain. J Neurophysiol. 2010;103:725–732. doi: 10.1152/jn.00722.2009. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Bracci E, Vreugdenhil M, Hack SP, Jefferys JG. On the synchronizing mechanisms of tetanically induced hippocampal oscillations. J Neurosci. 1999;19:8104–8113. doi: 10.1523/JNEUROSCI.19-18-08104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wison CL. Hippocampal and entorhinal cortex high-frequency oscillations in human epileptic brain and kainic acid treated rats. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel JJR. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented GABAergic inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Burt DR, Kamatchi GL. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Capogna M, Gahwiller BH, Thompson SM. Mechanism of μ-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero G, Uva L, Gnatkovsky V, Avoli M, de Curtis M. Independent epileptiform discharge patterns in the olfactory and limbic areas of the in vitro isolated guinea pig brain during 4-aminopyridine treatment. J Neurophysiol. 2010;103:2728–2736. doi: 10.1152/jn.00862.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephalographers. Electroenceph Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Compte A, Reig R, Descalzo VF, Harvey MA, Puccini GD, Sanchez-Vives MV. Spontaneous high-frequency (10–80 Hz) oscillations during up states in the cerebral cortex in vitro. J Neurosci. 2008;28:13828–13844. doi: 10.1523/JNEUROSCI.2684-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coursin DB. Convulsive seizures in infants with pyridoxine-deficient diet. J Am Med Assoc. 1954;154:406–408. doi: 10.1001/jama.1954.02940390030009. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Benini R, Biagini G, D’Arcangelo G, Barbarosie M, Tancredi V, Avoli M. Limbic network interactions leading to hyperexcitability in temporal lobe epilepsy. J Neurophysiol. 2002;87:634–639. doi: 10.1152/jn.00351.2001. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Louvel J, Köhling R, Mattia D, Bernasconi A, Olivier A, Turak B, Devaux A, Pumain R, Avoli M. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain. 2004;127:1626–1640. doi: 10.1093/brain/awh181. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, de Guzman P, Kano T, Avoli M. Ripple activity in the dentate gyrus of disinhibited hippocampus-entorhinal cortex slices. J Neurosci Res. 2005;80:92–103. doi: 10.1002/jnr.20440. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Panuccio G, Tancredi V, Avoli M. Repetitive low-frequency repetitive stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol Dis. 2005;19:119–128. doi: 10.1016/j.nbd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Biella G, Forti M, Panzica F. Multifocal spontaneous epileptic activity induced by restricted convulsing ejection in the piriform cortex of the isolated guinea pig brain. J Neurophysiol. 1994;71:2463–2476. doi: 10.1152/jn.1994.71.6.2463. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Progr Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Reevaluating the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia. 2009a;50:2514–2525. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Paré D. The parahippocampal cortex: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Biella G, Buccellati C, Folco G. Simultaneous investigation of the neuronal and vascular compartments in the guinea pig brain isolated in vitro. Brain Res Protocol. 1998;3:221–228. doi: 10.1016/s1385-299x(98)00044-0. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Re-considering the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia. 2009b;50:2514–2525. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- de Guzman P, D’Antuono M, Avoli M. Initiation of electrographic seizures by neuronal networks in entorhinal and perirhinal cortices in vitro. Neuroscience. 2004;123:875–886. doi: 10.1016/j.neuroscience.2003.11.013. [DOI] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Baldelli E, de Curtis M, Biagini G, Avoli M. Network hyperexcitability within the deep layers of the pilocarpine-treated rat entorhinal cortex. J Physiol. 2008;586:1867–1883. doi: 10.1113/jphysiol.2007.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Biagini G, Baldelli E, Mollinari C, Merlo D, Avoli M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16:843–860. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- Derchansky M, Jahromi SS, Mamani M, Shin DS, Sik A, Carlen PL. Transition to seizures in the isolated immature mouse hippocampus: a switch from dominant phasic inhibition to dominant phasic excitation. J Physiol. 2008;586:477–494. doi: 10.1113/jphysiol.2007.143065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. I Characteristics and topographical features. J Neurophysiol. 1969;32:649–662. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Alonso A. Muscarinic induction of synchronous population activity in the entorhinal cortex. J Neurosci. 1997;17:6729–6744. doi: 10.1523/JNEUROSCI.17-17-06729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson CT, Biella G, de Curtis M. Evidence for spatial modules mediated by temporal synchronization of carbachol-induced gamma rhythm in medial entorhinal cortex. J Neurosci. 2000;20:7846–7854. doi: 10.1523/JNEUROSCI.20-20-07846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson CT, Biella G, de Curtis M. Slow periodic events and their transition to fast gamma oscillations in the entorhinal cortex of the isolated guinea-pig brain. J Neurophysiol. 2003;90:39–46. doi: 10.1152/jn.01063.2002. [DOI] [PubMed] [Google Scholar]
- Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl 1):S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Reduced excitatory drive onto interneurons in the dentate gyrus after status epilepticus. J Neurosci. 2001;21:2048–2057. doi: 10.1523/JNEUROSCI.21-06-02048.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Axmacher N, Kolbaev S. Presynaptic ionotropic GABA receptors. Results Probl Cell Differ. 2008;44:69–85. doi: 10.1007/400_2007_040. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activityin rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Kuchibhotla KV, Glykys JC, Kahle KT, Swiercz WB, Feng G, Kuner T, Augustine GJ, Bacskai BJ, Staley KJ. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J Neurosci. 2010;30:11745–11761. doi: 10.1523/JNEUROSCI.1769-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JJR, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Khazipov R, Ben-Ari Y, Bernard C. Operative GABAergic inhibition in hippocampal CA1 pyramidal neurons in experimental epilepsy. Proc Natl Acad Sci USA. 1997;94:12151–12156. doi: 10.1073/pnas.94.22.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signaling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Florey E, McLennan H. Effects of an inhibitory factor (factor I) from brain on central synaptic transmission. J Physiol. 1955;130:446–455. doi: 10.1113/jphysiol.1955.sp005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Forti M, Biella G, Caccia S, de Curtis M. Persistent excitability changes in the piriform cortex of the isolated guinea-pig brain after transient exposure to bicuculline. Eur J Neurosci. 1997;9:435–451. doi: 10.1111/j.1460-9568.1997.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Fraser DD, Duffy S, Angelides KJ, Perez-Velasquez JL, Kettenmann H, MacVicar BA. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J Neurosci. 1995;15:2720–2732. doi: 10.1523/JNEUROSCI.15-04-02720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–445. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Kaneda K, Takada M. Synaptic interactions between pyramidal cells and interneurone subtypes during seizure-like activity in the rat hippocampus. J Physiol. 2004;557:961–979. doi: 10.1113/jphysiol.2003.059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Takada M. Comparable GABAergic mechanisms of hippocampal seizure-like activity in posttetanic and low-Mg2+ conditions. J Neurophysiol. 2006;95:2013–2019. doi: 10.1152/jn.00238.2005. [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Fukai T, Takada M. Distinct types of ionic modulation of GABA actions in pyramidal cells and interneurons during electrical induction of hippocampal seizurelike network activity. Eur J Neurosci. 2007;25:2713–2725. doi: 10.1111/j.1460-9568.2007.05543.x. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2004;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- Gigout S, Louvel J, Kawasaki H, D’Antuono M, Armand V, Kurcewicz I, Olivier A, Laschet J, Turak B, Devaux B, Pumain R, Avoli M. Role of gap junctions in human neocortical network synchronization. Neurobiol Dis. 2006;22:496–508. doi: 10.1016/j.nbd.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Kettenmann H, Schachner M. γ-Aminobutyric acid directly depolarizes cultured oligodendrocytes. J Neurosci. 1984;4:561–569. doi: 10.1523/JNEUROSCI.04-02-00561.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: historical background and an overview from a modern perspective. In: Lüders H, editor. Epilepsy Surgery. Raven Press; New York: 1991. pp. 689–703. [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press; New York: 1997. [Google Scholar]
- Gloveli T, Albrecht D, Heinemann U. Properties of low Mg2+ induced epileptiform activity in rat hippocampal and entorhinal cortex slices during adolescence. Dev Brain Res. 1995;87:145–152. doi: 10.1016/0165-3806(95)00069-p. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatkovsky V, Wendling F, de Curtis M. Spontaneous periodic oscillatory events are generated by superficial neurons in medial entorhinal cortex of the isolated guinea pig brain. Eur J Neurosci. 2007;26:302–311. doi: 10.1111/j.1460-9568.2007.05667.x. [DOI] [PubMed] [Google Scholar]
- Gnatkowsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory networks in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Grover LM, Lambert NA, Schwartzkroin PA, Teyler TJ. Role of HCO3− ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol. 1993;69:1541–1555. doi: 10.1152/jn.1993.69.5.1541. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick MJ, Connors BW, Prince DA. Mechanisms of neocortical epileptogenesis in vitro. J Neurophysiol. 1982;48:1321–1335. doi: 10.1152/jn.1982.48.6.1321. [DOI] [PubMed] [Google Scholar]
- Gutnick MJ, Prince DA. Thalamocortical relay neurons: antidromic invasion of spikes from a cortical epileptogenic focus. Science. 1972;176:424–426. doi: 10.1126/science.176.4033.424. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Sarett LH. On the efficacy of asparagine, glutamine, gamma-aminobutyric acid and 1-pyrroiidinone in preventing chemically induced seizures in mice. Clin Chim Acta. 1957;2:481–484. doi: 10.1016/0009-8981(57)90049-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T. Neurophysiology and Neurochemistry of Convulsion. Dainihon-Tosho Co; Tokyo: 1959. p. 222. [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Herrero AI, Del Olmo N, González-Escalada JR, Solís JM. Two new actions of topiramate: inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology. 2002;42:210–220. doi: 10.1016/s0028-3908(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Higashima M, Kinoshita H, Yamaguchi N, Koshino Y. Activation of GABAergic function necessary for afterdischarge generation in rat hippocampal slices. Neurosci Lett. 1996;207:101–104. doi: 10.1016/0304-3940(96)12496-4. [DOI] [PubMed] [Google Scholar]
- Houser CR. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–761. [PubMed] [Google Scholar]
- Houser CR, Escaplez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. Entorhinal-hippocampal interactions revealed by real-time imaging. Science. 1996;272:1176–1179. doi: 10.1126/science.272.5265.1176. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Wilson CL, Babb TL. Inhibition in synchronously firing human hippocampal neurons. Epilepsy Res. 1989;3:236–247. doi: 10.1016/0920-1211(89)90030-2. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR. Basic mechanism of focal epilepsies. Exp Physiol. 1990;75:127–162. doi: 10.1113/expphysiol.1990.sp003390. [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Johnston D, Brown TH. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981;16:294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- Jones RS, Lambert JDC. Synchronous discharges in the rat entorhinal cortex in vitro: site of initiation and the role of excitatory amino acid receptors. Neuroscience. 1990a;34:657–670. doi: 10.1016/0306-4522(90)90172-z. [DOI] [PubMed] [Google Scholar]
- Jones RS, Lambert JDC. The role of excitatory amino acid receptors in the propagation of epileptiform discharges from the entorhinal cortex to the dentate gyrus in vitro. Exp Brain Res. 1990b;80:310–322. doi: 10.1007/BF00228158. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Barnett SM, Sassower KC, Staley KJ. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na(+)-K(+)-2Cl(−) cotransporter NKCC1. J Child Neurol. 2009;24:572–576. doi: 10.1177/0883073809333526. [DOI] [PubMed] [Google Scholar]
- Kaila K, Voipio J, Paalasmaa P, Pasternack M, Deisz RA. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurons. J Physiol. 1993;464:273–289. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate- dependent K+ transient. J Neurosci. 1997;17:7662–7767. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilov I, Khazipov R, Esclapez M, Ben-Ari Y. Bicuculline induces ictal seizures in the intact hippocampus recorded in vitro. Eur J Pharmacol. 1997;319:R5–R6. doi: 10.1016/s0014-2999(96)00964-8. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, DeRijk TC, lopes Da Silva FH. GABA-A receptor β1-3 subunit gene expression in the hippocampus of kindled rats. Neurosci Lett. 1994;174:5–8. doi: 10.1016/0304-3940(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, DeRijk TC, Lopes da Silva PH. Expression of GABAA receptor subunit mRNAs in hippocampal pyramidal and granular neurons in the kindling model of epileptogenesis: an in situ hybridization study. Mol Brain Res. 1995;31:33–47. doi: 10.1016/0169-328x(95)00022-k. [DOI] [PubMed] [Google Scholar]
- Kilb W, Sinning A, Luhmann HJ. Model-specific effects of bumetanide on epileptiform activity in the in-vitro intact hippocampus of the newborn mouse. Neuropharmacology. 2007;53:524–533. doi: 10.1016/j.neuropharm.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci USA. 2006;103:19152–19157. doi: 10.1073/pnas.0608215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp A, Frahm C, Fidzinski P, Witte OW, Behr J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain. 2008;131:1516–1527. doi: 10.1093/brain/awn095. [DOI] [PubMed] [Google Scholar]
- Köhling R, Lucke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, Pannek H, Oppel F. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121:1073–1087. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- Köhling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K. GABA-mediated inhibitory mechanisms in relation to epileptic discharge. In: Jasper HH, van Gelder NM, editors. Basic Mechanisms of Neuronal Hyperexcitability. Liss; New York: 1983. pp. 249–280. [Google Scholar]
- Krnjevic K, Morris M, Reiffestein RJ. Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol. 1980;58:570–583. doi: 10.1139/y80-097. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Schwartz S. Some properties of unresponsive cells in the cerebral cortex. Exp Brain Res. 1967;3:306–319. doi: 10.1007/BF00237557. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyt E, Uva L, de Curtis M, Wendling F. Realistic modeling of entorhinal cortex field potentials and interpretation of epileptic activity in the guinea-pig isolated brain preparation. J Neurophysiol. 2006;97:363–377. doi: 10.1152/jn.01342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC. Postsynaptic potentials mediated by excitatory and inhibitory amino acids in interneurons of stratum pyramidale of the CA1 region of rat hippocampal slices in vitro. J Neurophysiol. 1991;66:1441–1454. doi: 10.1152/jn.1991.66.5.1441. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Williams S. Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience. 1990;36:349–359. doi: 10.1016/0306-4522(90)90431-3. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Kaila K. Ionic mechanisms of spontaneous GABAergic events in rat hippocampal slices exposed to 4-aminopyridine. J Neurophysiol. 1997;78:2582–2591. doi: 10.1152/jn.1997.78.5.2582. [DOI] [PubMed] [Google Scholar]
- Lasztocki B, Nyitrai G, Heja L, Kardos J. Synchronization of GABAergic inputs to CA3 principal cells precedes seizure-like even onset in juvenile rat hippocampal slices. J Neurophysiol. 2009;102:2538–2553. doi: 10.1152/jn.91318.2008. [DOI] [PubMed] [Google Scholar]
- Laschet JJ, Kurcewicz I, Minier F, Trottier S, Khallou-Laschet J, Louvel J, Gigout S, Turak B, Biraben A, Scarabin JM, Devaux B, Chauvel P, Pumain R. Dysfunction of GABAA receptor glycolysis-dependent modulation in human partial epilepsy. Proc Natl Acad Sci USA. 2007;104:3472–3477. doi: 10.1073/pnas.0606451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J., Jr Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou J, Chen Z, Chen S, Zhu F, Zhou L. Long-term expressional changes of Na+-K+-Cl− co-transporter 1 (NKCC1) and K+-Cl− co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE) Brain Res. 2008;1221:141–146. doi: 10.1016/j.brainres.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Librizzi L, de Curtis M. Epileptiform ictal discharges are prevented by periodic interictal spiking activity in the guinea-pig olfactory-limbic cortex. Ann Neurol. 2003;53:382–389. doi: 10.1002/ana.10471. [DOI] [PubMed] [Google Scholar]
- Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictal-like discharges in the rat entorhinal cortex. J Neurophysiol. 1998a;79:352–360. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Laminar organization of epileptiform discharges in the rat entorhinal cortex in vitro. J Physiol. 1998b;509:785–796. doi: 10.1111/j.1469-7793.1998.785bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvel J, Papatheodoropoulos C, Siniscalchi A, Kurciewicz I, Pumain R, Devaux B, Turak B, Chodkiewicz JP, Esposito V, Villemeure JG, Avoli M. GABA-Mediated synchronization in the human cortex: elevations in extracellular potassium and presynaptic mechanisms. Neuroscience. 2001;105:803–813. doi: 10.1016/s0306-4522(01)00247-0. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J Physiol. 2008;586:5701–5715. doi: 10.1113/jphysiol.2008.152348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Dzhala VI, Ben-Ari Y. Generation and propagation of 4-AP-induced epileptiform activity in neonatal intact limbic structures in vitro. Eur J Neurosci. 2000;12:2757–2768. doi: 10.1046/j.1460-9568.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986;44:619–639. [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC. Interneuron diversity series: hippocampal interneuron classifications-making things as simple as possible, not simpler. Trends Neurosci. 2003;26:564–271. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Tse FWY, Crichton SA, Kettenmann H. GABA activated Cl− channels in astrocytes of hippocampal slices. J Neurosci. 1989;9:3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurons in the rat hippocampus. J Physiol. 1988;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglóczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci. 2007;27:2058–2073. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mann EO, Mody I. The multifaceted role of inhibition in epilepsy: seizuregenesis through excessive GABAergic inhibition in autosomal dominant nocturnal frontal lobe epilepsy. Curr Opin Neurol. 2008;21:155–160. doi: 10.1097/WCO.0b013e3282f52f5f. [DOI] [PubMed] [Google Scholar]
- Martin DL, Olsen RW. GABA in the Nervous System: The View at Fifty Years. Lippincott, Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- Martina M, Royer S, Pare D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdale. J Neurophysiol. 2001;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 1964;9:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RKS. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RKS. Synchronization of inhibitory neurones in the guinea-pig hippocampus in vitro. J Physiol. 1994;477:35–45. doi: 10.1113/jphysiol.1994.sp020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RK. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U, Deisz RA, Dodt HU, Lux HD. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986;232:1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Molony CJ, Parmelee AH. Convulsions in young infats as the result of pyridoxine (vitamin B6) deficiency. J Am Med Assoc. 1954;154:405–406. doi: 10.1001/jama.1954.02940390029008. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Buzsáki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci (USA) 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Obrocea GV, Avoli M. Extracellular K+ accumulations and synchronous GABA-mediated potentials evoked by 4-aminopyridine in the adult rat hippocampus. Exp Brain Res. 1996;109:71–82. doi: 10.1007/BF00228628. [DOI] [PubMed] [Google Scholar]
- Munakata M, Watanabe M, Otsuki T, Nakama H, Arima K, Itoh M, Nabekura J, Iinuma K, Tsuchiya S. Altered distribution of KCC2 in cortical dysplasia in patients with intractable epilepsy. Epilepsia. 2007;48:837–844. doi: 10.1111/j.1528-1167.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- Munoz A, Mendez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–673. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci, USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Nardou R, Ben-Ari Y, Khalilov I. Bumetanide, an NKCC1 antagonist, does not prevent formation of epileptogenic focus but blocks epileptic focus seizures in immature rat hippocampus. J Neurophysiol. 2009;101:2878–2888. doi: 10.1152/jn.90761.2008. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Macdonald RL. GABAA receptor complex: structure and function. In: Egeberg J, Schousboe A, Krogsgaard-Larsen P, editors. Glutamate and GABA Receptors and Transporters: Structure, Function and Pharmacology. Taylor & Francis; London: 2002. pp. 202–219. [Google Scholar]
- Olsen RW, Sieghart W. International union of pharmacology. LXX Subtypes of γ-aminobutyric acida receptors: classification on the basis of subunit composition, pharmacology, and function update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Chang CS, Li G, Hanchar HJ, Wallner M. Fishing for allosteric sites on GABA(A) receptors. Biochem Pharmacol. 2004;68:1675–1684. doi: 10.1016/j.bcp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuccio G, Curia G, Colosimo A, Cruccu G, Avoli M. Epileptiform synchronization in the cingulate cortex. Epilepsia. 2009;50:521–536. doi: 10.1111/j.1528-1167.2008.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuccio G, D’Antuono M, de Guzman P, De Lannoy L, Biagini G, Avoli M. In vitro ictogenesis and parahippocampal networks in a rodent model of temporal lobe epilepsy. Neurobiol Dis. 2010;39:372–380. doi: 10.1016/j.nbd.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Apergis J, Parè D. Low-probability transmission of neocortical and entorhinal impulses through the perirhinal cortex. J Neurophysiol. 2004;91:2079–2089. doi: 10.1152/jn.01197.2003. [DOI] [PubMed] [Google Scholar]
- Perez Velazquez JL, Carlen PL. Synchronization of GABAergic interneuronal networks during seizure-like activity in the rat horizontal hippocampal slice. Eur J Neurosci. 1999;11:1–10. doi: 10.1046/j.1460-9568.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J Neurophysiol. 1989;61:953–970. doi: 10.1152/jn.1989.61.5.953. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA. The depolarization shift in “epileptic” neurons. Exp Neurol. 1968;21:467–485. doi: 10.1016/0014-4886(68)90066-6. [DOI] [PubMed] [Google Scholar]
- Prince DA, Jacobs K. Inhibitory function in two models of chronic epileptogenesis. Epilepsy Res. 1998;32:83–92. doi: 10.1016/s0920-1211(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Psarropoulou C, Avoli M. 4-Aminopyridine-induced spreading depression episodes in immature hippocampus: developmental and pharmacological characteristics. Neuroscience. 1993;55:57–68. doi: 10.1016/0306-4522(93)90454-n. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978;140:315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Bradburne RM, Harris AB. A preferential loss of GABAergic, symmetric synapses in epileptic foci: a quantitative ultrastructural analysis of monkey neocortex. J Neurosci. 1982;2:1725–1735. doi: 10.1523/JNEUROSCI.02-12-01725.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Reiffenstein RJ. Selective inhibitory synapse loss in chronic cortical slabs: a morphological basis for epileptic susceptibility. Can J Physiol Pharmacol. 1982;60:864–870. doi: 10.1139/y82-122. [DOI] [PubMed] [Google Scholar]
- Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA, De Lorenzo RJ. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc Natl Acad Sci USA. 1996;93:9665–9669. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985;54:1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. Epileptiform activity in the hippocampus produced by tetraethylammonium. J Neurophysiol. 1990;64:1077– 1088. doi: 10.1152/jn.1990.64.4.1077. [DOI] [PubMed] [Google Scholar]
- Salah A, Perkins KL. Effects of subtype-selective group I mGluR antagonists on synchronous activity induced by 4-aminopyridine/CGP 55845 in adult guinea pig hippocampal slices. Neuropharmacology. 2008;55:47–54. doi: 10.1016/j.neuropharm.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Mutani R, Prince DA. Orthodromic and antidromic effects of a cortical epileptiform focus on ventrolateral nucleus of the cat. J Neurophysiol. 1975;38:795–811. doi: 10.1152/jn.1975.38.4.795. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978;147:117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980;183:61–73. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sipilä ST, Huttu K, Yamada J, Afzalov R, Voipio J, Blaesse P, Kaila K. Compensatory enhancement of intrinsic spiking upon NKCC1 disruption in neonatal hippocampus. J Neurosci. 2009;29:6982–6988. doi: 10.1523/JNEUROSCI.0443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. ‘Dormant basket cell’ hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Smirnov S, Paalasmaa P, Uusisaari M, Voipio J, Kaila K. Pharmacological isolation of the synaptic and nonsynaptic components of the GABA-mediated biphasic response in rat CA1 hippocampal pyramidal cells. J Neurosci. 1999;19:9252–9260. doi: 10.1523/JNEUROSCI.19-21-09252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreafico R, Battaglia G, Arcelli P, Andermann F, Dubeau F, Palmini A, Olivier A, Villemure JG, Tampieri D, Avanzini G, Avoli M. Cortical dysplasia: an immunocytochemical study of three patients. Neurology. 1998;50:27–36. doi: 10.1212/wnl.50.1.27. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Hines M, Wilson WA. Axon terminal hyperexcitability associated with epileptogenisis in vitro. I Origin of ectopic spikes. J Neurophysiol. 1993a;70:961–975. doi: 10.1152/jn.1993.70.3.961. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Mott DD, Wilson WA. Axon terminal hyperexcitability associated with epileptogenisis in vitro. I Pharmacological regulation by NMDA and GABAA receptors. J Neurophysiol. 1993b;70:976–984. doi: 10.1152/jn.1993.70.3.976. [DOI] [PubMed] [Google Scholar]
- Sudbury J, Avoli M. Epileptiform synchronization in the rat insular and perirhinal cortices in vitro. Eur J Neurosci. 2007;26:3571–3582. doi: 10.1111/j.1460-9568.2007.05962.x. [DOI] [PubMed] [Google Scholar]
- Swann JW, Brady RJ. Penicillin-induced epileptogenesis in immature rat CA3 hippocampal pyramidal cells. Dev Brain Res. 1984;12:243–254. doi: 10.1016/0165-3806(84)90046-4. [DOI] [PubMed] [Google Scholar]
- Swartzwelder SH, Lewis DV, Anderson WW, Wilson WA. Seizure-like events in brain slices: supression by interictal activity. Brain Res. 1987;410:362–366. doi: 10.1016/0006-8993(87)90339-8. [DOI] [PubMed] [Google Scholar]
- Tamás G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, Cardinale F, Cossu M, Ferrario A, Galli C, Bramerio M, Citterio A, Spreafico R. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–1732. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG. Simulations of epileptiform activity in the hippocampal CA3 region in vitro. Hippocampus. 1994;4:281–285. doi: 10.1002/hipo.450040310. [DOI] [PubMed] [Google Scholar]
- Traub RD, Borck C, Collins SB, Jefferys JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Buhl EH, Jefferys JG, Faulkner HJ. On the mechanism of the gamma → beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J Neurosci. 1999;19:1088–1105. doi: 10.1523/JNEUROSCI.19-03-01088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, LeBeau FE, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci, USA. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Michelson-Law H, Bibbig AE, Buhl EH, Whittington MA. Gap junctions, fast oscillations and the initiation of seizures. Adv Exp Med Biol. 2004;548:110–122. doi: 10.1007/978-1-4757-6376-8_9. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ. The direct relationship between inhibitory currents and local field potentials. J Neurosci. 2009;29:15299–15307. doi: 10.1523/JNEUROSCI.2019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombin F, Gnatkovsky V, de Curtis M. Changes in action potential features during focal seizure discharges in the entorhinal cortex of the in vitro isolated guinea pig brain. J Neurophysiol. 2011 doi: 10.1152/jn.00207.2011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Regulation of GABAA receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intra-cerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Uusisaari M, Smirnov S, Voipio J, Kaila K. Spontaneous epileptiform activity mediated by GABA(A) receptors and gap junctions in the rat hippocampal slice following long-term exposure to GABA(B) antagonists. Neuropharmacology. 2002;43:563–572. doi: 10.1016/s0028-3908(02)00156-9. [DOI] [PubMed] [Google Scholar]
- Uva L, Librizzi L, Wendling F, de Curtis M. Propagation dynamics of epileptiform activity in the hippocampal-parahippocampal region of the isolated guinea pig brain. Epilepsia. 2005;46:1914–1925. doi: 10.1111/j.1528-1167.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Uva L, Avoli M, de Curtis M. Synchronous GABAA receptor-dependent potentials in limbic areas of the in vitro isolated adult guinea pig brain. Eur J Neurosci. 2009;29:911–920. doi: 10.1111/j.1460-9568.2009.06672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden S, Panzica F, de Curtis M. Carbachol induces fast oscillations in the medial but not in the lateral entorhinal cortex of the isolated guinea pig brain. J Neurophysiol. 1999;82:2441–2450. doi: 10.1152/jn.1999.82.5.2441. [DOI] [PubMed] [Google Scholar]
- van Groen T, Lopes da Silva FH. Organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat: II. An electrophysiological study. J Comp Neurol. 1986;251:111–120. doi: 10.1002/cne.902510108. [DOI] [PubMed] [Google Scholar]
- van Groen T, van Haren FJ, Witter MP, Groenewegen HJ. The organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat: I. A neuroanatomical tracing study. J Comp Neurol. 1986;250:485–497. doi: 10.1002/cne.902500407. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Tolner EA, Lopes da Silva FH, Gorter JA. Progression of temporal lobe epilepsy in the rat is associated with immunocytochemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp Neurol. 2004;187:367–379. doi: 10.1016/j.expneurol.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Hellstrom-Westas L, de Vries LS. Bumetanide for neonatal seizures: based on evidence or enthusiasm? Epilepsia. 2009;50:1292–1293. doi: 10.1111/j.1528-1167.2008.01894.x. [DOI] [PubMed] [Google Scholar]
- Velazquez JL, Carlen PL. Synchronization of GABAergic interneuronal networks during seizure-like activity in the rat horizontal hippocampal slice. Eur J Neurosci. 1999;11:4110–4118. doi: 10.1046/j.1460-9568.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl− cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuyl RA, Albus H. Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res. 1985;342:54–66. doi: 10.1016/0006-8993(85)91352-6. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Bracci E, Jefferys JG. Layer-specific pyramidal cell oscillations evoked by tetanic stimulation in the rat hippocampal area CA1 in vitro and in vivo. J Physiol. 2005;562:149–164. doi: 10.1113/jphysiol.2004.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther H, Lambert JD, Jones RS, Heinemann U, Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986;69:156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Bellanger JJ, Chauvel P. Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Eur J Neurosci. 2002;15:1499–1508. doi: 10.1046/j.1460-9568.2002.01985.x. [DOI] [PubMed] [Google Scholar]
- White JA, Chow CC, Ritt J, Soto-Treviño C, Kopell N. Synchronization and oscillatory dynamics in heterogeneous, mutually inhibited neurons. J Comput Neurosci. 1998;5:5–16. doi: 10.1023/a:1008841325921. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Stanford IM, Colling SB, Jefferys JG, Traub RD. Spatiotemporal patterns of gamma frequency oscillations tetanically induced in the rat hippocampal slice. J Physiol. 1997;502:591–607. doi: 10.1111/j.1469-7793.1997.591bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Inhibitory interneurons and network oscillations in vitro. TINS. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Williams S, Vachon P, Lacaille JC. Monosynaptic GABA-mediated inhibitory postsynaptic potentials in CA1 pyramidal of hyperexcitable hippocampal slices from kainic acid-treated rats. Neuroscience. 1993;52:541–554. doi: 10.1016/0306-4522(93)90404-4. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Engel J., Jr . Electrical stimulation of the human epileptic limbic cortex. In: Devinski O, Bekic A, Dogali M, editors. Electrical and Magnetic Stimulation of the Brain and Spinal Cord. Raven Press; New York: 1993. pp. 103–113. [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- Wittner L, Miles R. Factors defining a pacemaker region for synchrony in the hippocampus. J Physiol. 2007;584:867–883. doi: 10.1113/jphysiol.2007.138131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Prince DA. Dendritic mechanisms underlying penicillin-induced epileptiform activity. Science. 1979;204:1228–1231. doi: 10.1126/science.451569. [DOI] [PubMed] [Google Scholar]
- Wong RK, Stewart M. Different firing patterns generated in dendrites and somata of CA1 pyramidal neurones in guinea-pig hippocampus. J Physiol. 1992;457:675–687. doi: 10.1113/jphysiol.1992.sp019401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Traub RD, Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol. 1986;44:583–592. [PubMed] [Google Scholar]
- Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro”. Science. 2003;301:463. doi: 10.1126/science.1084237. [DOI] [PubMed] [Google Scholar]
- Wozny C, Gabriel S, Jandova K, Schulze K, Heinemann U, Behr J. Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol Dis. 2005a;19:451–460. doi: 10.1016/j.nbd.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Wozny C, Knopp A, Lehmann TN, Heinemann U, Behr J. The subiculum: a potential site of ictogenesis in human temporal lobe epilepsy. Epilepsia. 2005b;46(Suppl 5):17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA, slow synaptic inhibition in mouse hippocampus. J Neurophysiol. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann EC, Glaser GH. Hippocampal epileptic activity induced by localized ventricular perfusion with high-potassium cerebrospinal fluid. Exp Neurol. 1968;20:87–110. doi: 10.1016/0014-4886(68)90126-x. [DOI] [PubMed] [Google Scholar]