Abstract
Importance
Type 1 diabetes usually has a preclinical phase identified by circulating islet autoantibodies, but the rate of progression to diabetes after seroconversion to islet autoantibodies is uncertain.
Objective
To determine the rate of progression to diabetes after islet autoantibody seroconversion.
Design, Setting, and Participants
Data were pooled from prospective cohort studies performed in Colorado (recruitment, 1993-2006), Finland (recruitment, 1994-2009), and Germany (recruitment, 1989-2006) examining children genetically at risk for type 1 diabetes for the development of insulin autoantibodies, glutamic acid decarboxylase 65 (GAD65) autoantibodies, insulinoma antigen 2 (IA2) autoantibodies, and diabetes. Participants were all children recruited and followed up in the 3 studies (Colorado, 1962; Finland, 8597; Germany, 2818). Follow-up assessment in each study was concluded by July 2012.
Main Outcomes and Measures
The primary analysis was the diagnosis of type 1 diabetes in children with 2 or more autoantibodies. The secondary analysis was the diagnosis of type 1 diabetes in children with 1 autoantibody or no autoantibodies.
Results
Progression to type 1 diabetes at 10-year follow-up after islet autoantibody seroconversion in 585 children with multiple islet autoantibodies was 69.7% (95% CI, 65.1%-74.3%), and in 474 children with a single islet autoantibody was 14.5% (95% CI, 10.3%-18.7%). Risk of diabetes in children who had no islet autoantibodies was 0.4% (95% CI, 0.2%-0.6%) by the age of 15 years. Progression to type 1 diabetes in the children with multiple islet autoantibodies was faster for children who had islet autoantibody seroconversion younger than age 3 years (hazard ratio [HR], 1.65 [95% CI, 1.30-2.09; P < .001]; 10-year risk, 74.9% [95% CI, 69.7%-80.1%]) vs children 3 years or older (60.9% [95% CI, 51.5%-70.3%]); for children with the human leukocyte antigen (HLA) genotype DR3/DR4-DQ8 (HR, 1.35 [95% CI, 1.09-1.68; P=.007]; 10-year risk, 76.6% [95% CI, 69.2%-84%]) vs other HLA genotypes (66.2% [95% CI, 60.2%-72.2%]); and for girls (HR, 1.28 [95% CI, 1.04-1.58; P=.02];10-year risk, 74.8% [95% CI, 68.0%-81.6%]) vs boys (65.7% [95% CI, 59.3%-72.1%]).
Conclusions and Relevance
The majority of children at risk of type 1 diabetes who had multiple islet autoantibody seroconversion progressed to diabetes over the next 15 years. Future prevention studies should focus on this high-risk population.
Type 1 diabetes is a chronic autoimmune disease that often manifests during childhood and adolescence.1 The life-long requirement for insulin injections and the many complications that follow the diagnosis can be difficult for those affected.2 Type 1 diabetes usually has a preclinical phase that can be identified by the presence of autoantibodies to antigens of the pancreatic β cells.
Several studies that have performed cross-sectional screening of individuals genetically at risk of islet autoantibodies have demonstrated that the presence of circulating islet autoantibodies is associated with an increased risk of developing type 1 diabetes.3 These studies have also shown that risk is dependent on β-cell function and the diversity and titer of these autoantibodies, with greatest risk associated with the presence of 2 or more autoantibodies and impaired glucose tolerance.
Several intervention studies have been undertaken in which eligibility is based on these markers.4-7 However, follow-up studies of individuals identified by cross-sectional screening cannot determine a true progression rate because they do not identify when a patient develops islet autoantibodies. Importantly, patients who progress rapidly from initial seroconversion to type 1 diabetes are missed in these studies.
Recently, 3 long-term studies in Germany, Finland, and Colorado have followed up children from birth and have an opportunity to determine both the age of seroconversion and the age at diabetes onset.7-9 These studies have shown that islet autoantibody seroconversion is relatively common in the first years of life. However, information about diabetes progression rates after seroconversion and the factors that affect the rate of progression is still limited. In this study, we pooled data from these 3 studies to estimate rates of disease progression and associated characteristics based on islet autoantibody status.
METHODS
Study Populations
All 3 studies were approved by institutional review boards. Written informed consent was obtained from parents or legal guardians for each participant.
Data from prospective birth cohort studies were combined for this analysis. The Colorado Diabetes Autoimmunity Study in the Young (DAISY) study,8 the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study,7 and the German BABYDIAB9 and BABYDIET10 studies were undertaken to investigate the natural history of islet autoimmunity and type 1 diabetes in children with increased genetic risk of type 1 diabetes. The studies were homogeneous in the definition of islet autoantibody seroconversion and type 1 diabetes and were similar in the inclusion of at-risk populations and follow-up design. Additionally, the DIPP study included an intervention to evaluate efficacy of intranasally administered insulin to reduce progression to diabetes in children with multiple islet autoantibodies, and the high-risk study, BABYDIET, included investigation of whether delay of exposure to gluten could reduce the risk of developing islet autoantibodies in children who are genetically at risk. These interventions failed to show an effect on the progression rate to diabetes and islet autoimmunity,7,10 and all children underwent follow-up after completion of the intervention within a natural history protocol.
The DAISY study recruited newborns and infants at risk of type 1 diabetes with human leukocyte antigen (HLA) DR/DQ genotypes born at St Joseph’s Hospital (Denver) from 1993 through 2006 and also children who had a first-degree relative with type 1 diabetes who was treated at the Barbara Davis Center, as previously described.8 Children enrolled in the study were scheduled for follow-up and islet autoantibody measurement at age 9, 15, and 24 months and yearly thereafter or every 3 to 6 months if autoantibody positive.
The DIPP study recruited newborns and infants at risk of type 1 diabetes with HLA DR/DQ genotypes from 3 clinical centers in Oulu, Tampere, and Turku from 1994 through 2009, as previously described.7 Children recruited from Oulu and Tampere were scheduled for follow-up and islet autoantibody measurement at age 3, 6, 12, 18, and 24 months and yearly thereafter, and children recruited in Turku were scheduled for the same follow-up procedures every 3 months until 2 years of age and every 6 months thereafter.
The BABYDIAB study recruited newborns and infants who had a mother or father with type 1 diabetes (1989-2000), and the BABYDIET study recruited newborns who had a first-degree relative with type 1 diabetes (2000-2006), as previously described.9,10 Children recruited into the BABYDIAB or BABYDIET studies were scheduled for follow-up and islet autoantibody measurement at age 9 months, 2 years, and every 3 years thereafter. BABYDIET scheduled 150 high-risk children participating in dietary intervention for follow-up and islet autoantibody measurements every 3 months until 3 years of age and yearly thereafter.10 Children considered to be at high risk were those with the HLA genotypes DR3/4-DQ8, DR4-DQ8/DR4-DQ8, or DR3/3 and children who had 2 or more first-degree relatives with type 1 diabetes.
All 3 studies measured autoantibodies against insulin, glutamic acid decarboxylase 65 (GAD65), and insulinoma antigen 2 (IA2) from multiple samples taken throughout childhood to identify the age of islet autoantibody seroconversion. Outcome in the prospective studies was the development of islet autoantibodies with subsequent follow-up for type 1 diabetes. Islet autoantibody seroconversion was defined as a positive test result for 1 or more islet autoantibodies in at least 2 serial samples or in 1 sample followed by the development of diabetes before the next follow-up visit. All children with islet autoantibody seroconversion (2 positive samples) were included in our study analyses. Children who did not reach islet autoantibody seroconversion but had at least 1 sample tested from scheduled visits in either Colorado or Germany or at least 3 samples tested in the Finnish study (which had more scheduled visits) were included in our study analyses and were identified as islet autoantibody negative. The primary analysis included those who developed multiple autoantibodies. The secondary analysis included children with only 1 autoantibody or no autoantibodies. Autoantibodies against insulin, GAD65, and IA2 were determined in all follow-up samples with previously described methods.9,11,12 Zinc transporter 8 autoantibodies were additionally measured in children with islet autoantibodies from the Colorado and Germany cohorts and progression to diabetes in children with 2 or more of the 4 islet autoantibodies reported separately.13
The primary analysis was diabetes diagnosed using World Health Organization and American Diabetes Association criteria.14 Children participated in follow-up visits until July 2012 or until the development of diabetes. Families were asked to report the occurrence of diabetes symptoms. In children with islet autoantibodies, an annual oral glucose tolerance test was performed. Diabetes onset was defined as unequivocal hyperglycemia with acute metabolic decompensation; the observation on at least 2 occasions of a 2-hour plasma glucose greater than 200 mg/dL (to convert to millimoles per liter, multiply by 0.0555) after an oral glucose test; or a random blood glucose concentration greater than 200 mg/dL accompanied by unequivocal symptoms. Since 1997, fasting blood glucose greater than 126 mg/dL on 2 occasions was added to the diabetes diagnosis criteria.14 Families of children who dropped out of the study or refused to provide blood samples or perform oral glucose tolerance tests were regularly contacted by telephone and were asked if the child had developed diabetes. In case of loss to follow-up, local diabetes registries or cohort studies were used as a second source to obtain information on diabetes development of former study participants. Children who had not developed diabetes and could not be contacted for 3 or more years were considered lost to follow-up.
Statistical Analysis
Time-to-event analysis was used to examine progression from seroconversion to diabetes. Kaplan-Meier estimates were used to calculate risk and to compare probabilities of type 1 diabetes in children stratified by country. The time-to-event was calculated from the age at seroconversion to the age at diagnosis of diabetes or the age at last follow-up. For children who did not develop islet autoantibodies, the median age of seroconversion in children with multiple islet autoantibodies (2.1 years) was used as a proxy for seroconversion age. Risk was presented at 5-, 10-, and 15-year follow-up visits after seroconversion (or proxy), and by 15 years of age. The log-rank test was used to compare categories in the Kaplan-Meier estimates.
Associations between age at seroconversion, sex, HLA genotype, number of islet autoantibodies, and type 1 diabetes risk were analyzed by the Cox proportional hazards regression model, assessing hazard ratios (HRs) with corresponding 95% confidence intervals. Children with missing data were not included in the model. Proportionality in the model was tested by generating time-dependent covariates through creating and including interactions of the predictors and the logarithm of survival time. If any of the time-dependent covariates were significant, those predictors were not considered proportional. In order to account for potential heterogeneity among the 3 cohorts, we included random effects for the different studies in the Cox model, thus using a meta-analytical approach for individual participant data, which accounts for the variance both within and between studies. Specifically, a shared frailty model for clustered data with normally distributed random effects was calculated with residual maximum likelihood estimation of the variance parameter.
The age at islet autoantibody seroconversion and the follow-up time after seroconversion were compared between cohorts using the Mann-Whitney U test. Comparisons of islet autoantibody status, HLA genotype, and sex among children who developed diabetes within 10 years and those who did not develop diabetes for more than 10 years was performed by the Fisher exact test. For all analyses, a 2-tailed P value of .05 was considered significant. All statistical analyses were performed using SAS, version 9.2 (SAS Institute).
RESULTS
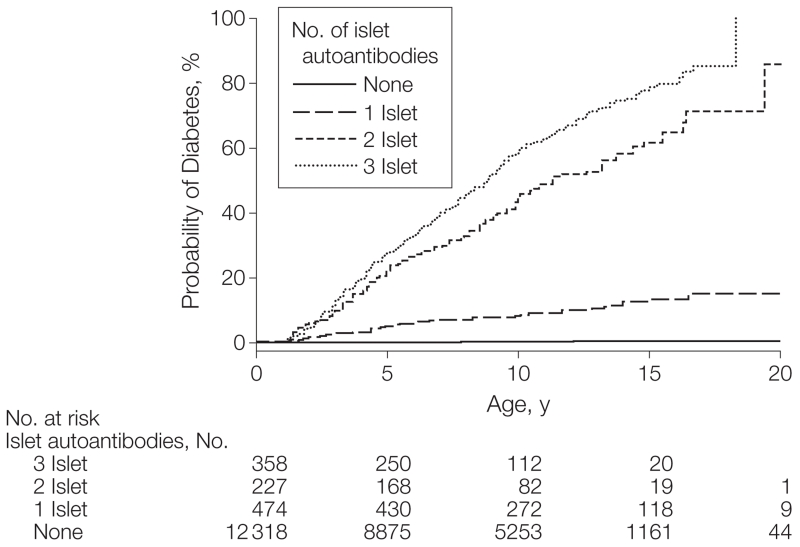
Altogether, 13 377 children enrolled into the prospective studies in Colorado (n=1962), Finland (n=8597), and Germany (n=2818) were tested for autoantibodies against insulin, GAD65, and IA2 at scheduled visits. A total of 1059 children (7.9%) seroconverted to islet autoantibody–positive (460; 43.4% girls), and 12 318 children (92.1%) remained islet autoantibody–negative (5807; 47.1% girls) (Table 1). Of the 1059 children with islet autoantibodies, 585 children (4.4%) developed multiple islet autoantibodies (249; 42.6% girls), including 69 (3.5%) from Colorado, 399 (4.6%) from Finland, and 117 from (4.2%) Germany. The remaining 474 children (3.5%) were single islet autoantibody–positive; 71 (3.6%) in Colorado, 293 (3.4%) in Finland, 110 (3.9%) in Germany. A total of 428 children developed diabetes, including 25 children who progressed to diabetes without an islet autoantibody–positive sample prior to diabetes onset (8 in Colorado, 13 in Finland, and 4 in Germany). Diabetes risk by 15 years of age was 0.4% (95% CI, 0.2%-0.6%) in children with no autoantibodies, 12.7% (95% CI, 8.9%-16.5%) in children with a single islet autoantibody, 61.6% (95% CI, 53%-70.2%) in children with 2 islet autoantibodies, and 79.1% (95% CI, 73.3%-85%) in children with 3 islet autoantibodies (Figure 1).
Table 1.
Description of Study Population
| No. /Total (%) of Participants | ||||
|---|---|---|---|---|
| All | Colorado | Finland | Germany | |
| Multiple islet autoantibody–positive | 585/13 377 (4.4) | 69/1962 (3.5) | 399/8597 (4.6) | 117/2818 (4.2) |
| Girls | 249/585 (42.6) | 34/69 (49.3) | 164/399 (41.1) | 51/117 (43.6) |
| Family history of type 1 diabetes | 201/585 (34.4) | 39/69 (56.5) | 45/399 (11.3) | 117/117 (100) |
| HLA DR3/DR4-DQ8 | 185/577 (32.1)a | 31/69 (44.9) | 119/399 (29.8) | 35/109 (32.1)a |
| Follow-up time, median (IQR), yb | 4.5 (2.3-7.2) | 5.6 (3.5-7.7) | 3.9 (2.2-6.9) | 5.3 (2.9-7.7) |
| Seroconversion age, median (IQR), y | 2.1 (1.3-4.1) | 3.1 (1.6-5.4) | 2.0 (1.3-4.0) | 2.1 (1.1-5.0) |
| Nonwhite race/ethnicity | 8/585 (1.4) | 6/69 (8.7) | 0/399 (0) | 2/117 (1.7) |
| Single islet autoantibody–positive | 474/13 377 (3.5) | 71/1962 (3.6) | 293/8597 (3.4) | 110/2818 (3.9) |
| Girls | 211/474 (44.5) | 34/71 (47.9) | 115/293 (39.2) | 62/110 (56.4) |
| Family history of type 1 diabetes | 161/474 (34.0) | 35/71 (49.3) | 17/293 (5.8) | 110/110 (100) |
| HLA DR3/DR4-DQ8 | 79/450 (17.5)a | 20/71 (28.2) | 51/276 (18.5)a | 8/103 (7.8)a |
| Follow-up time, median (IQR), yb | 5.5 (2.6-8.5) | 5.5 (2.9-7.8) | 5.5 (2.4-8.2) | 5.9 (2.8-10) |
| Seroconversion age, median (IQR), y | 4.8 (2.1-7.7) | 5.4 (2.6-9.2) | 3.9 (2.0-6.5) | 7.3 (3.3-9.8) |
| Nonwhite race/ethnicity | 9/474 (1.9) | 7/71 (9.9) | 0/293 (0) | 2/110 (1.8) |
| Autoantibody negative | 12 318/13 377 (92.1) | 1822/1962 (92.8) | 7905/8597 (91.9) | 2591/2818 (91.9) |
| Girls | 5807/12 318 (47.1) | 882/1822 (48.4) | 3662/7905 (46.3) | 1263/2591 (48.7) |
| Family history of type 1 diabetes | NA | 629/1822 (34.5) | NA | 2591/2591 (100) |
| HLA DR3/DR4-DQ8 | 1200/7298 (16.4)a | 404/1822 (22.2) | 613/4488 (13.7)a | 183/2188 (8.4)a |
| Follow-up time, median (IQR), yb | 8.9 (4.1-12.6) | 9.0 (3.3-14) | 8.9 (4.3-12.1) | 8.2 (4.8-13.5) |
| Nonwhite race/ethnicity | 204/13 377 (1.5) | 164/1822 (9.0) | 0/7905 (0) | 40/2591 (1.5) |
Abbreviations: HLA, human leukocyte antigen; IQR, interquartile range; NA, not available.
Full HLA DR-DQ genotype was unavailable in 8 children with multiple islet antibodies, 7 children with a single islet autoantibody, and 403 children with no islet autoantibodies from Germany; 17 children with a single islet autoantibody, and 3417 children with no islet autoantibodies from Finland.
Follow-up time from the age of seroconversion to the age at diabetes or last contact.
Figure 1.
Development of Diabetes in Children Stratified for Islet Autoantibody Outcome
The numbers at risk represent the children receiving follow-up at age 0, 5, 10, 15, and 20 years.
Children With Multiple Islet Autoantibodies
The median age at seroconversion in the 585 children with multiple islet autoantibodies was 2.1 years (range, 0.5-16 years; interquartile range [IQR], 1.3-4.1 years), and was slightly higher in children from Colorado (3.1 years) than in children from Finland (2.0 years) and Germany (2.1 years) (P=.003).
Median follow-up time after seroconversion in children with multiple islet autoantibodies was 4.5 years (range, 0-19.6 years; IQR, 2.3-7.2 years; 3021 total follow-up years), and was slightly shorter in children from Finland (3.9 years) than in children from Colorado (5.6 years) and Germany (5.3 years) (P=.04).
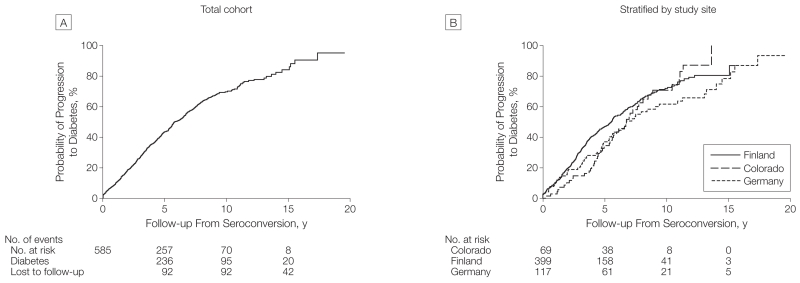
A total of 355 children (60.7%) with multiple islet autoantibodies progressed to diabetes (HR compared withchildren with no autoantibodies, 395.6 [95% CI, 263.2-594.4], P<.001) at a median follow-up time after seroconversion of 3.5 years (IQR, 1.7-6.0 years), and a median age of 6.1 years (IQR, 3.5-9.2 years). Progression to diabetes after seroconversion was 43.5% (95% CI, 39.4%-47.8%) at 5-year follow-up, 69.7% (95% CI, 65.1%-74.3%) at the 10-year follow-up, and 84.2% (95% CI, 77.7%-89.7%) at the 15-year follow-up (Figure 2). Ten-year risks were not significantly different (P=.08 for the log-rank test) across the 3 cohorts: Colorado, 70.8% (95% CI, 57.3%-83.2%); Finland, 71.9% (95% CI, 66.2%-77.3%); and Germany, 61.7% (95% CI, 51.3%-72.2%).
Figure 2.
Progression to Diabetes From the Time of Seroconversion in Children With Multiple Islet Autoantibodies
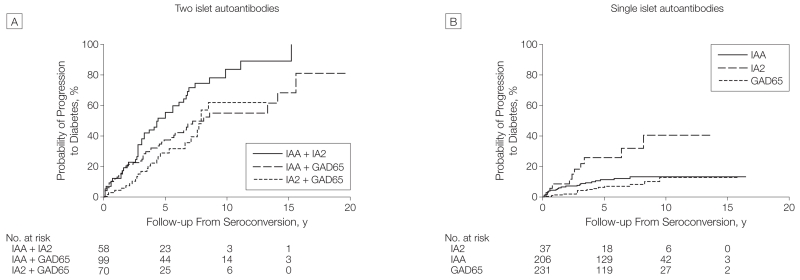
After 10 years of follow-up, 331 children with multiple autoantibodies had developed diabetes, 70 did not develop diabetes, and 184 had not reached 10 years of follow-up. Girls were more frequent among the children who developed diabetes within 10 years (151 of 331) than children who did not develop diabetes (21 of 70, P=.02). In the multivariable Cox proportional hazards regression model (Table 2), faster progression to diabetes after seroconversion was associated with younger age at seroconversion (<3 years vs ≥3 years; HR,1.65 [95% CI, 1.30-2.09], P<.001; 10-year risk of 74.9% [95% CI, 69.7%-80.1%] at age <3 years vs 60.9% [95% CI, 51.5%-70.3%] at age ≥3 years); HLA DR3/DR4-DQ8 genotype (HR, 1.35 [95% CI, 1.09-1.68], P=.007; 10-year risk of 76.6% [95% CI, 69.2%-84.0%] for HLA DR3/4-DQ8 vs 66.2% [95% CI, 60.2%-72.2%] for other HLA genotypes); and female sex (HR,1.28 [95% CI, 1.04-1.58], P=.02; 10-year risk of 74.8% [95% CI, 68.0%-81.6%] for girls vs 65.7% [95% CI, 59.3%-72.1%] for boys). Within the subgroup of children with 2 islet autoantibodies, progression to diabetes within 10 years after seroconversion was increased in children with the combination of autoantibodies against insulin and IA2 (83.6% [95% CI, 70.1%-97.1%]) than in children with autoantibodies against insulin and GAD65 (55.1% [95% CI, 41.4%-68.8%], P=.006) and children with autoantibodies against GAD65 and IA2 (62.0% [95% CI, 43.3%-90.7%], P = .002) (Figure 3A). In the Colorado and German cohorts, the addition of zinc transporter 8 autoantibodies identified 7 more children (6%) who progressed to diabetes but did not substantially alter the estimates of diabetes progression: 10-year risk of 68.9% (95% CI, 55.5%-82.3%) in Colorado and 65.3% (95% CI, 54.9%-75.7%) in Germany.
Table 2.
Predictors of Type 1 Diabetes After Seroconversion in Children With Multiple Islet Autoantibodies
| Variable | 10-Year Risk, % (95% CI) |
Bivariable Hazard Ratio (95% CI) |
P Value |
Multivariable Hazard Ratio (95% CI)a |
P Value |
|---|---|---|---|---|---|
| Serocoversion age, y | |||||
| <3 | 74.9 (69.7-80.1) | 1.72 (1.36-2.17) | <.001 | 1.65 (1.30-2.09) | <.001 |
|
|
|
||||
| ≥3 | 60.9 (51.5-70.3) | 1 [Reference] | 1 [Reference] | ||
|
| |||||
| HLA genotype | |||||
| DR3/DR4-DQ8 | 76.6 (69.2-84.0) | 1.40 (1.12-1.73) | .003 | 1.35 (1.09-1.68) | .007 |
|
|
|
||||
| Other | 66.2 (60.2-72.2) | 1 [Reference] | 1 [Reference] | ||
|
| |||||
| Sex | |||||
| Girls | 74.8 (68.0-81.6) | 1.30 (1.05-1.60) | .02 | 1.28 (1.04-1.58) | .02 |
|
|
|
||||
| Boys | 65.7(59.3-72.1) | 1 [Reference] | 1 [Reference] | ||
|
| |||||
| No. of autoantibodies | |||||
| 3 | 72.1 (66.5-77.7) | 1.27 (1.02-1.59) | .04 | 1.19 (0.95-1.49) | .14 |
|
|
|
||||
| 2 | 65.1 (56.3-73.9) | 1 [Reference] | 1 [Reference] | ||
Multivariable hazard ratio includes all listed variables (seroconversion age, human leukocyte antigen genotype, sex, number of autoantibodies).
Figure 3.
Progression to Diabetes in Children From the Time of Seroconversion According to Islet Autoantibody Type
IAA indicates insulin autoantibodies; IA2, insulinoma antigen 2 autoantibodies; and GAD65, glutamic acid decarboxylase 65 autoantibodies. The numbers at risk represent the children receiving follow-up at year 0, 5, 10, and 15.
Children With a Single Islet Autoantibody
The median age of seroconversion in the 474 children with a single islet autoantibody was 4.8 years (range, 0.5-18.4 years; IQR, 2.1-7.7 years). Of these, 206 had insulin autoantibodies; 231, GAD65 autoantibodies; and 37, IA2 autoantibodies. The median follow-up time after seroconversion in children with a single islet autoantibody was 5.5 years (range, 0.1-16.5 years; IQR, 2.6-8.5 years; 2779 total follow-up years). In total, 48 children (10%) progressed to diabetes (HR compared with children with no autoantibodies, 52.7 [95% CI, 32.4-85.7], P<.001) at a median follow-up time after seroconversion of 2.6 years (IQR, 0.6-4.1 years), and a median age of 5.2 years (IQR, 2.9-10 years). Progression to diabetes within 10 years after seroconversion was 14.5% (95% CI, 10.3%-18.7%). Ten-year risks were not significantly different (log-rank test, P=.69) across the 3 cohorts: Colorado, 17.7% (95% CI, 4.1%-31.3%); Finland, 13.3% (95% CI, 9.9%-17.7%); Germany, 14.7% (95% CI, 5.1%-25.3%). Progression to diabetes within 10 years was higher in children with IA2 autoantibodies (40.5% [95% CI, 17.7%-63.3%]) than in children with GAD65 autoantibodies (12.9% [95% CI, 5.1%-20.7%], P<.001) and children with insulin autoantibodies (13.1% [95% CI, 8.0%-18.2%], P=.005) (Figure 3B).
DISCUSSION
These data show that the detection of multiple islet autoantibodies in children who are genetically at risk marks a preclinical stage of type 1 diabetes. Only a minority of these children did not develop diabetes for more than a decade regardless of whether they had a family history of type 1 diabetes or a high-risk HLA genotype. Thus, the development of multiple islet autoantibodies in children predicts type 1 diabetes. While most children with multiple islet autoantibodies progressed to diabetes, progression time to diabetes after seroconversion was heterogeneous. In our study, it ranged from weeks to 18 years and has been reported to take more than 2 decades in some individuals.15 Variation in progression time was associated with the age of seroconversion, genetic markers, sex, and the type of islet autoantibody. A faster rate of progression in children with early seroconversion was previously reported in a subset of the German cohort.16 Variation in risk depending on islet autoantibody type is consistent with findings in studies using cross-sectional screening to identify patients with islet autoantibodies.17-21
Islet autoantibodies are generally considered markers, rather than mediators, of β-cell dysfunction. Exposure to islet autoantibodies is insufficient to cause disease because maternal transfer of autoantibodies to the fetus does not increase the risk of type 1 diabetes in offspring.22 Moreover, none of the known islet autoantigens are expressed on the β-cell surface, and no direct effect of islet autoantibodies on β-cell function has been reproducibly observed.23 However, a pathogenetic role of the autoantibodies cannot be excluded. The observation made in this study that multiple islet autoantibodies are associated with highest risk of type 1 diabetes, together with previous indications that risk is associated with the titer of some islet autoantibodies, is consistent with a role in pathogenesis.19,20,24 Autoantigens are released as a consequence of β-cell death, permitting islet autoantibodies to bind and form immune complexes that are likely to promote islet inflammation. Specific islet autoantibody–antigen complexes may also accelerate the spread of autoimmunity to multiple targets via an opsonization-like process. IA2 autoantibodies, which were associated with a higher risk than GAD65 autoantibodies or insulin autoantibodies, may be particularly effective in epitope spreading because they not only bind to IA2 but also to IA2-β, which is also expressed in the β cell and a target autoantigen.25
Our study has important limitations. Data on potentially relevant socioeconomic, environmental, and clinical factors were not collected in all 3 studies and could not be included in prediction analyses. It is possible that seroconversions were missed in children who may have had transient circulating autoantibodies between visits. This would mainly have affected estimates from the subset of children in the German study with visit intervals of up to 3 years. Relevant to this limitation, around 10% of children with islet autoantibodies who were screened at intervals of 12 months or less had transient antibodies, and, consistent with a previous report,26 almost all had single islet autoantibodies. The cohort enrolled only children who were genetically susceptible into follow-up, and entry criteria for islet autoantibody screening differed among the 3 studies. A sizeable portion of type 1 diabetes diagnoses will occur among children with lower genetic risk than those included in the cohort, and a relatively large number of patients with type 1 diabetes are diagnosed in adulthood. Thus, it is possible that the findings may not be generalizable to all presentations of childhood type 1 diabetes and type 1 diabetes in adults.
Type 1 diabetes is currently not preventable in children who develop multiple islet autoantibodies. Our findings highlight the need for research into finding interventions to stop the development of multiple islet autoantibodies and to stop or delay progression to type 1 diabetes. Children with islet autoantibodies who do not develop diabetes for more than 15 years and the factors associated with slower progression (such as sex and age at seroconversion) should also be studied, because it may be helpful for understanding natural protective mechanisms.
Acknowledgments
Funding/Support: Primary support for this work was provided by grants 1-2000-619 (BABYDIAB) and 4-1999-731 and 4-2001-435 (DIPP) from the Juvenile Diabetes Research Foundation; R01 DK32493 (DAISY) from the National Institutes of Health; 01KD89030 and 1KD9601 (BABYDIAB) from Bundesministerium für Bildung und Forschung; and ZI-310/14-1, ZI-310/14-2, ZI-310/14-3, and ZI-310/14-4(BABYDIET)from Deutsche Forschungsgemeinschaft.
Role of the Sponsor: The Juvenile Diabetes Research Foundation, National Institutes of Health, Bundesministerium für Bildung und Forschung, and Deutsche Forschungsgemeinschaft had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank Fran Dong, MS (Barbara Davis Center for Childhood Diabetes, University of Colorado), and Andreas Beyerlein, PhD (Institute of Diabetes Research, Helmholtz Zentrum München), for statistical analyses. Ms Dong and Dr Beyerlein did not receive additional compensation.
Footnotes
Author Contributions: Dr Ziegler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Ziegler, Rewers, and O. Simell had equal contribution as first authors.
Study concept and design: Ziegler, Rewers, O. Simell, T. Simell, Ilonen, Veijola, Bonifacio.
Acquisition of data: Ziegler, Rewers, O. Simell, T. Simell, Lempainen, Steck, Winkler, Ilonen, Veijola, Knip.
Analysis and interpretation of data: Ziegler, Rewers, O. Simell, T. Simell, Lempainen, Winkler, Ilonen, Veijola, Bonifacio, Eisenbarth.
Drafting of the manuscript: Ziegler, Bonifacio, Eisenbarth.
Critical revision of the manuscript for important intellectual content: Ziegler, Rewers, O. Simell, T. Simell, Lempainen, Steck, Winkler, Ilonen, Veijola, Knip, Bonifacio.
Statistical analysis: Ziegler, Rewers, Lempainen, Winkler, Veijola, Bonifacio.
Obtained funding: Ziegler, Rewers, O. Simell, Ilonen, Veijola.
Administrative, technical, or material support: Ziegler, Rewers, Lempainen, Winkler, Bonifacio.
Study supervision: Ziegler, Rewers, O. Simell, Ilonen, Veijola, Eisenbarth.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ziegler reports receiving grant funding from the Juvenile Diabetes Research Foundation, Deutsche Forschungsgemeinschaft, and Bundesministerium für Bildung und Forschung. Dr O. Simell reports receiving grant funding from the Juvenile Diabetes Research Foundation International. Dr Steck reports receiving grant support from the National Institutes of Health. Dr Veijola reports receiving grant support from the Juvenile Diabetes Research Foundation and Oulu University Hospital Research funds, and funding for travel from the Juvenile Diabetes Research Foundation; and serving as board member for Novo Nordisk Diabetes, Finland, and Medtronic Nordic; and having pending grants from the National Institutes of Health for the Environmental Determinants of Diabetes in the Young (TEDDY) and the Trial to Reduce Insulin-Dependent Diabetes Mellitus in the Genetically at Risk (TRIGR) international studies, Alma and K.A. Snellman Foundation, and the Foundation for Pediatric Research, Finland. Dr Bonifacio reports receiving grant funding from the Juvenile Diabetes Research Foundation. No other financial disclosures were reported.
REFERENCES
- 1.Eisenbarth GS. Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med. 1986;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Milton B, Holland P, Whitehead M. The social and economic consequences of childhood-onset type 1 diabetes mellitus across the lifecourse: a systematic review. Diabet Med. 2006;23(8):821–829. doi: 10.1111/j.1464-5491.2006.01796.x. [DOI] [PubMed] [Google Scholar]
- 3.Tarn AC, Thomas JM, Dean BM, et al. Predicting insulin-dependent diabetes. Lancet. 1988;1(8590):845–850. doi: 10.1016/s0140-6736(88)91601-7. [DOI] [PubMed] [Google Scholar]
- 4.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 6.Vehik K, Cuthbertson D, Ruhlig H, Schatz DA, Peakman M, Krischer JP. DPT-1 and TrialNet Study Groups. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care. 2011;34(7):1585–1590. doi: 10.2337/dc11-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372(9651):1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 8.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 1996;39(7):807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABY-DIAB study. Diabetes. 1999;48(3):460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 10.Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34(6):1301–1305. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kukko M, Kimpimäki T, Korhonen S, et al. Dynamics of diabetes-associated autoantibodies in young children with human leukocyte antigen-conferred risk of type 1 diabetes recruited from the general population. J Clin Endocrinol Metab. 2005;90(5):2712–2717. doi: 10.1210/jc.2004-1371. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Rewers M, Gianani R, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81(12):4264–4267. doi: 10.1210/jcem.81.12.8954025. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia. 2009;52(9):1881–1888. doi: 10.1007/s00125-009-1438-0. [DOI] [PubMed] [Google Scholar]
- 14.Puavilai G, Chanprasertyotin S, Sriphrapradaeng A, World Health Organization Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria. Diabetes Res Clin Pract. 1999;44(1):21–26. doi: 10.1016/s0168-8227(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 15.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care. 2010;33(6):1206–1212. doi: 10.2337/dc09-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hummel M, Bonifacio E, Schmid S, Walter M, Knopff A, Ziegler AG. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med. 2004;140(11):882–886. doi: 10.7326/0003-4819-140-11-200406010-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bingley PJ, Gale EA. European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia. 2006;49(5):881–890. doi: 10.1007/s00125-006-0160-4. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Boulware DC, Beam CA, et al. Type 1 Diabetes TrialNet Study Group. Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care. 2012;35(6):1213–1218. doi: 10.2337/dc11-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orban T, Sosenko JM, Cuthbertson D, et al. Diabetes Prevention Trial-Type 1 Study Group. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32(12):2269–2274. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53(2):384–392. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- 21.Decochez K, Truyen I, van der Auwera B, et al. Belgian Diabetes Registry. Combined positivity for HLA DQ2/DQ8 and IA-2 antibodies defines population at high risk of developing type 1 diabetes. Diabetologia. 2005;48(4):687–694. doi: 10.1007/s00125-005-1702-x. [DOI] [PubMed] [Google Scholar]
- 22.Koczwara K, Bonifacio E, Ziegler AG. Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes. 2004;53(1):1–4. doi: 10.2337/diabetes.53.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Vives M, Somoza N, Soldevila G, et al. Reevaluation of autoantibodies to islet cell membrane in IDDM: failure to detect islet cell surface antibodies using human islet cells as substrate. Diabetes. 1992;41(12):1624–1631. doi: 10.2337/diab.41.12.1624. [DOI] [PubMed] [Google Scholar]
- 24.Bonifacio E, Bingley PJ, Shattock M, et al. Quantification of islet-cell antibodies and prediction of insulindependent diabetes. Lancet. 1990;335(8682):147–149. doi: 10.1016/0140-6736(90)90013-u. [DOI] [PubMed] [Google Scholar]
- 25.Bonifacio E, Lampasona V, Bingley PJ. IA-2 (islet cell antigen 512) is the primary target of humoral autoimmunity against type 1 diabetes-associated tyrosine phosphatase autoantigens. J Immunol. 1998;161(5):2648–2654. [PubMed] [Google Scholar]
- 26.Colman PG, Steele C, Couper JJ, et al. Islet autoimmunity in infants with a type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia. 2000;43(2):203–209. doi: 10.1007/s001250050030. [DOI] [PubMed] [Google Scholar]