Abstract
Temporal lobe epilepsy (TLE) is a chronic epileptic disorder involving the hippocampal formation. Details on the interactions between the hippocampus proper and parahippocampal networks during ictogenesis remain, however, unclear. In addition, recent findings have shown that epileptic limbic networks maintained in vitro are paradoxically less responsive than non-epileptic control (NEC) tissue to application of the convulsant drug 4-aminopyridine (4AP). Field potential recordings allowed us to establish here the effects of 4AP in brain slices obtained from NEC and pilocarpine-treated epileptic rats; these slices included the hippocampus and parahippocampal areas such as entorhinal and perirhinal cortices and the amygdala. First, we found that both types of tissue generate epileptiform discharges with similar electrographic characteristics. Further investigation showed that generation of robust ictal-like discharges in the epileptic rat tissue is (i) favored by decreased hippocampal output (ii) reinforced by EC–subiculum interactions and (iii) predominantly driven by amygdala networks. We propose that a functional switch to alternative synaptic routes may promote network hyperexcitability in the epileptic limbic system.
Keywords: 4-Aminopyridine, Amygdala, CA3, Hippocampus, Ictogenesis, Pilocarpine, Subiculum, Temporal lobe epilepsy
Introduction
Temporal lobe epilepsy (TLE) is a partial epilepsy disorder involving the hippocampus proper and parahippocampal structures such as entorhinal (EC) and perirhinal (PC) cortices, amygdala, and temporal neocortex (Gloor, 1991; Mathern et al., 1997). TLE patients are often unresponsive to antiepileptic drugs and present with a typical pattern of brain damage known as Ammon’s horn sclerosis (Gloor, 1991; Du et al., 1993; Wiebe et al., 2001). Current animal models of TLE are based on local or systemic injection of convulsant drugs (e.g., kainic acid or pilocarpine) or on repetitive electrical stimulation of limbic pathways (reviewed in Pitkänen et al., 2006) to induce an initial status epilepticus (SE) followed by a chronic condition of spontaneous, recurrent, limbic seizures that are also poorly controlled by antiepileptic drugs (Glien et al., 2002; Chakir et al., 2006).
Evidence obtained from humans and rodents indicates that epileptic hyperexcitability results from seizure-induced brain damage leading to (i) synaptic reorganization (Sutula et al., 1989; Mikkonen et al., 1998; Houser, 1999; Gorter et al., 2001), (ii) loss of specific interneuron subtypes (Bernard et al., 2000; Maglóczky and Freund, 2005) and (iii) alterations in GABAergic inhibition (Sloviter, 1987; Brooks-Kayal et al., 1998). The latter changes along with NMDA receptor upregulation have been reported in pilocarpine-treated parahippocampal areas such as the subiculum (Knopp et al., 2005; de Guzman et al., 2006), the EC (Kobayashi et al., 2003; Kumar and Buckmaster, 2006; Yang et al., 2006; de Guzman et al., 2008) and the lateral amygdala (LA) (Benini and Avoli, 2005). In addition, modified GABAA receptor signaling due to changes in K+/Cl− cotransporter has been identified in the epileptic human subiculum in vitro (Cohen et al., 2002; Wozny et al., 2003; Huberfeld et al., 2007). It remains however to be defined how these alterations in cellular excitability lead to an epileptic brain capable of producing chronic recurrent seizures. Moreover, the roles played by parahippocampal areas in seizure initiation remain vague. Both aspects are presumably relevant for advancing our understanding of TLE pathogenesis.
Findings obtained from animal models of TLE suggest that changes in K+ channel subunit expression may be part of the epileptogenic process leading to neuronal hyperexcitability and the consequent manifestation of spontaneous epileptic seizures (Bernard et al., 2004; Zahn et al., 2008). In addition, contrary to what was reported by us in pilocarpine-treated mice (D’Antuono et al., 2002), treatment with the K+ channel blocker 4-aminopyridine (4AP), a well established model of in vitro ictogenesis (Avoli et al., 2002), failed to induce the generation of ictal-like discharges in slices obtained from kainic-treated epileptic rats (Zahn et al., 2008). We were therefore interested in studying the effects induced by bath-application of this convulsant in a limbic brain slice preparation in the rat pilocarpine model of TLE. Here, we employed field potential recordings to: (i) compare the effect of 4AP application in non-epileptic control (NEC) and pilocarpine-treated tissue; (ii) investigate the role of hippocampal–parahippocampal interactions in the generation of epileptiform discharges and (iii) establish whether the site of epileptiform discharge initiation is modified in epileptic versus NEC tissue.
Methods
All the experimental procedures were approved by the Canadian Council on Animal Care (cf. de Guzman et al., 2006, 2008). All efforts were made to minimize the number of animals used and their suffering.
Preparation of pilocarpine-treated rats
Adult male Sprague Dawley rats (250–300 g) were injected with the cholinergic agonist pilocarpine hydrochloride (380 mg/kg, i.p.). To prevent discomfort induced through peripheral muscarinic receptor stimulation, rats were treated with scopolamine methylnitrate (1 mg/kg, i.p.) 30 min prior to pilocarpine administration. Animal behavior was monitored for 4–6 h after pilocarpine injection and scored according to Racine’s classification (1972). SE, defined as repeated stages 3–5 seizures (Racine, 1972) without recovery in between, was arrested with diazepam (5–20 mg/kg, i.p.) if it did not resolve spontaneously within 2 h. Rats experiencing ≥60 min SE were defined as the experimental group. Previous studies have established that all pilocarpine-treated animals experiencing SE become epileptic (see for example Cavalheiro et al., 1991, reviewed in Pitkänen et al., 2006, and by Curia et al., 2008). We have previously reported that rats exposed to 60–120 min of SE develop spontaneous recurring behavioral seizures within ~3 weeks from treatment (Biagini et al., 2008, 2009). Therefore, animals were randomly monitored for the manifestation of spontaneous behavioral seizures, which was confirmed in all of them, and were used for electrophysiological studies 4–10 weeks following pilocarpine injection. Age-matched NEC rats—injected i.p. with scopolamine and saline—never manifested behavioral seizures.
Brain slice preparation and electrophysiological procedures
Both NEC and pilocarpine-treated animals were decapitated under deep isoflurane anesthesia, the brain was quickly removed and placed in ice-cold (~4 °C) oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) composed of the following (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and D-glucose 10 (pH=7.4, 305 mOsm/kg). Horizontal brain slices (450–500 μm thick) were obtained with the use of a vibratome (Leica VT1000S, Leica, Germany). Slices were transferred to an interface tissue chamber and superfused with oxygenated (95% O2, 5% CO2) ACSF at 32–34 °C (pH=7.4, 305 mOsm/kg). Chemicals were acquired from Sigma-Aldrich Canada, Ltd. (Oakville, Ontario). 4AP (50 μM) was bath-applied.
Two different slicing procedures were used to partially preserve connectivity between hippocampus and parahippocampal areas. In order to record from brain slices in which hippocampal output activity was unable to spread to the EC (cf., Avoli et al., 1996), the dorsal side of the brain was glued onto the vibratome stage without any prior manipulation of the tissue block. In these brain slices, which included the hippocampal formation throughout its dorso-ventral extent, fast CA3-driven interictal-like activity disclosed by 4AP application was restrained to the hippocampus proper and did not propagate to the EC (see Fig. 1Aa, arrows; cf., Avoli et al., 1996). We will thereafter refer to these slices as partially disconnected slices. In a different set of experiments, preservation of CA3 outputs to the EC and other parahippocampal areas was achieved by slicing the brain along a horizontal plane that was tilted by ~10° along a posterosuperior–anteroinferior plane passing between the lateral olfactory tract and the brain stem base (cf., Benini et al., 2003). To this end, only the most ventral slices were used since they present with the highest connectivity between hippocampus and parahippocampal structures (Avoli et al., 2002; Benini et al., 2003). These slices were comprised between −8.6 and −7.6 mm from the bregma (Paxinos and Watson, 1998), and included the most ventral hippocampus, the EC, the PC and the LA; in these experiments fast CA3-driven interictal-like activity induced by 4AP could propagate to the EC and the other para-hippocampal structures (see Fig. 3; cf., Benini et al, 2003).
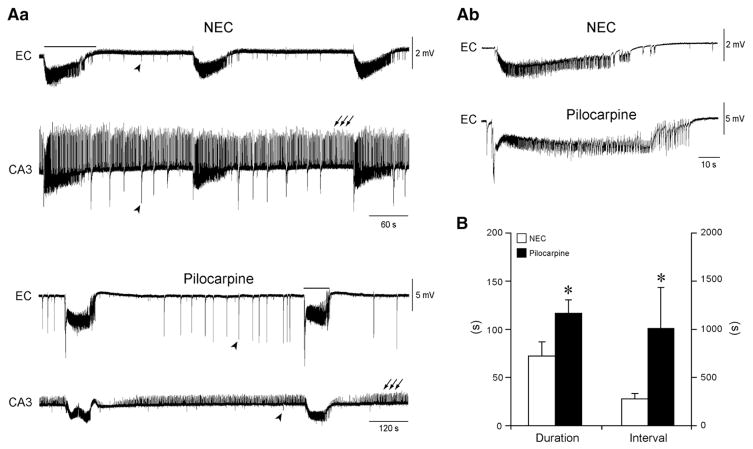
Fig. 1.
In vitro ictogenesis is maintained in pilocarpine-treated epileptic tissue. Aa: Epileptiform activity induced by 4AP (50 μM) simultaneously recorded from EC and CA3 of a NEC and a pilocarpine-treated ‘partially disconnected’ slice shows similar electrographic characteristics. Note, however, that epileptic tissue generates ictal-like events that are less frequent but more robust than those seen in NEC, as emphasized by the different time scale. Note also that CA3-driven interictal-like discharges (arrows) do not propagate to the EC, which only generates a pattern of slow interictal-like events (arrowheads). In (b) are the expanded traces of the ictal-like discharges indicated by the solid lines in panel (a). B: Summary of the results indicating that slices obtained from pilocarpine-treated epileptic animals generate ictal-like discharges that are longer in duration and occur less frequently than those generated by NEC tissue.
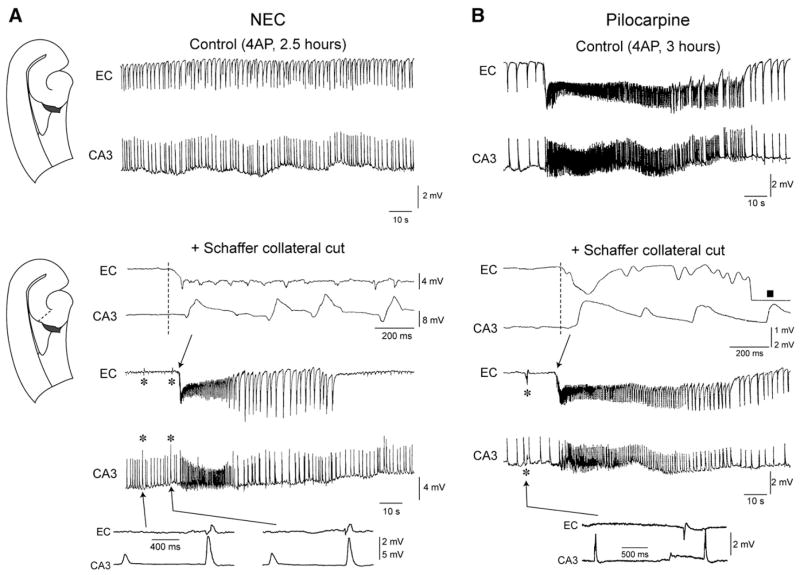
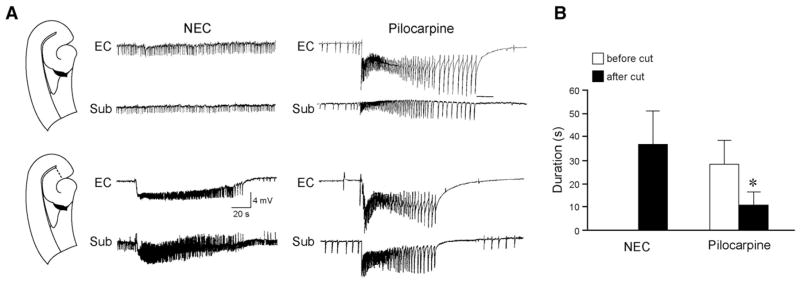
Fig. 3.
CA3–EC interaction is impaired in pilocarpine-treated epileptic tissue. Effects induced by cutting the Schaffer collaterals in NEC (A) and pilocarpine-treated slices (B). In the slice schematic, the site of lesion is indicated by the dashed line. Under control conditions, after 2.5 h of continuous 4AP application, NEC slices generate spontaneous interictal-like discharges only while both interictal-like and ictal-like activities still occur in pilocarpine-treated slices after 3 h of 4AP treatment. Note that in both types of tissue cutting the Schaffer collaterals abolishes interictal-like discharges in EC and discloses slow interictal-like events (asterisks). Further, this procedure recovers ictal-like activity in NEC slices, while no change is observed in pilocarpine-treated slices. Inserts on top of Schaffer collateral cut panels in A and B are expanded traces of ictal-like discharge onset that initiates in both cases in the EC (square in the EC trace of the enlarged ictal-like discharge generated by the epileptic tissue marks a period of signal saturation). Inserts on the bottom are expanded traces of the slow interictal-like events.
Field potential recordings were made with ACSF-filled glass pipettes (tip diameter <10 μm; resistance 5–10 MΩ) that were positioned in the deep layers of the EC and (i) CA3 (stratum pyramidale or radiatum) or (ii) subiculum, or (iii) the deep layers of the PC and the LA. Signals were fed to high-impedance amplifiers and displayed on a WindoGraph recorder (Gould Instruments, Cleveland, OH, USA) or sent to a computer interface device (Digidata 1322A, Molecular Devices, Palo Alto, CA, USA), acquired and stored in the hard drive using pClamp 8.0 software (Molecular Devices, Palo Alto, CA, USA). Traces were acquired at a sampling rate of 5 kHz and low-pass filtered on line at 2 kHz. Subsequent analysis of these data was performed with Clampfit 9 software (Molecular Devices, Palo Alto, CA, USA). Time-delay measurements for epileptiform discharge onset were obtained by taking as temporal reference the first deffection from the baseline in expanded traces.
Local application of kynurenic acid (5 mM in ACSF) to the EC or subiculum was performed by pressure-ejection through a broken pipette (tip diameter ~15 μm). To avoid diffusion of the drug from the perfused area to distant regions, slices in these experiments were positioned in such a way that the treated area was downstream with respect to the ACSF flow. In addition, we used bipolar, stainless steel electrodes to deliver electrical stimuli (100 μs; <350 μA) in the subiculum or in the EC (Fig. 5), and thus to rule out that the changes in epileptiform activity could reflect decreased network excitability due to kynurenic acid diffusion. The drop-applied solution was visualised by including phenol red to the perfused medium. Cuts within different areas were made with a microknife under visual guidance (cf. Benini et al, 2003).
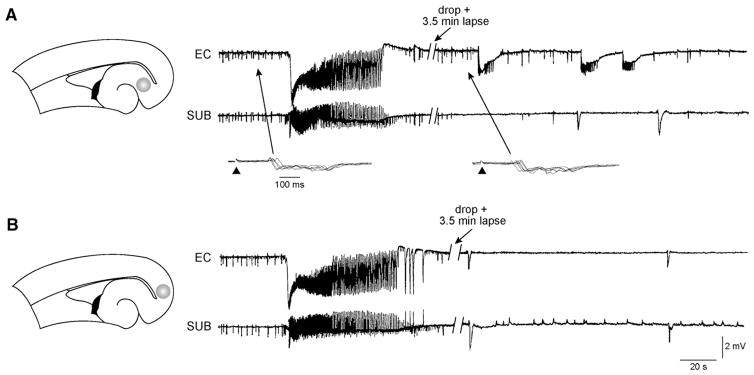
Fig. 5.
Reciprocal excitatory inputs between EC and subiculum contribute to reinforcement of ictal-like activity. A: Local application of kynurenic acidto the subiculum (grey area in the slice schematic on the left) blocks ictal-like events in this area and reduces their duration in the EC. This effect is seen at a time when spontaneous or stimulus induced-interictal-like discharges were similar to those seen during control condition (arrows and inserts), suggesting that the decreased duration of ictal-like activity in EC was not due to spread of kynurenic acid from the subiculum to this area. Arrowheads in the inserts indicate the time of electrical stimulation. B: After ~40 min washout, subsequent application of kynurenic acid to the EC in the same slice abolishes 4AP-induced ictal-like discharges recorded in both EC and subiculum (the site of local application is indicated by the grey area in the slice schematic on the left).
A direct comparison between the pattern of 4AP-induced epileptiform activity in the two animal groups was performed by recording simultaneously from the EC and CA3 subfield of partially disconnected slices that were obtained from both NEC and pilocarpine-treated animals; brain slices from these two types of tissue were maintained during the same experiment in a two-well interface recording chamber accommodating 2 to 4 slices from each animal per well. This approach allowed us to implement similar experimental conditions for both animal groups thus ensuring that no bias was introduced by possible subtle differences that may occur during the slice preparation and maintenance.
Database and statistical analysis
In all, we analyzed 4AP-induced epileptiform activity in 29 and 46 brain slices obtained from NEC and pilocarpine-treated epileptic animals, respectively. Within and between each experimental group, all the slices exhibited the same electrographic pattern of 4AP-induced epileptiform activity, regardless of the animal age or the time after SE. Therefore, data were pooled.
Measurements throughout the text are expressed as mean±SEM, and n represents the number of slices studied. Data were compared with the Student’s t-test. Results were considered significantly different if p<0.05.
Results
Field potential characteristics of 4AP-induced epileptiform activity in NEC and pilocarpine-treated epileptic brain tissue
First, we sought to determine whether 4AP-induced in vitro ictogenesis is maintained in pilocarpine-treated epileptic tissue. To this aim, we performed ‘parallel’ recordings from partially disconnected slices obtained from both NEC and pilocarpine-treated rats (n=9 and 10 slices, respectively, from 3 animals, each group; see Methods). Bath application of 4AP (50 μM) induced synchronous epileptiform activity with similar electrographic characteristics in both experimental groups (Fig. 1). As previously reported (Avoli et al., 1996, 2002; Brückner and Heinemann, 2000; Klueva et al., 2003), this activity consisted of three different patterns: (i) robust ictal-like discharges resembling tonic–clonic electrographic seizures that originated in the parahippocampal regions from where they could spread to the CA3 subfield (Fig. 1Aa, solid line); (ii) ‘slow’ interictal-like events of variable origin that occurred every ~20 s (Fig. 1Aa, arrowheads and Fig. 2A, asterisks) and resembled the so called ‘synchronous GABA-mediated potentials’; and (iii) ‘fast’ interictal-like activity (Fig. 1Aa, arrows and Fig. 2A) that occurred at ~1 Hz and originated in the CA3 subfield (not illustrated, but see Benini and Avoli, 2005). This latter type of discharge, which failed to propagate to the EC in partially disconnected slices (cf. Figs. 1 and 3), will be hereafter referred to as fast CA3-driven interictal-like activity.
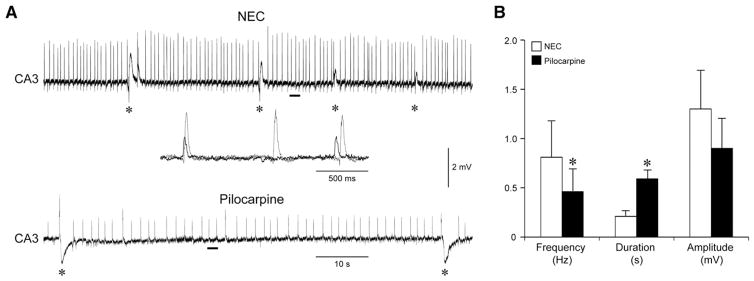
Fig. 2.
Interictal-like activity in the CA3 subfield is impaired in pilocarpine-treated slices. A: Field potential recordings obtained from the CA3 subfield of a NEC and a pilocarpine-treated slice. The CA3 subfield of both tissues generates fast interictal-like activity along with a pattern of slow events (asterisks) that resemble the so called ‘synchronous GABA-mediated potentials’. The insert shows superimposed expanded traces of the fast interictal-like pattern corresponding to the solid bars emphasizing the slower frequency and the smaller amplitude of the events generated by the epileptic tissue (black trace) compared to NEC (grey trace). Note the lower rate of occurrence of slow interictal-like discharges (asterisks) generated by this area in the pilocarpine-treated slice. B: Summary of the results obtained suggesting impairment of fast CA3-driven interictal-like activity in epileptic tissue.
Quantitative analysis of the duration and rate of occurrence of 4AP-induced synchronous discharges revealed, however, some differences. First, ictal-like events occurred less frequently in epileptic compared to NEC tissue (mean interval, NEC: 277.03±52.31 s; pilocarpine: 1011.79±425.55 s; n=9 and 6, respectively; p<0.05; Figs. 1Aa and B), but were more robust (mean duration, NEC: 72.29± 15.19 s; pilocarpine: 116.39±14.31 s; n=9 and 6, respectively; p<0.05; see expanded traces in Fig. 1Ab). In addition, epileptic tissue appeared to be less prone to induction of sustained ictal-like activity, since in this experimental set of ‘parallel’ recordings only 6 out of 10 slices obtained from pilocarpine-treated rats generated ictal-like discharges, whereas NEC tissue always exhibited it. This phenomenon did not correlate with the time after SE induction or with the age of the animal. Moreover, as reported by D’Antuono et al. (2002) (but also see Köhling et al., 1995; Nagao et al., 1994), CA3-driven interictal-like events in pilocarpine-treated slices (Figs. 2A and B) were less frequent (0.46±0.23 versus 0.81±0.37 Hz, p<0.05), of longer duration (0.59±0.10 versus 0.21±0.06 s, p<0.01) and of lower amplitude (0.9±0.3 versus 1.3±0.4 mV, peak-to-peak) than in NEC slices.
CA3–EC interaction is impaired in pilocarpine-treated epileptic slices
When the EC and CA3 were reciprocally connected (see Methods), fast CA3-driven interictal-like activity could propagate to the EC (Fig. 3). In addition, ictal-like discharges disappeared in NEC slices (n=11) within about 2 h of 4AP application, but continued to occur throughout the experiment (≥3 h) in most (14/19 slices, ~74%) pilocarpine-treated slices (cf., Fig. 3). In light of this and previous findings (Barbarosie and Avoli, 1997; Barbarosie et al., 2000), we hypothesized that the persistence of ictal-like activity in pilocarpine-treated slices resulted from altered EC–CA3 interactions. We therefore performed cuts of the Schaffer collaterals in reciprocally connected slices to establish the influence exerted by CA3-driven interictal-like discharges on ictal-like activity. In NEC slices (n=4), in which ictal-like discharges had stopped occurring overtime (Fig. 3A), this type of cut abolished the propagation of CA3-driven interictal-like events to the EC, and let ictal-like activity appear again in EC and hippocampus (Fig. 3A, “+Schaffer collateral cut” panel). Likewise, Schaffer collateral cut in pilocarpine-treated slices (n=5) abolished CA3-driven inter-ictal-like discharges in the EC. However, this procedure failed to induce any significant change on ictal-like discharges (Fig. 3B, “Schaffer collateral cut” panel) suggesting that functional impairment of the interaction between these two regions may contribute to the persistence of ictal-like activity generated by the EC.
EC–subiculum interactions sustain ictal-like discharges in pilocarpine-treated slices
We have reported that ictal-like activity in pilocarpine-treated mouse slices superfused with 4AP-containing medium propagates from the EC to the subiculum through the monosynaptic temporoammonic path, thus short-circuiting the hippocampal trisynaptic route (D’Antuono et al., 2002); this finding is in line with other studies in which a similar propagation pattern was reported during application of low-Mg2+-containing medium (Dreier and Heinemann, 1991) or local application of high K+ and bicuculline (Wozny et al., 2005a). Here, we further tested this type of propagation in rat brain slices during application of 4AP by comparing the effects produced by lesioning most of EC–subiculum connections in NEC and pilocarpine-treated tissue. As illustrated in the inserts of Fig. 4A, this was accomplished by performing a cut that was approximately normal to the alveus and extended through the subicular area toward the dentate upper blade.
Fig. 4.
EC–subiculum interactions reinforce ictal-like activity in pilocarpine-treated epileptic tissue. A: On the left, a slice schematic illustrates the extension of the knife cut performed to lesion EC–subiculum (Sub) connections (dashed line). The pattern of 4AP-induced epileptiform activity (upper traces) changes when EC–subiculum connections are severed by knife cut (lower traces). Interictal-like activity no longer propagates to the EC of both NEC (left) and pilocarpine-treated (right) slices. However, in NEC slices this procedure discloses ictal-like activity spreading to the subiculum, whereas in pilocarpine-treated tissue EC–subiculum separation causes a decrease in ictal-like discharge duration. B: Summary of the effects induced on ictal-like activity by cutting the connections between EC and subiculum in NEC and pilocarpine-treated slices.
As expected, in both NEC (n=5) and pilocarpine-treated (n=8) slices, this type of procedure abolished the propagation of CA3-driven interictal-like activity to the EC (Fig. 4A). In addition, in NEC slices (in which ictal-like discharges had stopped occurring overtime) severing EC–subiculum connections disclosed ictal-like activity that spread from the EC to the subiculum (Fig. 4A, left and Fig. 4B), whereas in pilocarpine-treated slices, which continued to generate ictal-like discharges throughout the experiment (duration: 28±3.8 s), it decreased the duration of these events (10.4±2.3 s) in both EC and subiculum (Fig. 4A, right and Fig. 4B). It should also be emphasized that ictal-like discharges recorded in subiculum and EC had similar duration both before and after the surgical manipulation. Further, the propagation of ictal-like discharges from the EC to the hippocampus was abolished by subsequent separation of the DG from the EC, suggesting that the trisynaptic route may be, at least in part, spared (not shown, but see also Wozny et al., 2005a).
In light of these findings, we hypothesized that subiculum–EC interactions sustain ictal-like synchronization in epileptic limbic networks. We therefore performed local application of the glutamatergic antagonist kynurenic acid (5 mM) in pilocarpine-treated slices (n=4) superfused with 4AP-containing medium to block the spread of reciprocal excitatory inputs between the subiculum and the EC. Local application of kynurenic acid to the subiculum (Fig. 5A, shaded area in the slice schematic) blocked ictal-like events in this region and reduced their duration in the EC (Fig. 5A). Such an effect could be seen at a time when spontaneous interictal-like discharges in the EC were similar to those observed in control condition and no change appeared in the responses generated by EC networks following electrical stimuli delivered in the subiculum (inserts in Fig. 5A) or in the EC (not illustrated); therefore, these results suggested that the decreased duration of ictal-like activity seen in EC during the first few minutes after drop application of kynurenic acid was not due to the spread of this drug from subiculum to EC. After a 20–30 min washout, we applied kynurenic acid to the EC (Fig. 5B, shaded area in slice schematic). Such procedure abolished ictal-like discharges in both EC and subiculum (Fig. 5B).
Initiation and spread of epileptiform activity in pilocarpine-treated parahippocampal areas
We and others have reported in previous studies (Benini et al., 2003; de Guzman et al., 2004; Klueva et al., 2003) that ictal-like discharges occurring in normal brain slices superfused with 4AP-containing medium can be initiated in the EC, PC or LA. Here, we addressed whether the ability of these parahippocampal networks to generate epileptiform activity is modified in pilocarpine-treated rats. To this aim, we focused on slow interictal-like activity and ictal-like discharges and measured time delays in the appearance of both types of event in the EC and PC with respect to LA.
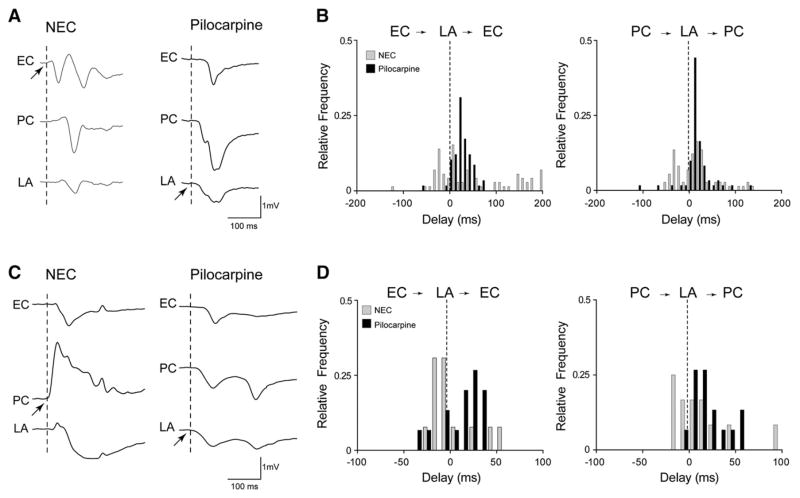
In keeping with our previous findings (Benini et al., 2003; de Guzman et al., 2004), epileptiform discharges could initiate in and spread to any of these limbic areas in both types of tissue. However, epileptic LA networks revealed a greater propensity to drive both types of epileptiform activity. Fig. 6A shows the most frequently observed propagation modalities of slow interictal-like discharges observed in NEC (n=4) and pilocarpine-treated (n=5) slices. The interplay between LA–EC and LA–PC is illustrated in Fig. 6B, where the frequency distributions of time delays are compared between NEC (grey bars) and epileptic tissues (black bars). In NEC slices, slow interictal-like events observed in the LA preceded those recorded in the EC in ~65% of the cases (n =49/75 events), whereas in pilocarpine-treated slices the LA drove the majority of them (n=55/61 events, ~90%). Similarly, slow interictal-like events recorded in the PC of NEC tissue were preceded by those seen in the LA in ~61% of the cases (n=46/75 events), whereas in pilocarpine-treated slices they propagated from the LA to the PC in ~88% of the cases (n=54/61 events). Fig. 6C illustrates the onset of ictal-like discharges that were simultaneously recorded in the EC, PC and LA of a NEC (left) and pilocarpine-treated (right) brain slice. Analysis of time delays of ictal-like activity (Fig. 6D) revealed that events recorded in the LA preceded those seen in the EC more frequently in pilocarpine-treated than in NEC tissue (NEC: 7/17 events from 4 slices, ~41%; pilocarpine: 10/15 events from 5 slices, ~67%), a phenomenon that could also be observed when analyzing the LA–PC interplay (NEC: 11/17 events from 4 slices, ~ 65%; pilocarpine: 13/15 events from 5 slices, ~87%).
Fig. 6.
Increased LA drive of epileptiform activity in pilocarpine-treated tissue. A. Slow interictal-like events recorded from the EC, PC and LA showing the different sites of onset (arrows) in NEC and pilocarpine-treated epileptic tissue, where the LA most frequently initiates this type of discharge. B. Frequency distribution histograms of time delays of interictal-like events generated by NEC and pilocarpine-treated slices, showing the increased propensity of the LA to drive this type of epileptiform activity in the latter. C. Expanded traces illustrating the different onset modalities of ictal-like events in NEC and pilocarpine-treated tissues. As indicated by the arrows, in these experiments the PC initiates the ictal-like event in the NEC slice, whereas the LA is the site of initiation in the pilocarpine-treated slice. D. Frequency distributions of time delays of ictal-like discharges generated by NEC and pilocarpine-treated slices, where the right shift of the distribution histograms indicates increased propensity of the epileptic LA to drive ictal-like activity.
Discussion
In this study we employed the pilocarpine model of TLE along with field potential recordings from an EC–hippocampal slice preparation to investigate whether in vitro ictogenesis and the role of para-hippocampal structures in epileptiform synchronization are changed in epileptic compared to NEC tissue. We have found that pilocarpine-treated slices challenged with 4AP can generate sustained ictal-like activity that, contrary to intact NEC slices, persists over time and is accompanied by functional impairment of CA3. Further investigation of these phenomena by means of pharmacological manipulations and sectioning procedure suggested that in pilocarpine-treated epileptic tissue: (i) decreased hippocampal output activity may favor the persistence of parahippocampal ictal-like activity; (ii) EC–subiculum interactions reinforce ictal-like synchronization; and (iii) epileptiform activity is predominantly driven by the LA.
In vitro ictogenesis induced by 4-aminopyrindine is maintained in pilocarpine-treated slices
In spite of the highly documented hyperexcitability characterizing the epileptic brain (Sloviter, 1987; Esclapez et al., 1999; Kobayashi et al., 2003; Kumar and Buckmaster, 2006; de Guzman et al., 2006, 2008), recent evidence has challenged the idea that epileptic tissue may respond to convulsant drug treatment by generating synchronous epileptiform discharges similar to those observed in control. We have previously reported that 4AP treatment can induce prolonged periods of sustained epileptiform activity in limbic brain slices obtained from pilocarpine-treated mice (D’Antuono et al., 2002). In contrast, Zahn et al. (2008) have described in brain slices from kainic acid-treated epileptic rats a paradoxically reduced susceptibility to induction of seizure-like activity by this drug, along with down-regulation of 4AP-sensitive K+ channel subunits. However, in vitro experiments were performed in their study at a later time-point than in ours (i.e. 10–40 versus 4–10 weeks after SE induced at similar age), when the reduced ictogenic potential of 4AP could be partially explained by age-dependent plastic neuronal changes along with the more prolonged epileptic condition.
Here, we have shown that ~60% of pilocarpine-treated slices could generate robust ictal-like activity in response to 4AP treatment. We may therefore hypothesize that the use of different TLE models (i.e. kainic acid versus pilocarpine) contributes to these contrasting observations. On the other hand, the observation that ~40% of these slices failed in generating ictal-like activity in response to 4AP treatment suggests the existence of complex plastic changes that impact the expression of 4AP-sensitive K+ conductances, similarly to what was described in the kainic acid model of TLE by Zahn et al. (2008).
CA3-driven interictal-like activity is impaired in pilocarpine-treated epileptic tissue
We have previously demonstrated that CA3-driven interictal activity controls ictogenesis in parahippocampal areas, where, accordingly, ictal-like discharges can be disclosed by lesioning the Schaffer collaterals (Barbarosie and Avoli, 1997; Barbarosie et al., 2000; Benini et al., 2003; de Guzman et al., 2004). These data suggested that the cell damage occurring in TLE may cause a permanent decrease of hippocampal output activity that would then release ictogenesis, thus establishing a chronic epileptic condition. In line with this hypothesis, we have previously reported that interictal-like discharges generated within the CA3 area occur less frequently in epileptic than in NEC rodents (Köhling et al., 1995; Nagao et al., 1994; D’Antuono et al., 2002). We further substantiated these findings by recently showing that in chronically epileptic rats the expression of FosB/ΔFosB is decreased in the CA3 subfield, which also appeared to be hypoactive in response to mossy fiber but not to local stimulation (Biagini et al., 2005). In addition, it has been reported that the excitatory drive provided by granule cells of the dentate gyrus onto the CA3 area is functionally impaired in kindled animals due to the emergence of activity-dependent fast inhibition (Gutiérrez and Heinemann, 2001).
Our data further support the potential anti-ictogenic role of CA3 outputs (Barbarosie and Avoli, 1997) by showing for the first time that 4AP-induced ictal-like activity generated by the EC is not affected in pilocarpine-treated slices when the Schaffer collaterals are severed by surgical cut; indeed, this evidence would suggest functional impairment of CA3/EC interactions in the epileptic brain. In support of this hypothesis, we have found that, contrary to what was observed in intact NEC slices, ictal-like discharges continue to occur in the pilocarpine-treated EC even when CA3–EC connectivity is preserved by the slicing procedure. It is noteworthy that we have recently described in adult pilocarpine-treated rats the development of a focal vascular lesion (Biagini et al., 2008) that strongly correlates with disruption of the trisynaptic pathway and the consequent decrease of CA3 excitability (Biagini et al., 2005; Ang et al., 2006); these data point at decreased CA3 output as a key factor favoring the generation of ictal-like discharges within epileptic parahippocampal networks.
EC–subiculum interactions reinforce ictal-like synchronization in epileptic limbic networks
We have shown here that surgical disconnection of the EC from the subiculum mimicked the effect of Schaffer collaterals cut in NEC slices whereas in pilocarpine-treated tissue it attenuated 4AP-induced ictal-like discharges, which continued to propagate to the hippocampus proper via the dentate gyrus (see also Wozny et al., 2005a). Therefore, although the perforant pathway appears to retain, at least in part, its functionality, this finding further substantiates the role of a functional switch from the trisynaptic to the temporoammonic route (Witter et al., 1989) in reinforcing ictal-like synchronization in epileptic limbic networks. The involvement of the subiculum in epileptiform synchronization is also evidenced by the ability of this region to generate spontaneous rhythmic discharges in human epileptic tissue (Cohen et al., 2002; Wozny et al., 2005b) even when not stricken by hippocampal sclerosis (Wozny et al., 2005b), suggesting that both synaptic and intrinsic neuronal alterations underlie the increased seizure susceptibility of this region. In line with these results, pyramidal neurons exhibit hyperexcitability in the epileptic rat subiculum (Knopp et al., 2005; de Guzman et al., 2006), where, similarly to what was reported from human brain specimens (Huberfeld et al., 2007) downregulation of the K+/Cl− cotransporter KCC2 accounts for a depolarized reversal potential of GABAA-mediated IPSPs (de Guzman et al., 2006). In addition, we have reported that subicular expression of FosB/ΔFosB is increased after pilocarpine-induced SE (Biagini et al., 2005), suggesting robust activation of this region during seizure activity.
The strategic position of the subiculum makes it an ideal candidate to control the flow of information between hippocampus and parahippocampal structures which in turn deliver powerful GABAergic inhibition through recurrent (Menendez de la Prida, 2003) and feed-forward (Finch et al., 1988; Behr et al., 1998) mechanisms. However, the feed-forward control exerted by the temporoammonic pathway changes from mainly inhibitory to excitatory in pilocarpine-treated animals (Wozny et al., 2005a). Accordingly, we have shown here that depressing subicular network excitability by local application of kynurenic acid restrained ictal-like discharges in the EC while the same procedure applied to the EC abolished ictal-like activity in both areas. Local GABAergic inhibition endows the subiculum with gating properties that control hippocampal output activity in NEC brain slices (Benini and Avoli, 2005). Hence, increased seizure susceptibility of the subiculum along with disruption of its physiological gating role in epileptic animals (Van Vliet et al., 2004; Knopp et al., 2008) should confer this structure a pivotal role in sustaining ictal-like synchronization through reinforcement of EC–subiculum interactions.
Amygdala networks drive epileptiform activity in pilocarpine-treated epileptic slices
Field potential recordings from reciprocally connected EC, PC and LA (von Bohlen und Halbach and Albrecht, 2002) revealed that the propensity of the latter to drive epileptiform discharges is increased in pilocarpine-treated slices. The involvement of amygdala networks in TLE is highly documented by several studies from both humans and animal models (Gloor, 1992; Pitkanen et al., 1998; Aroniadou-Anderjaska et al., 2008), where this limbic area often represents the primary focus of epileptic seizures. In the absence of pathological conditions, the amygdala is under strong inhibitory GABAergic control provided by local circuits (Le Gal La Salle, 1976; Le Gal La Salle and Ben-Ari, 1981; Rainnie et al., 1991; Kemppainen and Pitkänen, 2000; Martina et al., 2001). Investigations performed on epileptic tissue obtained from animal models of TLE suggest that the epileptic hyperexcitability seen in this area is contributed by interneuronal loss (Tuunanen et al., 1996, 1997). Accordingly, GABAergic inhibition is functionally impaired in the epileptic LA (Benini and Avoli, 2006; Fritsch et al., 2009).
We have previously reported that severing the Schaffer collaterals discloses ictal-like activity in the LA and, accordingly, local electrical stimulation of this area mimicking CA3-driven interictal-like activity depresses ictal-like discharges in the EC of NEC animals (Benini et al., 2003). Although the LA presents with dense reciprocal connections with the hippocampal formation, no direct CA3 input to this region has been identified so far (Pitkänen et al., 2000; see also Benini et al., 2003) and, in intact brain slices obtained from NEC animals, the CA1/subiculum serves as a bidirectional route of communication between the LA and the hippocampus (Pitkänen et al., 2000). Importantly, in spite of the extensive neuronal damage, this pathway is largely spared in kainic acid-treated epileptic animals (Kemppainen and Pitkänen, 2004). We may therefore hypothesize that, consequent to the chronic epileptic condition, impaired hippocampal output along with decreased inhibition contribute in concert to the released epileptogenicity of the LA, which would in turn entrain the hippocampal formation through the CA1/subiculum projection.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Grant MT-8109), the Savoy Foundation, and the Italian Ministry of University and Research (MIUR). MD was a fellow of the Fragile X Research Foundation of Canada. GP is a fellow of Epilepsy Canada.
References
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Köhling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal–entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, Kurcewicz I, Avoli M. CA3-released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol. 2000;83:1115–1124. doi: 10.1152/jn.2000.83.3.1115. [DOI] [PubMed] [Google Scholar]
- Behr J, Gloveli T, Heinemann U. The perforant path projection from the medial entorhinal cortex layer III to the subiculum in the rat combined hippocampal–entorhinal cortex slice. Eur J Neurosci. 1998;10:1011–1018. doi: 10.1046/j.1460-9568.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol (Lond) 2005;566:885–900. doi: 10.1113/jphysiol.2005.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benini R, Avoli M. Altered inhibition in lateral amygdala networks in a rat model of temporal lobe epilepsy. J Neurophysiol. 2006;95:2143–2154. doi: 10.1152/jn.01217.2005. [DOI] [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Bernard C, Cossart R, Hirsch JC, Esclapez M, Ben-Ari Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia. 2000;41(Suppl 6):S90–S95. doi: 10.1111/j.1528-1157.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Contri MB, Guerrini U, Sironi L, Gelosa P, Zini I, Ragsdale DS, Avoli M. Proepileptic influence of a focal vascular lesion affecting entorhinal cortex–CA3 connections after status epilepticus. J Neuropathol Exp Neurol. 2008;67:687–701. doi: 10.1097/NEN.0b013e318181b8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, D’Arcangelo G, Baldelli E, D’Antuono M, Tancredi V, Avoli M. Impaired activation of CA3 pyramidal neurons in the epileptic hippocampus. Neuromol Med. 2005;7:325–342. doi: 10.1385/NMM:7:4:325. [DOI] [PubMed] [Google Scholar]
- Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neurosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brückner C, Heinemann U. Effects of standard anticonvulsant drugs on different patterns of epileptiform discharges induced by 4-aminopyridine in combined entorhinal cortex–hippocampal slices. Brain Res. 2000;859:15–20. doi: 10.1016/s0006-8993(99)02348-3. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Chakir A, Fabene PF, Ouazzani R, Bentivoglio M. Drug resistance and hippocampal damage after delayed treatment of pilocarpine-induced epilepsy in the rat. Brain Res Bull. 2006;71:127–138. doi: 10.1016/j.brainresbull.2006.08.009. 28. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Meth. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Benini R, Biagini G, D’Arcangelo G, Barbarosie M, Tancredi V, Avoli M. Limbic network interactions leading to hyperexcitability in a model of temporal lobe epilepsy. J Neurophysiol. 2002;87:634–639. doi: 10.1152/jn.00351.2001. [DOI] [PubMed] [Google Scholar]
- de Guzman P, D’Antuono M, Avoli M. Initiation of electrographic seizures by neuronal networks in entorhinal and perirhinal cortices in vitro. Neuroscience. 2004;123:875–886. doi: 10.1016/j.neuroscience.2003.11.013. [DOI] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Biagini G, Baldelli E, Mollinari C, Merlo D, Avoli M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16:843–860. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Baldelli E, de Curtis M, Biagini G, Avoli M. Network hyperexcitability within the deep layers of the pilocarpine-treated rat entorhinal cortex. J Physiol. 2008;586:1867–1883. doi: 10.1113/jphysiol.2007.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. J Comp Neurol. 1999;408:449–460. doi: 10.1002/(sici)1096-9861(19990614)408:4<449::aid-cne1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Finch DM, Tan AM, Isokawa-Akesson M. Feedforward inhibition of the rat entorhinal cortex and subicular complex. J Neurosci. 1988;8:2213–2226. doi: 10.1523/JNEUROSCI.08-07-02213.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Rogawski MA, Braga MF. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neurosci. 2009;163:415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Löscher W. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43:350–357. doi: 10.1046/j.1528-1157.2002.18101.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: historical background and an overview from a modern perspective. In: Luders H, editor. Epilepsy Surgery. Raven; New York: 1991. pp. 698–703. [Google Scholar]
- Gloor P. Role of the amygdala in temporal lobe epilepsy. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 505–538. [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Heinemann U. Kindling induces transient fast inhibition in the dentate gyrus-CA3 projection. Eur J Neurosci. 2001;13:1371–1379. doi: 10.1046/j.0953-816x.2001.01508.x. [DOI] [PubMed] [Google Scholar]
- Houser CR. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–761. [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Damage to the amygdalo-hippocampal projection in temporal lobe epilepsy: a tract-tracing study in chronic epileptic rats. Neuroscience. 2004;126:485–501. doi: 10.1016/j.neuroscience.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Klueva J, Munsch T, Albrecht D, Pape HC. Synaptic and non-synaptic mechanisms of amygdala recruitment into temporolimbic epileptiform activities. Eur J Neurosci. 2003;8:2779–2791. doi: 10.1111/j.1460-9568.2003.02984.x. [DOI] [PubMed] [Google Scholar]
- Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–488. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Knopp A, Frahm C, Fidzinski P, Witte OW, Behr J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain. 2008;131:1516–1527. doi: 10.1093/brain/awn095. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Wen X, Buckmaster PS. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhling R, Lücke A, Nagao T, Speckmann EJ, Avoli M. Extracellular potassium elevations in the hippocampus of rats with long-term pilocarpine seizures. Neurosci Lett. 1995;201:87–91. doi: 10.1016/0304-3940(95)12136-r. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal La Salle G. Unitary responses in the amygdaloid complex following stimulation of various diencephalic structures. Brain Res. 1976;118:475–478. doi: 10.1016/0006-8993(76)90315-2. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Ben-Ari Y. Unit activity in the amygdaloid complex: a review. In: Ben-Ari Y, editor. The Amygdaloid Complex. Elsevier/North-Holland; Amsterdam: 1981. pp. 227–237. [Google Scholar]
- Maglóczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Paré D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol. 2001;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Armstrong DL. Hippocampal sclerosis. In: Engel J, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven; Philadelphia: 1997. pp. 133–155. [Google Scholar]
- Menendez de la Prida L. Control of bursting by local inhibition in the rat subiculum in vitro. J Physiol. 2003;549:219–230. doi: 10.1113/jphysiol.2003.039305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Kalvianen R, Tapiola T, Ylinen A, Vapalahti M, Paljärvi L, Pitkänen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- Nagao T, Avoli M, Gloor P. Interictal discharges in the hippocampus of rats with long-term pilocarpine seizures. Neurosci Lett. 1994;174:160–164. doi: 10.1016/0304-3940(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of Seizures and Epilepsy. Elsevier Academic Press; Burlington: 2006. [Google Scholar]
- Pitkanen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–2725. doi: 10.1111/j.1460-9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Decrease in somatostatin-immunoreactive neurons in the rat amygdaloid complex in a kindling model of temporal lobe epilepsy. Epilepsy Res. 1997;26:315–327. doi: 10.1016/s0920-1211(96)00900-x. [DOI] [PubMed] [Google Scholar]
- Van Vliet EA, Aronica E, Tolner EA, Lopes da Silva FH, Gorter JA. Progression of temporal lobe epilepsy in the rat is associated with immunocyto-chemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp Neurol. 2004;187:367–379. doi: 10.1016/j.expneurol.2004.01.016. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Albrecht D. Reciprocal connections of the hippocampal area CA1, the lateral nucleus of the amygdala and cortical areas in a combined horizontal slice preparation. Neurosci Res. 2002;44:91–100. doi: 10.1016/s0168-0102(02)00092-5. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro”. Science. 2003;301:463. doi: 10.1126/science.1084237. [DOI] [PubMed] [Google Scholar]
- Wozny C, Gabriel S, Jandova K, Schulze K, Heinemann U, Behr J. Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol Dis. 2005a;19:451–460. doi: 10.1016/j.nbd.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Wozny C, Knopp A, Lehmann TN, Heinemann U, Behr J. The subiculum: a potential site of ictogenesis in human temporal lobe epilepsy. Epilepsia. 2005b;46(Suppl 5):17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Woodhall GL, Jones RS. Tonic facilitation of glutamate release by presynaptic NR2B-containing NMDA receptors is increased in the entorhinal cortex of chronically epileptic rats. J Neurosci. 2006;26:406–410. doi: 10.1523/JNEUROSCI.4413-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn RK, Tolner EA, Derst C, Gruber C, Veh RW, Heinemann U. Reduced ictogenic potential of 4-aminopyridine in the perirhinal and entorhinal cortex of kainate-treated chronic epileptic rats. Neurobiol Dis. 2008;29:186–200. doi: 10.1016/j.nbd.2007.08.013. [DOI] [PubMed] [Google Scholar]