Summary
Background
Renal-cell carcinoma is highly vascular, and proliferates primarily through dysregulation of the vascular endothelial growth factor (VEGF) pathway. We tested sunitinib and sorafenib, two oral anti-angiogenic agents that are effective in advanced renal-cell carcinoma, in patients with resected local disease at high risk for recurrence.
Methods
In this double-blind, placebo-controlled, randomised, phase 3 trial, we enrolled patients at 226 study centres in the USA and Canada. Eligible patients had pathological stage high-grade T1b or greater with completely resected non-metastatic renal-cell carcinoma and adequate cardiac, renal, and hepatic function. Patients were stratified by recurrence risk, histology, Eastern Cooperative Oncology Group (ECOG) performance status, and surgical approach, and computerised double-blind randomisation was done centrally with permuted blocks. Patients were randomly assigned (1:1:1) to receive 54 weeks of sunitinib 50 mg per day orally throughout the first 4 weeks of each 6 week cycle, sorafenib 400 mg twice per day orally throughout each cycle, or placebo. Placebo could be sunitinib placebo given continuously for 4 weeks of every 6 week cycle or sorafenib placebo given twice per day throughout the study. The primary objective was to compare disease-free survival between each experimental group and placebo in the intention-to-treat population. All treated patients with at least one follow-up assessment were included in the safety analysis. This trial is registered with ClinicalTrials.gov, number NCT00326898.
Findings
Between April 24, 2006, and Sept 1, 2010, 1943 patients from the National Clinical Trials Network were randomly assigned to sunitinib (n=647), sorafenib (n=649), or placebo (n=647). Following high rates of toxicity-related discontinuation after 1323 patients had enrolled (treatment discontinued by 193 [44%] of 438 patients on sunitinib, 199 [45%] of 441 patients on sorafenib), the starting dose of each drug was reduced and then individually titrated up to the original full doses. On Oct 16, 2014, because of low conditional power for the primary endpoint, the ECOG-ACRIN Data Safety Monitoring Committee recommended that blinded follow-up cease and the results be released. The primary analysis showed no significant differences in disease-free survival. Median disease-free survival was 5·8 years (IQR 1·6–8·2) for sunitinib (hazard ratio [HR] 1·02, 97·5% CI 0·85–1·23, p=0·8038), 6·1 years (IQR 1·7–not estimable [NE]) for sorafenib (HR 0·97, 97·5% CI 0·80–1·17, p=0·7184), and 6·6 years (IQR 1·5–NE) for placebo. The most common grade 3 or worse adverse events were hypertension (105 [17%] patients on sunitinib and 102 [16%] patients on sorafenib), hand-foot syndrome (94 [15%] patients on sunitinib and 208 [33%] patients on sorafenib), rash (15 [2%] patients on sunitinib and 95 [15%] patients on sorafenib), and fatigue (110 [17%] patients on sunitinib and 44 [7%] patients on sorafenib). There were five deaths related to treatment or occurring within 30 days of the end of treatment; one patient receiving sorafenib died from infectious colitis while on treatment and four patients receiving sunitinib died, with one death due to each of neurological sequelae, sequelae of gastric perforation, pulmonary embolus, and disease progression. Revised dosing still resulted in high toxicity.
Interpretation
Adjuvant treatment with the VEGF receptor tyrosine kinase inhibitors sorafenib or sunitinib showed no survival benefit relative to placebo in a definitive phase 3 study. Furthermore, substantial treatment discontinuation occurred because of excessive toxicity, despite dose reductions. These results provide a strong rationale against the use of these drugs for high-risk kidney cancer in the adjuvant setting and suggest that the biology of cancer recurrence might be independent of angiogenesis.
Funding
US National Cancer Institute and ECOG-ACRIN Cancer Research Group, Pfizer, and Bayer.
Introduction
Roughly a third of the 214 000 people diagnosed worldwide with primary renal-cell carcinoma each year will ultimately die from metastatic disease.1 The standard of care for primary resected renal-cell carcinoma at risk for recurrence has historically been observation.
Risk of disease recurrence can be estimated based on algorithms incorporating clinical and histological features with TNM staging.2,3,4 Advances in genomic, epigenetic, and expression profiling, which can refine estimates of recurrence risk, are not available for clinical decision making. Previous adjuvant trials of interleukin 2, hormonal therapy, or chemotherapy in patients with high-risk resected disease have all been negative.5 Trials of adjuvant interferon, with potentially anti-angiogenic or immuno-stimulatory effects, were also negative.5
Renal-cell carcinoma is arguably the most biologically rational setting in which to assess the adjuvant role of anti-angiogenic therapies, given their single-agent activity in patients with advanced disease. The vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (VEGFR inhibitors) sunitinib and sorafenib extend the progression-free survival of patients with advanced disease.6,7 The sequential use of these and other inhibitors in advanced disease has improved median overall survival from 13 months to more than 29 months.8,9 Extensive testing of VEGFR inhibitors in the adjuvant setting is underway, and these agents are being used in the community in this clinical setting. This study is the first randomised trial to compare disease-free survival with adjuvant sorafenib or sunitinib versus placebo in patients with resected primary renal-cell carcinoma at high risk for recurrence.
Methods
Study design and participants
The E2805 double-blind, placebo-controlled, randomised, phase 3 trial was led by the Eastern Cooperative Oncology Group (ECOG-ACRIN) with participation from the Southwest Oncology Group (SWOG), Cancer and Leukemia Group B (Alliance), and the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG). The study was done at 226 study centres in the USA and Canada. An independent data monitoring committee monitored the study. Approval for the study was granted by the institutional review board at each centre. The trial was supported by the Cancer Trials Evaluation Program (CTEP) of the National Cancer Institute. The study protocol is available online.
Patients were recruited through ClinicalTrials.gov and through participating institutions. Potentially eligible patients presenting at participating sites were offered enrolment. We did not collect information about screening failures. Eligible patients had histologically proven, completely resected high-risk clear cell or non-clear cell renal-cell carcinoma within 12 weeks of removal of the primary tumour. The high-risk designation included the following criteria in accordance with the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edition (2002): pT1b G3–4 N0 (or pNX where clinically N0) M0 to T(any) G(any) N + (fully resected) M0. Patients were required to have a left ventricular ejection fraction of 50% within 4 weeks before randomisation. Paraffin-embedded tumour samples were required for central review. Patients needed to be treatment-naive for kidney cancer, have good ECOG performance status (0 or 1), and normal liver and haematological function. The criterion for kidney function was a creatinine clearance of more than 30 mL per min. Exclusion criteria were uncontrolled hypertension, a pre-existing thyroid disorder, or known HIV infection. Patients with medullary kidney cancer and collecting duct kidney cancer were also excluded. Participants gave written informed consent.
Randomisation and masking
Patients were randomised (1:1:1) to the three groups in a double-blind manner with permuted blocks. Randomisation was done with the ECOG-ACRIN web-based randomisation system, which was accessed by enrolling sites through the NCI OPEN interface. Patients were stratified by histology (clear vs non-clear cell), surgery (laparoscopic vs open), ECOG performance status (0 vs 1), and risk category (intermediate high risk vs high or very high risk). The risk categories were defined based on modified UCLA International Staging Criteria and pathological grading.10 When the study activated, bottles of sorafenib, sorafenib placebo, sunitinib, and sunitinib placebo were labelled by the National Cancer lnstitute’s Pharmaceutical Monitoring Branch with a blinded drug identification number given to them by ECOG-ACRIN as specified by the randomisation algorithm. When a patient was randomly assigned, an arm assignment of X was communicated to sites, along with the patient’s identifier. At the same time, an electronic blinded drug order was sent overnight to DARTS, the Pharmaceutical Monitoring Branch’s distribution application. The application linked the patient identifier with a blinded drug ID. The Pharmaceutical Monitoring Branch labelled the bottles and shipped them to the treating facility. Each patient in every group received supplies of two drugs: sorafenib or its placebo and sunitinib or its placebo. Patients could be assigned to receive subitinib plus sorafenib placebo (sunitinib group), sorafenib plus sunitinib placebo (sorafenib group), or sunitinib placebo plus sorafenib placebo (placebo group). They were instructed to take sunitinib or sunitinib placebo for 4 weeks of every 6 week cycle and sorafenib or its placebo every day.
Procedures
The treatment regimen was 54 weeks of either sunitinib taken orally at 50 mg per day for the first 28 days of each 6 week cycle, or sorafenib taken orally at 400 mg twice per day throughout all cycles, or placebo. All patients took four 12·5 mg pills of sunitinib or sunitinib placebo per day for 4 weeks of every 6 weeks and two 200 mg pills of sorafenib or sorafenib placebo twice per day throughout. On May 22, 2009, to address toxicity issues, the starting doses were amended to 37·5 mg (for sunitinib or matching placebo) or 400 mg (for sorafenib or matching placebo) for the first one or two cycles of therapy. At the time of the dose amendment, the starting dose was changed to three 12·5 mg pills of sunitinib or sunitinib placebo per day for 4 weeks of every 6 weeks and two 200 mg pills of sorafenib or sorafenib placebo per day throughout. Patients who had tolerable grade 2 side-effects at worst were escalated to full doses. Dose reductions were allowed for grade 3 or 4 toxic effects as assessed with the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Dose reductions were in increments of 12·5 mg sunitinib or sunitinib placebo and 400 mg sorafenib or sorafenib placebo, with doses allowed as low as 25 mg sunitinib or 400 mg sorafenib every other day. Patients were assessed every 6 weeks for toxic effects and were imaged every three cycles (4·5 months) during treatment, then every 6 months for 2 years, and then once per year for 10 years during follow-up. Imaging was non-contrast chest CT and MRI of the abdomen and pelvis with gadolinium or CT of the chest, abdomen and pelvis with intravenous contrast to establish that there was no disease recurrence in the most likely sites. Patients also had multi-gated assessments of cardiac function prior to registration and at 3, 6, and 12 months or for toxic cardiac affects. Medication compliance was assessed with a pill diary and pill count. Sorafenib and its placebo and sunitinib and its placebo were provided by CTEP through agreements with Bayer and Pfizer.
Outcomes
The primary endpoint was disease-free survival, defined as the time from randomisation to recurrence, development of second primary cancer, or death from any cause. Patients alive without disease recurrence at the time of analysis were censored on the date of last disease evaluation. Secondary endpoints included overall survival, disease-free survival for clear cell renal cancer, and toxic effects as assessed by NCI CTCAE version 3.0. The effect of treatment on cardiac function has been reported previously.11 Data about a prospective quality-of-life assessment for fatigue were collected (patient-reported quality of life and assessment of a new measure of quality of life, PROMIS Fatigue-SF1) and will be published separately. A final secondary objective was to prospectively collect tumour and biological specimens to assess their characteristics and associations with various angiogenesis markers, genetic mutations and polymorphisms, DNA methylation profiles, drug metabolising, enzymes, and circulating endothelial cells, which will be reported in a later publication.
Statistical analysis
Data were analysed by the study statistician (JM) and represent the clinical database as of Aug 27, 2015. The study was designed to show a 25% reduction in the hazard rate in patients treated with either agent directly compared with placebo at a one-sided per-comparison significance level of 1·25%. In the original design, the planned full information was 498 events in the two groups being compared, which would provide 80% power, allowing for interim analyses, the first of which was scheduled to occur once about 34% of information had been collected (169 of 498 disease-free survival events had occurred). Enrolment of 1332 patients was planned to obtain the necessary number of events.
Because of higher than expected treatment discontinuation in the experimental groups, an amendment to expand accrual to 1923 patients was activated in July, 2009. The revised design proposed to enrol these patients over 4 years and follow them up for 6·6 additional years. This revised design provided 81% power to test the original hypothesis of a 25% reduction in the hazard rate, assuming a discontinuation rate on the experimental arms of 23·4%, again at a one-sided per-comparison significance level of 1·25%. The revised statistical plan also included an assessment of the effect of the dosing change on the treatment discontinuation rate. The design parameters before and after the amendment are shown in the appendix (p 1).
Interim efficacy analyses were planned to occur once per year starting after roughly 34% information had been collected, starting after the study closed to accrual. At each interim analysis, the information proportion was computed and the analysis was done exactly 8 weeks before the meeting of the ECOG-ACRIN Data Safety Monitoring Committee. Significance levels at these interim analyses were established with a truncated O’Brien-Fleming error spending rate function. Boundaries for analyses before 50% of the 498 necessary disease-free survival events had been observed were truncated at 0·00025, with the significance levels at subsequent analyses adjusted to preserve the overall type I error rate. At each analysis, one-sided p values comparing each of the two agents to placebo were calculated with a stratified log-rank test. Each of the two p values was then compared with the nominal significance level corresponding to an overall significance level of 0·0125. If either p value was smaller than the corresponding nominal significance level, the other p value was to be compared with the nominal significance level corresponding to an overall significance level of 0·025.
Monitoring for early stopping in favour of the null hypothesis was done with repeated confidence intervals. At each interim analysis, a nominal (1 − [2 × α]) confidence interval was calculated. The futility stopping rules12 were not considered in the power calculation for the study. Monitoring by the Data Safety Monitoring Committee began in September, 2010. On Oct 16, 2014, the committee concluded that further blinded follow-up was highly unlikely to alter the evidence, and recommended that the study results be released. By this time, all patients had completed treatment. Follow-up for survival continues.
We used descriptive statistics to characterise patients at entry. To assess differences in efficacy outcomes between groups, we used the stratified log-rank test (stratified on the four factors used to balance randomisation). We estimated hazard ratios (HR) and associated CIs by use of Cox proportional hazards models, stratified by the factors used at randomisation. We estimated IQRs for disease-free survival, and not estimable (NE) shows that the estimate cannot be made at the 75th percentile of the distribution because of variability. We used Fisher’s exact test to test for differences in categorical distributions. We did efficacy analyses with the intention-to-treat population and safety analyses in all patients who received at least one dose of study drug and had follow-up data available. We deemed a two-sided significance level of 2·5% as significant for comparisons between groups, and we used a 5% level for two-way comparisons. We did analyses with SAS version 9.2 and R version 3.0.2.
This trial is registered with ClinicalTrials.gov, number NCT00326898.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The trial accrued 1943 patients between April 24, 2006, and Sept 1, 2010, with 647 patients assigned to sunitinib, 649 assigned to sorafenib, and 647 assigned to placebo (figure 1). 18 patients in the sunitinib group, 17 patients in the sorafenib group, and 14 patients in the placebo group did not receive study drug, but were included in the main efficacy analysis. Median follow-up was 5·8 years (IQR 4·9–6·9). As shown in table 1 and in accordance with the natural history of the disease, most patients participating in this trial were male and white. 634 (33%) participants were women and 251 (13%) were from minority (Hispanic or non-white) populations. The groups were well balanced with respect to sex, race and ethnic origin, age, and performance status. Most patients were in good health, with ECOG performance status of 0. About half of patients were stratified at randomisation into the higher-risk stratum (table 1), meaning that the expected risk for recurrence was 39·7–61·8% at 5 years.10 165 (8%) patients were node positive at locally reported pathological assessment (47 [7%] of 647 patients assigned sunitinib, 51 [8%] of 649 assigned sorafenib, and 67 [10%] of 646 assigned placebo).
Figure 1.
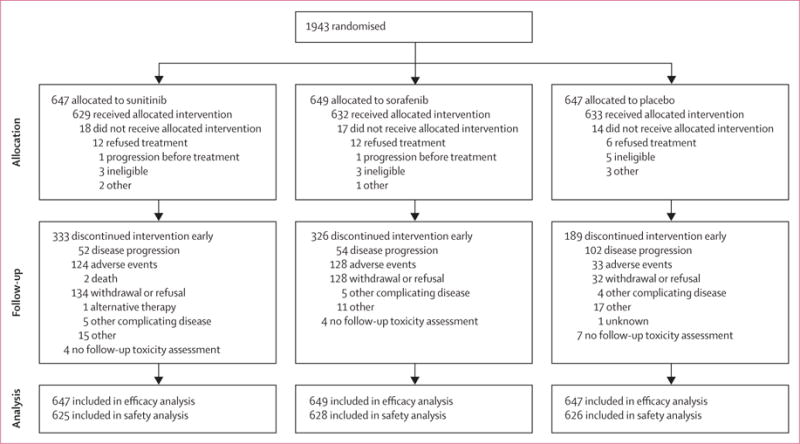
Trial profile
Table 1.
Baseline characteristics
| Sunitinib (n=647) | Sorafenib (n=649) | Placebo (n=647) | |
|---|---|---|---|
| Sex | |||
| Male | 429 (66%) | 437 (67%) | 443 (68%) |
| Female | 218 (34%) | 212 (33%) | 204 (32%) |
|
| |||
| Race | |||
| White | 598 (92%) | 589 (91%) | 585 (90%) |
| African American | 27 (4%) | 27 (4%) | 31 (5%) |
| Asian | 11 (2%) | 17 (3%) | 15 (2%) |
| Other/unknown | 11 (2%) | 16 (2%) | 16 (2%) |
|
| |||
| Ethnic origin | |||
| Hispanic | 30 (5%) | 36 (6%) | 41 (6%) |
| Non-Hispanic | 576 (89%) | 565 (87%) | 560 (87%) |
| Other/unknown | 41 (6%) | 48 (7%) | 46 (7%) |
|
| |||
| Age (years) | 56 (49–64) | 55 (48–63) | 57 (49–64) |
|
| |||
| ECOG performance status | |||
| 0 | 510 (79%) | 511 (79%) | 508 (79%) |
| 1 | 137 (21%) | 138 (21%) | 139 (21%) |
|
| |||
| Surgical approach as stratified | |||
| Open | 394 (61%) | 391 (60%) | 389 (60%) |
| Laparoscopic | 253 (39%) | 258 (40%) | 258 (40%) |
|
| |||
| Surgical approach as reported by surgeon | |||
| Open | 376 (58%) | 361 (56%) | 374 (58%) |
| Laparoscopic | 271 (42%) | 288 (44%) | 272 (42%) |
|
| |||
| Type of nephrectomy (reported by surgeon) | |||
| Radical | 614 (95%) | 605 (93%) | 617 (95%) |
| Partial | 33 (5%) | 44 (7%) | 29 (4%) |
|
| |||
| Histology | |||
| Clear cell | 513 (79%) | 519 (80%) | 509 (79%) |
| Papillary | 39 (6%) | 52 (8%) | 59 (9%) |
| Chromophobe | 40 (6%) | 43 (7%) | 28 (4%) |
| Mixed | 31 (5%) | 21 (3%) | 31 (5%) |
| Unclassified | 23 (4%) | 14 (2%) | 19 (3%) |
| Sarcomatoid features | 51 (8%) | 58 (9%) | 61 (10%) |
|
| |||
| UCLA International Staging System risk stratification | |||
| Intermediate high | 323 (50%) | 324 (50%) | 326 (50%) |
| Very high | 324 (50%) | 325 (50%) | 321 (50%) |
|
| |||
| AJCC stage | |||
| I | 57 (9%) | 61 (9%) | 64 (10%) |
| II | 159 (25%) | 167 (26%) | 154 (24%) |
| III | 422 (65%) | 409 (63%) | 424 (66%) |
| IV | 9 (1%) | 12 (2%) | 4 (1%) |
Data are n (%) or median (IQR). ECOG=Eastern Cooperative Oncology Group. AJCC=American Joint Committee on Cancer.
Figure 2 shows the primary outcome, disease-free survival, for sunitinib, sorafenib, and placebo. Median disease-free survival was 70 months (5·8 years, IQR 1·6–8·2) for sunitinib, 73·4 months (6·1 years, IQR 1·7–NE) for sorafenib, and 79·6 months (6·6 years, IQR 1·5–NE) for placebo. Disease-free survival did not differ significantly between groups. For sunitinib versus placebo, the HR was 1·02 (97·5% CI 0·85–1·23, stratified log-rank p=0·8038) and for sorafenib versus placebo, the HR was 0·97 (97·5% CI 0·80–1·17, stratified log-rank p=0·7184). Events were recorded for 284 patients on sunitinib (5 year disease-free survival 54·3%, 97·5% CI 49·7–59·3), 284 patients on sorafenib (54·0%, 49·4–58·9), and 287 patients (56·4%, 51·9–61·2) on placebo. Types of events and sites of recurrence are shown in the appendix (p 9).
Figure 2. Disease-free survival.
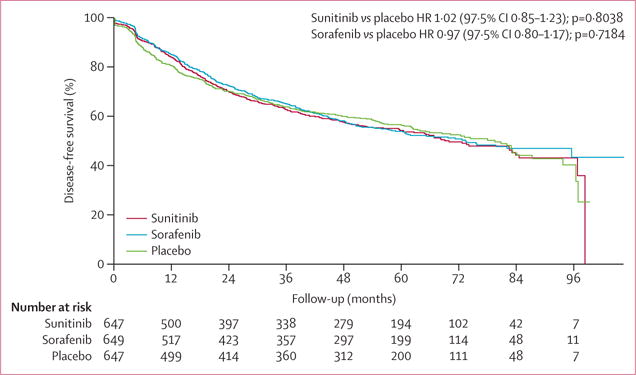
HR=hazard ratio.
Clear cell renal-cell carcinoma (typically 85% of renal-cell carcinoma cases) is almost always associated with VHL mutation, and with dysregulated angiogenesis.13 In a prespecified subset analysis, we considered this population to be the most likely to benefit. There were no differences associated with treatment (sunitinib vs placebo, HR 1·02, 97·5% CI 0·85–1·22, stratified log-rank p=0·8931; sorafenib vs placebo, HR 0·99, 97·5% CI 0·83–1·19, stratified log-rank p=0·8734). Median disease-free survival in the clear cell subset (1622 [83%] patients as stratified) was 66·9 months (5·6 years, IQR 1·6–8·2) for sunitinib, 66·9 months (5·6 years, 1·8–NE) for sorafenib, and 74·4 months (6·6 years, 1·5–8·1) for placebo (appendix p 10).
In our analysis of overall survival, events had been recorded in 156 patients on sunitinib (5 year overall survival 77·9%, 97·5% CI 74·1–81·9), 138 patients on sorafenib (80·5%, 76·8–84·2), and 141 patients on placebo (80·3%, 76·7–84·0) (figure 3). Overall survival did not differ significantly between the groups. All groups did better than predicted at the time of study design; median overall survival has not yet been reached in any group (figure 3).
Figure 3. Overall survival.
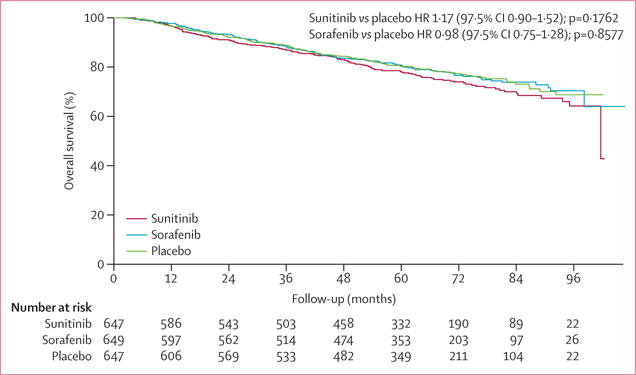
HR=hazard ratio.
Patients on sunitinib or sorafenib received a median of eight cycles (IQR 2–9) and those on placebo received a median of nine cycles (IQR 7–9). The proportions of patients receiving the intended dose at cycle 3 were 262 (42%) of 629 patients receiving sunitinib, 193 (31%) of 630 receiving sorafenib, and 560 (89%) of 633 receiving placebo. Patients were compliant, taking a high proportion of the prescribed dose, but the prescribed doses were frequently reduced due to adverse events (appendix p 2).
The effect of the mid-study change in dosing to reduce treatment discontinuation was significant (appendix p 11). Among patients starting at full dose, the overall rates of treatment discontinuation due to adverse events or patient withdrawal or refusal were 193 (44%) of 438 patients on sunitinib, 199 (45%) of 441 patients on sorafenib, and 47 (11%) of 444 patients on placebo. The overall discontinuation rates among patients starting at reduced dose were 65 (34%) of 191 patients on sunitinib, 56 (30%) of 189 patients on sorafenib, and 18 (10%) of 189 patients on placebo. The change in dosing scheme resulted in a significant reduction in discontinuation due to adverse events or patient refusal for the sunitinib (Gray’s test p=0·0142) and sorafenib (p=0·0001), but no change in the placebo group (p=0·6959).
Some side-effects associated with sorafenib and sunitinib occurred at a higher than expected rate. Hand-foot syndrome (affecting 94 [15%] of 625 patients on sunitinib, 208 [33%] of 628 patients on sorafenib, and seven [1%] of 626 patients on placebo) and hypertension (affecting 105 [17%] patients on sunitinib, 102 [16%] patients on sorafenib, and 26 [4%] patients on placebo) were the most common drug-related effects. Adverse events occurring in more than 1% of patients are shown in table 2. A complete table of adverse events is available in the appendix (p 3). Haematological adverse events were recorded if they were grade 4 or worse and occurred in less than 1% of patients. Fatigue was common and was corroborated by patient-reported outcomes, which will be reported in full in a future publication. Grade 3 or worse adverse events were reported by 394 (63%) of 625 patients on sunitinib, 450 (72%) of 628 patients on sorafenib, and 159 (25%) of 626 patients on placebo. Although the reduction in starting dose somewhat ameliorated this effect, the proportion of grade 3 or worse adverse events in patients starting at reduced dose still exceeded 55% in both the sunitinib and sorafenib groups (appendix p 9).
Table 2.
Grade 3 or worse adverse events
| Sunitinib (n=625) | Sorafenib (n=628) | Placebo (n=626) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| Hypertension | 104 (17%) | 1 (<1%) | ·· | 102 (16%) | ·· | ·· | 26 (4%) | ·· | ·· |
| Fatigue | 106 (17%) | 4 (1%) | ·· | 44 (7%) | ·· | ·· | 19 (3%) | ·· | ·· |
| Pruritus/itching | 1 (<1%) | ·· | ·· | 11 (2%) | ·· | ·· | 1 (<1%) | ·· | ·· |
| Rash/desquamation | 15 (2%) | ·· | ·· | 93 (15%) | 2 (<1%) | ·· | 3 (<1%) | ·· | ·· |
| Hand-foot syndrome | 94 (15%) | ·· | ·· | 208 (33%) | ·· | ·· | 7 (1%) | ·· | ·· |
| Anorexia | 12 (2%) | ·· | ·· | 5 (1%) | ·· | ·· | ·· | ·· | ·· |
| Dehydration | 12 (2%) | ·· | ·· | 6 (1%) | ·· | ·· | 1 (<1%) | ·· | ·· |
| Diarrhoea without prior colostomy | 62 (10%) | ·· | ·· | 58 (9%) | ·· | ·· | 3 (<1%) | ·· | ·· |
| Dyspepsia | 15 (2%) | ·· | ·· | 6 (1%) | ·· | ·· | 1 (<1%) | ·· | ·· |
| Infection/neutropenia | 18 (3%) | ·· | 1 (<1%) | 26 (4%) | 2 (<1%) | 1 (<1%) | 12 (2%) | ·· | ·· |
| Mucositis/stomatitis | 24 (4%) | 1 (<1%) | ·· | 14 (2%) | ·· | ·· | 1 (<1%) | ·· | ·· |
| Nausea | 23 (4%) | ·· | ·· | 8 (1%) | ·· | ·· | 1 (<1%) | ·· | ·· |
| Vomiting | 14 (2%) | ·· | ·· | 7 (1%) | ·· | ·· | 2 (<1%) | ·· | ·· |
| Sensory neuropathy | 5 (1%) | ·· | ·· | 12 (2%) | ·· | ·· | 3 (<1%) | ·· | ·· |
| Pain | 45 (7%) | 1 (<1%) | ·· | 56 (9%) | 1 (<1%) | ·· | 18 (3%) | 2 (<1%) | ·· |
| Worst degree | 359 (57%) | 31 (5%) | 4 (1%) | 428 (68%) | 21 (3%) | 1 (<1%) | 135 (22%) | 24 (4%) | ·· |
Worst degree shows the number of patients for whom the indicated grade was their worst. Data are n (%). Only adverse events occurring in more than 1% of the population are shown.
We did several post-hoc subgroup analyses (figure 4). Because of the substantial treatment discontinuation rate, we did post-hoc analyses of the association between treatment administration and disease-free survival. As shown in the forest plots (figure 4), the absence of a treatment effect was seen both in patients who started at full dose and in those who started at reduced dose, although the hazard ratio comparing sorafenib to placebo tended to favour placebo among patients starting at reduced dose (p for arm-by-dose group interaction=0·0109). In view of the number of exploratory subset analyses done, these results could have occurred by chance and should be viewed with caution. None of the other arm-by-subgroup interaction tests were significant at the 0·025 level. The suggested benefit of sorafenib in races or ethnicities other than white is undergoing further analysis in more detailed exploratory subgroup analyses. We also used post-hoc landmark analyses to assess disease-free survival with respect to the number of months (appendix p 12), the total dose received, and the time to relapse after completion of active treatment. No outcomes differed significantly between groups.
Figure 4. Disease-free survival in exploratory subgroups.
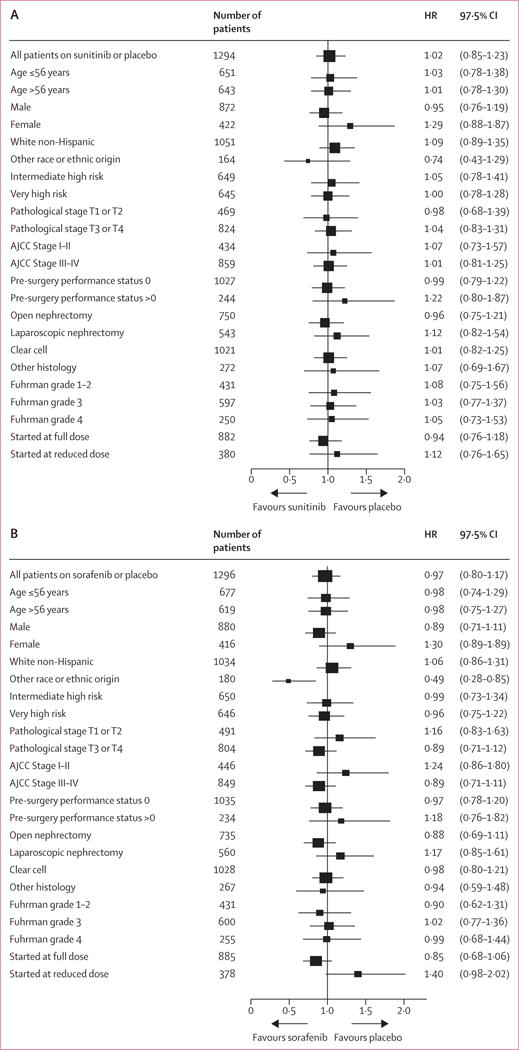
Forest plots show hazard ratios and 97·5% CIs for the comparison of sunitinib with placebo (A) and sorafenib with placebo (B). The x axis of the forest plot is scaled in accordance with the natural logarithm of the HR. The size of the squares is proportional to the inverse of the variance of the log hazard ratio, such that smaller squares correspond to largest variance. Race or ethnic origin was self-reported; other race or ethnic origin includes all patients who reported either race or ethnicity as other than white, non-Hispanic, or who did not report race or ethnicity. Intermediate high-risk disease (as stratified at randomisation) was defined as either pathological T1b, grade 3–4, pathological T2, grade 1–4, or pathological T3a, grade 1–2, provided that the pathological T2a grade was not due to adrenal involvement. Patients with other pathological T3–4 disease or any node-positive disease were categorised in the very high-risk group. HR=hazard ratio.
Discussion
This is the first trial to report on VEGFR inhibitors as adjuvant therapy for patients with locally advanced high-risk kidney cancer. The study included a histologically representative and diverse population, whose overall outcomes were better than expected, providing reassurance that the results are relevant to the population at large. The median time to disease recurrence did not differ between those who received sorafenib or sunitinib after surgery and those who received placebo. It should be noted that when protocol-specified early stopping occurs, final effect estimates are generally biased. However, given the solid absence of an effect, this potential bias is unlikely to have affected the interpretation of results for this study.
VEGFR inhibitors are active as single agents in patients with advanced renal-cell carcinoma,6,7,14–16 but their role in the earlier stages of disease is unclear. Thus, renal-cell carcinoma is uniquely suited to investigations of the adjuvant role of anti-angiogenic therapy as an isolated modality. Despite the positive effect of anti-angiogenic agents in patients with advanced disease, our results did not show any benefit from these agents relative to placebo when given in the adjuvant setting.
These findings are similar to those of adjuvant chemotherapy trials in other tumours, in which the benefit of adding anti-angiogenic therapy in metastatic disease is not seen in the adjuvant setting. For example, the addition of bevacizumab to chemotherapy in metastatic colorectal, breast, and non-small-cell lung cancers showed improvements in outcomes compared with chemotherapy alone.17–19 These benefits were not replicated when bevacizumab was used in the adjuvant setting.20–22 The possibility that effective chemotherapy might have obscured the benefit of anti-angiogenic therapy is refuted by our results in E2805.
Dose intensity and treatment duration are appropriate concerns with these drugs. That patients might have had too great a dose reduction is countered by the high occurrence of hypertension, a pharmacodynamic marker of drug effect.23,24 Our analysis of dosing supports the notion that, in terms of disease-free survival, a duration of therapy greater than 6 months did not differ from 3–6 months or less than 3 months of therapy.
Treatment duration has sometimes been a factor in maximising the benefit of targeted therapy (as with anti-oestrogens in breast cancer, and imatinib in gastrointestinal stromal tumours). However, in such cases, shorter durations of therapy did show some efficacy, which is not the case in our results. Future survival analyses will help to elucidate such concerns. Potentially more productive will be a series of correlative studies using the patients’ tissues and plasma from this study to look for evidence of subsets with varying outcomes that might be assessed for future therapeutic interventions.
In the adjuvant setting, the issue has been raised as to whether the micrometastases that presumably result in recurrent disease have a blood supply that is as susceptible to the effect of anti-angiogenic therapy as is the case in macrometastases. Given the absence of benefit from VEGFR inhibitor therapy, it seems reasonable to conclude that inhibition of VEGF-driven angiogenesis does not produce sustained, if any, anti-tumour effects in micrometastatic lesions. Notably, results from preclinical mouse kidney cancer xenograft models supported a paradoxical effect whereby VEGFR inhibitors accelerate the rate of metastases.25,26 Explanations for this finding included upregulation of non-VEGF pro-angiogenic and proliferative factors, as well as hypoxia-driven epithelial-to-mesenchymal transformation.27–30 Although such findings raised concern that adjuvant therapy might prove ineffective, there was no evidence for this process in metastatic disease.8,31 In view of this conflicting information and the striking benefit seen with these inhibitors in metastatic disease, placebo-controlled adjuvant studies were deemed crucial. Indeed, even with their well-documented limitations, these mouse xenograft models probably identified a process that is relevant to this human trial. These potentially valid models might provide insight into the pathophysiology of VEGFR inhibitor treatment of renal-cell carcinoma to inform future studies.
Even though E2805 did not establish a role for sorafenib or sunitinib in the adjuvant setting, its placebo-controlled design has provided a definitive answer that will help to prevent costs and toxic effects associated with inappropriate use of these agents. It should be noted that a large number of patients in E2805 are likely to remain disease free, but were exposed to toxic effects with no benefit. Additionally, those patients who developed recurrent cancer had no benefit from this adjuvant therapy, and conceivably might have compromised their future benefit from VEGFR inhibitors post-relapse. Thus, the patients on this trial should be thanked for their willingness to take risks in order to inform future generations.
The results of this study argue strongly against the use of anti-angiogenic therapy in the adjuvant setting of patients with primary resected renal-cell carcinoma. Other adjuvant approaches, such as checkpoint inhibitor immunotherapy, can be explored in research studies with observation or placebo control groups.
Supplementary Material
Research in context.
Evidence before this study
A systemic literature review was not done as part of the planning for this trial; however, we based the design of the trial on previous adjuvant trials in renal-cell carcinoma (Ariser and Oncophage) and selected our risk populations on the basis of published models of risk recurrence (the UCLA international staging system). At the time of study design, sorafenib and sunitinib were the only small molecule vascular endothelial growth factor tyrosine kinase inhibitors approved for the treatment of advanced renal-cell carcinoma (approved by the US Food and Drug Administration in 2004 and 2005, respectively), based on improvement in progression-free survival of first-line sunitinib versus interferon (26·4 months with sunitinib vs 21·8 months with interferon) and second-line sorafenib versus placebo (5·5 months with sorafenib vs 2·8 months with placebo). This trial aimed to test the role of sorafenib and sunitinib in prolonging disease-free survival in resected high-risk renal-cell cancer. Given the single-agent activity of anti-angiogenic therapy in advanced renal-cell carcinoma, this disease was arguably the most biologically rational setting in which to assess the adjuvant role of anti-angiogenic therapy. No adjuvant trials in renal-cell carcinoma had been implemented as standard practice.
Added value of this study
The current study is the only randomised phase 3 trial of anti-angiogenic therapy in the adjuvant setting in completely resected renal-cell cancer. The trial showed no benefit in disease-free survival and the hazard ratio of both agents approached 1·0 compared with a placebo control. Moreover, there was a high prevalence of grade 3 or worse side-effects in the active treatment groups. A reduction in the initial starting dose improved compliance, but the proportion of patients with grade 3 or worse adverse effects still exceeded 55% in both the sunitinib and sorafenib groups.
Implications of all the available evidence
This study supports the ineffectiveness of anti-angiogenic therapy in adjuvant therapy for solid tumours. The results of our study are consistent with the findings of studies of anti-angiogenic regimens containing chemotherapy in breast, lung, and colon cancers. The biological implication of this result is that angiogenesis might not be critical to the growth or survival of micrometastases. Treatment with sorafenib or sunitinib exposed patients to many toxic effects. Based on these findings, adjuvant trials in kidney cancer should be focused on interventions directed at pathways other than angiogenesis.
Acknowledgments
This study was funded by Public Health Service Grants to the ECOG-ACRIN Cancer Research Group (CA21115, CA66636, CA23318, CA180820, CA180794, CA15488, CA180867, CA27525, CA80775, CA21076, CA180799, CA14958, CA189859, CA17145, CA16116, CA180802, CA49883, CA49957, CA180847, CA13650, CA180790, CA107868, CA180821, CA31946, CA41287, CA32291, CA180836, CA11789, CA077202, CA180863, CA180888, CA180858, CA32102, CA105409, CA189953, CA20319). Support, including masked study drug, was also provided by Pfizer and Bayer. We thank Subramanian Hariharan for his advice in the design and conduct of the trial, and Tanya Mustacchio, who coordinated data management.
Footnotes
Contributors
NBH was the study chair. JM did the statistical analysis. NBH and JM designed the trial with co-development from RGU, KTF, CGW, CK, MJ, JPD, MBA, MP, GW, DC, LW, MC, RC, and RSDP. DC and LW designed the quality of life proposal. CGW, SM, TMK, WJS, YNW, TKC, RP, IP, MK, and WS enrolled the most patients. All authors analysed and interpreted the data. JM created the figures. NBH and JM wrote the first draft of the manuscript, with revisions and approval of final manuscript from all authors except RC.
Declaration of interests
JPD reports personal fees for Pfizer consultation. All other authors declare no competing interests.
For the study protocol see http://ecog-acrin.org/resources/publications/e2805
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed Dec 2, 2015)
- 2.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 3.Leibovich BC, Cheville JC, Lohse CM, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005;174:1759–63. doi: 10.1097/01.ju.0000177487.64651.3a. [DOI] [PubMed] [Google Scholar]
- 4.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 5.Pal S, Haas NB. Adjuvant therapy for renal cell carcinoma: past, present, and future. Oncologist. 2014;19:851–59. doi: 10.1634/theoncologist.2014-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt T. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108:1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 10.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466–72. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 11.Haas NB, Manola J, Ky B, et al. Effects of adjuvant sorafenib and sunitinib on cardiac function in renal cell carcinoma patients without overt metastases: results from ASSURE, ECOG 2805. Clin Cancer Res. 2015;21:4048–54. doi: 10.1158/1078-0432.CCR-15-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennison C, Turnbull BW. Interim analyses: the repeated confidence interval approach. J R Stat Soc Series B Stat Methodol. 1989;51:305–34. [Google Scholar]
- 13.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 14.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–68. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 15.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–39. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–28. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–19. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol. 2013;31:359–64. doi: 10.1200/JCO.2012.44.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–42. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 22.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 23.Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol. 2006;24:1363–69. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 24.Rini BP, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason G, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–39. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schor-Bardach R, Alsop DC, Pedrosa I, et al. Does arterial spin-labeling MR imaging-measured tumor perfusion correlate with renal cell cancer response to antiangiogenic therapy in a mouse model? Radiology. 2009;25:731–42. doi: 10.1148/radiol.2521081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67:441–61. doi: 10.1124/pr.114.010215. [DOI] [PubMed] [Google Scholar]
- 28.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffioen AW, Mans LA, de Graaf AM, et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res. 2012;18:3961–71. doi: 10.1158/1078-0432.CCR-12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammers HJ, Verheul HM, Salumbides B, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9:1525–35. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz LH, Mazumdar M, Wang L, et al. Response assessment classification in patients with advanced renal cell carcinoma treated on clinical trials. Cancer. 2003;98:1611–19. doi: 10.1002/cncr.11712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


