Abstract
This paper aimed to review currently available cohort studies of subjects with mood disorders such as major depressive disorder (MDD) and bipolar disorder (BD). Using the PubMed and KoreaMed databases, we reviewed eight major cohort studies. Most studies recruited participants with MDD and BD separately, so direct comparison of factors associated with diagnostic changes was difficult. Regular and frequent follow-up evaluations utilizing objective mood ratings and standardized evaluation methods in a naturalistic fashion are necessary to determine detailed clinical courses of mood disorders. Further, biological samples should also be collected to incorporate clinical findings in the development of new diagnostic and therapeutic approaches. An innovative cohort study that can serve as a platform for translational research for treatment and prevention of mood disorders is critical in determining clinical, psychosocial, neurobiological and genetic factors associated with long-term courses and consequences of mood disorders in Korean patients.
Keywords: Cohort study, Mood disorder, Bipolar disorder, Major depressive disorder, Review
INTRODUCTION
Mood disorders are relatively common mental illnesses that are notorious for high disease burden and chronicity.1,2 Disease course and treatment responses in mood disorders varies from person to person because they are associated with an interplay of socio-cultural and genetic-biological factors. Major mood disorders, including major depressive disorder (MDD) and bipolar disorder (BD), have profound social impacts due to increased suicide risk and decreased functional levels and quality of life.3,4,5,6 Despite recent advancements in the diagnosis and treatment of mood disorders, it is still difficult to predict treatment response and disease courses. Delayed diagnosis and diagnostic conversion from MDD to BD are making clinical decision making more difficult. Furthermore, long-term effects of currently available treatments on mood disorders are unclear. Novel approaches for early detection of and intervention for mood disorders are necessary.
A better understanding of long-term clinical courses is crucial for finding clinically meaningful biomarkers for early diagnosis and intervention. Cohort studies can provide valuable information on the clinical courses of mood disorders and the effects of treatments on the course of mood disorders.7 Various prior cohort studies of mood disorders are available, yet each study has limitations in explaining the complexity of clinical presentation of mood disorders.
In this paper, as part of a preliminary investigation for a prospective cohort study of Korean patients with mood disorders, we reviewed all currently available findings from major cohort studies of patients with mood disorders, including MDD and BD Through this review process, we propose an efficient study design that is suitable for capturing the real-world clinical setting in Korea.
METHODS
We searched the currently available literature based on cohort studies of mood disorders using databases such as PubMed and KoreaMed. The search for relevant publications was carried out using the terms 'cohort study', 'design', 'methodology', 'mood disorder', 'depression', and 'bipolar disorder'. We had screened all abstracts of publications through the two databases. As this review focused on adult populations, we excluded literature involving children, adolescents and the elderly. We included cohort studies that followed clinical courses of mood disorders using relatively standardized methodology. We excluded any literature that was not directly associated with mood disorders or conducted in a single center. After a careful review process, we selected eight major cohort studies using standardized evaluation methods for this review. We explored the design and methodology of each study and summarized the studies' major findings and limitations.
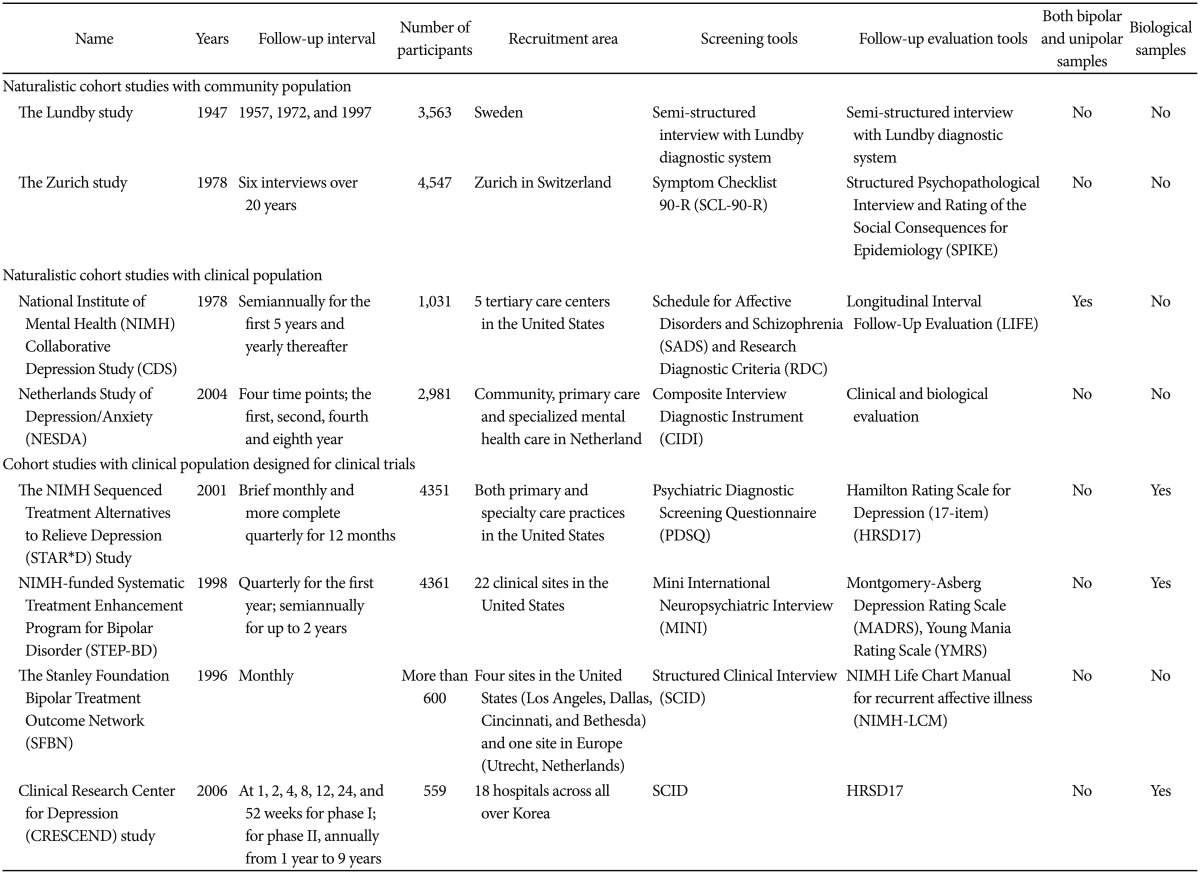
RESULTS (Table 1)
Table 1. Summary of cohort studies for mood disorders.
Naturalistic cohort study with community population
The Lundby study
The Lundby study is a longitudinal cohort study of a geographically defined population consisting of 3563 subjects. This study was designed to investigate the distribution of personality traits and mental disorders.8 The first field investigation started in 1947, and another investigation began in 1957 to include additional participants who had moved into the geographic area. Ninety-nine percent of the subjects were interviewed by psychiatrists. The interviews, most of which were conducted face-to-face, were carried out in 1947, 1957, 1972 and 1997.
At each time point, subjects participated in a semi-structured interview consisting of questions about their sociodemographic background, somatic health, mental health, medical service use, and life events that had taken place during the period since the last interview. Along with to the face-to-face interview, investigators contacted key informants to obtain additional reliable information. Also, investigators had access to case notes and several registers, including the Patient Register, which contained information about all inpatient care in Sweden from 1972 to 1997.9 The final evaluations of the clinical diagnoses took place after collecting all available information and determining a best-estimate diagnosis.
Since the DSM system10 was not available at the time of the study, the main categories of the Lundby diagnostic system are: depression, anxiety disorders, tiredness, mixed neurosis, schizophrenia, other psychoses, organic syndrome, and dementia. This is a hierarchical diagnostic system; that is, only one diagnosis per episode was registered. The Lundby diagnosis of depression remained unchanged during the study period.
After excluding subjects with preexisting alcohol problems or depression, 344 subjects (116 males, 228 females) who experienced their first depressive episode between 1947 and 1997 were examined to explore long-term courses of depression.11 The median age of the first onset of depression was around 35 years for individuals followed for 30–49 years. The recurrence rate was approximately 40% and ranged from 17% to 76% depending on the follow-up duration. A transition to diagnoses other than depression occurred for 21% of the total sample, with alcohol disorders in 7% and BD in 2%. Only 5% committed suicide; here, male gender and severity of depression were significant risk factors.
Limitation
The main limitation of the Lundby study is the diagnostic system applied. Taking into consideration the high comorbidity of mood disorders, a hierarchical system is not sufficient to evaluate the complex nature of mood disorders. Also, the interval of follow-up evaluations was too sparse to explore clinical courses in detail.12
The Zurich study
The Zurich study13 selected 4547 participants, 2201 males and 2346 females aged 19 and 20, respectively, from the canton of Zurich in Switzerland in 1978. At baseline, they were screened with the Symptom Checklist 90-R (SCL-90-R)14 in order to select for participants with high global severity indices (GSI) who would be more likely to develop psychiatric syndromes. A stratified random sample of 591 individuals (292 males, 299 females), two-thirds of whom were high scorers (defined as those scoring above the 85th percentile on the GSI), was selected for a follow-up interview. Following the initial screening in 1978, six subsequent interview waves were conducted across 20 years.
Psychiatric residents and clinical psychologists carried out the follow-up interviews using the Structured Psychopathological Interview and Rating of the Social Consequences for Epidemiology (SPIKE). The SPIKE evaluates a broad range of psychiatric and somatic syndromes and symptoms through questions regarding duration, frequency, treatment and subjective impairment and distress. All psychiatric diagnoses were made based on DSM-III-R and DSM-IV criteria.10
Major findings
The Zurich cohort study provided a valuable opportunity to examine the chronicity and diagnostic stability of mood disorders.15 The presence of MDD predicted heart complications and an increase in long-term body weight variability.16 Comorbid anxiety and depression tended to be more stable than either syndrome alone, suggesting the importance of comorbidity in clinical courses. Melancholic depression showed a more severe clinical course than atypical depression. Chronic depression, during which a major depressive episode lasts longer than 2 years, was associated with an earlier onset, worse clinical symptoms, more frequent psychiatric comorbidity, and poorer functional level and physical condition relative to episodic depression.17 Combined agitated and retarded major depressive states were associated with a transition to BD, while pure agitated depression was less frequently associated with BD.18 Relative to MDD, BD was more closely associated with the development of alcohol and benzodiazepine use disorders.19
Limitation
The Zurich cohort study explored clinical courses of mood disorders among patients ages 20 to 40, which limits the generalizability of the findings to people over 40 years old. The relatively infrequent evaluations and lack of biological measures limit the applicability of study findings.
Naturalistic cohort study with clinical population
National Institute of Mental Health Collaborative Depression Study
The NIMH CDS is the lengthiest longitudinal study of clinical courses of mood disorders. Inpatients and outpatients who were experiencing an active affective episode were recruited from 5 tertiary care centers in the United States between 1978 and 1981.20 A total of 1,031 subjects with MDD, manic disorder, schizoaffective disorder and intermittent depressive disorder were enrolled, and long term follow-up evaluations were conducted for 31 years.
At the initial intake, a semi-structured interview using the Schedule for Affective Disorders and Schizophrenia (SADS)21 and the Research Diagnostic Criteria (RDC)22 was conducted to confirm subjects' diagnoses. Functional levels, a personality battery, and family history were also collected. The follow-up evaluations were based on the Longitudinal Interval Follow-up Evaluation (LIFE) ratings.23 The LIFE ratings determine the symptom severity of each affective disorder on a 6-point Likert scale based on whether symptoms meet RDC's definite criteria (1: usual self; 2: residual; 3: partial remission; 4: marked; 5: definite criteria; and 6: definite criteria, severe). The Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool (LIFE-RIFT)24 evaluates subjects' functional levels in the domains of work, interpersonal relations, recreation, and global satisfaction on a 5-point Likert scale (1=no impairment; 5=severe impairment). Trained raters interviewed participants every 6 months for the first 5 years and yearly thereafter, using variations of the LIFE and LIFE-LIFT ratings.
Major finding
A major strength of the CDS is that patients having diverse affective spectrum diseases, including MDD, bipolar I disorder, and bipolar II disorder, were recruited together and evaluated using uniform evaluation methods. As a result, we were able to see differences between the courses of these affective spectrum diseases. Both MDD and BD were chronic and recurrent disorders that caused significant psychosocial disability.25,26,27 Most subjects experienced subthreshold residual symptoms even when they were not in an acute episode, which had negative effects on their prognosis.28 Comorbid anxiety, substance use disorder, double depression and longer episodes predicted negative treatment responses.29 In BD, depressive episodes and symptoms dominated overall clinical courses and were more disabling than (hypo)manic episodes and symptoms.27,30,31,32 Strikingly, one-fourth of subjects with MDD eventually became diagnosed with BD.33 The main risk factor for the transition was subthreshold manic/hypomanic symptoms.
The CDS established the foundation for biological and clinical research of mood disorders through a comprehensive long-term evaluation using phenomenological, diagnostic, and genetic approaches. Major findings from the CDS have contributed to the study design of treatment strategies for mood disorders, such as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D)34 and the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD).35
Limitations
Only Caucasians were included in the genetic studies, which limits the generalizability of the study findings. In addition, an early-age onset population was not included, so the lifetime course of mood disorder from the early period of mood disorders was not explored. Although the study was done prospectively, each follow-up evaluation using the LIFE and LIFE-RIFT was a retrospective evaluation that took place every 6 months or 1 year. Consequently, the follow-up evaluations may have been prone to recall biases. Although LIFE had a high intraclass correlation coefficient, more detailed and objective assessments for mood symptoms and suicidality at each follow-up period were not included in the study. This could have compensated for this major limitation of the LIFE and LIFE-RIFT evaluation.
Netherlands Study of Depression/Anxiety
The NESDA is a multi-site naturalistic cohort study describing the long-term course and consequences of depressive and anxiety disorders.36 The NESDA followed 2981 participants ages 18 to 65 for eight years. In order to increase the generalizability of the study, subjects were recruited from various health care settings (community, primary care, and specialized mental health care) and at various stages of the disorder's developmental history (normal, high familial risk, subthreshold disorders, first and recurrent episodes). Among all participants, 1701 subjects had depression (with or without a comorbid anxiety disorder), had a lifetime diagnosis, or were at risk due to a family history of subthreshold symptoms.
The Composite Interview Diagnostic Instrument (CIDI)-Lifetime Version37 was used to confirm the diagnoses based on DSM-IV criteria.10 Information from a detailed clinical interview, self-report questionnaire, medical examination, brain functional magnetic resonance imaging (fMRI), cognitive computer task and blood and saliva samples were collected at baseline.
The follow-up evaluations were conducted at four time points: one year, two years, four years, and eight years after the initial assessments. The one-year follow-up assessment consists of questionnaires to determine demographic changes, recent life events and the course and consequences of anxiety and depression symptoms. The two-year follow-up assessment includes a face-to-face clinic visit in addition to the questionnaires given at the one-year follow-up assessment. During the clinic visit, participants complete a clinical interview, computerized cognitive tasks, and an additional questionnaire regarding seasonality.
Major findings
So far, findings from the two-year follow-up assessments are available. The comorbid depression-anxiety group showed a more chronic course than the pure depression group. Predictors of poor clinical course were severity and duration of index episode, comorbidity, earlier onset age, and older age. The NESDA incorporates a comprehensive clinical and biological evaluation that offers a unique opportunity to explore the long-term course of depression. Contrary to other cohort studies of populations with mood disorders, the NESDA increases the generalizability of study findings by including a variety of clinical populations. In addition, the study utilizes various biological measures, such as cognitive and neuroimaging evaluations, which can be valuable biomarkers in determining clinical outcomes of mood disorders.
Limitations
The main limitation of the NESDA study is the lack of frequent evaluations during the observational period. Since follow-up assessments are conducted at one-year, two-year, four-year, and eight-year time points, we cannot evaluate detailed mood fluctuations during each time interval. Recall biases are also inevitable in this study design.
Cohort studies with clinical population designed for clinical trials
The NIMH Sequenced Treatment Alternatives to Relieve Depression Study
The NIMH-funded STAR*D study is a multisite, prospective, randomized, multistep clinical trial of patients with non-psychotic MDD.34 The STAR*D aimed to determine effectiveness of different treatments, including non-pharmacological treatments, for MDD that did not respond to the initial treatment. The STAR*D also aimed to describe the incidence, nature, and course of symptoms and functioning for those entering the 12-month naturalistic follow-up.38
At baseline, the Psychiatric Diagnostic Screening Questionnaire (PDSQ)39 was used to confirm DSM-IV based psychiatric diagnoses. The 17-item Hamilton Rating Scale for Depression (HRSD17),40 the 16-item Quick Inventory of Depressive Symptomatology-Clinician Rating (QIDS-CR) and the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR)41 were used to determine the severity of depression symptoms. Clinical Global Impression-Improvement,42 QIDS-CR, QIDS-SR and questionnaires regarding side effects and functional levels were used for follow-up evaluation. The primary outcome measure was the HRSD17, which was administered at the end of each treatment level by independent phone interviewers.
The STAR*D used broad inclusion and minimal exclusion criteria; subjects between ages 18 and 75 who met the criteria for single or recurrent non-psychotic MDD with a HRSD score less than or equal to 14 were included in the study. Participants with an adequate symptomatic response entered the 12-month naturalistic follow-up phase, which involved brief monthly as well as more thorough quarterly assessments. The study also collected subjects' blood samples for genetic analysis.
Major findings
The STAR*D study provided crucial information on deciding treatment sequence through detailed treatment responses and clinical presentations of individuals with non-psychotic MDD.43 It also revealed clinical factors associated with treatment responses and prognosis. A total of 4351 subjects from both primary and specialty care practices were enrolled in the study. Remission rates for treatment levels 1 through 4 were 36.8%, 30.6%, 13.7% and 13.0%, respectively.44 There was no difference in effectiveness between any treatments at any treatment level. Patients with longer index episodes, more concurrent psychiatric or general medical disorders, and/or lower measures of baseline function were less likely to achieve remission. There were no major differences in outcomes between patients treated in primary versus specialist care,45 nor were there significant differences in depression rating scores obtained through clinician ratings versus self-report. This study also evaluated various clinical trials, including non-pharmacological treatment for treatment-resistant depression, none of which showed superiority over other treatment modalities.46,47,48
In addition to clinical trials, the STAR*D also explored various clinical characteristics that could affect treatment responses and the prognosis of MDD. As reflected in the DSM-5 diagnostic system, the study confirmed the importance of anxiety in clinical courses of MDD.49 About 20% of participants with MDD had chronic depression,50 which exhibited poorer prognosis. Recent manic-like symptoms and family histories of BD were associated with conversion to BD, while irritability and antidepressant resistance were not associated with the conversion.51,52 General medical comorbid conditions were not uncommon in MDD, and they affected the severity of the depression.53,54 Age of onset55 and episode duration56 did not change the likelihood of remission. Using blood samples collected from subjects, genetic association studies integrating detailed clinical observations and genetic variances were conducted.57,58,59,60,61,62,63,64
Limitations
Because the STAR*D study aimed to conduct clinical trials from the outset, there may have been a bias in the recruitment processes. Detailed clinical observation is one of the major strengths of the STAR*D study, but it also can limit initial study participation. In addition, populations with psychotic depression, not uncommon in clinical practice, were not included in the study.
The NIMH-funded Systematic Treatment Enhancement Program for Bipolar Disorder
The STEP-BD is a multisite and prospective treatment study for BD.35 Similar to the STAR*D project for non-psychotic MDD, the STEP-BD projects aimed to determine the effectiveness of various treatment modalities for BD in both the acute and maintenance phases. Like the STAR*D project, the STEP-BD also aimed to observe a naturalistic clinical course for 12 months. It incorporated both randomized controlled clinical studies and uncontrolled, evidence-based clinical care.65
Considering the complexity of the clinical presentation of BD, the STEP-BD was designed to include the full spectrum of bipolar patients seeking clinical care. All patients with bipolar subtypes, including bipolar I, II, bipolar not otherwise specified (NOS), and cyclothymia, who were in any phase of the illness for longer than 15 years were able to take part in the study. Regardless of whether patients participated in the naturalistic study or the randomized trials, they received identical ongoing assessments of treatment and outcome information. Blood samples were also collected for the genetic analyses.
Diagnoses were confirmed through the administration of the Mini International Neuropsychiatric Interview (MINI)66 and a standardized affective disorder evaluation (ADE)67 by a clinical specialist (a psychologist or social worker) and the treating psychiatrist, respectively. For symptom evaluation, various questionnaires were used to consider the complex symptom presentation of BD; interviewers administered the Montgomery-Asberg Depression Rating Scale (MADRS),68 Young Mania Rating Scale (YMRS),69 Liebowitz Social Phobia Scale,70 Panic Disorder Severity Scale,71 and Yale-Brown Obsessive Compulsive Scale.72 Subjects also completed various self-report questionnaires, including the Beck Depression Inventory Version II (BDI-II),73 NEO Five-Factor Inventory,74 ADHD Symptom Checklist, Eating Disorder Inventory, Fear Questionnaire, Beck Anxiety Inventory,75 Beck Hopelessness Scale, and SF-36 Health Survey.76 These questionnaires and rating scales were utilized for both the baseline and follow-up assessments. The YMRS and the MADRS were the primary assessments of mood symptoms. Independent study assessments were scheduled at baseline and quarterly for the first year and then semiannually for up to 2 years.
Major findings
A total of 4,361 subjects with bipolar spectrum disorder across 22 clinical sites in the United States were enrolled in the study. The STEP-BD study explored the efficacy of antidepressant use for BD, which has been controversial. In general, antidepressant use had neither benefit nor risk relative to the use of mood stabilizer alone.77 In a randomized study, patients who successfully recovered with both antidepressants and mood stabilizers were assigned to either the antidepressant plus mood stabilizer or the mood stabilizer alone treatment groups; no significant difference in the recurrence rate or the duration of remission was observed between the two groups.78 However, in naturalistic studies, adjunctive antidepressant use was associated with increased mania symptom severity and did not appear to reduce the time to recovery compared to treatment with mood stabilizer alone.79 Antidepressant use also exacerbated the clinical course of rapid cycling BD but was not associated with the development of suicidality.80
Regarding treatment-resistant bipolar depression, a randomized study was conducted to compare the effectiveness of lamotrigine, inositol, and risperidone. Although significant statistical differences were found in the primary analysis, lamotrigine was more effective in terms of CGI and functional levels in the post-hoc analysis.81 The study also explored the efficacy of psychosocial intervention for BD. Intensive psychosocial intervention, such as interpersonal and social rhythm therapy, cognitive behavior therapy, and family-focused therapy, enhanced functioning and life satisfaction in patients with BD.82
In a naturalistic study, patients with BD showed a high disease burden even during standard clinical care. Among the 1469 patients who were symptomatic at study entry, 858 (58%) subsequently achieved remission during follow-up.83 Nearly 50% of these patients experienced recurrence during the 2 years of follow-up. Residual mood symptoms at initial recovery were associated with increased risk of recurrence. Although this is generally consistent with other reports, it suggests that the predominance of depressive relapse over manic relapse is substantially lower than was reported in the CDS study.
The study also confirmed the complex disease presentation of BD. BD showed high rates of psychiatric and medical comorbid conditions,84 which were associated with poorer clinical courses. Mixed features were common,85 and anxiety comorbidity increased suicide risk86 and worsened clinical courses.87 Polypharmacy was also very common,88 and about 10% of patients were treated with the second-generation atypical antipsychotics.89 Similar to the STAR*D study, genetic studies are actively being done in combination with sophisticated phenotype definition using the database.90,91,92,93,94
Limitations
Also similar to the STAR*D study, participants in the STEP-BD may not be representative of the general population with BD. In order to reflect the real-world clinical setting, patients who visited and sought treatment at the study sites were recruited. Thus, this study's population may be different from the population of the epidemiological study. Additionally, although the study team made an effort to recruit people within the minority population, Asian and Latino subjects were underrepresented.
The Stanley Foundation Bipolar treatment outcome Network
The NIMH SFBN95 is a multisite clinical trials network that has been established to determine the relative efficacy of treatments for BDs that can address a wide range of variants and comorbidities of disease, which are the main characteristics of BD. Subjects were recruited from four sites in the United States (Los Angeles, Dallas, Cincinnati, and Bethesda) and one site in Europe (Utrecht, Netherlands). All participants were community-based subjects diagnosed with either BD I, II or not otherwise specified (NOS). The network was designed to conduct multi-level randomized controlled clinical trials. More than 600 subjects were recruited, and 125 of these subjects participated in the randomized controlled clinical trials.
At baseline, all patients received the Structured Clinical Interview (SCID) for DSM-IV, Research Version (SCID-P).96 Socio-demographic and clinical information were collected through self-report questionnaires and an individual interview with a clinician.
The NIMH Life Chart Manual for recurrent affective illness (NIMH-LCM)97 was used for long-term assessment. In the LCM self-version of prospective ratings (LCM-S/P),98 each subject was instructed to record daily mood changes as well as functional levels and important life events. The clinician integrated information from the LCM-S/P and interview and finalized the NIMH Clinician version of the Prospective ratings (the NIMH-LCM-C/P).98 Moreover, additional information was collected through the 30-item Inventory for Depressive Symptomatology (IDS)99 for depression, the Young Mania Rating Scale (YMRS) for mania, the Positive and Negative Syndrome Scale (PANSS)100 for psychosis, the Global Assessment of Functional Scale (GAF)101 for social and occupational functioning, and the Life Functioning Questionnaire (LFQ)102 for quality of life.
Major findings
The SFBN included three double blind, randomized, controlled studies on adjunctive agents for BD103: 1) adjunctive use of bupropion, sertraline and venlafaxine; 2) omega-3 versus placebo; and 3) modafinil versus placebo. Adjunctive use of antidepressants confirmed the risk of (hypo)manic switch in antidepressant use, which occurred less often in bipolar II disorder compared to bipolar I disorder.104 Among the three antidepressants examined, venlafaxine was associated with the highest risk of (hypo)manic switch.105 In the study on adjunctive use of omega-3, there was no significant difference in mood systems between the use of omega-3 and the placebo,106 while modafinil trials showed significant improvement of depressive symptoms compared to the placebo trials.107 Also, two open randomized trials (tranylcypromine vs. lamotrigine; sibutramine vs. topiramate) were conducted; tranylcypromine showed promising efficacy in treating depressive symptoms compared to lamotrigine.108 Both sibutramine and topiramate, however, had significant side effects, such as weight loss. Topiramate had a higher discontinuation rate. In addition to randomized trials, numerous open-label, naturalistic case series on adjunctive use of anticonvulsants (gabapentin, zonisamide, levetiracetam, topiramate, lamotrigine, tiagabine and oxcarbazepine)109,110,111,112 and atypical antipsychotics (olanzapine, risperidone, quetiapine, clozapine and aripiprazole)113,114 were reported.
The study also confirmed the chronic and recurrent nature of BD. A majority of subjects had a considerable degree of residual illness-related morbidity, with a three-fold greater amount of time spent depressed than manic.103 The study also found that there was a time delay of more than a decade between the onset of symptoms that met the full criteria of the mood disorder and the onset of first treatment. Early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorders were associated with the early onset of BD.115 Both medical and psychiatric comorbidity were very common among the subjects.116 The severity of mood disorders was associated with having experienced mood episodes more than 10 times, a family history of drug abuse, and poor occupational functioning. The study also found that gender affected disease presentation.117,118,119
Limitations
The study did not collect any biological samples to determine a biomarker for the clinical courses of BD. Thorough evaluation through academic or tertiary-referral centers may limit recruiting samples that can represent bipolar disorder in general. More importantly, subjects in the study had the illness for an average of 20 years, so study findings may not be generalizable to those in a relatively early phase of the illness. In particular, considering the fact that most illness variables were gathered through self-report questionnaire and interviews, reliability regarding the disease course may be lower than expected.
The Clinical Research Center for Depression study
The Clinical Research Center for Depression (CRESCEND) study is a 9-year observational collaborative prospective cohort study examining clinical outcomes in patients with depressive disorders in Korea.120 From January 2006 to August 2008, 1,183 participants were enrolled in the CRESCEND study from 18 hospitals throughout Korea. The CRESCEND study endorsed a wide inclusion criteria; participants were over 7 years old and met the criteria for either MDD, dysthymic disorder, or depressive disorder, not otherwise specified (NOS), as determined by the SCID.96
The CRESCEND study included two phases with a follow-up period of 9 years. In phase I, which lasted a year, each eligible participant visited the hospital and was assessed at 1, 2, 4, 8, 12, 24, and 52 weeks after baseline. During phase II, an extension of phase I, all participants were given an annual evaluation from year 1 (or the end of phase I) to year 9.
A diagnostic evaluation, retrospective personal history of medical or psychiatric illnesses and treatment, and socio-epidemiologic clinical data were collected at baseline. Clinical, social, and functional outcomes of the treatment were evaluated with clinician- and self-administered measures during each visit. The clinician-administered measures included the CGI,42 the HRSD17,40 the Hamilton Anxiety Rating Scale (HAMA),121 the Brief Psychiatric Rating Scale (BPRS),122 and the Social and Occupational Functioning Assessment Scale (SOFAS).10 The self-administered measures consisted of the BDI-II,73 the Scale for Suicidal Ideation (SSI),123 and the abbreviated version of the World Health Organization Quality of Life assessment instrument (WHO-QOL-BREF).124
Major findings
The CRESCEND study is the first long-term prospective collaborative observational cohort study investigating the natural course and outcome for depressive patients in Korea. Baseline data from the study showed distinct characteristics of depression in Korea. Subjects with depression in Korea showed an older age of onset, more frequent histories of suicide attempts and lower rates of family history of depression than previous studies in the Western countries.125 These characteristics may be associated with biological and sociocultural characteristics of Korean MDD, with important implications for treatment plans and courses. The phase II follow-up assessments are currently in progress. The remission rate after 12 weeks of antidepressant treatment was 31.4%; female subjects without a history of prior suicide attempt and with low baseline anxiety were found to have a higher remission rate.126,127
Limitations
The study included all spectrums of depression; however, it did not include patients with BDs. About three-fourths of the participants in the CRESCEND study were recruited from outpatient settings, which may cause selection bias.
CONCLUSION
So far, various cohort studies on mood disorders provided crucial information on the complex course of mood disorders, building a foundation for clinical and biological research. However, there are several limitations in directly applying the study findings to real world clinical settings. In particular, most studies explored clinical courses of populations with depression and BD separately, even though there has been controversy over whether the two diseases are within the same spectrum or have distinct neurobiological backgrounds. Considering the heterogeneous illness presentation and the transition from unipolar depression to bipolar disorder, which is not uncommon, it would be preferable to apply loose criteria using standardized evaluation methods to include a broad range of patients with mood disorders. In order to understand the detailed clinical course, close observation is necessary, and follow-ups should occur more frequently. Previous clinical trials have generally gathered detailed information on the course, but there may have been a potential bias in their recruitment process. Regular naturalistic follow-up evaluations will give us the best opportunity to observe the clinical course more closely. Moreover, it is ideal to have both an objective mood rating and a functional level evaluation during the evaluation visits. In addition, it is important to observe the early stages of illnesses to determine factors associated with chronicity or recurrence. In order to apply clinical information from naturalistic observations to early detection and treatment, biological samples should be collected at the same time. Aside from the CRESCEND study, the majority of participants in cohort studies were Caucasian, so the biopsychosocial characteristics of Asian populations with mood disorders have not been thoroughly investigated. An innovative cohort study that can serve as a platform for translational research for treatment and prevention of mood disorders is critical in determining clinical, psychosocial, neurobiological, and genetic factors that are associated with long-term course and consequences of mood disorders in Korean patients.
Acknowledgments
This study was supported by the Korea Health 21 R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HM14C2606).
References
- 1.Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 3.Jeon HJ, Lee JY, Lee YM, Hong JP, Won SH, Cho SJ, et al. Lifetime prevalence and correlates of suicidal ideation, plan, and single and multiple attempts in a Korean nationwide study. J Nerv Ment Dis. 2010;198:643–646. doi: 10.1097/NMD.0b013e3181ef3ecf. [DOI] [PubMed] [Google Scholar]
- 4.Jeon HJ, Lee JY, Lee YM, Hong JP, Won SH, Cho SJ, et al. Unplanned versus planned suicide attempters, precipitants, methods, and an association with mental disorders in a Korea-based community sample. J Affect Disord. 2010;127:274–280. doi: 10.1016/j.jad.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Jeon HJ, Walker RS, Inamori A, Hong JP, Cho MJ, Baer L, et al. Differences in depressive symptoms between Korean and American outpatients with major depressive disorder. Int Clin Psychopharmacol. 2014;29:150–156. doi: 10.1097/YIC.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 6.Jeon HJ, Park JI, Fava M, Mischoulon D, Sohn JH, Seong S, et al. Feelings of worthlessness, traumatic experience, and their comorbidity in relation to lifetime suicide attempt in community adults with major depressive disorder. J Affect Disord. 2014;166:206–212. doi: 10.1016/j.jad.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Munoz A, Gange SJ. Methodological issues for biomarkers and intermediate outcomes in cohort studies. Epidemiol Rev. 1998;20:29–42. doi: 10.1093/oxfordjournals.epirev.a017970. [DOI] [PubMed] [Google Scholar]
- 8.Hagnell O, Ojesjo L. A prospective study concerning mental disorders of a total population investigated in 1947, 1957 and 1972. The Lundby study III (preliminary report) Acta Psychiatr Scand Suppl. 1975;263:1–11. doi: 10.1111/j.1600-0447.1975.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 9.The National Board of Health and Welfare; National Board of Health and Welfare, editors. Patient Register. Stockholm: National Board of Health and Welfare; 2004. [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 11.Mattisson C, Bogren M, Horstmann V, Munk-Jorgensen P, Nettelbladt P. The long-term course of depressive disorders in the Lundby Study. Psychol Med. 2007;37:883–891. doi: 10.1017/S0033291707000074. [DOI] [PubMed] [Google Scholar]
- 12.Nettelbladt P, Bogren M, Mattisson C, Ojesjo L, Hagnell O, Hofvendahl E, et al. Does it make sense to do repeated surveys?--the Lundby Study, 1947-1997. Acta Psychiatr Scand. 2005;111:444–452. doi: 10.1111/j.1600-0447.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Angst J, Dobler-Mikola A, Binder J. The Zurich study--a prospective epidemiological study of depressive, neurotic and psychosomatic syndromes. I. Problem, methodology. Eur Arch Psychiatry Neurol Sci. 1984;234:13–20. doi: 10.1007/BF00432878. [DOI] [PubMed] [Google Scholar]
- 14.Derogatis LR, Cleary PA. Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br J Soc Clin Psychol. 1977;16:347–356. doi: 10.1111/j.2044-8260.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 15.Burns T, Eichenberger A, Eich D, Ajdacic-Gross V, Angst J, Rossler W. Which individuals with affective symptoms seek help? Results from the Zurich epidemiological study. Acta Psychiatr Scand. 2003;108:419–426. doi: 10.1046/j.0001-690x.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 16.Eich D, Neuhaus C, Gamma A, Angst J, Rossler W, Ajdacic-Gross V, et al. Is depression a risk factor for heart complaints? Longitudinal aspects in the Zurich study. Eur Arch Psychiatry Clin Neurosci. 2007;257:396–401. doi: 10.1007/s00406-007-0747-x. [DOI] [PubMed] [Google Scholar]
- 17.Merikangas KR, Zhang H, Avenevoli S, Acharyya S, Neuenschwander M, Angst J, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003;60:993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- 18.Angst J, Gamma A, Benazzi F, Ajdacic V, Rossler W. Does psychomotor agitation in major depressive episodes indicate bipolarity? Evidence from the Zurich Study. Eur Arch Psychiatry Clin Neurosci. 2009;259:55–63. doi: 10.1007/s00406-008-0834-7. [DOI] [PubMed] [Google Scholar]
- 19.Merikangas KR, Herrell R, Swendsen J, Rossler W, Ajdacic-Gross V, Angst J. Specificity of bipolar spectrum conditions in the comorbidity of mood and substance use disorders: results from the Zurich cohort study. Arch Gen Psychiatry. 2008;65:47–52. doi: 10.1001/archgenpsychiatry.2007.18. [DOI] [PubMed] [Google Scholar]
- 20.Katz MM, Klerman GL. Introduction: overview of the clinical studies program. Am J Psychiatry. 1979;136:49–51. doi: 10.1176/ajp.136.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 22.Manni E, Bortolami R, Pettorossi VE, Lucchi ML, Callegari E. Afferent fibers and sensory ganglion cells within the oculomotor nerve in some mammals and man. II. Electrophysiological investigations. Arch Ital Biol. 1978;116:16–24. [PubMed] [Google Scholar]
- 23.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 24.Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol Med. 1999;29:869–878. doi: 10.1017/s0033291799008570. [DOI] [PubMed] [Google Scholar]
- 25.Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 27.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62:1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- 28.Judd LL, Schettler PJ, Akiskal HS, Coryell W, Leon AC, Maser JD, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386–394. doi: 10.1001/archpsyc.65.4.386. [DOI] [PubMed] [Google Scholar]
- 29.Coryell W, Solomon DA, Fiedorowicz JG, Endicott J, Schettler PJ, Judd LL. Anxiety and outcome in bipolar disorder. Am J Psychiatry. 2009;166:1238–1243. doi: 10.1176/appi.ajp.2009.09020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judd LL, Akiskal HS, Schettler PJ, Coryell W, Maser J, Rice JA, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2003;73:19–32. doi: 10.1016/s0165-0327(02)00324-5. [DOI] [PubMed] [Google Scholar]
- 31.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 32.Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 33.Judd LL. Major depressive disorder: longitudinal symptomatic structure, relapse and recovery. Acta Psychiatr Scand. 2001;104:81–83. doi: 10.1034/j.1600-0447.2001.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 34.Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003;26:457–494. x. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 35.Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 36.Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler RC, Abelson J, Demler O, Escobar JI, Gibbon M, Guyer ME, et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI) Int J Methods Psychiatr Res. 2004;13:122–139. doi: 10.1002/mpr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman M, Mattia JI. The reliability and validity of a screening Questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60:677–683. doi: 10.4088/jcp.v60n1006. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 42.Busner J, Targum SD, Miller DS. The Clinical Global Impressions scale: errors in understanding and use. Compr Psychiatry. 2009;50:257–262. doi: 10.1016/j.comppsych.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Alpert JE, Biggs MM, Davis L, Shores-Wilson K, Harlan WR, Schneider GW, et al. Enrolling research subjects from clinical practice: ethical and procedural issues in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial. Psychiatry Res. 2006;141:193–200. doi: 10.1016/j.psychres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 45.Gaynes BN, Rush AJ, Trivedi M, Wisniewski SR, Balasubramani GK, Spencer DC, et al. A direct comparison of presenting characteristics of depressed outpatients from primary vs. specialty care settings: preliminary findings from the STAR*D clinical trial. Gen Hosp Psychiatry. 2005;27:87–96. doi: 10.1016/j.genhosppsych.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519–1530. doi: 10.1176/ajp.2006.163.9.1519. quiz 1665. [DOI] [PubMed] [Google Scholar]
- 47.McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163:1531–1541. doi: 10.1176/ajp.2006.163.9.1531. quiz 1666. [DOI] [PubMed] [Google Scholar]
- 48.Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 49.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 50.Gilmer WS, Trivedi MH, Rush AJ, Wisniewski SR, Luther J, Howland RH, et al. Factors associated with chronic depressive episodes: a preliminary report from the STAR-D project. Acta Psychiatr Scand. 2005;112:425–433. doi: 10.1111/j.1600-0447.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 51.Perlis RH, Uher R, Ostacher M, Goldberg JF, Trivedi MH, Rush AJ, et al. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry. 2011;68:351–360. doi: 10.1001/archgenpsychiatry.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perlis RH, Fava M, Trivedi MH, Alpert J, Luther JF, Wisniewski SR, et al. Irritability is associated with anxiety and greater severity, but not bipolar spectrum features, in major depressive disorder. Acta Psychiatr Scand. 2009;119:282–289. doi: 10.1111/j.1600-0447.2008.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yates WR, Mitchell J, Rush AJ, Trivedi MH, Wisniewski SR, Warden D, et al. Clinical features of depressed outpatients with and without co-occurring general medical conditions in STAR*D. Gen Hosp Psychiatry. 2004;26:421–429. doi: 10.1016/j.genhosppsych.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Bryan CJ, Songer TJ, Brooks MM, Thase ME, Gaynes BN, Klinkman M, et al. A comparison of baseline sociodemographic and clinical characteristics between major depressive disorder patients with and without diabetes: a STAR*D report. J Affect Disord. 2008;108:113–120. doi: 10.1016/j.jad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Kozel FA, Trivedi MH, Wisniewski SR, Miyahara S, Husain MM, Fava M, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2008;16:58–64. doi: 10.1097/JGP.0b013e31815a43d7. [DOI] [PubMed] [Google Scholar]
- 56.Gilmer WS, Gollan JK, Wisniewski SR, Howland RH, Trivedi MH, Miyahara S, et al. Does the duration of index episode affect the treatment outcome of major depressive disorder? A STAR*D report. J Clin Psychiatry. 2008;69:1246–1256. doi: 10.4088/jcp.v69n0807. [DOI] [PubMed] [Google Scholar]
- 57.Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry. 2010;67:369–379. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- 58.Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 59.Warden D, Rush AJ, Wisniewski SR, Lesser IM, Kornstein SG, Balasubramani GK, et al. What predicts attrition in second step medication treatments for depression?: a STAR*D Report. Int J Neuropsychopharmacol. 2009;12:459–473. doi: 10.1017/S1461145708009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cabanero M, Laje G, Detera-Wadleigh S, McMahon FJ. Association study of phosphodiesterase genes in the Sequenced Treatment Alternatives to Relieve Depression sample. Pharmacogenet Genomics. 2009;19:235–238. doi: 10.1097/FPC.0b013e328320a3e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry. 2007;164:1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 62.Perlis RH, Purcell S, Fava M, Fagerness J, Rush AJ, Trivedi MH, et al. Association between treatment-emergent suicidal ideation with citalopram and polymorphisms near cyclic adenosine monophosphate response element binding protein in the STAR*D study. Arch Gen Psychiatry. 2007;64:689–697. doi: 10.1001/archpsyc.64.6.689. [DOI] [PubMed] [Google Scholar]
- 63.Perlis RH, Moorjani P, Fagerness J, Purcell S, Trivedi MH, Fava M, et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. The FKBP5-gene in depression and treatment response--an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowden CL, Perlis RH, Thase ME, Ketter TA, Ostacher MM, Calabrese JR, et al. Aims and results of the NIMH systematic treatment enhancement program for bipolar disorder (STEP-BD) CNS Neurosci Ther. 2012;18:243–249. doi: 10.1111/j.1755-5949.2011.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 67.Sachs GS. Use of clonazepam for bipolar affective disorder. J Clin Psychiatry. 1990;51(Suppl 31-34) discussion 50-53. [PubMed] [Google Scholar]
- 68.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA) Br J Psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 69.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 70.Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 71.Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter collaborative panic disorder severity scale. Am J Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- 72.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 73.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 74.McCrae RR, John OP. An introduction to the five-factor model and its applications. J Pers. 1992;60:175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 75.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 76.Ware JE., Jr Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 77.Harel EV, Levkovitz Y. Effectiveness and safety of adjunctive antidepressants in the treatment of bipolar depression: a review. Isr J Psychiatry Relat Sci. 2008;45:121–128. [PubMed] [Google Scholar]
- 78.Ghaemi SN, Ostacher MM, El-Mallakh RS, Borrelli D, Baldassano CF, Kelley ME, et al. Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71:372–380. doi: 10.4088/JCP.08m04909gre. [DOI] [PubMed] [Google Scholar]
- 79.Goldberg JF, Perlis RH, Ghaemi SN, Calabrese JR, Bowden CL, Wisniewski S, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164:1348–1355. doi: 10.1176/appi.ajp.2007.05122032. [DOI] [PubMed] [Google Scholar]
- 80.Bauer MS, Wisniewski SR, Marangell LB, Chessick CA, Allen MH, Dennehy EB, et al. Are antidepressants associated with new-onset suicidality in bipolar disorder? A prospective study of participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) J Clin Psychiatry. 2006;67:48–55. doi: 10.4088/jcp.v67n0108. [DOI] [PubMed] [Google Scholar]
- 81.Nierenberg AA, Ostacher MJ, Calabrese JR, Ketter TA, Marangell LB, Miklowitz DJ, et al. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163:210–216. doi: 10.1176/appi.ajp.163.2.210. [DOI] [PubMed] [Google Scholar]
- 82.Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Kogan JN, Sachs GS, et al. Intensive psychosocial intervention enhances functioning in patients with bipolar depression: results from a 9-month randomized controlled trial. Am J Psychiatry. 2007;164:1340–1347. doi: 10.1176/appi.ajp.2007.07020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 84.Magalhaes PV, Kapczinski F, Nierenberg AA, Deckersbach T, Weisinger D, Dodd S, et al. Illness burden and medical comorbidity in the Systematic Treatment Enhancement Program for Bipolar Disorder. Acta Psychiatr Scand. 2012;125:303–308. doi: 10.1111/j.1600-0447.2011.01794.x. [DOI] [PubMed] [Google Scholar]
- 85.Goldberg JF, Perlis RH, Bowden CL, Thase ME, Miklowitz DJ, Marangell LB, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166:173–181. doi: 10.1176/appi.ajp.2008.08050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon NM, Zalta AK, Otto MW, Ostacher MJ, Fischmann D, Chow CW, et al. The association of comorbid anxiety disorders with suicide attempts and suicidal ideation in outpatients with bipolar disorder. J Psychiatr Res. 2007;41:255–264. doi: 10.1016/j.jpsychires.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Otto MW, Simon NM, Wisniewski SR, Miklowitz DJ, Kogan JN, Reilly-Harrington NA, et al. Prospective 12-month course of bipolar disorder in out-patients with and without comorbid anxiety disorders. Br J Psychiatry. 2006;189:20–25. doi: 10.1192/bjp.bp.104.007773. [DOI] [PubMed] [Google Scholar]
- 88.Ghaemi SN, Hsu DJ, Thase ME, Wisniewski SR, Nierenberg AA, Miyahara S, et al. Pharmacological Treatment Patterns at Study Entry for the First 500 STEP-BD Participants. Psychiatr Serv. 2006;57:660–665. doi: 10.1176/ps.2006.57.5.660. [DOI] [PubMed] [Google Scholar]
- 89.Brooks JO, 3rd, Goldberg JF, Ketter TA, Miklowitz DJ, Calabrese JR, Bowden CL, et al. Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the Systematic Treatment Enhancement Program for Bipolar Disorder. J Clin Psychiatry. 2011;72:240–247. doi: 10.4088/JCP.09m05214yel. [DOI] [PubMed] [Google Scholar]
- 90.Huang J, Perlis RH, Lee PH, Rush AJ, Fava M, Sachs GS, et al. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am J Psychiatry. 2010;167:1254–1263. doi: 10.1176/appi.ajp.2010.09091335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mansour HA, Talkowski ME, Wood J, Pless L, Bamne M, Chowdari KV, et al. Serotonin gene polymorphisms and bipolar I disorder: focus on the serotonin transporter. Ann Med. 2005;37:590–602. doi: 10.1080/07853890500357428. [DOI] [PubMed] [Google Scholar]
- 92.Drago A, Giegling I, Schafer M, Hartmann AM, Friedl M, Konte B, et al. AKAP13, CACNA1, GRIK4 and GRIA1 genetic variations may be associated with haloperidol efficacy during acute treatment. Eur Neuropsychopharmacol. 2013;23:887–894. doi: 10.1016/j.euroneuro.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Drago A, Crisafulli C, Serretti A. The genetics of antipsychotic induced tremors: a genome-wide pathway analysis on the STEP-BD SCP sample. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:975–986. doi: 10.1002/ajmg.b.31245. [DOI] [PubMed] [Google Scholar]
- 94.Howrigan DP, Laird NM, Smoller JW, Devlin B, McQueen MB. Using linkage information to weight a genome-wide association of bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:462–471. doi: 10.1002/ajmg.b.31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Post RM, Nolen WA, Kupka RW, Denicoff KD, Leverich GS, Keck PE, Jr, et al. The Stanley Foundation Bipolar Network. I. Rationale and methods. Br J Psychiatry Suppl. 2001;41:s169–s176. doi: 10.1192/bjp.178.41.s169. [DOI] [PubMed] [Google Scholar]
- 96.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research. New York: New York State Psychiatric Institute, New York; 2002. [Google Scholar]
- 97.Denicoff KD, Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE, et al. Validation of the prospective NIMH-Life-Chart Method (NIMH-LCM-p) for longitudinal assessment of bipolar illness. Psychol Med. 2000;30:1391–1397. doi: 10.1017/s0033291799002810. [DOI] [PubMed] [Google Scholar]
- 98.Born C, Amann BL, Grunze H, Post RM, Schärer L. Saving time and money: a validation of the self ratings on the prospective NIMH Life-Chart Method (NIMH-LCM) BMC Psychiatry. 2014;14:130. doi: 10.1186/1471-244X-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 100.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 101.Krzyminska E, Rossa G, Krzyminski S. [The Global Deterioration Scale (GDS) and Functional Assessment Staging (FAST) in the diagnosis of Alzheimer type dementia] Psychiatr Pol. 1993;27:129–138. [PubMed] [Google Scholar]
- 102.Altshuler L, Mintz J, Leight K. The Life Functioning Questionnaire (LFQ): a brief, gender-neutral scale assessing functional outcome. Psychiatry Res. 2002;112:161–182. doi: 10.1016/s0165-1781(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 103.Post RM, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Keck PE, Jr, et al. An overview of recent findings of the Stanley Foundation Bipolar Network (Part I) Bipolar Disord. 2003;5:310–319. doi: 10.1034/j.1399-5618.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 104.Altshuler LL, Suppes T, Black DO, Nolen WA, Leverich G, Keck PE, Jr, et al. Lower switch rate in depressed patients with bipolar II than bipolar I disorder treated adjunctively with second-generation antidepressants. Am J Psychiatry. 2006;163:313–315. doi: 10.1176/appi.ajp.163.2.313. [DOI] [PubMed] [Google Scholar]
- 105.Post RM, Altshuler LL, Frye MA, Suppes T, Rush AJ, Keck PE, Jr, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord. 2001;3:259–265. [PubMed] [Google Scholar]
- 106.Keck PE, Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60:1020–1022. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 107.Frye MA, Grunze H, Suppes T, McElroy SL, Keck PE, Jr, Walden J, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry. 2007;164:1242–1249. doi: 10.1176/appi.ajp.2007.06060981. [DOI] [PubMed] [Google Scholar]
- 108.Nolen WA, Kupka RW, Hellemann G, Frye MA, Altshuler LL, Leverich GS, et al. Tranylcypromine vs. lamotrigine in the treatment of refractory bipolar depression: a failed but clinically useful study. Acta Psychiatr Scand. 2007;115:360–365. doi: 10.1111/j.1600-0447.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 109.McElroy SL, Suppes T, Keck PE, Frye MA, Denicoff KD, Altshuler LL, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biol Psychiatry. 2000;47:1025–1033. doi: 10.1016/s0006-3223(99)00316-9. [DOI] [PubMed] [Google Scholar]
- 110.Suppes T, Brown ES, McElroy SL, Keck PE, Jr, Nolen W, Kupka R, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affect Disord. 1999;53:95–98. doi: 10.1016/s0165-0327(98)00077-9. [DOI] [PubMed] [Google Scholar]
- 111.Suppes T, Chisholm KA, Dhavale D, Frye MA, Altshuler LL, McElroy SL, et al. Tiagabine in treatment refractory bipolar disorder: a clinical case series. Bipolar Disord. 2002;4:283–289. doi: 10.1034/j.1399-5618.2002.01201.x. [DOI] [PubMed] [Google Scholar]
- 112.Hummel B, Walden J, Stampfer R, Dittmann S, Amann B, Sterr A, et al. Acute antimanic efficacy and safety of oxcarbazepine in an open trial with an on-off-on design. Bipolar Disord. 2002;4:412–417. doi: 10.1034/j.1399-5618.2002.02228.x. [DOI] [PubMed] [Google Scholar]
- 113.McElroy SL, Frye M, Denicoff K, Altshuler L, Nolen W, Kupka R, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord. 1998;49:119–122. doi: 10.1016/s0165-0327(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 114.Suppes T, McElroy SL, Keck PE, Altshuler L, Frye MA, Grunze H, et al. Use of quetiapine in bipolar disorder: a case series with prospective evaluation. Int Clin Psychopharmacol. 2004;19:173–174. doi: 10.1097/00004850-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 115.Leverich GS, McElroy SL, Suppes T, Keck PE, Jr, Denicoff KD, Nolen WA, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51:288–297. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- 116.McElroy SL, Altshuler LL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- 117.Frye MA, Altshuler LL, McElroy SL, Suppes T, Keck PE, Denicoff K, et al. Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry. 2003;160:883–889. doi: 10.1176/appi.ajp.160.5.883. [DOI] [PubMed] [Google Scholar]
- 118.Altshuler LL, Kupka RW, Hellemann G, Frye MA, Sugar CA, McElroy SL, et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry. 2010;167:708–715. doi: 10.1176/appi.ajp.2009.09010105. [DOI] [PubMed] [Google Scholar]
- 119.Shivakumar G, Bernstein IH, Suppes T, Stanley Foundation, Keck PE, McElroy SL, et al. Are bipolar mood symptoms affected by the phase of the menstrual cycle? J Womens Health (Larchmt) 2008;17:473–478. doi: 10.1089/jwh.2007.0466. [DOI] [PubMed] [Google Scholar]
- 120.Park MH, Kim TS, Yim HW, Jeong SH, Lee C, Lee CU, et al. Clinical characteristics of depressed patients with a history of suicide attempts: results from the CRESCEND study in South Korea. J Nerv Ment Dis. 2010;198:748–754. doi: 10.1097/NMD.0b013e3181f4aeac. [DOI] [PubMed] [Google Scholar]
- 121.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 122.Overall J, Gorham D. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:779–812. [Google Scholar]
- 123.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 124.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 125.Kim TS, Jeong SH, Kim JB, Lee MS, Kim JM, Yim HW, et al. The clinical research center for depression study: baseline characteristics of a korean long-term hospital-based observational collaborative prospective cohort study. Psychiatry Investig. 2011;8:1–8. doi: 10.4306/pi.2011.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim JM, Kim SW, Stewart R, Kim SY, Yoon JS, Jung SW, et al. Predictors of 12-week remission in a nationwide cohort of people with depressive disorders: the CRESCEND study. Hum Psychopharmacol. 2011;26:41–50. doi: 10.1002/hup.1168. [DOI] [PubMed] [Google Scholar]
- 127.Yang SJ, Kim SY, Stewart R, Kim JM, Shin IS, Jung SW, et al. Gender differences in 12-week antidepressant treatment outcomes for a naturalistic secondary care cohort: the CRESCEND study. Psychiatry Res. 2011;189:82–90. doi: 10.1016/j.psychres.2010.12.027. [DOI] [PubMed] [Google Scholar]