Abstract
Purpose
To investigate the properties of angiogenin (ANG) as a potential tool for the diagnosis and grading of dry eye syndrome (DES) by analyzing tear protein profiles.
Methods
Tear samples were collected with capillary tubes from 52 DES patients and 29 normal individuals as controls. Tear protein profiles were analyzed with an immunodot blot assay as a screening test. To confirm that the tear ANG levels were in inverse proportion to the disease severity grade, the ANG and lactoferrin (LF) tear contents of normal controls and DES patients were compared in an enzyme-linked immunosorbent assay.
Results
In the immunodot blot assay, the ANG area was lower in patients with grades 3 and 4 DES than in normal controls. The areas of basic fibroblast growth factor, transforming growth factor β2, and interleukin 10 were significantly greater than those of normal controls only in grade 4 DES patients, but these proteins were not linearly correlated with dry eye severity. Upon enzyme-linked immunosorbent assay analysis, the mean concentrations of ANG and LF decreased significantly as dry eye severity increased, except between grades 1 and 2. In addition, the ratios of ANG and LF to total tear proteins were correlated significantly with DES severity.
Conclusions
ANG level was significantly lower in DES patients than in normal controls, and was significantly correlated with the worsening severity of DES, except between grades 1 and 2, as was LF. Therefore, ANG may be a useful measure of DES severity through proteomic analysis.
Keywords: Angiogenin, Dry eye syndrome, Lactoferrin, Tear
Dry eye syndrome (DES) is generally known as a multifactorial disease, which includes lacrimal gland disorder, neurotrophic deficiency, and meibomian gland dysfunction [1]. Many previous studies have shown that inflammation is also involved in the pathogenesis of DES and ultimately leads to chronic ocular surface disease [2,3,4,5,6,7]. Furthermore, several inflammatory cytokines and chemokines are correlated with DES severity [8]. In the clinical setting, DES grading is based on its symptoms and signs, but effective biological markers for determining DES grade are not yet known.
Recent proteomic analyses have identified nearly 500 proteins in human tears [9,10]. Some proteins are known to have biological roles in DES [11]. For example, lactoferrin (LF) is known to be related with the secretory function of the lacrimal gland, and its concentration is reduced in the tears of DES patients.
Angiogenin (ANG) is a 14.124 kDa single-chain polypeptide that is well-known to induce angiogenesis by activating vessel endothelial and smooth muscle cells [12,13]. However, a recent report indicated that ANG had an immunomodulatory effect via inhibition of nuclear factor kappa B translocation in human corneal fibroblasts [14]. Sack et al. [15] also reported an increase in the concentration of ANG in tears collected from a closed eye, suggesting that ANG may act as a defensive agent in proinflammatory conditions induced by prolonged eye closure.
In this study, we analyzed several tear proteins, including LF and ANG, in normal individuals and DES grade 1 through 4 patients to investigate the correlation between the concentration of tear proteins and DES grade through immunodot blot and enzyme-linked immunosorbent assay (ELISA).
Materials and Methods
Participants
A total of 81 participants, including 52 patients with DES (21 males and 31 females; mean age, 52.37 ± 10.18 years; range, 32 to 75 years) and 29 age- and sex-matched healthy individuals as controls (17 males and 12 females; mean age, 50.69 ± 10.37 years; range, 24 to 68 years), were enrolled in this study. DES was diagnosed by a physician based on the results of a patient questionnaire and clinical examinations according to the International Dry Eye Workshop report [16]. In the 52 patients with DES not associated with Sjögren's syndrome, coexisting lid margin disease, or altered tear distribution and clearance, DES severity was categorized into 1 of 4 levels, according to the Delphi panel classification (DES grade 1, n = 10; grade 2, n = 10; grade 3, n = 22; grade 4, n = 10).
Symptoms were assessed with the McMonnies Dry Eye Questionnaire [17]. Then, each individual underwent a complete examination of the ocular surface in the following order: tear break-up time (TBUT), corneal staining with f luorescein, conjunctival staining with lissamine green, and Schirmer's test without anesthesia. Corneal staining was evaluated using the National Eye Institute method, and conjunctival staining was evaluated using the Oxford Scheme. Measurements during ocular exams were performed by a single physician (JCK).
The exclusion criteria included an age less than 18 years, previous usage of ophthalmic medications except artificial tears, a history of surgical intervention, chemical injury, a corneal pathology such as a corneal infection, complaints of ocular pain or discomfort due to any recent history of ocular disease besides DES, use of contact lenses in the previous 6 months, connective tissue disease (other than rheumatoid arthritis), autoimmune disease, diabetes mellitus, and Parkinson's disease. All control individuals had no abnormalities in their ocular surface examinations, i.e., a Schirmer test I value greater than 10 mm/5 min, a TBUT value greater than 10 seconds, no complaint of ocular discomfort, and no usage of ocular medications. The research followed the tenets of the Declaration of Helsinki and informed consent was obtained from the participants after explanation of the nature and possible consequences of the study. The research was approved by an institutional review board (no. C2012136-831).
Tear collection
Samples of pure, undiluted tear fluid were collected from all 81 participants in 50-µL glass capillaries placed with one end at the lateral canthus in contact with the tear film of the right eye. Each tear sample was placed in a 1.5-mL Eppendorf tube and immediately stored at -70℃ until further examination. To keep tear stimulation at a low level, contact with the conjunctival epithelium was minimized. No additional solution that could induce irritation and stimulate tear fluids was used during tear collection. From each participant, approximately 10 µL of unstimulated tears were collected by a single physician (SWW). The total tear protein concentration was evaluated with a Nano-Drop spectrophotometer (ND-1000; Nano-Drop Technologies, Wilmington, DE, USA). As the concentrations of ANG and LF in an individual's tear fluids used for ELISA may vary, we normalized the concentration of each protein to the total tear protein concentration for both groups.
Immunodot blot assay
We used 3 µL of tear sample from each normal individual and DES patient, and diluted this aliquot to a volume of 1 mL with buffer. Tear protein profiling of all participants was performed with an immunodot blot assay following the manufacturer's instructions. Tear proteins such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor, transforming growth factor (TGF)-β2, tumor necrosis factor-α, interleukin (IL)-10, IL-17, angiostatin, stromal cell-derived factor-1α, Fas ligand, and ANG were investigated with a human cytokine antibody array kit (RayBiotech, Atlanta, GA, USA). Collected tear samples were centrifuged to eliminate any cell debris and were applied to a membrane. The tear samples were applied to each membrane for 2 hours at room temperature, after which the non-specific areas of the membranes were blocked for 30 minutes. All membranes were washed three times for 5 minutes with wash buffer 1, and then washed twice for 5 minutes with wash buffer 2. Tear proteins were detected by diluted biotin-conjugated antibodies added to each membrane for 2 hours of incubation. After washing, diluted horseradish peroxidase-conjugated streptavidin was added to each membrane. The membranes were visualized using a ChemiDoc XRS (Bio-Rad Laboratories, Berkeley, CA, USA). The density of each blot was measured with a personal molecular imager FX (Bio-Rad Laboratories) supported by imaging analysis software (Quantity One, Bio-Rad Laboratories). Positive controls marked within each array were included for immunodot blot assay validation.
Enzyme-linked immunosorbent assay
We used 3-µL tear samples for ANG and LF analysis. Each tear sample was diluted to a total volume of 300 µL with buffer. To confirm the results of the immunodot blot assay, the amounts of ANG and LF in tears from normal controls and DES patients were compared quantitatively by ELISA using a human LF detection kit (Bethyl Laboratories, Montgomery, TX, USA) and a human ANG ELISA detection kit (Abcam, Cambridge, England) following the manufacturers' instructions.
In brief, standards or samples were added to 96-well plates and incubated for 1 hour at 4℃. The plates were washed four times, and a biotin antibody for ANG or LF detection was added to each well. The plates were covered, incubated for 1 hour at room temperature, and then washed four times. A streptavidin solution for ANG or an horseradish peroxidase solution was added to each well. The plates were covered and incubated at room temperature for 45 minutes with ANG or 30 minutes with LF. After the washing step was repeated, the 3,3',5,5'-tetramethylbenzidine liquid substrate solution was added to each well. The plates were incubated for 30 minutes at room temperature in the dark, and the stop solution was added to each well. The optical density was measured at 450 nm on a microplate photometer SpectraMax 340PC using SoftMax Pro 5.4.1 (MDS Inc., Toronto, ON, Canada). The results are reported as the mean ± standard deviation.
Statistical analysis
All data were analyzed with statistical software SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). For all tests, the significance level was set at p < 0.05. Based on the normality of the data distribution, comparative analyses among individual samples of proteins were performed using the Mann-Whitney U-test, Student's t-test, and the chi-square test. Correlations between the protein concentrations and dry eye severity were assessed using Spearman's correlation test. Data collection was extended to reach a conditional power of 0.8.
Results
Demographics of the participants
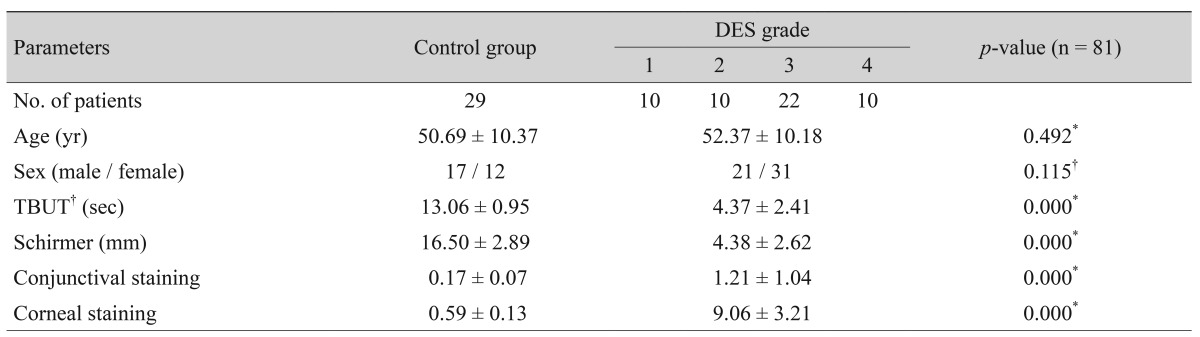
Demographic data from the patients and healthy controls, including age, sex, and clinical test results, are summarized in Table 1.
Table 1. Demographics and clinical test results of the study population.
Values are presented as number or mean ± standard deviation.
DES = dry eye syndrome; TBUT = tear break-up time.
*Independent Student's t-test; †Chi-square test.
Immunodot blot assay
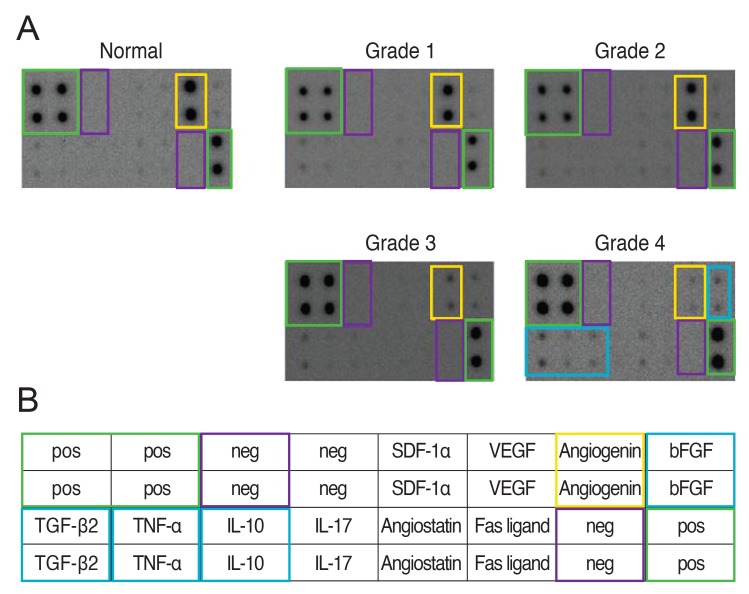
The ANG areas in DES grades 3 and 4 patients were significantly lower than those of normal controls and DES patients with lower grades. However, there were no significant differences between normal controls and DES patients with lower grades. The ANG blot densities were 508.15 ± 60.80 in normal controls, 511.39 ± 63.34 in DES grade 1 patients, 460.40 ± 35.72 in DES grade 2 patients, 49.05 ± 23.57 in DES grade 3 patients, and 38.70 ± 11.71 in DES grade 4 patients. The blot density of the positive control was 390.22.
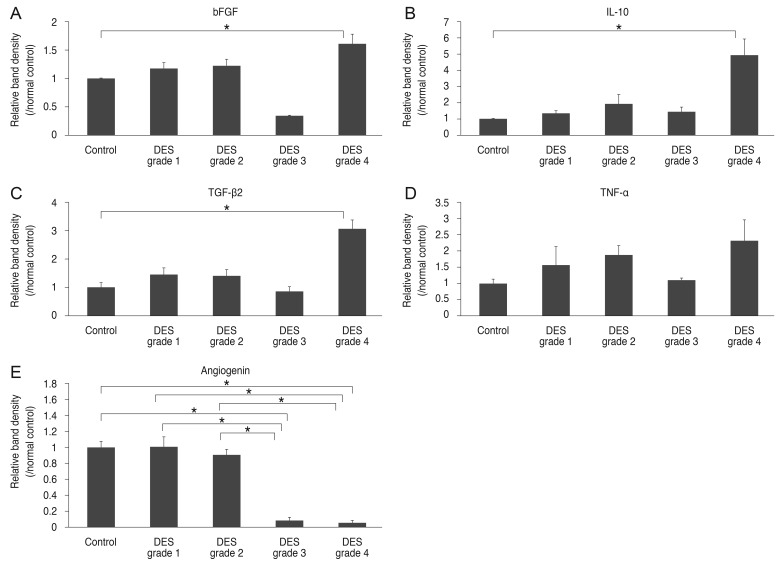
The areas of bFGF, TGF-β2, and IL-10 were significantly greater than those of normal controls only in grade 4 DES patients, but these proteins did not correlate significantly with dry eye severity. IL-17, angiostatin, stromal cell-derived factor-1α, Fas ligand, and vascular endothelial growth factor did not differ significantly among participants, regardless of DES grade. Figs. 1A and 1B and 2A-2E summarize the results of tear protein profiling from the immunodot blot assay.
Fig. 1. Immunodot blot assay of tear proteins in dry eye syndrome (DES) patients and normal participants as controls. (A) The relative amount of angiogenin tended to decrease as the DES grade increased. The densities of basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-β2, and interleukin (IL)-10 increased remarkably only in grade 4 DES patients compared to normal controls. (B) Custom Human Cytokines Antibody Array Map. pos = positive; neg = negative; SDF-1α = stromal cell derived factor-1alpha; VEGF = vascular endothelial growth factor.
Fig. 2. The relative density, presented as the average amount of each cytokine expressed in each dry eye syndrome (DES) severity grade group relative to that in the normal control group. (A-C) The areas of basic fibroblast growth factor (bFGF) (A), interleukin (IL)-10 (B), and transforming growth factor (TGF)-β2 (C) were significantly greater than those of normal controls only in grade 4 DES patients. (D) There was no significant difference of tumor necrosis factor (TNF)-α expression according to the grades of DES. (E) The areas of angiogenin in DES grade 3 and 4 patients were significantly lower than those of normal controls and DES patients of grades 1 and 2 (*p < 0.05).
Enzyme-linked immunosorbent assay
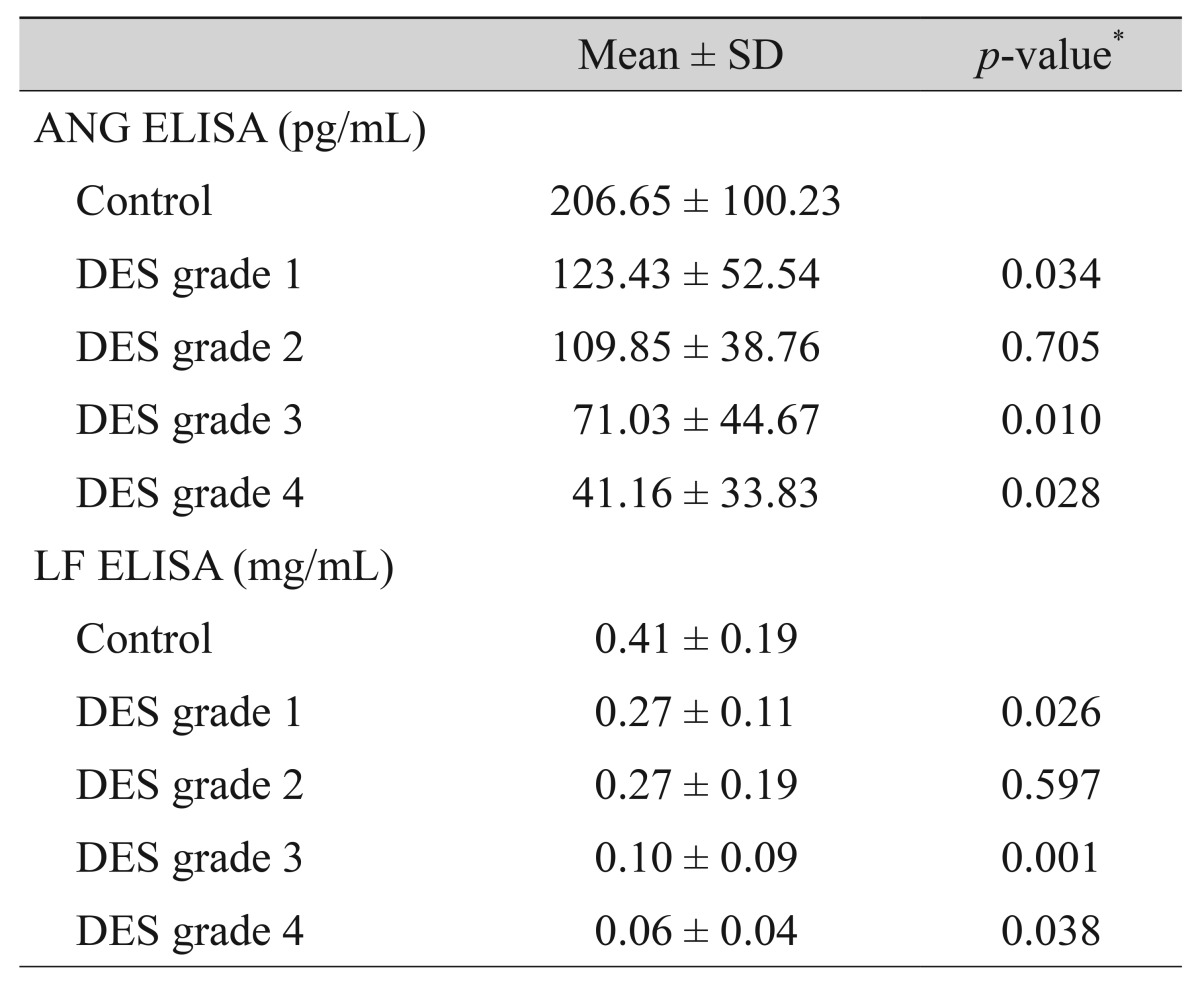
The mean concentrations of ANG in the tears of normal controls (n = 29) and DES grade 1 (n = 10), and 2 (n = 10) patients were 206.65 ± 100.23 pg/mL, 123.43 ± 52.54 pg/mL, and 109.85 ± 38.76 pg/mL, respectively. The concentrations of ANG in the tears of DES grade 3 (n = 22) and 4 (n = 10) patients were 71.03 ± 44.67 pg/mL and 41.16 ± 33.83 pg/mL, respectively. The mean concentrations of LF in the tears of normal controls and DES grade 1, and 2 patients were 0.41 ± 0.19 mg/mL, 0.27 ± 0.11 mg/mL, and 0.27 ± 0.19 mg/mL, respectively. The concentrations of LF in the tears of DES grade 3 and 4 patients were 0.10 ± 0.09 mg/mL and 0.06 ± 0.04 mg/mL, respectively. ANG and LF concentrations in DES patients were lower than those of normal controls and decreased significantly with increasing DES severity, except in grades 1 and 2. The group-to-group differences in LF and ANG were statistically significant (p < 0.05), except for the differences between DES severity grades 1 and 2. Table 2 summarizes the mean concentrations of ANG and LF in control and DES tears.
Table 2. Mean concentrations of ANG and LF in ELISA.
ELISA = enzyme-linked immunosorbent assay; Mean = mean concentration value; SD = standard deviation; ANG = angiogenin; DES = dry eye syndrome; LF = lactoferrin.
*Mann-Whitney test between the groups in the rows above and below the position of the p-value.
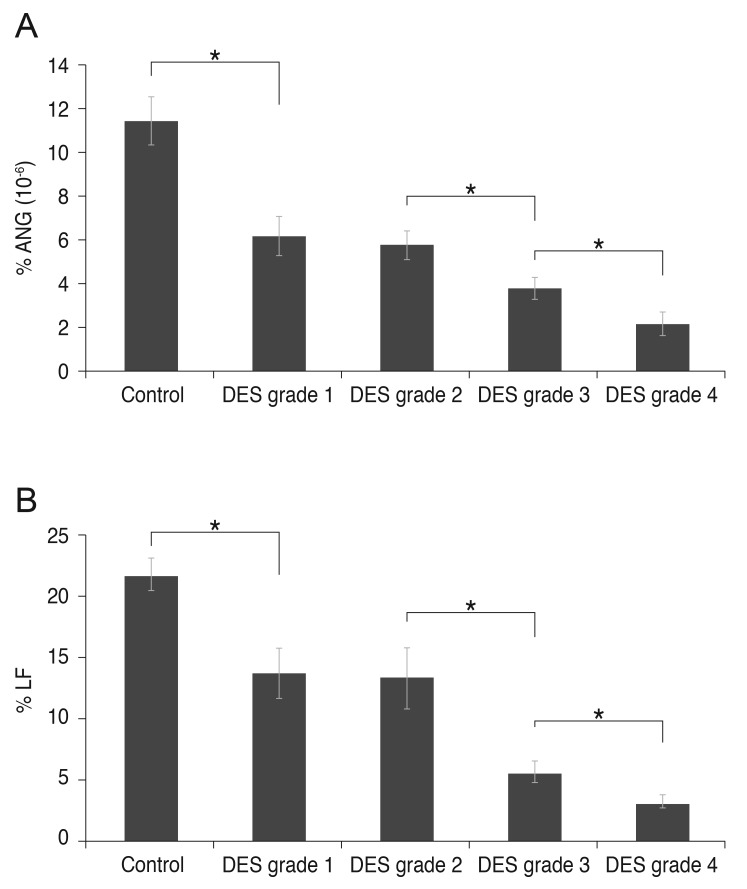
The ratios of ANG and LF to total tear proteins were measured to supplement the concentration results in normal controls and DES patients. The percentage concentration of ANG/total tear protein was 11.48 ± 5.90 (×10-6)% in the normal control group, 6.14 ± 2.93 (×10-6)% in DES grade 1, 5.74 ± 2.12 (×10-6)% in DES grade 2, 3.78 ± 2.27 (×10-6)% in DES grade 3, and 2.15 ± 1.62 (×10-6)% in DES grade 4 patients. These data were significantly correlated with DES severity. The percentage concentration of LF/total tear protein was 21.69 ± 7.58% in the normal control group, 13.65 ± 6.70% in DES grade 1, 13.36 ± 7.74% in DES grade 2, 5.54 ± 4.68% in DES grade 3, and 3.05 ± 2.31% in DES grade 4 patients. These data also were significantly correlated with DES severity. The group-to-group differences in the percentage concentrations of LF and ANG were statistically significant ( p < 0.05), except for those between DES severity grades 1 and 2.
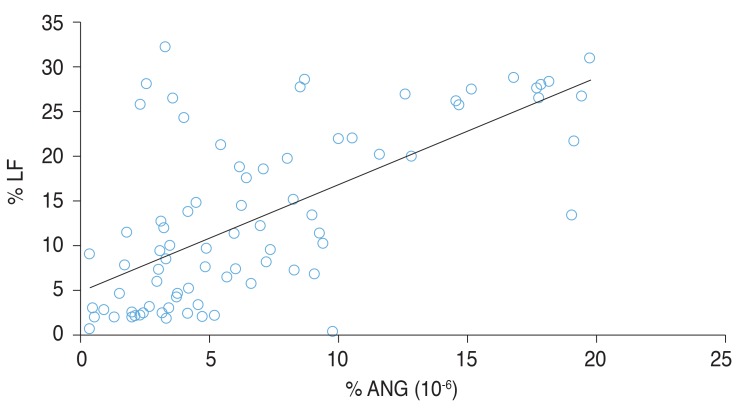
Fig. 3 shows the significant correlation between the percentage concentrations of ANG (Fig. 3A) and LF (Fig. 3B), and DES severity. Fig. 4 shows the significant correlation between the percentage concentrations of ANG and LF in DES patients [LF (%) = 1.0 × ANG (%) × 106 + 4.7828].
Fig. 3. Enzyme-linked immunosorbent assay analysis of (A) angiogenin (ANG) and (B) lactoferrin (LF) in dry eye syndrome (DES) patients (classified according to grade) and normal controls. The percentage concentrations of ANG and LF relative to total tear proteins were measured. Statistically significant correlations between DES severity and the percentage concentrations of both ANG (A) and LF (B) were noted. The group-to-group differences of ANF and LF also were statistically significant, except between DES grades 1 and 2 (*p < 0.05).
Fig. 4. Scatter plot showing the correlation between angiogenin (ANG) and lactoferrin (LF) in enzyme-linked immunosorbent assay analysis. In the correlation analysis, the percentage concentrations of ANG and LF relative to total proteins were significantly correlated with the dry eye syndrome grade (r = 0.661, p < 0.001).
Discussion
Several diagnostic tests for DES, such as fluorescein staining, TBUT measurement, Schirmer's test, Rose Bengal staining, impression cytology, and tear clearance rate measurement, have been widely used. However, these commonly used diagnostic tests are reported to be poorly associated with patients' symptoms [18], and no definite standards for measuring the therapeutic response to treatment in patients with DES have been established. Recently, several studies have evaluated and graded DES via proteomic analysis; a proteomic approach is becoming more common as a novel diagnostic tool for DES.
Many proteins have the potential to be expressed in human tears. These proteins can be associated with both physiologic functions and pathologic mechanisms [19]. Tear protein levels have been compared between evaporative dry eye patients and controls. In a previous study, LF and lipophilin A-C were lower in evaporative dry eye patients than in controls [11]. On the other hand, DES patients had higher levels of serum albumin and IL-17 than normal patients [11,20]. As DES is understood to be a multifactorial disorder and inflammation of the ocular surface is considered to be closely related with DES [3], alterations in tear protein concentrations in dry eye patients may reflect the inflammatory status of the eye.
We hypothesized that the tear protein levels of DES patients would differ from those of normal controls, and evaluated the correlation between the DES severity and the concentrations of certain proteins. In the present study, concentrations of bFGF, TGF-β2, and IL-10 were significantly greater than those of normal controls only in grade 4 DES patients, but these proteins did not correlate significantly with dry eye severity. As TGF-β2 and IL-10 are reported to be anti-inflammatory proteins secreted by CD4+ regulatory T cells upon inflammation of the ocular surface [21], and bFGF is reported to potentiate inflammatory mediator-induced leukocyte recruitment in chronic inflammation [22], we concluded that the results of our immunodot blot assay were similar to those of the previous studies. However, the concentrations of bFGF, TGF-β2, tumor necrosis factor-α, and IL-10 were not significantly correlated with DES severity.
In contrast, ANG was linearly associated with DES severity, except between grades 1 and 2. We performed a further study of ANG and LF by ELISA to confirm that the ANG levels in participants' tears were inversely proportional to the disease severity grade in DES patients, as LF has been reported to indicate reflect the severity of ocular surface damage due to DES [23]. In the present study, LF and ANG decreased proportionately as the DES severity of DES increased. Therefore, we concluded that both ANG and LF are specific proteins that are significantly correlated with DES severity, except between grades 1 and 2. This study has valuable implications for the practical use of proteomic analysis in diagnosing DES severity.
Although there have been few studies regarding ANG in the field of ophthalmology, various biological functions have been disclosed. We can infer the characteristics and functions of ANG from studies that have investigated the effects of LF and the association between LF and DES.
LF is a glycoprotein present in human breast milk and is secreted into tears by the lacrimal and meibomian glands [24]. The tear LF level has been reported to be an indicator of lacrimal secretory function [3]. LF has several functions, including anti-inflammatory effects that reduce tumor necrosis factor-α and T-cell proliferation, increase IL- 10 levels, and actions that promote cell growth and DNA synthesis [25,26]. Previous studies also have reported that tear LF levels correlated with the severity of conjunctivocorneal epithelial lesions in DES patients [23]. As DES is a multifactorial disease of the tears and the ocular surface, including an inflammatory process that alters the tear protein composition, it is worth considering LF as a therapeutic agent that may reduce DES severity. Indeed, Dogru et al. [27] reported that oral LF supplements improved tear stability and the state of the ocular surface epithelium, confirming the above-mentioned hypothesis.
Possible interactions between ANG and LF in the immune and inflammatory systems have been reported previously [28,29], and we found a significant positive correlation between ANG and LF in the present study. Although the roles of ANG in inflammatory cascades are not clear, we could hypothesize that ANG and LF interact with each other in DES progression. We also may suppose that the gradual decrease in ANG levels with the increasing DES severity reflects the progression of the inflammatory response, inferring from a previous study regarding the action of ANG as a defensive agent in pro-inflammatory conditions [15]. Together, these findings suggest that ANG could be used as a diagnostic tool for grading DES and its associated inflammatory symptoms. In addition, as ANG is a natural protein present in the body, it is expected that an exogenous ANG supplement may improve DES symptoms, much like supplements of LF and albumin do.
It is necessary to investigate the possible actions of ANG in the eye and the safety of its biological use through in vitro and in vivo studies of therapeutic exogenous supplements. In general, ANG has been reported to induce the synthesis of ribosomal RNA and neovascularization [13]. Furthermore, there have been several reports regarding the action of ANG in the posterior segments of the eye. Skeie et al. [30] reported that ANG may participate in pathologic angiogenesis in eyes with choroidal neovascularization. In contrast, Marek et al. [31] postulated that ANG is not associated with the pathogenesis of diabetic retinopathy, contrary to vascular endothelial growth factor, which is the main mediator responsible for pathological neovascularization in diabetic retinopathy. As the action of ANG is still a matter of debate, further studies are needed regarding the action of ANG in various regions of the eye.
There are some limitations of this study to consider. First, ANG levels did not differ significantly between the normal controls and DES grade 1 and 2 patients in the immunodot blot assay. These results do not support the conclusion of the present study that ANG could be used for the diagnosis and grading of DES. On the other hand, unlike the results of the immunodot blot assay, the ELISA results indicated that the concentration of ANG was negatively correlated with dry eye severity, and the group-to-group difference in ANG was statistically significant, except between DES grades 1 and 2. These results were quite consistent with those for LF. Therefore, we believe that the present study is important and will be valuable because it is the first to discuss the correlation among ANG, LF, and DES severity.
Second, we analyzed the participants' clinical test results and the tear fluids collected from their right eyes, because it was uncertain whether both eyes would have the same DES grade. We recognize that this study is limited in evaluating the true state of dry eye severity, as only one eye was studied, and there may be variability in dry eye conditions between both eyes. However, we thought that this methodology was appropriate, because the goal of this study was to evaluate the correlation between ANG levels and dry eye severity by analyzing tear fluids and the diagnostic test results.
Thirdly, although we tried to categorize the DES patients based on completely consistent test results using the Delphi classification, there were some discrepancies among the results of the TBUT, Schirmer's test, and corneal staining. In such cases, we placed priority on the corneal staining, as it is more reliable than both TBUT and Schirmer's test [32,33].
In conclusion, ANG and LF concentrations were significantly lower in DES patients than in normal controls, and were significantly correlated with DES severity, except between grades 1 and 2. Therefore, ANG may be a useful measure of DES severity through proteomic analysis. Also, ANG may be expected to act as a therapeutic agent in DES patients, inferring from the results of previous and recent studies of LF. Further research should be carried out to evaluate the safety of ANG for clinical use.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A2A2A01004643).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Javadi MA, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011;6:192–198. [PMC free article] [PubMed] [Google Scholar]
- 2.Bhavsar AS, Bhavsar SG, Jain SM. A review on recent advances in dry eye: pathogenesis and management. Oman J Ophthalmol. 2011;4:50–56. doi: 10.4103/0974-620X.83653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC. Anti-inf lammatory therapy of dry eye. Ocul Surf. 2003;1:31–36. doi: 10.1016/s1542-0124(12)70005-8. [DOI] [PubMed] [Google Scholar]
- 5.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 6.Rolando M, Barabino S, Mingari C, et al. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eyes. Cornea. 2005;24:951–954. doi: 10.1097/01.ico.0000157421.93522.00. [DOI] [PubMed] [Google Scholar]
- 7.Farris RL. Tear osmolarity: a new gold standard? Adv Exp Med Biol. 1994;350:495–503. doi: 10.1007/978-1-4615-2417-5_83. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Schwalb TA, Addeo JV, et al. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: the effect of topical Cyclosporin A therapy. Cornea. 1998;17:654–663. doi: 10.1097/00003226-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 9.de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7:R72. doi: 10.1186/gb-2006-7-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green-Church KB, Nichols KK, Kleinholz NM, et al. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008;14:456–470. [PMC free article] [PubMed] [Google Scholar]
- 11.Versura P, Nanni P, Bavelloni A, et al. Tear proteomics in evaporative dry eye disease. Eye (Lond) 2010;24:1396–1402. doi: 10.1038/eye.2010.7. [DOI] [PubMed] [Google Scholar]
- 12.Weremowicz S, Fox EA, Morton CC, Vallee BL. Localization of the human angiogenin gene to chromosome band 14q11, proximal to the T cell receptor alpha/delta locus. Am J Hum Genet. 1990;47:973–981. [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai) 2008;40:619–624. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Kim KW, Min KM, et al. Angiogenin reduces immune inflammation via inhibition of TANK-binding kinase 1 expression in human corneal fibroblast cells. Mediators Inflamm. 2014;2014:861435. doi: 10.1155/2014/861435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sack RA, Conradi L, Krumholz D, et al. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci. 2005;46:1228–1238. doi: 10.1167/iovs.04-0760. [DOI] [PubMed] [Google Scholar]
- 16.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 17.McMonnies CW. Key questions in a dry eye history. J Am Optom Assoc. 1986;57:512–517. [PubMed] [Google Scholar]
- 18.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 19.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 20.Oh JY, Kim MK, Choi HJ, et al. Investigating the relationship between serum interleukin-17 levels and systemic immune-mediated disease in patients with dry eye syndrome. Korean J Ophthalmol. 2011;25:73–76. doi: 10.3341/kjo.2011.25.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006;168:835–846. doi: 10.2353/ajpath.2006.050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pflugfelder SC, Stern ME Symposium Participants. Immunoregulation on the ocular surface: 2nd Cullen Symposium. Ocul Surf. 2009;7:67–77. doi: 10.1016/s1542-0124(12)70297-5. [DOI] [PubMed] [Google Scholar]
- 23.Danjo Y, Lee M, Horimoto K, Hamano T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol (Copenh) 1994;72:433–437. doi: 10.1111/j.1755-3768.1994.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conneely OM. Antiinflammatory activities of lactoferrin. J Am Coll Nutr. 2001;20(5 Suppl):389S–395S. doi: 10.1080/07315724.2001.10719173. [DOI] [PubMed] [Google Scholar]
- 26.Brock JH. Lactoferrin: 50 years on. Biochem Cell Biol. 2012;90:245–251. doi: 10.1139/o2012-018. [DOI] [PubMed] [Google Scholar]
- 27.Dogru M, Matsumoto Y, Yamamoto Y, et al. Lactoferrin in Sjogren's syndrome. Ophthalmology. 2007;114:2366–2367. doi: 10.1016/j.ophtha.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Murata M, Wakabayashi H, Yamauchi K, Abe F. Identification of milk proteins enhancing the antimicrobial activity of lactoferrin and lactoferricin. J Dairy Sci. 2013;96:4891–4898. doi: 10.3168/jds.2013-6612. [DOI] [PubMed] [Google Scholar]
- 29.Schmaldienst S, Oberpichler A, Tschesche H, Horl WH. Angiogenin: a novel inhibitor of neutrophil lactoferrin release during extracorporeal circulation. Kidney Blood Press Res. 2003;26:107–112. doi: 10.1159/000070992. [DOI] [PubMed] [Google Scholar]
- 30.Skeie JM, Zeng S, Faidley EA, Mullins RF. Angiogenin in age-related macular degeneration. Mol Vis. 2011;17:576–582. [PMC free article] [PubMed] [Google Scholar]
- 31.Marek N, Raczynska K, Siebert J, et al. Decreased angiogenin concentration in vitreous and serum in proliferative diabetic retinopathy. Microvasc Res. 2011;82:1–5. doi: 10.1016/j.mvr.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Cho P, Brown B, Chan I, et al. Reliability of the tear breakup time technique of assessing tear stability and the locations of the tear break-up in Hong Kong Chinese. Optom Vis Sci. 1992;69:879–885. doi: 10.1097/00006324-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Abusharha AA, Pearce EI. The effect of low humidity on the human tear film. Cornea. 2013;32:429–434. doi: 10.1097/ICO.0b013e31826671ab. [DOI] [PubMed] [Google Scholar]