Summary
Background
The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) was developed for use in clinical trials and longitudinal patient assessment.
Objectives
To characterize disease severity using the CDASI and assess responsiveness of this instrument to clinically meaningful changes in disease activity.
Methods
Patients with cutaneous dermatomyositis at the University of Pennsylvania (UPenn, n = 93) and Stanford University (Stanford, n = 106) were prospectively evaluated using the CDASI, physician global assessment (PGA) Likert scales, and visual analog scale (VAS). Data was analyzed using logistic regression models and receiver operating characteristic curves to select cut-offs.
Results
Baseline CDASI activity scores for the patients evaluated at UPenn ranged from 0 to 47 (median 17), and baseline PGA VAS scores ranged from 0 to 9.6 (median 1.1). At UPenn a CDASI activity score of 19 differentiated mild from moderate and severe disease. At Stanford baseline CDASI scores ranged from 0 to 48 (median 21), baseline PGA VAS scores ranged from 0 to 9.7 (median 4.2) and CDASI activity scores of 14 or less characterized mild disease. When a 2 cm change in the PGA VAS was regarded as a clinically significant improvement, a 4-point (UPenn) or 5-point (Stanford) change in CDASI reflected a minimal clinically significant response.
Conclusions
The CDASI is a valid and responsive measure that can be used to characterize cutaneous dermatomyositis severity and detect improvement in disease activity. Variations in cutoffs may be due to differences in disease severity between the two populations or inter-rater variations in use of the external gold measures.
Introduction
Dermatomyositis (DM) is among the idiopathic inflammatory myopathies, a heterogeneous group of chronic systemic autoimmune diseases characterized by proximal muscle inflammation.1 The reported incidence is 9.63 cases per million.2 This rare autoimmune disease, especially its cutaneous manifestations, has significant impact on patients’ quality of life.3 Optimal management necessitates a validated instrument to reliably assess disease progression and treatment efficacy. A validated disease-specific skin-based assessment tool can also be used in clinical trials to facilitate objective assessment and comparison between studies.
Dependable outcome measures allow for objective and consistent clinical assessment of dermatologic disease. The development and validation of outcome measures for dermatologic disorders including cutaneous lupus erythematosus, psoriasis, atopic dermatitis, vitiligo and rosacea, have made it possible to interpret and compare clinical trials.4, 5 The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), a skin-based outcome measure, was developed to systematically assess the extent of cutaneous disease in patients with DM.6
An ideal outcome measure is comprehensive, sensitive to change, accurate, biologically sensible, practically useful and credible to practitioners, researchers, and patients.7 Excellent inter-rater reliability and intra-rater reliability of the CDASI have been reported as well as a statistically significant (P < 0.05) correlation with the gold standard, the physician global measure.6 The CDASI was later simplified and the modified version (CDASI ver02) was found to have equally good correlation with the physician global measure.8 The reliability and validity of the CDASI was assessed in comparison to other outcome instruments. Comparison of the CDASI to the Cutaneous Assessment Tool for Myositis – Binary Method (CAT-BM), another outcome measure for dermatomyositis, revealed that both are significant predictors of the physician global measure, but CDASI has superior inter-rater reliability, intra-rater reliability and responsiveness.9 In this study we sought to classify dermatomyositis severity using CDASI activity scores and to evaluate responsiveness of the CDASI to clinically significant changes in disease activity.
Materials and Methods
Study design
Adult patients with clinical and histologic evidence of DM seen in outpatient dermatology clinics at the Hospital of the University of Pennsylvania (UPenn) and Stanford University were screened for enrollment in a DM database. A total of 93 UPenn patients and 106 Stanford patients who were eligible and willing to participate enrolled in the database between May 2007 and November 2012. Study visits were conducted during the patients’ visits to the dermatology clinic. At each study visit a board-certified dermatologist (VPW at UPenn and DFF at Stanford) assessed the patient’s skin using the CDASI ver02 and three physician global assessment (PGA) tools, namely a 5-point Likert of overall disease severity, a 3-point Likert of change in disease activity, and a visual analog scale (PGA VAS). Data acquired at UPenn were entered into REDcap, a secure web-based database, and data from Stanford were stored in a secure Excel file. The study was approved by the University of Pennsylvania and Stanford University Institutional Review Boards and is in accordance with the Declaration of Helsinki principles. All participants gave written informed consent before enrollment.
Outcome measures
Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI)
The CDASI (Fig. 1) is a partially validated DM-specific instrument designed to capture the extent of cutaneous disease. Disease involvement in 15 different anatomical locations is rated using three activity (erythema, scale, erosion/ulceration) and two damage (poikiloderma, calcinosis) measures. The presence and severity of gottron’s papules, periungual changes and alopecia are also captured. The resulting activity and damage score range from 0 to 100 and 0 to 32 respectively. Higher scores indicate greater disease severity.
Figure 1.

Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI)
Physician Global Assessment
PGA tools are often used as external measures in validation studies.10,11,12 In this study a 5-point Likert (“static”) scale (none, mild, moderate, severe or extremely severe) and 0 – 10 cm visual analog scale (PGA VAS) were used to rate disease severity. The VAS is a continuous scale where 0 cm is no disease activity and 10 cm represents extremely severe disease. At each visit the physician also compared disease activity to the previous study visit and indicated any change using a 3-point PGA Likert (“dynamic”) scale (improved, no change, worse).
Severity analysis
Study participants with at least one complete visit were included in this analysis. Due to the small population of study participants with severe and extremely severe disease, the analysis was aimed at differentiating mild from moderate and severe disease.
Responsiveness analysis
Study participants with two or more complete visits were included in the responsiveness analysis. Improvement in disease activity was recorded using the PGA 3-point Likert scale however it does not define how much of a change is needed therefore we used a model using the PGA VAS as the gold standard. Patients with an improvement in VAS scores of 2 cm or more were considered responders while those with a change of less than 2 cm were considered non-responders.13,14 The strong intra-rater reliability of the PGA VAS and elimination of the dependence on recall suggests that results from this alternative external measure may better approximate the minimally clinically significant change so this model was used to assess the CDASI’s sensitivity to clinically relevant change.9
Statistical Methods
Logistic regression using generalized estimating equations (GEE) were used to account for the correlation among repeated measures within patients; an exchangeable correlation structure was assumed. A receiver operating characteristic curve was then used to select cut-offs. Separate site-specific analyses were performed due to interactions between study site and CDASI score and differences in severity prevalence between sites for both the severity and responsiveness analyses.
Results
Study Population
At the UPenn Connective Tissue Disease Clinic 121 patients were considered for enrollment in the DM database. Of these, 9 were excluded due to disease overlap, unclear diagnosis or diagnosis of Juvenile DM. In addition, 6 of the patients who were screened declined to participate. 106 patients were enrolled in the DM database, 13 did not complete their first visit and were not included in any of the analyses. At the Stanford clinic, 125 patients were screened, and 9 were excluded due to disease overlap or unclear diagnosis. Of these 116 patients, 10 had incomplete data and were excluded.
The UPenn analysis included 93 patients, 51% of whom had classic DM and 49% had skin-predominant DM at diagnosis (Table 1). Skin-predominant subtype includes patients who had either amyopathic or hypomyopathic DM. Baseline CDASI activity scores for the patients evaluated at this site ranged from 0 to 47 (median 17), and baseline PGA VAS scores ranged from 0 to 9.6 (median 1.1) (Figs 2–3). The Stanford analysis included 106 patients of whom 78% had classic and 22% had skin-predominant disease (Table 1). At this site baseline CDASI scores ranged from 0 to 48 (median 21), and baseline PGA VAS scores ranged from 0 to 9.7 (median 4.2) (Figs 2–3). The combined group of 199 patients was 79% female and 78% Caucasian with 65% classic DM and 35% skin-predominant. The patients included for the responsiveness analysis were only those who completed two or more visits, which totaled 128 (68 patients from the UPenn database and 60 from the Stanford database). This subset of patients was clinically similar to the total patient population with 65% classic and 35% skin-predominant DM.
Table 1.
Patient demographics
| University of Pennsylvania N = 93 |
Stanford University N = 106 |
All Study Participants N = 199 |
|
|---|---|---|---|
| Age | |||
| Median (Range) | 52 (20 – 82) | 50 (17 – 87) | 51 (17 – 87) |
| Sex | |||
| Female | 79 (85%) | 78 (74%) | 157 (79%) |
| Male | 14 (15%) | 28 (26%) | 42 (21%) |
| Ethnicity | |||
| Caucasian | 82 (88%) | 74 (70%) | 156 (78%) |
| Hispanic | 5 (5%) | 12 (11%) | 17 (9%) |
| African American | 5 (5%) | 5 (5%) | 10 (5%) |
| Other | 1 (1%) | 15 (14%) | 16 (8%) |
| Disease Subtype | |||
| Classic | 47 (51%) | 83 (78%) | 130 (65%) |
| Skin-predominant1 | 46 (49%) | 23 (22%) | 69 (35%) |
| Study visits per patient | |||
| Median (Range) | 3 (1 – 12) | 2 (1 – 12) | 2 (1 – 12) |
| Baseline CDASI Activity Score | |||
| Median (Range) | 17 (0 – 47) | 21 (0 – 48) | 19 (0 – 48) |
| Baseline PGA VAS Score | |||
| Median (Range) | 1.1 (0 – 9.6) | 4.2 (0 – 9.7) | 3.2 (0 – 9.7) |
Skin-predominant subtype includes patients who had either amyopathic and hypomyopathic DM.
Figure 2.
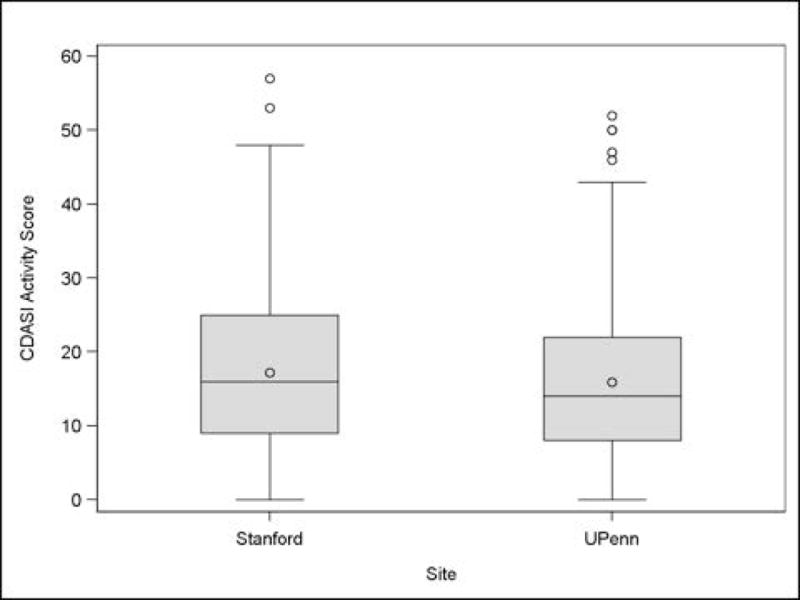
Distribution of CDASI scores by site
Figure 3.
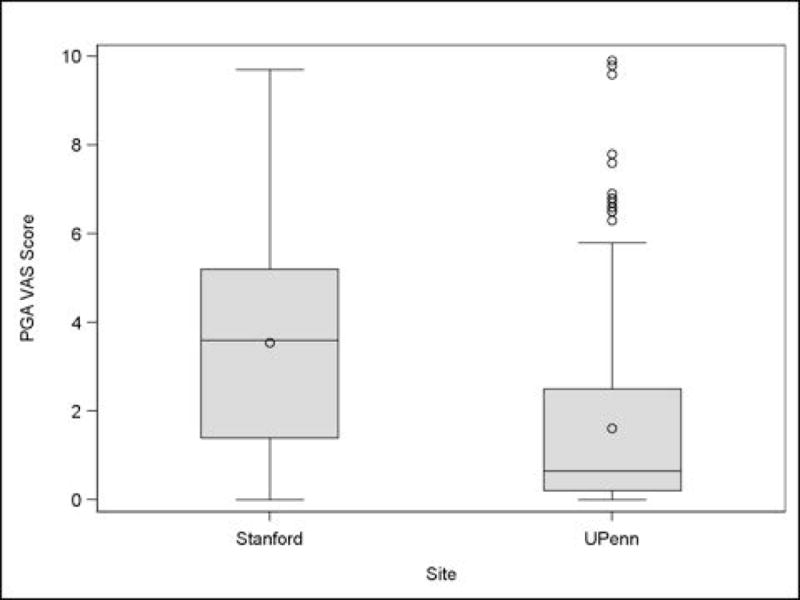
Distribution of PGA VAS scores by site
Combined Severity Analysis
Each patient in this analysis had 1 to 12 complete study visits resulting in a total of 688 visits at which patient skin was assessed using the PGA 5-point Likert and CDASI. At 60% of these visits patient disease severity was classified as mild using the PGA 5-point Likert and 40% were moderate or severe. There was a statistically significant site-specific difference in the PGA 5-point Likert relative to the CDASI score (P < 0.0001), and clinical site effect (P = 0.0004) but no statistically significant interaction between CDASI and site (P = 0.54). Although there was not a statistically significant interaction between CDASI and clinical site we maintained separate models for consistency with the responsiveness model.
University of Pennsylvania Severity Analysis
A total of 378 study visits were completed at UPenn. Of these, 74% of assessments were classified as mild on the PGA 5-point Likert and 26% were moderate or severe. A receiver operating characteristic curve (AUC 0.91) revealed a cut-off of 19 in the CDASI (Sensitivity, 88%; Specificity, 85%; Correctly identified 87%) to best differentiate mild from moderate and severe disease (Table 2). At this site mild disease corresponded with CDASI activity scores of 19 or less, while moderate and severe disease corresponded with scores greater than 19.
Table 2.
Severity cut-off points
| University of Pennsylvania | |||
|---|---|---|---|
| CDASI Score | Sensitivity (%) | Specificity (%) | Correctly identified (%) |
| 18 | 85.3 | 85.9 | 85.4 |
| 19 | 88.2 | 84.8 | 87.3 |
| 20 | 90.7 | 82.8 | 88.6 |
| Stanford University | |||
|---|---|---|---|
| CDASI Score | Sensitivity (%) | Specificity (%) | Correctly identified (%) |
| 13 | 73.8 | 83.5 | 79.4 |
| 14 | 77.6 | 81.8 | 80.0 |
| 15 | 80.6 | 75.6 | 77.7 |
Stanford University Severity Analysis
At Stanford 310 study visits were completed, 43% of which were mild and 57% were moderate or severe. Using a receiver operating characteristic curve (AUC 0.88) the cut-off between mild and moderate and severe disease was 14 (Sensitivity, 78%; Specificity 82%; Correctly identified 80%) (Table 2). At this site mild disease corresponded with scores of 14 or less. CDASI activity scores greater than 14 represented moderate and severe disease activity.
Combined Responsiveness Analysis
Overall, 128 patients and 484 pairs of visits were complete with physician assessments using the VAS and CDASI. Of these, 16% were responders and 84% were non-responders. The interaction between the CDASI and clinical site on the response defined by the change in the VAS was statistically significant (P = 0.015), requiring separate site-specific analyses, as discussed above.
University of Pennsylvania Responsiveness Analysis
At UPenn, 280 pairs of visits were complete with VAS and CDASI assessments; 12% of which were responders and 88% were non-responders. The ROC curve (AUC 0.65) revealed a change of 4 points (Sensitivity 59%, Specificity 71%, Correctly identified 70%) corresponded with a clinical response (Table 3).
Table 3.
Clinically significant change in CDASI using PGA VAS scale
| University of Pennsylvania | |||
|---|---|---|---|
| Change in CDASI | Sensitivity (%) | Specificity (%) | Correctly identified (%) |
| 3 | 64.7 | 63.0 | 63.2 |
| 4 | 58.8 | 71.1 | 69.6 |
| 5 | 52.9 | 75.2 | 72.5 |
| Stanford University | |||
|---|---|---|---|
| Change in CDASI | Sensitivity (%) | Specificity (%) | Correctly identified (%) |
| 4 | 81.4 | 70.8 | 73.0 |
| 5 | 79.1 | 77.0 | 77.4 |
| 6 | 74.4 | 80.1 | 78.9 |
Stanford University Responsiveness Analysis
The responsiveness analysis using the PGA VAS included 204 pairs of visits at Stanford; 21% of which were responders and 79% were non-responders. A cut-off of 5 (Sensitivity 79%, Specificity 77%, Correctly identified 77%) (Table 3) was derived from an ROC curve (AUC 0.83) indicating a clinically significant change in CDASI.
Discussion
Clinical research necessitates outcome measures that are valid, reliable, and responsive to clinically relevant changes in disease state. The CDASI was developed to facilitate comparative trials and longitudinal assessment of cutaneous DM. Validation of this instrument began with several studies demonstrating its content validity, construct validity, inter-rater reliability, intra-rater reliability and sensitivity to change.6,8,9 Goreshi et al compared the intraclass correlation coefficient (ICC) of the CDASI, CAT-BM, PGA VAS and PGA Likert and found that the CDASI had superior inter-rater reliability (Activity ICC: CDASI 0.748, CAT-BM 0.516, PGA VAS 0.721, PGA Likert 0.653) while the PGA VAS had higher intra-rater reliability than other measures (Activity ICC: CDASI 0.868, CAT-BM 0.714, PGA VAS 0.911, PGA Likert 0.737).9 This study also demonstrated that the CDASI was most sensitive to change.9
Data from our two-center prospective study revealed statistically significant interactions between the clinical site and CDASI for predicting the PGA VAS in the responsiveness analysis that required separate site-specific analyses. This resulted in distinct but similar values for the characterization of disease severity and clinical response. The analysis of data collected at Stanford suggests that CDASI scores of 14 distinguish mild disease from moderate and severe disease. Results from UPenn suggest that patients with mild disease are those with CDASI scores of 19 or less while those with moderate and severe disease have scores greater than 19. When responsiveness of the CDASI is assessed using a 2-point change in the PGA VAS score, the minimally clinically significant change in the CDASI score is 5 points at Stanford and 4 points at UPenn. Overall these results confirm that CDASI scores vary with disease severity and that the instrument is responsive to clinical change.
Since CDASI activity scores were similar between sites and it is the more systematically derived score, it is likely that the severity of disease was also similar between sites although we were not able to directly test this. Inter-rater variations in the use of the PGA 5-point Likert most likely accounts for the differences in severity cutoffs between sites. Although physician global measures are frequently used as the “gold standard” in research studies, their limitations are recognized.10,15 Lack of reliable external “gold standard” measures for use in validation studies presents a challenge. In addition, recent work by our group (abstract) on the use of severity measures for cutaneous lupus erythematosus suggest that establishing responsiveness with external gold measures is particularly difficult in mild disease.15 Despite these limitations this study provides evidence that the CDASI is a valid and responsive tool for the evaluation of cutaneous DM.
For use of this tool in clinical trials we suggest that CDASI activity scores of 19 or less characterize mild disease. The actual cut-off may fall between 14 and 19 points. A change in CDASI of 4 or 5 points represents a minimally clinically significant change. Future randomized controlled trials will be important to further examine the CDASI in a controlled trial setting.
What’s already known about this topic?
Validated outcome measures are essential for comparative trials.
CDASI was created for assessment of cutaneous dermatomyositis in clinical trials, translational research and clinical practice.
Previous studies have demonstrated the validity and reliability of this instrument.
What does this study add?
Cutaneous dermatomyositis disease severity can be characterized with CDASI.
Clinical change can be assessed by changes in CDASI activity scores.
Practical and standardized use of the CDASI is now possible.
Acknowledgments
This project is supported by the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development and by the National Institutes of Health (NIH K24-AR 02207 – VPW, UL1000003 – KJP).
Footnotes
Conflict of interest disclosures: The senior author developed the CDASI, and the copyright is owned by the University of Pennsylvania.
References
- 1.Rider LG, Werth VP, Huber AM, et al. Measures of Adult and Juvenile Dermatomyositis, Polymyositis, and Inclusion Body Myositis. Arthritis Care Res. 2011;63:118–57. doi: 10.1002/acr.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeder MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26–30. doi: 10.1001/archdermatol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goreshi R, Chock M, Foering K, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65:1107–16. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaines E, Werth BP. Development of outcome measures for autoimmune dermatoses. Arch Dermatol Res. 2008;300:3–9. doi: 10.1007/s00403-007-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008;159:887–94. doi: 10.1111/j.1365-2133.2008.08711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer AJ, Thode HC, Hollander JE. Research fundamentals: selection and development of clinical outcome measures. Acad Emerg Med. 2000;7:397–401. doi: 10.1111/j.1553-2712.2000.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 8.Yassaee M, Fiorentino D, Okawa J, et al. Modification of the Cutaneous Dermatomyositis Disease Area and Severity Index, an outcome instrument. Br J Dermatol. 2010;162:669–73. doi: 10.1111/j.1365-2133.2009.09521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goreshi R, Okawa J, Rose M, et al. Evaluation of reliability, validity, and responsiveness of the CDASI and the CAT-BM. J Invest Dermatol. 2012;132:1117–24. doi: 10.1038/jid.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corzillius M, Fortin P, Stucki G. Responsiveness and sensitivity to change of SLE disease activity measures. Lupus. 1999;8:655–659. doi: 10.1191/096120399680411416. [DOI] [PubMed] [Google Scholar]
- 11.Hundley JL, Carroll CL, Lang W, et al. Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol. 2006;54:217–220. doi: 10.1016/j.jaad.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: Results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801–815. doi: 10.1016/s0190-9622(03)01475-0. [DOI] [PubMed] [Google Scholar]
- 13.Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25:406–411. doi: 10.1016/s0885-3924(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 14.Bonilla-Martinez ZL, Albrecht J, Troxel AB, et al. The cutaneous lupus erythematosus disease area and severity index: a responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008;144:173–180. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YC, Propert KJ, Werth VP. The limitations in measuring improvement in disease activity in patients with mild cutaneous lupus erythematosus. J Invest Dermatol. 2013;133:S32. [Google Scholar]


