Summary
Background
It has already been shown that patients with more severe CLE activity have a poorer quality of life (QoL). Racial and ethnic disparities have been reported in disease activity and outcomes in SLE, but similar information is not available in CLE.
Objective
The main objective of the current study was to evaluate the impact of lupus-related skin damage on skin-specific QoL, as well as differences stratified by ethnic backgrounds.
Methods
Data collected included sex, race, diagnosis, CLASI scores, and Skindex-29. These parameters were analysed at the initial and last visits. CLASI damage scores (dyspigmentation and scarring) and CLASI activity scores were collected, grouped by ethnicity, and correlated with Skindex-29. 223 patients were analysed at baseline, with 141 of these patients completing more than one study visit.
Results
The majority were Caucasians (63.7%), followed by African Americans (29.1%) and Asian Americans (4.0%). African American patients accounted for a disproportionate percentage of both localised (50% of cases) and generalised (48.9% of cases) DLE. Median CLASI damage scores significantly differed between our African American, Caucasian, and Asian American patients, at both first (8.5, 4.0, 7.0) (Kruskal-Wallis p<0.0001) and last visit (10.0, 6.0, 8.5) (Kruskal-Wallis p<0.01) (Dunn's Multiple Comparison p<0.0001, p<0.01). CLASI damage scores in African Americans correlated with CLASI activity scores (Spearman's r=0.45, p=0.0003).
Conclusion
There was no significant correlation between CLASI damage scores and Skindex domains overall. Individually, dyspigmentation and scarring also did not have a significant effect on QoL. In conclusion, disease damage does not affect QoL, as measured by the Skindex-29. Ethnic differences in CLE patients were found: African American patients with CLE, do exhibit a high rate of DLE, experience damage early in their disease course, frequently in conjunction with disease activity.
Introduction
There is a spectrum of clinical presentations for the skin disease seen in lupus erythematosus (LE), present in those with isolated cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE). The prevalence of systemic lupus (SLE) worldwide ranges from 17 to 48 per 100,000, and cutaneous lupus is thought to be as prevalent as systemic disease.1 Skin involvement occurs in up to 70% of patients with SLE and is diagnosed when four or more of the eleven American College of Rheumatology (ACR) criteria are present.2-3
Studies of SLE have highlighted the racial and ethnic disparities in socioeconomics, demographics, disease activity, and outcomes in the SLE population. The LUMINA [Lupus in Minorities, Nature versus nurture (LUMINA)] study collected longitudinal data in a multiethnic, multicenter cohort of patients with SLE that met ACR criteria.3-5 Multicenter, multiethnic longitudinal studies have been developed to further evaluate the disparities in Quality of Life (QoL) between different ethnic groups with SLE. 5-6 It has already been demonstrated that racial and ethnic variations exist in disease activity in SLE and that the degree of disease activity in systemic lupus is a risk factor for disease damage.7
CLE has also been studied among different geographic populations, focusing on the impact on QoL, as well as demographic profiles.8 Prior to the development of a validated disease severity measure, studies of CLE had been limited. The development of a validated disease severity measure, the CLASI (Cutaneous Lupus Activity and Severity Index), has facilitated prospective data collection of cutaneous lupus severity and damage in CLE.9-13 At the University of Pennsylvania, a web-based database of a population of patients with both cutaneous and systemic lupus was established in 2006. This resource has enabled the development of a longitudinal cohort study of a multiracial, multiethnic LE patient population. Our group has shown that the use of this web-based database is an effective method of collecting data about CLE and analysing its disease profile, disease activity and response, and the effect of disease activity on quality of life (QoL) in lupus.10, 14-16
Research carried out by our group, has examined the effect of various parameters on Quality of Life in CLE patients. Using our CLE database, preliminary work from our group found no significant difference in the quality of life among CLE patients of different racial backgrounds.10 Recent published work in SLE has demonstrated a difference in cutaneous damage outcomes in different ethnicities.7 Studies by our group have shown that patients with cutaneous lupus have an impaired quality of life, and in particular suffer from emotional impairment. Disease severity, as measured by CLASI activity scores, has been shown to have a negative effect on quality of life, but the relationship between CLASI damage scores and quality of life is weak.10-11
The main objective of this study was to demonstrate the impact that lupus related disease damage has on quality of life and the differences observed within a population of CLE patients from different racial and ethnic backgrounds. Other diseases with damage effects similar to lupus, such as dyspigmentation, have demonstrated a negative effects on quality of life.17 Therefore we anticipated that the damage experienced in cutaneous lupus patients, namely dyspigmentation and scarring, should also have a negative effect on quality of life, and that a difference should be seen between patients of different racial backgrounds.
To our knowledge, no study has previously examined the differences in lupus-related skin damage, using the CLASI damage score, in CLE patients of different races, and assessed the impact that this damage has on quality of life, as measured by the Skindex-29. Access to a large cohort of CLE patients, the validated CLASI scoring tool, and a validated QoL measure, the Skindex-29, has enabled us to examine the relationships between racial background and CLASI damage scores, as well as the effect of CLASI damage scores on QoL.
Materials and Methods
Patient data was gathered from the autoimmune skin disease clinic at the Hospital of the University of Pennsylvania, and then entered into our lupus database. All patients with a diagnosis of CLE or SLE were invited to participate in this study. Their diagnosis was based on the Gilliam classification and/or American College of Rheumatology criteria, respectively. 3, 18 This database study was approved by our institutional review board. At the time of this study, 251 patients had been enrolled in our database. Data utilised was from study patients’ initial study visit and last study visit, if patients’ had more than a single visit. Gender, race, ethnicity, diagnosis details, CLASI damage and activity scores, and Skindex-29 scores were collected. The age of diagnosis and the year of onset of cutaneous disease was information provided by the patient or by the referring physician.
CLASI
The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index) is a validated physician scoring tool of cutaneous lupus related disease activity and damage.9,21 Disease activity is scored on a scale of 0 – 70, using the parameters of erythema and scale, with higher scores indicating worse disease activity. Damage is scored on a scale of 0-56, using the parameters of dyspigmentation and scarring, with higher scores indicating worse disease damage. Scores are given for different anatomical locations, and are based on the worst area involved. The CLASI activity score demonstrates disease responsiveness and has excellent inter-rater and intra-rater reliability.20,23
Skindex- 29
The Skindex-29 is a previously validated, multi-dimensional, skin specific quality of life measure and has been used in a number of chronic cutaneous conditions, including lupus.9,22,24 The multi-dimensional component of the Skindex is comprised of 3 main domains: emotions, functioning and symptoms.24 A fourth domain has been added for specific lupus – related questions. Scores for each domain range from 0 to 100, with 100 indicating a poorer quality of life.
Statistical Analysis
The CLASI damage scores of our patients were analysed at first and last visits, by race. Only CLASI damage scores for African American, Caucasian, and Asian patients were analysed, while other groups with small numbers were excluded from the analysis. Data from our Asian population was subsequently excluded during further comparative analysis, and this was also due to small sample size.
CLASI damage scores among races were compared using a non-parametric Kruskal–Wallis test, for both first and last visits. This was repeated in the analysis of time between visits for each racial background. Pairwise differences in the damage scores for each race were then compared using the Dunn's Multiple Comparison Test. Differences in the age at diagnosis and disease duration between African American subjects and Caucasian subjects were detected using a Wilcoxon rank-sum test.
CLASI damage scores were correlated to CLASI activity scores, and both CLASI damage and CLASI activity scores were each correlated to the Skindex-29 domains, for the entire patient population, and for Caucasian and African Americans, using a Spearman's correlation. The effect of race on the association of CLASI damage scores and Skindex-29 scores in African Americans and Caucasians was analyzed using linear regression analysis. The effect of dyspigmentation and scarring on the emotion domain of the Skindex in African Americans and Caucasians, as measured by the total dyspigmentation and scarring scores, was also evaluated using Spearman's correlation. A Kruskal-Wallis test was used in the analysis of the effect of distribution of dyspigmentation and scarring on Skindex-29 emotion scores of patients with different locations of disease damage. Differences in the racial variation of the distribution of dyspigmentation and scarring were detected using a Chi-squared test.
Results
Patients’ characteristics
251 patients were enrolled in our database at the time of analysis in November 2011. Of these patients, 223 patients had sufficient data recorded in the database to be included in the overall analysis of the prevalence of lupus sub-types in our population. During the initial review of the database, 19 patients were identified and excluded from the study because they did not have an initial study visit completed. An additional 9 patients were later identified and excluded from the study due to incomplete data, with no CLASI scores recorded, or did not have any data regarding gender, ethnicity or disease diagnosis entered in the database.
Data gathered from the remaining 223 study patients’ initial study visit included gender, race, ethnicity, diagnosis details, CLASI activity and damage scores, and Skindex 29 scores. Of the 223 patients, whose data was analysed, 141 patients completed more than one study visit. Data from the last study visit, which included CLASI damage and Skindex-29 scores, was also analysed for these 141 patients. Basic demographic data from the 223 patients is presented in Table 1. The majority of our predominantly female patient population was Caucasian, followed by African American and Asian. There was an earlier mean age of diagnosis seen among African American patients when compared to Caucasians. 11 patients in the database had a single diagnosis of SLE, and these patients were predominantly Caucasian. 35 of the 65 African American patients had a concomitant diagnosis of CLE and SLE: 33 of these patients were female. African American patients who had CLE and SLE had a younger mean age of diagnosis (32.7 years), as compared with African American patients with CLE alone (37.5 years).
Table 1.
The Profile of CLE Patient Population
| Ethnicity | African American | Caucasian | Asian | Other |
|---|---|---|---|---|
| Patients n(%) | 65(29.1) | 142(63.5) | 9(4.1) | 7(3.2) |
| Gender (% female) | 83.1 | 81.0 | 77.8 | 71.4 |
| Mean age at Diagnosis ([Years (SDM), Range] | 34.8 (12.49), 12-60 | 41.8 (15.51), 10-82 | 31.1 (17.07), 15-61 | 33.2 (22.76), 19-78 |
| Mean Disease Duration) [Years (SDM), Range] | 10.8 (8.54), 0.42-31.03 | 7.2(7.48), 0-29.56 | 7.7(4.98), 0.68-17.93 | 3.9(3.91), 0.26-10.02 |
Caucasians, followed by African Americans were the two largest ethnicities in our patient population. The majority of our patients were female. There was a significant difference in the mean age at diagnosis, and mean disease duration between Caucasians and African Americans (Age p<0.0048; Disease duration p<0.0042; Mann-Whitney).
The majority of our patients had a diagnosis of Chronic Cutaneous Lupus (CCLE), followed by Subacute Cutaneous Lupus (SCLE) and Acute Cutaneous Lupus (ACLE) respectively. The CCLE subtypes and racial distribution for these 3 subtypes of LE is presented (Table 2). Almost all patients with SCLE were Caucasian: there were no African American patients in our population with a diagnosis of SCLE. The ACLE subtype was also predominantly Caucasian. However, there was a more even distribution between African Americans and Caucasians in the CCLE population. There was a predominance of African American patients with both localised and generalised DLE, while there were more Caucasian patients with tumid LE.
Table 2.
Distribution of CLE Subtypes by Ethnicity
| African American n(%) | Caucasian n(%) | Asian n(%) | Other n(%) | |
|---|---|---|---|---|
| SCLE (n=59) | 0(0.0) | 57(96.6) | 1 (1.7) | 1(1.7) |
| ACLE (n=16) | 5(31.2) | 8(50.0) | 1(6.3) | 2(12.5) |
| CCLE (n=135) | 59(43.7) | 66(48.9) | 7(5.2) | 3(9.8) |
| Generalized DLE (n=47) | 23(48.9) | 17(36.2) | 4(8.5) | 5(10.6) |
| Localized DLE (n=54) | 27(50.0) | 25(44.4) | 2(3.7) | 0(0.0) |
| Tumid LE (n=19) | 3(15.8) | 15(78.9) | 1(5.2) | 0(0.0) |
| LE Panniculitis (n=3) | 1(33.3) | 2(66.7) | 0(0.0) | 0(0.0) |
| Overlap (n=8) | 3(37.5) | 5(62.5) | 0(0.0) | 0(0.0) |
| Chillblain LE (n=4) | 2(50.0) | 2(50) | 0(0.0) | 0(0.0) |
| SLE (n=11) | 1(9.1) | 10(90.9) | 0(0.0) | 0 (0.0) |
Almost all patients with Subacute Cutaneous Lupus (SCLE) were Caucasian. While there was majority of Caucasians seen in the subtypes of CLE, there was a predominance of African Americans in the DLE population.
There was no difference in the time between first and last visit for each population (p=0.93) Table 3), however there was a significant difference in the mean number of years between disease diagnosis and time of initial presentation to our clinic in African Americans and Caucasians (African Americans 10.8 years, Caucasians 7.2 years (p=0.004) (Table 2).
Table 3.
Ethnic Variation in Median CLASI Damage Scores at First and Last Visit
| Ethnicity | Median CLASI dmg score (visit 1) | Median CLASI dmg score (last visit) | Median time between visit 1 and last visit (weeks) |
|---|---|---|---|
| AA | 8.5 | 10.0 | 104.4 |
| Caucasian | 4.0 | 6.0 | 106 |
| Asian | 7.0 | 8.5 | 119 |
There was no difference in the time between first and last visits, for each ethnic population (p=0.93)
Ethnic Variation of CLASI Damage Scores
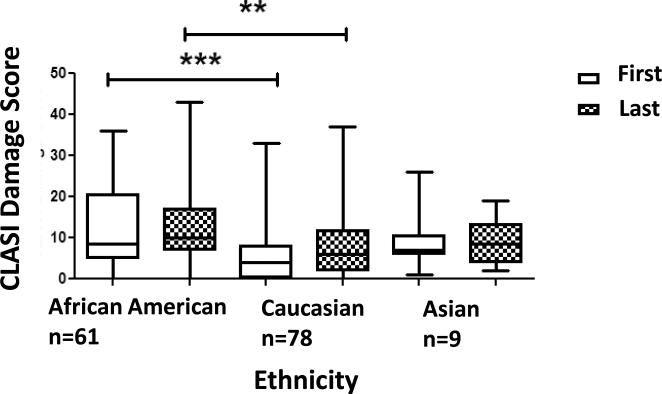
There was a significant difference between racial backgrounds in their median CLASI damage scores at first and last visit (p<0.0001, p<0.0036) (Figure 1). African Americans had significantly greater median damage scores at first visit as compared with Caucasians (p<0.0001). The median CLASI damage scores of African American showed little change between first and last visits, however there was a greater change seen in the CLASI damage scores of Caucasians between first and last visit, with an increase in median CLASI damage scores. There remained a significant difference in the median CLASI damage scores of African Americans and Caucasians, at last visit (Figure 1) (p<0.001), despite a significant difference in time between first and last visit for each population (Table 3).
Figure 1. The Distribution of the Median CLASI Damage Scores at First and Last Visits in Different Ethnicities.
African Americans had higher damage scores at visit 1 and did not change much over time, in contrast to Caucasians, who had low damage scores at visit 1 and increased at the last visit. There was a significant difference in median CLASI damage scores between each population of different racial backgrounds at first and last visit (p<0.0001, p=0.0036) and specifically between African Americans and Caucasians at first and last visits (p<0.0001, p<0.001).
The Association Between CLASI Damage and Skindex-29 Scores
The relationship between the CLASI damage score and each domain of the Skindex-29 (emotions, functioning, and symptoms) was analysed for each racial population. Asian Americans, patients of Native American and Hispanic descent, and patients of undisclosed race were excluded from further analysis, due to their small population size.
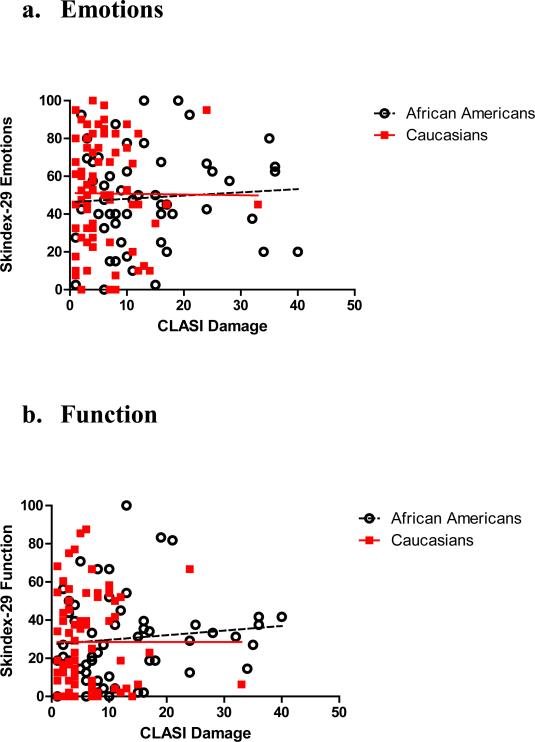
When analysing for the entire patient population, CLASI damage scores did not correlate with Skindex-29 emotion scores (r=0.02, p=0.85) (Figure 2a). There was no correlation between damage and Skindex-29 emotion scores for either Caucasian or African American patients (r=-0.01, r=0.06) (p=0.92, p=0.64). Similarly, CLASI damage scores did not correlate with Skindex function scores (r=0.11, p=0.20) (Figure 2b). In terms of racial variation in correlations, there was no correlation between CLASI damage and Skindex-29 function scores in either Caucasian or African Americans (r=0.03, r=0.13) (p=0.83, p=0.35). Skindex-29 symptoms scores correlated with CLASI damage scores (r= 0.25, p= 0.003) (Figure 2c). In African Americans, we found that CLASI damage scores significantly correlated with Skindex-29 symptoms scores (r=0.36, p=0.01). However, in Caucasians, CLASI damage scores did not correlate with symptoms scores (r=0.09, p=0.46).
Figure 2. Correlations of CLASI Damage Scores and Skindex-29 Domains in Different Ethnicities.
CLASI Damage scores did not correlate with the Skindex emotion or function domains. There was a correlation between CLASI Damage scores and Skindex-29 Symptoms scores, in African Americans but not in Caucasians.
Linear regression analysis was done to evaluate the role of racial background in the relationship between CLASI damage scores in association with each of the Skindex-29 domains (Figure 2a-c). There was no significant difference due to race on the CLASI damage scores and any of the Skindex-29 scores.
The effect of the individual parameters that comprise the CLASI damage score, namely dyspigmentation and scarring, on the Skindex domains was analysed and not found to have any correlation (Figure 3). This was true in both African Americans and Caucasians. The only exception to this was for scarring alopecia alone, which did correlate with Skindex-29 emotions scores (r=0.28, p=0.03).
Figure 3. The Effect of Distribution of Dyspigmentation and Scarring on Skindex-29 Emotion Scores.
There was no significant difference seen in the Skindex-29 emotion scores based on the distribution of dyspigmentation and scarring.
The Relationship Between CLASI Activity and Damage Scores and Skindex-29 Scores
Overall CLASI damage scores correlated with CLASI activity scores (r=0.16, p=0.04). There was a correlation found between CLASI damage and activity scores in African Americans (r=0.45, p=0.0003) (Figure 4). There was however no correlation between lupus related skin damage and activity in Caucasians (r=0.07, p=0.57). CLASI Activity scores correlated with each of the Skindex-29 domains, emotions (r= 0.24, p = 0.0013), function (r = 0.29, p < 0.0001) and symptoms (r = 0.36, p < 0.0001).
Figure 4. The Correlation between CLASI Damage and Activity Scores in African Americans and Caucasians.
There was a strong correlation between disease activity and damage in African Americans, but this was not true in Caucasians.
Discussion
In our study the demographic profile of our lupus population was consistent with the literature, with a predominantly female population and with discoid lupus (DLE) the predominant CCLE subtype.1,5 Our study population was also predominantly Caucasian, consistent with previous studies of our referral population and not an accurate demonstration of the true racial and ethnic distribution in CLE.1 The age at diagnosis was significantly lower in African Americans (34.8 years) as compared with Caucasians (41.74 years). This mean age of diagnosis in African American patients is younger than in a previously reported mixed but predominantly Caucasian CLE population.1
There was a significant difference in the degree of lupus-related skin damage seen in different races. African Americans with CLE present initially with greater disease damage than Caucasians, with little change in their degree of damage between first and last visits. This differed from Caucasians, who had less damage on initial presentation but demonstrated an increase in disease damage by the last visit. This finding may indicate that lupus-related skin damage occurs earlier in African Americans than Caucasians, or that African Americans presented later in their disease course than Caucasians.
In work previously published by our group, disease severity, defined by CLASI activity scores, was shown to be a significant factor affecting Quality of Life, as measured by the Skindex-29, particularly the emotions domain.10,23 We hypothesized that lupus related skin damage should have a negative effect on the emotional and functional domains of the Skindex. We found that CLASI activity scores had higher correlations with the Skindex-29, as compared with CLASI damage scores, particularly in the psychosocial domains (emotions and functioning), which was an unexpected finding. This finding may be a function of scaling, but confirms our groups' previous results. 10,23 We did find a correlation, however, between CLASI damage scores and the Skindex-29 symptoms scores and there were differences in these correlations for African American and Caucasian patients. Disease damage correlated with the symptoms domain of the Skindex in African Americans, and this was surprising. The questions in the symptoms domain of the Skindex-29 are those pertaining to skin discomfort (i.e. itching, burning and/or painful skin), and are intuitively synonymous with disease activity. We also found a correlation between damage and activity, and specifically in African Americans. Any correlation between CLASI damage scores and the symptoms domain of the Skindex likely reflects the co-existence of disease activity with damage in African Americans, and this is possibly true in other racial populations. The lack of correlation of damage with symptoms in Caucasians suggests that Caucasians tend to get damage later, after resolution of the disease activity. This would suggest disease damage does not impact Skindex-29 scores, unlike what has been seen with cutaneous lupus activity.10
We did not observe any correlation between dyspigmentation and QoL in African Americans or Caucasians with CLE, regardless of its distribution or facial involvement. This was interesting to us as this finding contrasts with vitiligo, a disease characterized by dyspigmentation, which exhibits emotional and functional impairment, as measured by the Skindex-2917,25 and where it has been shown that quality of life is more affected in dark-skinned patients.
Similarly, we did not observe any correlation between the severity of scarring and QoL in African Americans or Caucasians regardless of distribution, except for scarring alopecia. This contrasts with the effect of lupus-related disease activity. Previous studies demonstrated that lupus disease activity with facial involvement and inflammatory alopecia correlated with worse QoL outcomes.10
Our study was limited by several factors. Our patient population comprised predominantly Caucasians and African Americans. Other limiting factors of our study include the lack of consideration for the different grades of skin pigmentation within each race, and the potential confounder of previous psychological conditions, which may alter Skindex-29 results. Further studies of CLASI damage scores, the individual effects of dyspigmentation and scarring, and their effects on Skindex-29 scores should be carried out in different racial and ethnic populations of CLE patients.
To our knowledge, this is the first database study looking at cutaneous lupus-related skin damage and its effect on quality of life, and its racial variations. This study demonstrates that African Americans present initially with greater disease damage than Caucasians and in conjunction with disease activity. The degree of damage and co-existence of damage with activity in African Americans is an important aspect of disease measurement and understanding.
It is disease activity, and not damage or its parameters of scarring and dyspigmentation that affect quality of life in most patients with cutaneous lupus. Dyspigmentation in cutaneous lupus does not appear to have the same effect that other diseases of dyspigmentation have on quality of life. The overall greater impact of disease activity relative to damage on QoL, as evidenced by our work, was surprising and future directions for research should include studies of quality of life measures that are sensitive to changes in disease damage.
What is already known about this topic?
It has been shown in SLE that there is a difference in cutaneous damage outcomes in different ethnicities.
Patients with cutaneous lupus have an impaired quality of life, and in particular suffer from emotional impairment.
Cutaneous lupus disease severity, measured by CLASI activity scores, has been shown to have a negative effect on quality of life.
What does this study add?
African Americans with cutaneous lupus present initially with greater disease damage than Caucasians and in conjunction with disease activity.
It is disease activity, as measured by the CLASI, and not damage or its parameters of scarring and dyspigmentation that affect quality of life.
Dyspigmentation in cutaneous lupus does not appear to have the same effect that other diseases of dyspigmentation have on quality of life.
Acknowledgements
This material is based upon work supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development) and by the National Institutes of Health (NIH K24-AR 02207) to VPW. The Copyright for the CLASI is owned by the University of Pennsylvania.
Funding/Support: This material is based upon work supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development) and by the National Institutes of Health (NIH K24-AR 02207) to VPW (Werth).
Footnotes
Author Contributions: Drs. Verma and Werth, and Ms. Okawa had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Verma, and Werth. Acquisition of data: Verma, Werth, and Okawa. Analysis and interpretation of data: Verma, Werth, and Propert. Drafting of the manuscript: Verma, Werth, and Propert. Critical revision of the manuscript for important intellectual content: Verma, Werth, and Propert. Statistical analysis: Propert, Verma, and Werth. Obtained funding: Werth. Administrative, technical, or material support: Verma, Werth, and Okawa. Study supervision: Werth.
Financial Disclosures: Dr. Verma, Dr. Propert and Ms. Okawa do not have any conflicts of interest or any relevant financial relationships to report. Dr. Werth reports that she serves as a consultant for Lupus Foundation of America, Pfizer, Medimmune, Genentech, Novartis, Celgene, Stiefel, Rigel, Astion, Amgen, Infinity Pharmaceuticals, Sanofi-Aventis, and UBC; has research grants from Amgen, Celgene, and Rigel; and has partial stock ownership of UV Therapeutics; and that University of Pennsylvania holds the copyright for the Cutaneous Lupus Erythematosus Disease Area and Severity Index and the Cutaneous Disease and Activity Severity Index.
References
- 1.Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145(3):249–53. doi: 10.1001/archdermatol.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tebbe B, Orfanos CE. Epidemiology and socioeconomic impact of skin disease in lupus erythematosus. Lupus. 1997;6(2):96–104. doi: 10.1177/096120339700600204. [DOI] [PubMed] [Google Scholar]
- 3.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 5.Fernández M, Alarcón GS, Calvo-Alén J, et al. LUMINA Study Group A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007 May 15;57(4):576–84. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 6.Moldovan I, Katsaros E, Carr FN, Cooray D, Torralba K, Shinada S, Ishimori ML, Jolly M, Wallace DJ, Weisman MH, Nicassio PM. The patient reported outcomes in Lupus (PATROL) study: role of depression in health-related quality of life in a southern California lupus cohort. Lupus. 2011 Oct;20(12):1285–92. doi: 10.1177/0961203311412097. [DOI] [PubMed] [Google Scholar]
- 7.Pons-Estel GJ, Alarcón GS, González LA, et al. Lumina Study Group Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res (Hoboken) 2010 Mar;62(3):393–400. doi: 10.1002/acr.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraz LB, Almeida FA, Vasconcellos MR, et al. The impact of lupus erythematosus cutaneous on the Quality of life: the Brazilian-Portuguese version of DLQI. Qual Life Res. 2006 Apr;15(3):565–70. doi: 10.1007/s11136-005-2638-9. [DOI] [PubMed] [Google Scholar]
- 9.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005 Nov;125(5):889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein R, Moghadam-Kia S, LoMonico J, et al. Quality of Life in Cutaneous Lupus Erythematosus. J Am Acad Dermatol. 2011;64(5):849–58. doi: 10.1016/j.jaad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaines E, Bonilla-Martinez Z, Albrecht J, et al. Quality of life and disease severity in a cutaneous lupus erythematosus pilot study. Arch Dermatol. 2008 Aug;144(8):1061–2. doi: 10.1001/archderm.144.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonilla-Martinez Z, Albrecht J, Taylor L, et al. The CLASI is a useful clinical instrument to separately follow activity and damage during therapy of cutaneous lupus erythematosus. Arch Dermatol. 2008;144:173–180. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krathen MS, Dunham J, Gaines E, et al. The Cutaneous Lupus Erythematosus Disease Activity and Severity Index (CLASI) expansion for rheumatology and dermatology. Arthritis Care & Research. 2008;59:338–344. doi: 10.1002/art.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghadam-Kia S, Chilek K, Gaines E, et al. Cross-sectional analysis of a collaborative Web-based database for lupus erythematosus-associated skin lesions: prospective enrollment of 114 patients. Arch Dermatol. 2009 Mar;145(3):255–60. doi: 10.1001/archdermatol.2008.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piette EW, Foering KP, Chang AY, et al. Impact of Smoking in Cutaneous Lupus Erythematosus. Arch Dermatol. 2012 Mar;148(3):317–22. doi: 10.1001/archdermatol.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foering K, Goreshi R, Klein R, et al. Prevalence of self-report photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2012 Feb;66(2):220–8. doi: 10.1016/j.jaad.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linthorst Homan MW, Spuls PI, de Korte J, et al. The Burden of vitiligo: Patient characteristics associated with quality of life. J Am Acad Dermatol. 2009 Sep;61(3):411–20. doi: 10.1016/j.jaad.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Purvisha P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373–385. doi: 10.1016/s0733-8635(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005 Nov;125(5):889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonilla-Martinez Z, Albrecht J, Taylor L, et al. The CLASI is a useful clinical instrument to separately follow activity and damage during therapy of cutaneous lupus erythematosus. Arch Dermatol. 2008 Feb;144:173–180. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krathen MS, Dunham J, Gaines E, et al. The Cutaneous Lupus Erythematosus Disease Activity and Severity Index (CLASI) expansion for rheumatology and dermatology. Arthritis Care & Research. 2008;59:338–344. doi: 10.1002/art.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chren M-M, Lasek RJ, Flocke SA, Zyzanski SJ. Improved Discriminative and Evaluative Capability of a Refined Version of Skindex, a Quality – of – Life Instrument for Patients With Skin Diseases. Arch Dermatol. 1997;133:1433–1440. [PubMed] [Google Scholar]
- 23.Klein R, Moghadam-Kia S, LoMonico J, Okawa J, Coley C, Taylor L, Troxel AB, Werth VP. Development of the CLASI as a Tool to Measure Disease Severity and Responsiveness to Therapy in Cutaneous Lupus Erythematosus. Arch Dermatol. 2011;147(2):203–208. doi: 10.1001/archdermatol.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chren MM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107(5):707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 25.Samponga F, Raskovic D, Guerra L, et al. Identification of categories at risk for high quality of life impairment in patients with vitiligo. Br Journal Dermatol. 2008;159:351–359. doi: 10.1111/j.1365-2133.2008.08678.x. [DOI] [PubMed] [Google Scholar]