Abstract
Brucellosis remains a significant zoonotic threat worldwide. Humans and animals acquire infection via their oropharynx and upper respiratory tract following oral or aerosol exposure. After mucosal infection, brucellosis develops into a systemic disease. Mucosal vaccination could offer a viable alternative to conventional injection practices to deter disease. Using a nasal vaccination approach, the ΔznuA B. melitensis was found to confer potent protection against pulmonary Brucella challenge, and reduce colonization of spleens and lungs by more than 2500-fold, with more than 50% of vaccinated mice showing no detectable brucellae. Furthermore, tenfold more brucellae-specific, IFN-γ-producing CD8+ T cells than CD4+ T cells were induced in the spleen and respiratory lymph nodes. Evaluation of pulmonary and splenic CD8+ T cells from mice vaccinated with ΔznuA B. melitensis revealed that these expressed an activated effector memory (CD44hiCD62LloCCR7lo) T cells producing elevated levels of IFN-γ, TNF-α, perforin, and granzyme B. To assess the relative importance of these increased numbers of CD8+ T cells, CD8−/− mice were challenged with virulent B. melitensis, and they showed markedly increased bacterial loads in organs in contrast to similarly challenged CD4−/− mice. Only ΔznuA B. melitensis- and Rev-1-vaccinated CD4−/− and wild-type mice, not CD8−/− mice, were completely protected against Brucella challenge. Determination of cytokines responsible for conferring protection showed the relative importance of IFN-γ, but not IL-17. Unlike wild-type mice, IL-17 was greatly induced in IFN-γ−/− mice, but IL-17 could not substitute for IFN-γ’s protection, although an increase in brucellae dissemination was observed upon in vivo IL-17 neutralization. These results show that nasal ΔznuA B. melitensis vaccination represents an attractive means to stimulate systemic and mucosal immune protection via CD8+ T cell engagement.
Keywords: Brucella, vaccines, nasal immunization, T cells
INTRODUCTION
Brucellosis is the most common zoonotic disease. 1 The causative bacteria of this disease belong to the genus Brucella, a highly homogenous group of 10 - 12 Gram-negative species sharing greater than 94% DNA homology 2, with B. abortus, B. melitensis, and B. suis being the primary species responsible for human disease. 1 Acute disease is severely debilitating causing a febrile illness with flu-like symptoms, and if left untreated, can persist for weeks to months. Chronic disease manifests with arthritis, endocarditis, neurological complications, or testicular or bone abscess formation1. Human brucellosis poses significant economic and health concerns in Northen Africa, Middle East, Western Europe, Latin America, Sub-Saharan Africa, and Central Asia with more than 500,000 cases reported annually worldwide. 3 Where endemic, the disease burden is often underestimated by as much as 20-fold. 4 In livestock, brucellosis is responsible for reproductive loss resulting from abortion, birth of weak offspring, or infertility5. Brucellosis contributes to significant economic losses due to loss of work days and diminished animal and dairy production. 5
Brucella infections generally involve crossing a mucosal surface of the host. 6 For livestock, the predominant route of Brucella exposure is by ingestion or inhalation of microorganisms present in the aborted fetus which can be as high as 1013 organisms per gram of tissue. 7 Human infection is mainly acquired via the ingestion of contaminated foods such as unpasteurized dairy products or raw meat.1, 8 Inhalation or mucosal exposure to aerosolized bacteria from contact with the infected animal’s vaginal secretions, urine, feces, or blood (especially amongst livestock producers, abattoir workers, and veterinarians) can also cause disease transmission.8 What is shared between animal and human Brucella transmission is the naso-oropharyngeal mucosa being impacted by Brucella, and not so much the intestinal mucosa 9, despite oral ingestion of contaminated foods. Given the relevance of mucosal exposure for infection by Brucella, a vaccine aimed at immunizing head and neck mucosal tissues has a high potential of success.
Epidemiological evidence implicates human disease is related to the persistence of brucellosis in livestock emphasizing the importance of livestock vaccination as a means to control both animal and human brucellosis.10 Currently, live, attenuated Brucella abortus S19, B. abortus RB51, and B. melitensis Rev-1 vaccines are used to control livestock brucellosis. 8 However, these vaccines have some disadvantages including S19 and Rev-1 can induce abortion in pregnant animals, and retention of their lipopolysaccharide (LPS) makes it difficult to differentiate vaccinated from naturally infected animals using serological methods.6,10 These livestock vaccines are approximately 70% efficacious and are pathogenic to humans. 6 A superior vaccine would need to eliminate these problems.
Although Brucella primarily infects via a mucosal surface 8, relatively few studies have tested oral11–14 and nasal vaccination methods.15–17 Despite oral vaccination being able to confer significant protection against brucellae dissemination following oral14 or nasal11, 13 challenge, varied protection of the lungs was observed following nasal challenge.11, 13 In many ways, the nasal challenge method mimics aspects of natural Brucella infections by infecting via the naso-oropharyngeal tissues. Attempts to render protection using a nasal vaccination approach also resulted in minimal to no respiratory or systemic protection.15–17 While parenthetically it seems that mucosal vaccination methods did not work in these studies11, 13–17, our evidence suggests these vaccines were unable to stimulate potent protective T cell responses, and hence, unsuccessful.
We have previously reported that a single oral dose of our live, attenuated ΔznuA B. melitensis vaccine conferred superior protection of the lungs as well as prevention of systemic dissemination following nasal B. melitensis 16M challenge.12 In this study, 83% and 58% of the vaccinated mice showed no detectable brucellae in their spleens and lungs, respectively.12 Although oral vaccination was highly effective, one caveat was that it required a large vaccine dose similar to what others have found.11,13 One alternative to oral vaccination is to exploit the nasal route in conferring protection in the respiratory tract. The advantages over the oral route include readily accessible mucosal tissue, the need of less vaccine, and the lack of exposure of the vaccine to low pH or to the digestive enzymes present in the gastrointestinal tract. Nasal vaccination is highly capable of stimulating both mucosal and systemic immune responses.
An effective brucellosis vaccine needs to stimulate IFN-γ and TNF-α by responding T cells since protection against Brucella infections requires cell-mediated immunity.12, 18,19 However, how the relative contributions by CD4+ and CD8+ T cells aid to protect against Brucella infections are less well understood. Several studies 20–22 have suggested that CD4+ and CD8+ T cells are both necessary in protection against brucellosis, whereas others advocated the importance of CD4+ T cells23–26 or CD8+ T cells.15, 27–29 Such T cell biases may arise from attributes of the vaccine used or modes of delivery. Some vaccine approaches, including subunit vaccines, lend themselves to a T cell bias, particularly to CD8+ T cells.27, 29, 30 Aside from differences in immunization regimens, the route of vaccination may greatly influence the types of T cell responses induced. Most brucellosis vaccine studies use the intraperitoneal (i.p.) route of infection to reproduce the systemic aspects of infection. 31 However, the i.p. route bypasses the natural mucosal mode of infection and may not recapitulate the induced immune defense mechanisms. This became evident from Mycobacterium tuberculosis (M. tb) vaccine studies.32
Herein, we demonstrate that a single, nasal dose of ΔznuA B. melitensis mutant induces Ag-specific CD4+ and CD8+ T cell responses that confer potent protection against pulmonary Brucella challenge with CD8+ T cells being required for complete protection. This is supported by greater bacterial burden in CD8−/− mice, but not in CD4−/− mice. In fact, ΔznuA B. melitensis-vaccinated CD4−/− and wild-type mice showed complete protection against Brucella challenge in contrast to CD8−/− mice, supporting the notion that CD8+ T cells are essential for mucosal protection against brucellosis.
RESULTS
ΔznuA B. melitensis is attenuated in BALB/c mice
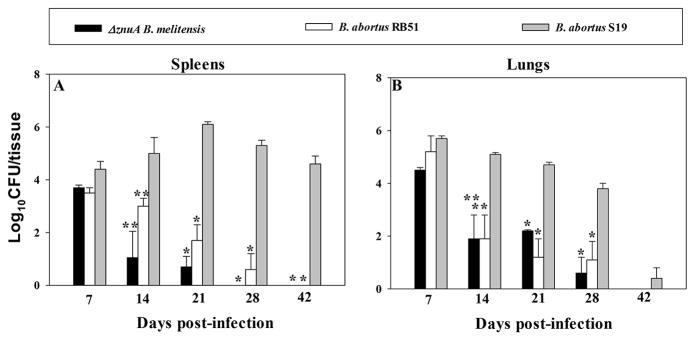
The ΔznuA B. melitensis vaccine was previously shown to be cleared as efficiently from the host as RB51 when given by the oral route. 12 Hence, we inquired into ΔznuA B. melitensis virulence following nasal administration. Groups of BALB/c mice (n=60) were nasally dosed with 109 CFUs of ΔznuA B. melitensis, B. abortus S19, or B. abortus RB51 to measure colonization of spleens and lungs at 7, 14, 21, 28, and 42 days post-vaccination. The ΔznuA B. melitensis vaccine was rapidly cleared from the spleen within 3 – 4 weeks (wks), and RB51 remained for at least 4 wks (Figure 1A). In contrast, both of these vaccines persisted for at least 28 days in the lungs, and were undetectable by 42 days (Figure 1B). Mice vaccinated with S19 showed long-lasting, elevated infection of the spleen for > 42 days, but nearly cleared from the lungs within this same time frame (Figure 1A and B). Thus, these results show that ΔznuA B. melitensis and RB51 are cleared rapidly from the host unlike S19.
Figure 1. ΔznuA B. melitensis is effectively cleared from the host by 6 weeks post-infection.
BALB/c mice (4 mice/group/time point) were nasally dosed with 1×109 CFUs of ΔznuA B. melitensis, RB51, or S19 vaccines. On days 7, 14, 21, 28, and 42 individual (A) spleens and (B) lungs were evaluated for colonization (CFUs). Values are the means of individual mice ± SEM, and differences in colonization were determined when compared to S19 vaccine, *P≤0.001, **P<0.05.
ΔznuA B. melitensis confers potent protection in BALB/c and IFN-γ−/− mice
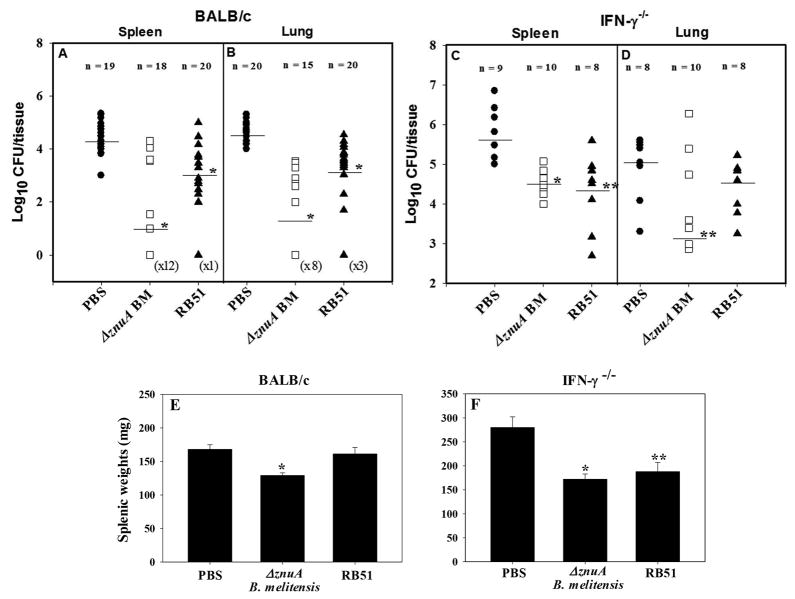
To assess its protective efficacy, groups of BALB/c mice were nasally vaccinated once on day 0 with ΔznuA B. melitensis, RB51, or PBS. Following a rest for 6 wks, mice were nasally challenged with 5 × 104 CFUs of virulent B. melitensis strain 16M, and 4 weeks after challenge, mouse spleens and lungs were examined for extent of colonization by wild-type (wt) B. melitensis strain 16M. The ΔznuA B. melitensis vaccine conferred significant (P≤0.001) protection against systemic dissemination by virulent B. melitensis relative to PBS-dosed mice evident by the 2500-fold reduction in colonization (Figure 2A). In contrast, RB51-vaccinated mice showed only 15.8-fold reduction (P≤0.001; Figure 2A). Nasal vaccination was also able to confer potent protection in the lungs of ΔznuA B. melitensis-immunized mice (1600-fold reduction in colonization; P≤0.001) in contrast to RB51-vaccinated BALB/c mice (40-fold reduction; P≤0.001) (Figure 2B). Additionally, ΔznuA B. melitensis- vaccinated BALB/c mice showed significantly lower splenic weight than PBS-immunized mice (P≤0.001; Figure 2E)
Figure 2. ΔznuA B. melitensis vaccine protects against wild-type B. melitensis 16M challenge.
(A, B, E) BALB/c mice were nasally immunized once with 1×109 CFUs ΔznuA B. melitensis (ΔznuA BM; 18 mice/group), RB51 (20 mice/group), or sterile PBS (20 mice/group). (C, D, F) IFN-γ−/− mice were also nasally immunized once with 1×109 CFUs ΔznuA B. melitensis (10 mice/group), RB51 (8 mice/group), or sterile PBS (9 mice/group). After 6 weeks, mice were nasally challenged with 5 × 104 CFUs of wild-type B. melitensis 16M. Four weeks post-challenge, their spleens and lungs were assessed for (A–D) brucellae colonization (CFUs; the limit of detection was 50 CFU/organ) and (E, F) splenic weights. Results are expressed as mean ± SEM of two - three independent experiments. *P≤0.001, **P<0.05 represent the significant differences in log CFUs or splenic weights versus PBS-dosed control group.
IFN-γ is crucial to subdue Brucella infections, otherwise, these become lethal in the absence of functional IFN-γ gene. 33 Hence, we queried whether in the absence of IFN-γ, other proinflammatory cytokines may be induced to compensate and control brucellosis. In this regard, IFN-γ−/− mice were nasally vaccinated with either ΔznuA B. melitensis or RB51, and both vaccines were able to afford significant protection against systemic infection against virulent B. melitensis 16M (20- and 25-fold reduction in colonization, respectively; P≤0.001, P<0.05; Figure 2C). Yet, only the ΔznuA B. melitensis vaccine could confer protection in the IFN-γ−/− lungs (50-fold reduction in colonization; P<0.05; Figure 2D). Both ΔznuA B. melitensis- and RB-51- vaccinated IFN-γ−/− mice showed reduced splenic weight relative to the PBS control group (P≤0.001; P<0.05; Figure 2F).
IFN-γ-producing CD8+ T cells are important for protection against pulmonary B. melitensis challenge
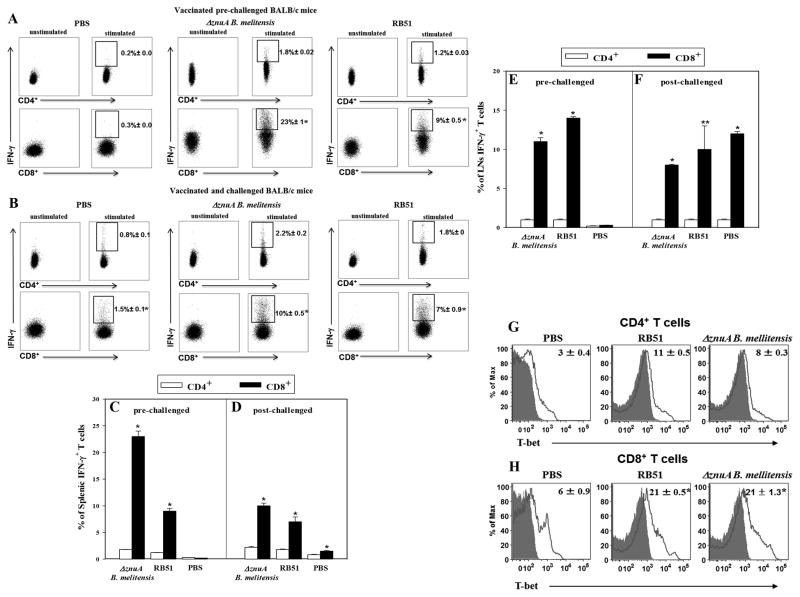
To date, studies have found that protection against Brucella infections are largely dependent on CD4+ T cells. 26, 27, 29 Yet, these studies evaluated T cell responses after systemic infection, but not when challenged by a mucosal route. Hence, flow cytometry analysis was performed 21 days after vaccination and 28 days after challenge with wt B. melitensis 16M to determine which T cells served as the source of for IFN-γ. There were 12- and 7.5-fold greater IFN-γ+ splenic CD8+ T cells than IFN-γ+ CD4+ T cells in ΔznuA B. melitensis- and RB51-vaccinated BALB/c mice, respectively (P≤0.001; Figure 3A and C). Similarly, the percentage of lymph node (LN; combined head and neck LNs [HNLN] and lower respiratory [or mediastinal] LNs [LRLN]) IFN-γ+ CD8+ T cells were greater than IFN-γ+ CD4+ T cells in both vaccine groups (P≤0.001; Figure 3E). Intracellular expression of T-bet, the transcriptional activator of IFN-γ and a key regulator for Th1 differentiation 38, was significantly elevated by ~2-fold in splenic CD8+ T cells compared to CD4+ T cells in both vaccinated groups (P≤0.001; Figure 3G and H). Analysis of IFN-γ expression 4 wks after virulent B. melitensis challenge also revealed those splenic IFN-γ+ CD8+ T cells were ~4-fold greater than CD4+ T cells by both ΔznuA B. melitensis- and RB51-vaccinated mice (P≤0.001; Figure 3B and D), and 8-, 10-, and 12-fold greater for respiratory LNs for ΔznuA B. melitensis-, RB51-, and PBS-vaccinated mice, respectively (P≤0.001, P<0.05; Figure 3F).
Figure 3. The ΔznuA B. melitensis and RB51 vaccines stimulate enhanced production of IFN-γ by splenic and respiratory lymph node (LN) CD8+ T cells before and after challenge with wild-type B. melitensis.
BALB/c mice (18/group) were nasally vaccinated with 109 CFUs of ΔznuA B. melitensis, RB51, or sPBS. Three wks after vaccination, half of the mice were evaluated for (A, C, E) IFN-γ production and (G, H) T-bet expression (red line). Whole lymphocyte cultures (pooled from 2–3 mice/culture and at least three cultures/experiment) were established from (A, C, G, H) spleens and (E) respiratory LNs (head and neck LNs [HNLNs] and lower respiratory LNs [LRLNs]) and restimulated with media or 109 CFUs of HKRB51 for 12 hr followed by 25 ng/ml PMA and 1 μg/ml ionomycin and 10 μg/ml brefeldin A for 6 hr. (B, D, F) Six wks after vaccination, the remaining half of the mice was nasally challenged with 5 × 104 CFUs of wild-type B. melitensis 16M. Four weeks post-challenge, whole (B, D) splenic and (F) respiratory LN lymphocytes (pooled from 2–3 mice/culture and at least three cultures/experiment) were restimulated exactly as done for the prechallenged mice. Restimulated splenic lymphocytes were evaluated for IFN-γ production and T-bet expression using standard intracellular staining methods for measurement by flow cytometry. Results are depicted as the mean ± SEM of triplicate cultures from two independent experiments. Significant differences in IFN-γ production were determined: *P≤0.001, **P<0.05 (versus CD4+ T cells).
ΔznuA B. melitensis induces proinflammatory cytokines
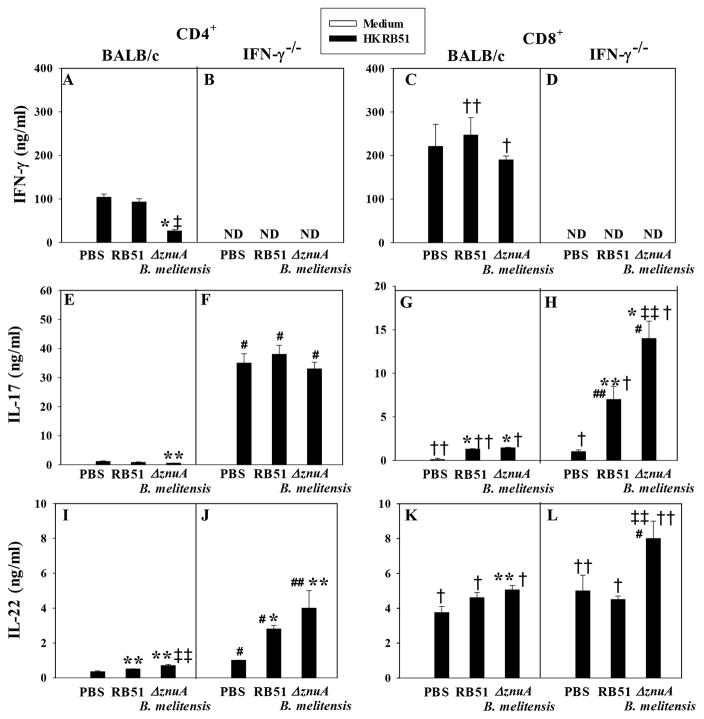
Protection to Brucella infections has been shown to be Th1 cell-dependent. 33 Yet, the role of Th17 cells has been less explored. 19,35,36 When whole lymphocyte cultures were evaluated following Ag restimulation, both RB51− and ΔznuA B. melitensis-vaccinated BALB/c mice showed elevated IFN-γ production (P≤0.001) with some increases in IL-17 (P≤0.001) and IL-22 (P≤0.001), when compared to lymphocytes from PBS-treated mice (Supplementary Figure 1A, E, and I). Four wks after virulent challenge, the ΔznuA B. melitensis-vaccinated BALB/c mice showed elevated IFN-γ and modest IL-17 production (P<0.05), while RB51-vaccinated mice had a markedly enhanced production of IL-22 (P≤0.001) relative to PBS-treated animals (Supplementary Figure 1C, G, and K).
Based on the findings from the whole cultures implicating the importance of IFN-γ, IL-17, and IL-22, additional studies were conducted to assess the relative contributions by CD4+ and CD8+ T cells from vaccinated BALB/c mice following nasal Brucella challenge, the types of proinflammatory cytokines induced, their source, and potency. Purified splenic CD4+ and CD8+ T cells from PBS−, RB51− and ΔznuA B. melitensis-vaccinated and challenged BALB/c and IFN-γ−/− mice were stimulated in vitro with heat-killed RB51 (HKRB51), a source of Brucella proteins without LPS. Increases in IFN-γ (Figure 4A and C) and IL-22 (Figure 4I and K) production were observed with modest changes in IL-17 (Figure 4E and G). CD8+ T cells from both ΔznuA B. melitensis- and RB51-vaccinated BALB/c mice showed elevated production of IFN-γ relative to their respective CD4+ T cells (P≤0.001, P<0.05; Figure 4A and C). Likewise, CD8+ T cells from vaccinated mice showed modest increases in IL-17 (P<0.05, P≤0.001; Figure 4E and G) and IL-22 compared to their CD4+ T cells (P≤0.001; Figure 4I and K).
Figure 4. ΔznuA B. melitensis and RB51 vaccines stimulate enhanced IFN-γ and IL-22 production by BALB/c CD8+ T cells and IL-17 production by IFN-γ−/− CD4+ T cells after challenge with wild-type B. melitensis 16M.
(A, C, E, G, I, K) BALB/c and (B, D, F, H, J, L) IFN-γ−/− mice (18/group) were nasally vaccinated with 109 CFUs of ΔznuA B. melitensis, RB51, or sPBS and nasally challenged six weeks later with 5 × 104 CFUs of wild-type B. melitensis 16M. Four weeks post-challenge, CD4+ and CD8+ T cells were isolated (pooled from 2–3 mice/culture and at least three cultures/experiment) and cultured in the presence of syngenic mitomycin C-treated Ag-presenting cells. T cells were restimulated with media or 109 CFUs of HKRB51 for 3 days, and cell culture supernatants were evaluated for (A–D) IFN-γ, (E–H) IL-17, and (I–L) IL-22 production by cytokine-specific ELISAs. Results depict the mean ± SEM of triplicate cultures from two independent experiments; ND, not detected. Significant differences in IFN-γ, IL-17, and IL-22 production were determined: *P≤0.001, **P<0.05 (versus PBS-dosed mice); †P≤0.001, P<0.05 (differences between CD4+ and CD8+ T cells for the same vaccine group); and ‡P≤0.001, ‡‡P<0.05 (differences between RB51 and ΔznuA B. melitensis-vaccinated mice); # P≤0.001, ## P<0.05 (differences between BALB/c and IFN-γ−/− mice to the same vaccine).
In the absence of IFN-γ, examination of Ag-restimulated whole lymphocytes revealed that IL-17 was significantly augmented in RB51− and ΔznuA B. melitensis-vaccinated IFN-γ−/− mice relative to similarly treated BALB/c mice (P≤0.001; Supplementary Figure 1E and F). Following challenge, IL-17 was significantly enhanced by all IFN-γ−/− treatment groups (PBS, RB51, and ΔznuA B. melitensis) compared to similarly treated BALB/c mice (P≤0.001, P<0.05; Supplementary Figure 1G and H). To determine the T cells impacted in vaccinated and challenged IFN-γ−/− mice, IL-17 from CD4+ T cells was found to be significantly augmented by 32-, 47-, and 66-fold in PBS−, RB51-, and ΔznuA B. melitensis-vaccinated IFN-γ−/− mice, respectively (P≤0.001), relative to CD4+ T cells from similarly vaccinated BALB/c mice (Figure 4E and F). CD8+ T cells from RB51− and ΔznuA B. melitensis-vaccinated IFN-γ−/− mice also showed increased IL-17 production by 5- and 10-fold, respectively (P≤0.001, P<0.05) relative to CD8+ T cells from similarly vaccinated BALB/c mice (Figure 4G and H). IFN-γ−/− CD4+ T cells from PBS-treated and vaccinated mice showed a significant increase in IL-17 as compared to CD8+ T cells (P≤0.001; Figure 4F and H). With respect to IL-22 production, CD4+ T cells from PBS−, RB51− and ΔznuA B. melitensis-vaccinated IFN-γ−/− mice showed more IL-22 than CD4+ T cells from BALB/c mice (P≤0.001, P<0.05; Figure 4I and J). Only CD8+ T cells from ΔznuA B. melitensis-vaccinated IFN-γ−/− mice showed enhanced IL-22 production compared to CD8+ T cells from similarly vaccinated BALB/c mice (P≤0.001; Figure 4K and L). Stimulation of IL-22 was significantly greater for CD8+ T cells in PBS-treated (P<0.05) and vaccinated IFN-γ−/− mice (P≤0.001, P<0.05) than that produced by CD4+ T cells (Figure 4J and L).
IL-17 has minimal impact upon protection conferred by nasal ΔznuA B. melitensis vaccination
To determine whether IL-17 contributes to protection against pulmonary B. melitensis infection, groups of BALB/c mice were nasally vaccinated with ΔznuA B. melitensis, RB51, or PBS. To neutralize IL-17, half of the mice was treated with an anti-mouse IL-17 monoclonal antibody (mAb) on days −1, 0, 7, 14, and 21, and the other half was treated with IgG1 isotype control Ab. Mouse spleens and lungs were examined 4 wks post-challenge for their extent of colonization by virulent B. melitensis 16M. Groups of mice vaccinated with ΔznuA B. melitensis or RB51 plus IgG1 showed significantly reduced colonization of the spleens and lungs (P≤0.001) relative to PBS-dosed plus IgG1-treated mice (Table 1). In fact, the ΔznuA B. melitensis plus IgG1-treated group conferred greatest protection (P<0.05) for spleens and lungs compared to RB51 plus IgG1-treated group (Table 1). Upon neutralization of IL-17, a slight reduction in protection of the spleen was observed for the ΔznuA B. melitensis-vaccinated (P<0.05) and a slim increase in virulence for PBS-dosed BALB/c mice (P≤0.001). As for the lungs, IL-17 neutralization had no significant impact for ΔznuA B. melitensis- or RB51-vaccinated mice, but brucellae colonization of the lungs for PBS plus anti-IL-17-treated group did show a modest increase in colonization (P≤0.001; Table 1). IL-17 neutralization also had no impact upon the splenic weights (Table 1).
Table 1.
The role of IL-17 in protection to B. melitensis 16M infection in BALB/c and IFN-γ−/− (H-2d) mice nasally vaccinated with ΔznuA B. melitensis and RB51a,b
| Vaccine and treatment | Log10 CFU of B. melitensis in spleen (mean ± SEM) | Log10 CFU of B. melitensis in lung (mean ± SEM) | Spleen wt (mg) (mean ± SEM) | |||
|---|---|---|---|---|---|---|
| BALB/c | IFN-γ −/− | BALB/c | IFN-γ −/− | BALB/c | IFN-γ −/− | |
| PBS + IgG | 4.10 ± 0.10 | 5.60 ± 0.20 | 4.20 ± 0.20 | 4.70 ± 0.10 | 206 ± 22 | 332 ± 25 |
| PBS + α–IL-17 | *4.80 ± 0.10 | 5.80 ± 0.20 | *4.70 ± 0.10 | **5.20 ± 0.05 | 230 ± 35 | **557 ± 60 |
| ΔznuA B. melitensis + IgG | *0.00 ± 0.00 | *4.30 ± 0.10 | *1.90 ± 0.50 | **3.90 ± 0.20 | 162 ± 16 | *180 ± 4 |
| ΔznuA B. melitensis + α–IL-17 | *‡‡1.20 ± 0.50 | ‡‡5.40 ± 0.40 | *2.20 ± 0.40 | **4.00 ± 0.20 | 172 ± 16 | 220 ± 21 |
| RB51+ IgG | ¶¶ **2.00 ± 0.70 | ¶¶*3.40 ± 0.30 | ¶¶ *3.00 ± 0.10 | **3.50 ± 0.50 | **151 ± 4 | 193 ± 14 |
| RB51 + α–IL-17 | *1.70 ± 0.50 | †**4.90 ± 0.10 | **2.70 ± 0.40 | 4.60 ± 0.20 | 165 ± 12 | 210± 27 |
Mice were orally immunized with 109 CFUs of ΔznuA B. melitensis and RB51, rested for 6 wks, and then treated with 250 μg of anti-IL-17 mAb or normal IgG1 24 h prior to and at time of nasal challenge with wt B. melitensis. Mice were given three additional antibody treatments on days 7, 14, and 21, and spleens and lungs were evaluated for differences in colonization and splenic weights.
* P ≤ 0.05, ** P ≤ 0.001 compared to PBS-immunized mice treated with IgG1; † P ≤ 0.001 compared to RB51-immunized mice treated with IgG1; ‡‡ P ≤ 0.001 compared to ΔznuA B. melitensis-immunized mice treated with IgG1; ¶¶ P ≤ 0.05 compared to ΔznuA B. melitensis-immunized mice treated with IgG1.
Since ΔznuA B. melitensis- and RB51-vaccinated IFN-γ−/− mice showed reduced colonization (although not to the extent obtained with immunocompetent BALB/c mice) against B. melitensis challenge and their T cells were found to produce elevated levels of IL-17, we inquired into the relevance of IL-17 upon protection. As for BALB/c mice, IL-17 neutralization was done with IFN-γ−/− mice (Table 1). Both ΔznuA B. melitensis- and RB51-vaccinated IFN-γ−/− mice showed enhanced susceptibility to infection noted by their increased splenic colonization by 12.6- (P<0.05) and 32-fold (P<0.05), respectively, relative to their vaccinated, IgG1-treated groups; however, IL-17 neutralization had no impact upon brucellae colonization in spleens from PBS-treated mice, yet it did significantly (P<0.05) enhance splenic size as noted by 68% increase in splenic weights (Table 1). In the lungs, no changes in brucellae colonization were observed for RB51− or ΔznuA B. melitensis-vaccinated mice neutralized of their IL-17. Control PBS-dosed mice treated with anti-IL-17 mAb did show significantly (P<0.05) increased brucellae colonization in the lungs relative to isotype control Ab-treated mice (Table 1). Collectively, these data show that IL-17 was only modestly produced by immunocompetent mice, and its neutralization did not alter extent of brucellae colonization; however, neutralization of IL-17 did have an impact by the increased brucellae colonization of ΔznuA B. melitensis- and RB51-vaccinated IFN-γ−/− spleens.
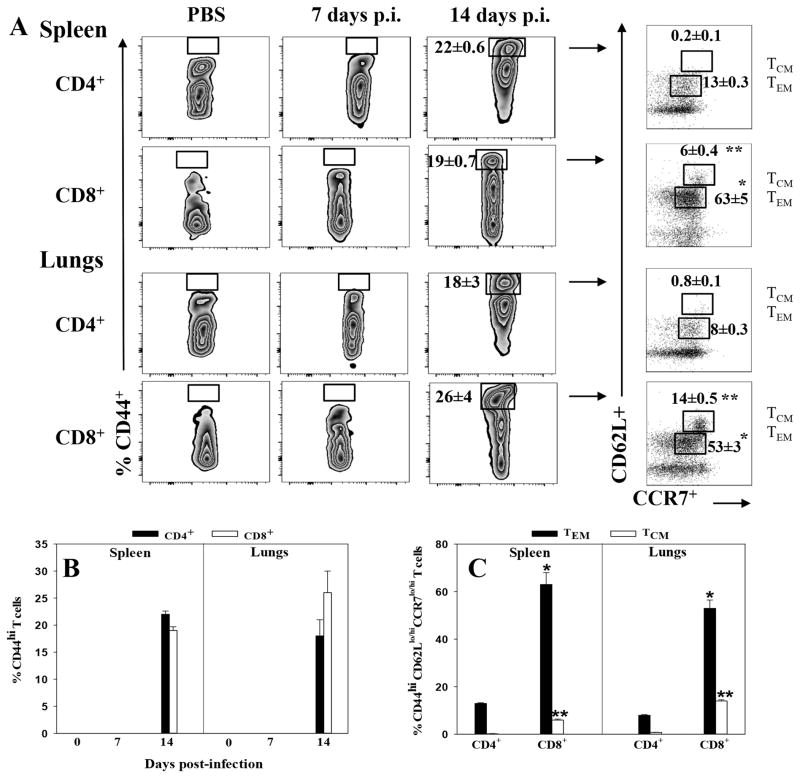
Memory CD4+ and CD8+ T cells are present 14 days after nasal ΔznuA B. melitensis infection
No studies have examined the development of memory T cells following mucosal Brucella infection. We queried about the emergence and activation of memory T cells following nasal ΔznuA B. melitensis vaccination. Both splenic and pulmonary CD4+ and CD8+ T cells were assessed for memory phenotype prior to and 7 and 14 days after infection. At 14 days post-infection, an accumulation of CD4+ and CD8+ T cells expressing an activated effector memory (TEM) phenotype, CD44hiCD62Llo CCR7lo, was detected (Figure 5A and B). The percentage of memory CD44hi CD62LloCCR7lo CD8+ T cell population was ~5- and ~7-fold greater in the spleen and lungs, respectively, when compared to memory CD4+ T cells (Figure 5B). The CD44hiCD8+ T cells were substantially enhanced by more than 50% in both tissues, and because of their elevated expression of CD62L and CCR7, these are considered as central memory (TCM) cells. Unlike CD8+ T cells, CD4+ TCM (CD62LhiCCR7hi) cells were not detected (Figure 5A and C).
Figure 5. Splenic and pulmonary effector and central memory CD44hi CD4+ and CD8+ T cells emerge 14 days post-vaccination with ΔznuA B. melitensis.
Groups of BALB/c mice were nasally vaccinated with 109 CFUs of ΔznuA B. melitensis (n=8) or PBS (n=6) or treated with PBS on day 0. Splenic and lung lymphocytes were isolated from individual mice 1 and 2 wks later. These were evaluated for expression of CD44, CD62L, and CCR7 by flow cytometry. Gated CD44hiCD4+ or CD44hiCD8+ T cells were examined for CD62L and CCR7 expression: low (lo) CD62L and CCR7 were designated as effector memory T (TEM) cells, and high (hi) levels of CD62L and CCR7, as central memory T (TCM) cells. (A) Splenic and lung CD4+ and CD8+ T cells were gated for CD44hi expression on naive and ΔznuA B. melitensis-vaccinated mice at 7 and 14 days post-infection. Both TCM and TEM CD8+ T cells were notably induced in the lungs. (B) Emergence of CD44hi expression on CD4+ and CD8+ T cells in the spleen and lungs on day 14 of ΔznuA B. melitensis-vaccinated mice, but not in naive or day 7 vaccinated mice. (C) Distribution of TEM and TCM cells among splenic and pulmonary memory CD44hi CD4+ and CD8+ T cells 14 days post-vaccination with ΔznuA B. melitensis. Results are depicted as the mean ± SEM of individual mice from two independent experiments. Significant differences in percentage of TEM or TCM between CD4+ and CD8+ T cells were determined: *P≤0.001, **P<0.05.
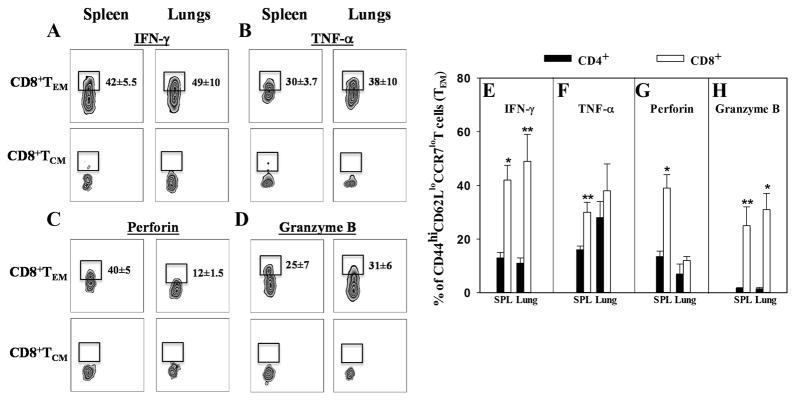
Upon restimulation with HKRB51, CD8+ and CD4+ TEM cells were found to be the primary source of proinflammatory cytokines, IFN-γ, TNF-α (Figure 6A, B, E, and F), and lytic molecules, perforin and granzyme B (Figure 6C, D, G, and H). In the spleens and lungs, CD8+ TEM cells exhibited 3- and 4-fold increases in IFN-γ production, respectively (P≤0.001; P<0.05) relative to CD4+ T cells (Figure 6E). Splenic TNF-α and perforin production by CD8+ TEM cells were significantly increased by 2- and 3-fold, respectively (P≤0.001; P<0.05) relative to CD4+ T cells (Figure 6F and G). Granzyme B production by CD8+ TEM cells was also significantly enhanced by more than 10-fold in both tissues (P≤0.001; P<0.05) relative to CD4+ T cells (Figure 6H).
Figure 6. CD8+ TEM cells are the major producers of proinflammatory cytokines and cytolytic molecules in response to Ag stimulation.
ΔznuA B. melitensis-vaccinated BALB/c mice in Fig. 5 were evaluated for (A, E) IFN-γ, (B, F) TNF-α, (C, G) perforin, and (D, H) granzyme B production. (A–D) Splenic and lung CD8+ T cells from ΔznuA B. melitensis-vaccinated mice were gated for CD44hi CD62LloCCR7lo expression at 14 days post-infection. (EH) Splenic and lung whole lymphocyte cultures from individual mice were restimulated with media or 109 CFUs of HKRB51 for 4hr in the presence of 25 ng/ml PMA and 1 μg/ml ionomycin and 10 μg/ml brefeldin A. Restimulated lymphocytes were evaluated for IFN-γ, TNF-α, perforin, and granzyme B production using standard intracellular staining methods for flow cytometric measurements. Results depict the mean ± SEM of individual cultures from two independent experiments. Significant differences in production were determined: *P≤0.001, **P<0.05 (between CD4+ and CD8+ T cells).
CD8−/− mice, but not CD4−/− mice, showed elevated tissue colonization following wt B. melitensis challenge
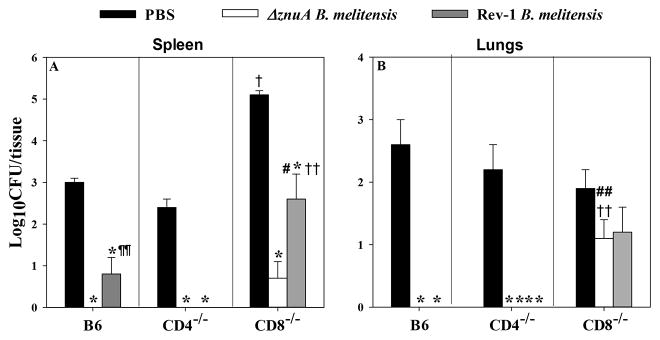
To examine which T cell subsets are important for protection against pulmonary challenge, studies were performed in CD4−/− and CD8−/− mice. B6, CD4−/−, and CD8−/− mice were nasally vaccinated with ΔznuA B. melitensis, Rev-1, or PBS, and 6 wks later, they were nasally challenged with wt B. melitensis 16M. Four wks post-challenge, lungs and spleens were evaluated for extent of brucellae colonization. B6, CD4−/−, and CD8−/− mice vaccinated with ΔznuA B. melitensis or Rev-1 conferred significant protection against splenic B. melitensis 16M colonization relative to PBS-treated mice (P≤0.001; Figure 7A). However, PBS-dosed CD8−/− mice showed exacerbated colonization of their spleens relative to PBS-dosed B6 or CD4−/− mice (Figure 7A; P≤0.001). In the absence of CD8+ T cells, Rev-1-vaccinated mice bore significantly greater bacterial loads than B6 and CD4−/− mice (P≤0.001, P<0.05; Figure 7A). Unexpectedly, ΔznuA B. melitensis-vaccinated CD8−/− mice could still confer protection as evidenced by the slight increase in splenic bacterial load relative to similarly vaccinated B6 and CD4−/− mice. No detectable bacteria were observed in ΔznuA B. melitensis-vaccinated B6 and CD4−/− mice (Figure 7A), and ΔznuA B. melitensis-vaccinated B6 showed significantly greater protection than Rev-1-vaccinated mice (P<0.05).
Figure 7. ΔznuA B. melitensis-induced protection to pulmonary wild-type B. melitensis 16M infection is abrogated in mice deficient in CD8+ T cells.
C57BL/6, CD4−/− and CD8−/− mice were nasally vaccinated with 109 CFUs of ΔznuA B. melitensis (n=8), Rev-1 (n=8), or PBS (n=8) on day 0. Mice were subsequently challenged nasally with wild-type B. melitensis 16M at 6 weeks post-vaccination. Four weeks after virulent challenge, individual spleens and lungs were evaluated for extent of brucellae colonization. The limit of detection was 50 CFU/organ. Results are expressed as mean ± SEM. *P ≤ 0.001, **P ≤ 0.05 represent the significant differences in log CFUs versus PBS-dosed control group for each mouse strain; †P≤0.001, ††P<0.05, differences between C57BL/6 and CD4−/− or CD8−/− mice to the same vaccine; #P≤0.001, ##P<0.05, differences between CD4−/− and CD8−/− mice to the same vaccine; and ¶¶P<0.05, differences between ΔznuA B. melitensis and Rev-1 vaccinated B6 mice.
B6 and CD4−/− mice vaccinated with ΔznuA B. melitensis or Rev-1 were able to control brucellae colonization of their lungs (P≤0.001, P<0.05; Figure 7B) demonstrating that CD8+ T cells impede infection of the lungs. The protective effect conferred by ΔznuA B. melitensis vaccine was abated in CD8−/− mice whereby wt brucellae remained in their lungs. In fact, ΔznuA B. melitensis- or Rev-1-vaccinated CD8−/− mice showed no significant differences (P<0.05) in brucellae colonization relative to PBS-treated mice (Figure 7B). Hence, CD8+ T cells are protective against pulmonary virulent B. melitensis infection when mice are nasally vaccinated.
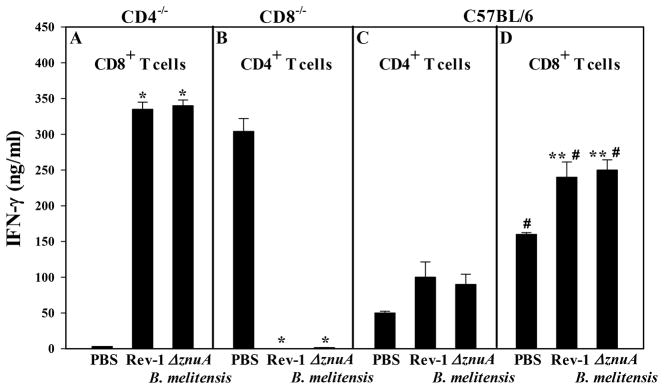
To determine whether the protection obtained in vaccinated CD4−/− and B6 mice correlates to IFN-γ production, HKRB51 restimulation was performed 4 wks after virulent B. melitensis challenge using splenic T cell subsets from PBS-treated or vaccinated mice. CD8+ T cells from Rev-1- or ΔznuA B. melitensis-vaccinated CD4−/− mice produced elevated levels of IFN-γ (Figure 8A), which accounts for the potent protection conferred by these vaccines against pulmonary challenge (Figure 7B). In contrast, no IFN-γ-producing CD4+ T cells were detected in ΔznuA B. melitensis- or Rev-1-vaccinated CD8−/− mice (Figure 8B) despite IFN-γ secretion by CD4+ T cells from PBS-treated CD8−/− mice (Figure 8B), although the bacterial loads were greater in PBS-treated CD8−/− mice (Figure 7A). Consistent with the findings that CD8+ T cells are the primary source of IFN-γ, analysis of T cell responses from B6 mice vaccinated and challenged with virulent B. melitensis revealed that all B6 treatment groups showed enhanced IFN-γ production derived from their CD8+ T cells rather than their CD4+ T cells (P≤0.001; Figure 8C and D). Thus, these studies show that CD8+ T cells contribute to protection against pulmonary infection with wt B. melitensis 16M when this protection is conferred by ΔznuA B. melitensis and Rev-1 vaccines. Furthermore, CD8+ T cells are able to sustain IFN-γ production in the absence of CD4+ T cells unlike mice having to rely solely on CD4+ T cells as in the case with the CD8−/− mice.
Figure 8. CD8+ T cells from ΔznuA B. melitensis- or Rev-1-vaccinated and challenged CD4−/− mice show elevated IFN-γ production.
Splenic lymphocytes were isolated from the same C57BL/6, CD4−/−, and CD8−/− mice that were nasally vaccinated and subjected to pulmonary infection with wild-type B. melitensis strain 16M as described in Figure 5. Splenic CD4+ and CD8+ T cells were isolated (pooled from 2–3 mice/culture and at least three cultures/experiment) and co-cultured in the presence of mitomycin C-treated C57BL/6 Ag-presenting cells and 109 CFUs HKRB51 or media for 3 days. Harvested supernatants were evaluated for IFN-γ production by ELISA. Results are expressed as the mean ± SEM of triplicate cultures. Significant differences in IFN-γ production were determined: *P≤0.001, **P<0.05 (versus PBS-dosed mice); #P≤0.001 (differences between CD4+and CD8+ T cells to the same vaccine).
DISCUSSION
Since most Brucella exposures occur at mucosal surfaces 1, a vaccine that capitalizes on mucosal immune responses would be advantageous. Instead, most efforts with live vaccines for brucellosis have relied upon parenteral immunization methods to protect against disseminated infection, rather than focusing efforts to stop Brucella at the site of infection. Consequently, parenteral vaccination proved ineffective for brucellae clearance from the lungs. 37, 38 Furthermore, the few studies that have tested mucosal routes of vaccination to protect against pulmonary disease were discouraging. One such study tested oral immunization with γ-irradiated Brucella neotomae, and found that bacterial colonization of spleen and lung tissues was reduced only by 15.8-fold. 11 In another study, oral vaccination with Brucella melitensis WR201 was able to confer lung protection by only 3-fold. 13 RB51 or RB51SOD given nasally failed to show protection against pulmonary disease. 16 Nasal co-administration of different TLR agonists 17 or adjuvants 15 with RB51 only had a modest impact upon systemic dissemination providing a 10-fold reduction in colonization of the lungs. Given these findings, we considered whether the route of vaccination as well as the type of live vaccine can impact systemic and mucosal protection. Our ΔznuA B. melitensis prototype vaccine proved to be potent in protecting against pulmonary B. melitensis infections. This vaccine reduced splenic colonization, a measure of systemic infection, by more than 2500-fold. A profound finding was that 67% of ΔznuA B. melitensis-vaccinated animals had Brucella-free spleens, a striking contrast to only 5% of RB51-vaccinated animals. In fact, nasal immunization with ΔznuA B. melitensis was able to confer protection against brucellae replication in the lungs at a level unsurpassed by any experimental or livestock vaccine. This was manifested by these vaccinated mice showing more than 1600-fold reduction in colonization relative to naive controls, with 53% of vaccinated animals showing no live brucellae in their lungs.
Oral vaccination with ΔznuA B. melitensis conferred effective lung protection 12, but nasal delivery afforded even better protection. This suggested that mucosal vaccination either directly via the respiratory tract or indirectly via the gut stimulates effective immunity for protection against pulmonary Brucella challenges. These findings resembled those obtained with vaccines for tuberculosis. Nasal, but not intramuscular, immunization with a recombinant adenovirus-based tuberculosis vaccine provided protection against pulmonary M.tb challenge. 32 Likewise, nasal vaccination with bacillus Calmette-Guèrin (BCG) conferred significantly better protection from M. tb challenge than s.c. vaccination. 39 Although sterilizing immunity (no detectable CFUs) in the lungs afforded by ΔznuA B. melitensis was similar following oral or nasal vaccination (58% vs. 53%, respectively), the overall protection for the remaining animals was better by more than 10-fold when this vaccine is given nasally. Hence, our studies show that protection against pulmonary Brucella infections is affected by both delivery method and vaccine composition.
How Brucella infects orally has been subject of some debate. 9 Despite the experimental evidence that Brucella is able to penetrate the intestinal barrier following oral inoculation 40, 41, the specific mechanism remains to be determined. In fact, intragastric infection of mice was found to be relatively inefficient and required a high infectious dose. 40, 42 Yet, in humans and livestock, oral exposure continues as the major route of infection. Other mucosal sites have been implicated as well, e.g., the oropharynx and upper respiratory tract, as the predominant sites of Brucella entry 43,44, and reaffirmed by the finding of brucellae being harbored in the HNLNs following oral infection. 45 Since delivery of the vaccines into the nasal mucosa can result in their deposition in the oropharynx 46, nasal vaccination may present as an optimal route for vaccine delivery and perhaps stimulation of the naso-oropharyngeal lymphoid tissues.
Protection to brucellosis is Th1 cell-dependent because of the inability to control brucellae persistence in mice or humans with IFN-γ deficiencies. IFN-γ−\− mice are unable to inhibit Brucella growth 33, and when left unchecked, these mice can eventually develop osteoarticular complications. 47 In the current studies, protection conferred by ΔznuA B. melitensis showed dependence on IFN-γ and other factors since similarly vaccinated IFN-γ−\− mice showed reduced B. melitensis colonization following challenge. In fact, ΔznuA B. melitensis- and RB51-vaccinated IFN-γ−/− mice displayed significant reduction in systemic brucellae dissemination. We questioned whether the observed control of the infection in vaccinated IFN-γ−/− mice may be the result of IL-17 since it was greatly augmented in response to vaccination and challenge. While IL-17 is thought to be required for protection against extracellular pathogens 48, recent work suggests that IL-17 is also needed to combat intracellular pathogens by driving the induction of IL-12 to generate Th1-type responses. 49, 50 Indeed, IL-17 facilitates vaccine-induced protection against M. tb challenge. 51, 52 Yet, only a couple of studies have considered a role for IL-17 in Brucella infections. 35, 36 Pasquevich et al. 36 demonstrated that protection with orally delivered unlipidated outer membrane protein 19 (Omp 19) was mediated by IL-17. This protective effect was lost in vaccinated mice neutralized of their IL-17. In the course of a Brucella infection, IL-17 was found to be tightly regulated by IL-10, and detected only in IL-10−/− mice. 35 We were not able to detect IL-10 in any of our BALB/c or IFN-γ−/− Ag-restimulated cultures.
Neutralization of IL-17 in ΔznuA B. melitensis-vaccinated BALB/c mice had no major impact on brucellae colonization of the spleens and lungs of ΔznuA B. melitensis- or RB51-vaccinated mice, although it did result in a significant increase in brucellae colonization of the lungs in unvaccinated mice. The relevance of IL-17 manifested as an increase in brucellae dissemination upon IL-17 neutralization of IFN-γ−/− mice. This occurred for both ΔznuA B. melitensis- and RB51-vaccinated IFN-γ−/− mice with 12.6- and 32-fold increases, respectively, in wt B. melitensis splenic colonization, but no significant differences in lung colonization. These data suggest that IL-17 may be more important for controlling brucellae dissemination when IFN-γ is absent or in limited quantities. Increases in lung colonization only became evident when IL-17 was neutralized in unvaccinated IFN-γ−/− mice. Studies using IL-17Rα−\− mice showed that IL-17 was not necessary for protection from systemic B. abortus challenge. 19 Although there may be fundamental differences in how the immune responses are induced by systemic 19 versus mucosal infections, IL-17 has been thought to compensate when Th1 cells are impaired as observed for Listeria monocytogenes infections. 53
IL-22 is another cytokine known for host defense against extracellular bacterial pathogens 54, but its role in protection against an intracellular bacterial pathogens is more controversial. IL-22 was found to be dispensable for Fransicella tularensis 49 and Listeria monocytogenes 55 infections. However, IL-22 was important for recall responses to F. tularensis as demonstrated by its secretion by memory peripheral blood CD4+ and CD8+ T cells restimulated with F. tularensis Ags. 56 Similarly, IL-22 was not required for protection against tuberculosis since IL-22 neutralization did not enhance susceptibility to aerosol M. tb infection. 57 Yet, IL-22 produced by NK cells induced optimal protection by augmenting Ag-specific T cell responses after M. tb challenge. 58 In the case for chlamydial lung infections, strong evidence showed that IL-22 was essential for protection. 59 Neutralization of IL-22 prior to and at the time of nasal infection with Chlamydia muridarum resulted in greater lung pathology, and was associated with the down regulation of Th1 and Th17 cell responses. Hence, the relevance of IL-22 in Brucella infections was examined. Our previous data showed that IL-22 was potently induced following oral vaccination with either ΔznuA B. melitensis or RB51 vaccines, as well as, after pulmonary challenge with wt B. melitensis. 12 Similarly, in the present study, IL-22 was found to be strongly induced in vaccinated and challenged BALB/c and IFN-γ−/− mice. In determining the source of IL-22, CD8+ T cells were the major producers in both BALB/c and IFN-γ−/− mice. Such data implicate IL-22’s involvement in immune protection against Brucella infections, possibly by the activation and/or recruitment of Th1 cells; however, this remains to be determined.
Th1-type responses, characterized by the production of IFN-γ, are essential for immune protection against Brucella infections. 18,19,33 Of the cell types that produce IFN-γ, CD4+ and CD8+ T cells are considered to be the primary sources during Brucella infections. 33 Heretofore, the consensus from published studies was those IFN-γ-producing CD4+ T cells are essential for protection against Brucella infections. 23,24,26 Scrutiny of these data suggests that this may vary based on the types of vaccine and the routes of immunization. Studies employing the i.p. route of vaccination showed the essential role of IFN-γ-producing CD4+ T cells to control B. melitensis infections. 23,24 Conversely, the majority of data supporting the importance of CD8+ T cells in resolving Brucella infections were mostly derived from studies testing parenterally administered subunit vaccines followed by systemic challenges. 28–30 Other studies showed that both CD4+ and CD8+ T cells were essential for protection when the subunit vaccines were given by various routes: i.p. 21, s.c. 22, or oral. 21 In addition, i.v. or i.p. immunization with the live attenuated B. abortus S19 or RB51 vaccine showed that CD4+ and CD8+ T cells worked synergistically to resolve Brucella infections. 20 Alternatively, the type of vaccine used may also influence the type of T cells needed for protection. Orally administered live, attenuated B. melitensis WR201 vaccine showed that CD8+ T cells were dispensable since vaccinated CD8−/− mice remained protected against nasal B. melitensis challenge. 25 For M. tb, it has been shown that routes of immunization can influence the adaptive immune responses against pulmonary challenge. Nasal vaccination with a recombinant adenovirus vaccine expressing M. tb Ag85A was able to effectively activate both CD4+ and CD8+ T cells in the lungs, particularly within the airway lumen. By comparison, i.m. immunization induced primarily CD8+ T cell responses in the peripheral lymphoid tissues. 32 Collectively, what can be concluded from these studies is that the vaccine composition, routes of vaccination, and route of challenge can significantly impact the types of T cells required for protection.
The results from this current study point to the relevance of the type of vaccine used and the route of immunization. Our studies with ΔznuA B. melitensis showed elevations in IFN-γ producing Ag-specific CD8+ T cells in both the spleens and regional LNs supported by the concurrent increased expression of the Th1-type transcription factor, T-bet. Although increases in CD8+ T cells were also observed when RB51 was mucosally administered, protection against nasal B. melitensis challenge remained insufficient relative to ΔznuA B. melitensis-vaccinated mice suggesting that RB51 vaccine lacks the potency of our vaccine. This relatively reduced efficacy by RB51 may be in part due to the lack of induced Abs to Brucella’s O-Ag. Unlike ΔznuA B. melitensis, RB51 vaccine is a rough strain and does not induce Abs directed to the O-side chain of the surface LPS. Passive transfer of Abs induced by RB51 vaccination failed to confer protection against virulent B. abortus challenge, in contrast to antisera derived from S19-vaccinated mice, provided significant protection. 60 Another attribute of nasal vaccination was the observed increase in the percentages of CD8+ T cells that were significantly augmented relative to CD4+ T cells. Ag recall responses by splenic and LN CD8+ T cells from ΔznuA B. melitensis-vaccinated mice resulted in greater IFN-γ production by 12- and 10-fold, respectively, than by CD4+ T cells. CD8+ T cells from RB51-immunized mice also showed greater capacity for IFN-γ production than CD4+ T cells.
Most immune analyses to date have not focused directly on examining long-term T cell responses, particularly, memory responses following live Brucella vaccination or infection. Recently, Durward et al 61 and Durward-Diioia et al 62 have characterized memory CD8+ T cells during chronic brucellosis following systemic challenge with virulent Brucella. They found that although both memory precursor and long-lived memory CD8+ T cells are present during chronic brucellosis, these T cells manifested an exhaustive phenotype and a lack of IFN-γ production, which may allow chronic brucellae persistence. In our previous study 12, oral ΔznuA B. melitensis vaccination required a two-month interval to clear the live vaccine, and in essence, the observed protection against wt B. melitensis challenge reflected a memory response, although the responsible T cells were not phenotyped. In this context, it has been shown that the protective memory response is mediated by TEM cells 63,64 that migrate to inflamed peripheral tissues imparting an immediate effector function. In contrast, TCM cells home to T cell areas of secondary lymphoid organs, having little or no effector function, but readily proliferate and differentiate to effector cells in response to antigenic stimulation. 63 To begin to characterize the memory T cells following mucosal vaccination, both splenic and pulmonary memory CD44hi CD4+ and CD8+ T cells were found to be induced subsequent ΔznuA B. melitensis vaccination. The CD8+ TEM cell population (CD62LloCCR7lo) was by more than 5-fold greater than CD4+ TEM cells. Importantly, these CD8+ TEM cells secreted greater amount of proinflammatory cytokines, IFN-γ and TNF-α, and lytic molecules, perforin and granzyme B, after Ag restimulation than memory CD4+ T cells. In addition, the CD8+ TCM (CD62LhiCCR7hi) cell subset was also induced, but mostly induced in the lungs of ΔznuA B. melitensis-vaccinated mice. Although these induced less in the spleen, TCM cells have been found in nonlymphoid organs, such as the liver and lungs. 65 Not only was the IFN-γ production by CD8+ T cells increased, but this elevation correlated with protection, i.e., mice deficient in CD8+ T cells showed little protection. Mice deficient in CD4+ or CD8+ T cells were tested for their resistance to pulmonary Brucella infection following nasal vaccination. Our findings revealed that ΔznuA B. melitensis- and Rev-1-vaccinated CD4−/− mice retained the capacity to control pulmonary Brucella infections and subsequent systemic dissemination by the presence of IFN-γ+ CD8+ T cells, and the level of protection was indistinguishable from that of similarly vaccinated C57BL/6 mice. In contrast, protection conferred by ΔznuA B. melitensis and Rev-1vaccines was abrogated in CD8−/− mice. The remaining CD4+ T cells could not compensate for the absence of IFN-γ-producing CD8+ T cells resulting in increased brucellae dissemination and increased brucellae colonization of the lungs. In fact, IFN-γ production by CD4+ T cells in CD8−/− mice was severely impaired. The CD4+ T cells from unvaccinated CD8−/− mice showed markedly enhanced IFN-γ production in response to pulmonary Brucella infection, but these mice were still susceptible to Brucella dissemination. In the lungs, both ΔznuA B. melitensis- and Rev-1-vaccinated CD8−/− mice sustained elevated bacterial loads. Collectively, for the three nasal vaccines tested, the data demonstrate CD8+ T cells’ importance for protection against pulmonary Brucella infections. The data support our contention that the mucosal route of vaccination is superior to parenteral vaccinations and that the potency of the CD8 T cell response is critical in conferring immunity to brucellosis.
METHODS
Brucella challenge and vaccine strains
Construction of the ΔznuA B. melitensis mutant has been previously described. 12 For nasal vaccination of mice, ΔznuA B. melitensis, B. melitensis Rev-1, B. abortus RB51, and B. abortus S19 vaccines were grown overnight in shaker flasks in Brucella broth (BB) at 37°C. A total of 2 to 3 ml of these broth cultures was plated on multiple 15-cm-diameter petri dishes containing Brucella agar. Virulent B. melitensis 16M was grown in a similar fashion. After 3 days of incubation at 37°C with 5% CO2, plates were harvested with saline. Cells were pelleted, washed twice in sterile phosphate buffered saline (sPBS), and diluted to 109 CFUs/30 μl for the vaccines and in 5×104 CFUs/30 μl for B. melitensis 16M in sPBS. For vaccination or for challenge, anesthetized mice were given 30 μl of suspended Brucella using a micropipette to administer dropwise into the external nares of mice. The actual viable inoculum CFUs were confirmed by serial dilution tests on potato infusion agar (PIA).
Animals
All animal experiments with live Brucella were performed in animal biosafety level 3 facilities. Female BALB/c mice were obtained from Frederick Cancer Research Facility (National Cancer Institute, MD), and C57BL/6 and CD4−/− and CD8−/− mice on a C57BL/6 background were obtained from The Jackson Laboratory. The colony of IFN-γ−/− mice on a BALB/c background was obtained as previously described. 12 All animals were maintained in individually ventilated cages under HEPA-filtered barrier conditions of 12 h of light and 12 h of darkness in the animal biosafety level 3 facility and provided with food and water ad libitum. Experiments were conducted with 7- to 9-week-old age-matched mice. All animal care and procedures were in accordance with institutional policies for animal health and well-being and approved by Montana State University Institutional Animal Care and Use Committee (IACUC) and approved by University of Florida IACUC.
In vivo colonization studies
Brucellae persistence was measured following nasal administration of ΔznuA B. melitensis, RB51, and S19. At 1, 2, 3, 4, and 6 weeks after dosing (n=4/time point), spleens and lungs were collected and bacterial colonies were enumerated from water lysed tissue homogenates. Tissue homogenates were prepared as previously described. 12 A total of 20 μl of undiluted and serial 10-fold dilutions of homogenates were grown in cultures on PIA. After incubation for 3 to 5 days at 37°C in 5% CO2, Brucella colonies were enumerated, and CFUs per spleen or lungs were calculated.
Vaccine efficacy studies
BALB/c mice nasally vaccinated on day 0 with ΔznuA B. melitensis (n = 18), RB51 (n = 20), or PBS (n = 20); IFN-γ−/− mice were also vaccinated with ΔznuA B. melitensis (n = 10), RB51 (n = 8), or PBS (n = 9). To measure the relative contribution of CD4+ and CD8+ T cells, CD4−/− and CD8−/− mice were nasally vaccinated on day 0 with ΔznuA B. melitensis (n=8), Rev-1 (n=8), or PBS (n=8). Mice were rested for 6 wks and then challenged nasally with wt B. melitensis strain 16M. Four weeks after virulent challenge, individual spleens and lungs were recovered, subjected to homogenization and lysis as described above, and CFUs were enumerated.
To assess the role of IL-17 in protection against B. melitensis challenge in vaccinated BALB/c and IFN-γ−/− mice, IL-17 was neutralized in vivo upon treatment with 250 μg/dose of anti-IL-17 mAb (clone 17F3; BioXcell, West Lebanon, NH) on days −1, 0, 7, 14, and 21 after challenge. Control animals were similarly treated using IgG1 (MOPC-21; BioXcell) isotype control Ab instead.
Cytokine ELISA
Cytokine levels were measured from supernatants collected from purified CD4+ or CD8+ T cell cultures established 4 wks after wt B. melitensis 16M challenge of BALB/c (18/group) and IFN-γ−/− (18/group) mice previously dosed with PBS or vaccinated with ΔznuA B. melitensis or RB51. Splenic, HNLN, and LRLN lymphocytes were pressed through a disposable cell strainer (BD Falcon) into complete medium (CM; RPMI 1640 [Invitrogen-Life Technologies, Grand Island, NY], 10% fetal bovine serum [Atlanta Biologicals, GA], 10mM HEPES buffer, 10mM nonessential amino acids, 10mM sodium pyruvate; [Invitrogen-Life Technologies, Grand Island, NY]). Cells were pelleted at 1700 x g for 10 min; resuspended in 10 ml ACK red blood cell lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) for 5 min; pelleted and washed with PBS; and then resuspended in CM and enumerated. To obtain purified CD4+ and CD8+ T cells, these were subjected to magnetic bead separation using negative isolation kits (Dynal Biotech). Purity was assessed to be >95% CD4+ or CD8+ T cells by flow cytometry analysis. Whole lymphocytes or 2.5×106 CD4+ or CD8+ T cells plus 2.5×106 mitomycin C-treated syngenic antigen-presenting cells (T cell-depleted splenic lymphocytes 66) were cultured in 24-well tissue plates at 5×106 cells/ml alone or restimulated with heat-killed RB51 (HKRB51; 1 × 109 CFUs/ml) for 3 days at 37°C. Capture ELISAs were performed on supernatants for IFN-γ, IL-17, and IL-22 using mAb pairs as previously described. 12
Flow cytometry
To measure intracellular IFN-γ and T-bet expression levels, splenic, HNLN, and LRLN lymphocytes from BALB/c mice nasally vaccinated with ΔznuA B. melitensis, RB51, or PBS were evaluated 21 days after vaccination and 28 days after wt B. melitensis 16M challenge. For intracellular IFN-γ detection, splenic and combined HNLN and LRLN lymphocytes from individual mice were stimulated in vitro with 109 CFUs of HKRB51 for 12 hr followed by 25 ng/ml PMA and 1 μg/ml ionomycin and simultaneously treated with 10 μg/ml brefeldin A (BioVision, Milpitas, CA) during the last 6 hr of culture. Cells were then stained with FITC-anti-CD4 and PE-anti-CD8 T cell mAbs (eBioscience, San Diego, CA), washed, and then were fixed with 2% paraformaldehyde. Afterwards, cells were permeabilized with 0.2% saponin, and stained with PerCP-Cy5.5-labeled anti-IFN-γ (eBioscience). For T-bet expression, fixed splenic lymphocytes permeabilized in 90% methanol and labeled with APC-anti-T-bet mAbs (eBioscience). Fluorescence was acquired on LSRFortessa flow cytometer (BD Biosciences) with BD FACSDiva software. All samples were analyzed using FlowJo software (Tree Star).
Memory T cell phenotyping was performed on splenic and lung lymphocytes from naive and individual BALB/c mice 7 and 14 days after nasal vaccination with ΔznuA B. melitensis. Splenic lymphocytes were prepared as described above. Lungs lymphocytes were isolated from minced lungs digested with Liberase™ TL Research Grade (Roche Life Science) in RPMI medium at 37°C for 45 min. 67. For detection of intracellular IFN-γ (PE), TNF-α (APC), perforin (APC), and granzyme B (PE), splenic and lungs lymphocytes from individual mice were stimulated in vitro as described above. Washed lymphocytes were stained with AmCy-anti-CD8, PE-Cy7-anti-CD4, PB-anti-CD44, APC-Cy7-anti-CD62L, and PE-Cy5-anti-CCR7 (eBioscience).
Statistical Analysis
To evaluate differences among cytokine responses and in vivo levels of infection by RB51, ΔznuA B. melitensis, or wt B. melitensis 16M, and in vivo splenic weights, an analysis of variance (ANOVA) followed by Tukey’s method was used, and results were discerned to the 95% confidence interval.
Supplementary Material
Acknowledgments
This work was supported by U. S. Public Health Grant R01 AI-093372-01, USDA-NIFA 2013-01165, and in part by funds from the University of Florida and by the Emerging Pathogens Institute.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests exist.
Supplementary information is available at Immunology & Cell Biology’s website.
References
- 1.Corbel M. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whatmore AM. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect Genet Evol. 2009;9:1168–1184. doi: 10.1016/j.meegid.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 4.Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PloS Negl Trop Dis. 2012;6:e1865. doi: 10.1371/journal.pntd.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth F, Zinsstag J, Orkhon D, Chimed-Ochir G, Hutton G, Cosivi O, et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull World Health Organ. 2003;81:867–876. [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. Progress in Brucella vaccine development. Front Biol (Beijing) 2013;8:60–77. doi: 10.1007/s11515-012-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander B, Schnurrenberger PR, Brown RR. Numbers of Brucella abortus in the placenta, umbilicus and fetal fluid of two naturally infected cows. Vet Rec. 1981;108:500. doi: 10.1136/vr.108.23.500. [DOI] [PubMed] [Google Scholar]
- 8.Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, Fretin D, et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med. 2011;102:118–131. doi: 10.1016/j.prevetmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Gorvel JP, Moreno E, Moriyon I. Is Brucella an enteric pathogen? Nat Rev Microbiol. 2009;7:250. doi: 10.1038/nrmicro2012-c1. [DOI] [PubMed] [Google Scholar]
- 10.Olsen SC. Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech. 2013;32:207–217. doi: 10.20506/rst.32.1.2201. [DOI] [PubMed] [Google Scholar]
- 11.Dabral N, Moreno-Lafont M, Sriranganathan N, Vemulapalli R. Oral immunization of mice with gamma-irradiated Brucella neotomae induces protection against intraperitoneal and intranasal challenge with virulent B. abortus 2308. PlosOne. 2014;9:e107180. doi: 10.1371/journal.pone.0107180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapp B, Skyberg JA, Yang X, Thornburg T, Walters N, Pascual DW. Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infect Immun. 2011;79:4165–4174. doi: 10.1128/IAI.05080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izadjoo MJ, Bhattacharjee AK, Paranavitana CM, Hadfield TL, Hoover DL. Oral vaccination with Brucella melitensis WR201 protects mice against intranasal challenge with virulent Brucella melitensis 16M. Infect Immun. 2004;72:4031–4039. doi: 10.1128/IAI.72.7.4031-4039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquali P, Rosanna A, Pistoia C, Petrucci P, Ciuchini F. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect Immun. 2003;71:2326–2330. doi: 10.1128/IAI.71.5.2326-2330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-González E, García-Hernàndez AL, Flores-Mejía R, López-Santiago R, Moreno-Fierros L. The protoxin Cry1Ac of Bacillus thuringienis improves the protection conferred by intranasal immunization with Brucella abortus RB51 in a mouse model. Vet Microbiol. 2015;175:382–388. doi: 10.1016/j.vetmic.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Surendran N, Sriranganathan N, Lawler H, Boyle SM, Hiltbold EM, Heid B, et al. Efficacy of vaccination strategies against intranasal challenge with Brucella abortus in BALB/c mice. Vaccine. 2011;29:2749–2755. doi: 10.1016/j.vaccine.2011.01.090. [DOI] [PubMed] [Google Scholar]
- 17.Surendran N, Sriranganathan N, Boyle SM, Hiltbolt EM, Tenpenny N, Walker M, et al. Protection to respiratory challenge of Brucella abortus strain 2308 in the lung. Vaccine. 2013;31:4103–4110. doi: 10.1016/j.vaccine.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 18.Zhan Y, Cheers C. Control of IL-12 and IFN-γ in response to live or dead bacteria by TNF and other factors. J Immunol. 1998;161:1447–1453. [PubMed] [Google Scholar]
- 19.Skyberg JA, Thornburg T, Rollins MC, Huarte E, Jutila M, Pascual DW. Murine and bovine γδ T cells enhance innate immunity against Brucella abortus infections. PlosOne. 2011;6:e21978. doi: 10.1371/journal.pone.0021978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Vemulapalli R, Zeytun A, Schurig GG. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect Immun. 2001;69:5502–5508. doi: 10.1128/IAI.69.9.5502-5508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquevich KA, Estein SM, Garcia Samartino C, Zwerdling A, Coria LM, Barrionuevo P, et al. Immunization with recombinant Brucella species outer membrane protein OMP16 or OMP19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection. Infect Immun. 2009;77:436–445. doi: 10.1128/IAI.01151-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goel D, Rajendran V, Ghosh PC, Bhatnagar R. Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against Brucella abortus 544. Vaccine. 2013;31:1231–1237. doi: 10.1016/j.vaccine.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 23.Vitry MA, Hanot Mambres D, De Trez C, Akira S, Ryffel B, Letesson JJ, et al. Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J Immunol. 2014;192:3740–3752. doi: 10.4049/jimmunol.1302561. [DOI] [PubMed] [Google Scholar]
- 24.Vitry MA, De Trez C, Goriely S, Dumoutier L, Akira S, Ryffel B, et al. Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect Immun. 2012;80:4271–4280. doi: 10.1128/IAI.00761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yingst SL, Izadjoo M, Hoover DL. CD8 knockout mice are protected from challenge by vaccination with WR201, a live attenuated mutant of Brucella melitensis. Clin Dev Immunol. 2013;2013:686919. doi: 10.1155/2013/686919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira SC, Harms JS, Banai M, Splitter GA. Recombinant Brucella abortus proteins that induce proliferation and gamma-interferon secretion by CD4+ T cells from Brucella-vaccinated mice and delayed-type hypersensitivity in sensitized guinea pigs. Cell Immunol. 1993;172:262–268. doi: 10.1006/cimm.1996.0241. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira SC, Splitter GA. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 28.Cassataro J, Velikovsky CA, de la Barrera S, Estein SM, Bruno L, Bowden R, et al. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun. 2995;73:6537–6546. doi: 10.1128/IAI.73.10.6537-6546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu XD, Chen ST, Li JY, Yu DH, Zhang Y, Cai H. An IL-15 adjuvant enhances the efficacy of a combined DNA vaccine against Brucella by increasing the CD8+ cytotoxic T cell response. Vaccine. 2010;28:2408–2415. doi: 10.1016/j.vaccine.2009.12.076. [DOI] [PubMed] [Google Scholar]
- 30.Jain S, Afley P, Dohre SK, Saxena N, Kumar S. Evaluation of immunogenicity and protective efficacy of a plasmid DNA vaccine encoding ribosomal protein L9 of Brucella abortus in BALB/c mice. Vaccine. 2014;32:4537–4542. doi: 10.1016/j.vaccine.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Rajashekara G, Glover DA, Krepps M, Splitter GA. Temporal analysis of pathogenic events in virulent and avirulent Brucella melitensis infections. Cell Microbiol. 2005;7:1459–1473. doi: 10.1111/j.1462-5822.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 32.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 33.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunol. 2001;103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarevic V, Glimcher LH. T-bet in disease. Nature Immunol. 2011;12:597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corsetti PP, de Almeida LA, Carvalho NB, Azevedo V, Silva TM, Teixeira HC, et al. Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PloS One. 2013;8:e74729. doi: 10.1371/journal.pone.0074729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasquevich KA, Ibanez AE, Coria LM, Samartino SG, Estein SM, Zwerdling A, et al. An oral vaccine based on U-Omp 19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in mice. PloS One. 2011;6:e16203. doi: 10.1371/journal.pone.0016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoover DL, Crawford RM, Van de Verg LL, Izadjoo MJ, Bhattacharjee AK, Paranavitana CM, et al. Protection of mice against brucellosis by vaccination with Brucella melitensis WR201 (16MΔpurEK) Infect Immun. 1999;67:5877–5884. doi: 10.1128/iai.67.11.5877-5884.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahl-McDonagh MM, Arenas-Gamboa AM, Ficht TA. Aerosol infection of BALB/c mice with Brucella melitensis and Brucella abortus and protective efficacy against aerosol challenge. Infect Immun. 2007;75:4923–4932. doi: 10.1128/IAI.00451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Wang J, Zganiacz A, Xing Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect Immun. 2004;72:238–246. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paixão TA, Roux CM, den Hartigh A, Sankaran-Walters S, Dandekar S, Santos R, et al. Establishment of systemic Brucella melitensis infection through the digestive tract requires urease, the type IV secretion system, and lipopolysaccharide O antigen. Infect Immun. 2009;77:4197–4208. doi: 10.1128/IAI.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackermann MR, Cheville NF, Deyoe BL. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet Pathol. 1988;25:28–35. doi: 10.1177/030098588802500104. [DOI] [PubMed] [Google Scholar]
- 42.Sangari FJ, Seoane A, Rodriguez MC, Aguero J, Garcia Lobo JM. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect Immun. 2007;75:774–780. doi: 10.1128/IAI.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plommet M. An International symposium. Texas A&M Univ. Press; Collage Station and London: 1977. Bovine Brucellosis; pp. 116–134. [Google Scholar]
- 44.Zachou K, Papamichalis PA, Dalekos GN. Severe pharyngitis in stockbreeders: an unusual presentation of brucellosis. Occup Med (Lond) 2008;58:305–307. doi: 10.1093/occmed/kqn020. [DOI] [PubMed] [Google Scholar]
- 45.von Bargen K, Gagnaire A, Arce-Gorvel V, de Bovis B, Baudimont F, Chasson L, et al. Cervical lymph nodes as a selective niche for Brucella during oral infections. PLoS One. 2015;10:e121790. doi: 10.1371/journal.pone.0121790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2001;51:21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 47.Skyberg JA, Thornburg T, Kochetkova I, Layton W, Callis G, Rollins MF, et al. IFN-γ deficient mice develop IL-1-dependent cutaneous and musculoskeletal inflammation during experimental brucellosis. J Leukoc Biol. 2012;92:375–387. doi: 10.1189/jlb.1211626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 51.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 52.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6:972–984. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells. J Immunol. 2008;180:4109–4115. doi: 10.4049/jimmunol.180.6.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong QW, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 55.Graham AC, Carr KD, Sieve AN, Indramohan M, Break TJ, Berg RE. IL-22 production is regulated by IL-23 during Listeria monocytogenes infection but is not required for bacterial clearance or tissue protection. PloS One. 2011;6:e17171. doi: 10.1371/journal.pone.0017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paranavitana C, Zelazowska E, DaSilva L, Pittman PR, Nikolich M. Th17 cytokines in recall responses against Francisella tularensis in humans. J Interferon Cytokine Res. 2010;30:471–476. doi: 10.1089/jir.2009.0108. [DOI] [PubMed] [Google Scholar]
- 57.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhiman R, Periasamy S, Barnes PF, Garg Jaiswal A, Paidipally P, Barnes AB, et al. NK1.1+ cells and IL-22 regulate vaccine-induced protective immunity against challenge with Mycobacterium tuberculosis. J Immunol. 2012;189:897–905. doi: 10.4049/jimmunol.1102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Y, Gao X, Yang J, Shekhar S, Wang S, Fan Y, et al. Interleukin-22 promotes T helper 1 (Th1)/Th17 immunity in chlamydial lung infection. Mol Med. 2014;20:109–119. doi: 10.2119/molmed.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adone R, Francia M, Pistoia C, Petrucci P, Pesciaroli M, Pasquali P. Protective role of antibodies induced by Brucella melitensis B115 against B. melitensis and Brucella abortus infections in mice. Vaccine. 2012;30:3992–3995. doi: 10.1016/j.vaccine.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Durward M, Radhakrishnan G, Harms J, Bareiss C, Magnani D, Splitter GA. Active evasion of CTL mediated killing and low quality responding CD8+ T cells contribute to persistence of brucellosis. Plos One. 2012;7:e34925. doi: 10.1371/journal.pone.0034925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durward-Diioia M, Harms J, Khan M, Hall C, Smith JA, Splitter GA. CD8+ T cell exhaustion, suppressed gamma interferon production, and delayed memory response induced by chronic Brucella melitensis infection. Infect Immun. 2015;83:4759–4771. doi: 10.1128/IAI.01184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 64.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, Lefrançois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unsoeld H, Pircher H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J Virol. 2005;79:4510–4513. doi: 10.1128/JVI.79.7.4510-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walters N, Trunkle T, Sura M, Pascual DW. Enhanced immunoglobulin A response and protection against Salmonella enterica serovar Typhimurium in the absence of the substance P receptor. Infect Immun. 2005;73:317–324. doi: 10.1128/IAI.73.1.317-324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abboud G, Tahiliani V, Desai P, Varkoly K, Driver J, Hutchinson TE, Salek-Ardakani S. Natural killer cells and Innate IFN-γ participate in host defense against respiratory vaccinia virus infection. J Virol. 2015 doi: 10.1128/JVI.01894-15. pii: JVI.01894–15 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.