Abstract
The high resolution magnetic resonance (MR) brain images contain some non-brain tissues such as skin, fat, muscle, neck, and eye balls compared to the functional images namely positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI) which usually contain relatively less non-brain tissues. The presence of these non-brain tissues is considered as a major obstacle for automatic brain image segmentation and analysis techniques. Therefore, quantitative morphometric studies of MR brain images often require a preliminary processing to isolate the brain from extra-cranial or non-brain tissues, commonly referred to as skull stripping. This paper describes the available methods on skull stripping and an exploratory review of recent literature on the existing skull stripping methods.
Keywords: Skull stripping, Brain segmentation, Brain extraction, MRI brain, Brain structure segmentation
Introduction
The application of digital image processing in medicine has increased the scope of diagnosis due to better visualization and quantitative analysis. The dawn of digital age has empowered medical imaging in such a way that computer-based medical image processing techniques have gained popularity in the past few decades. The rapid progress witnessed in computerized medical image analysis and computer-aided diagnosis has promoted many imaging techniques to find applications in medical image processing. Among the various imaging techniques, MRI (magnetic resonance image) is the most widely used imaging technique in the medical field. It is a noninvasive, nondestructive, flexible imaging tool that does not require ionizing radiation such as X-rays. It reveals information about the anatomy of human soft tissue that is not externally visible [1]. MRI has a high spatial resolution and hence provides more information on the anatomical structure, allowing quantitative pathological or clinical studies.
MR Brain Images
MRI is particularly suitable for brain studies, because it can image both interior and exterior brain structures with a high degree of anatomical details, using which even the minute changes in these structures that develop over a time period can be detected. MRI scans can produce cross-sectional images in any direction from top to bottom, side to side, or front to back. Therefore, the three dimensional MR brain images have become more popular in medical applications and are being used for research related to diagnosis, treatment, surgical planning, and image-guided surgeries.
There are primarily three types of MR brain images, T1-weighted, T2-weighted, and PD-weighted, which focus on different contrast characteristics of the brain tissues [2]. MR brain images have some advantages over other imaging modalities. MR images of the brain and other cranial structures are clearer and more detailed than the other imaging methods. These details make MRI an invaluable tool in early diagnosis and evaluation of many brain-related deceases. MRI has the ability to image the brain in any plane without physically moving the patient whereas CT scans are limited to one plane, the axial plane [3, 4].
The brain MRI is widely used to diagnose the brain diseases such as acoustic neuroma, Alzheimer’s disease, amyotrophic lateral sclerosis, aneurysm in the brain, arteriogram, arteriovenous malformation-cerebral, blood clots, brain abscess, brain tumor-children, central pontine myelinolysis, cerebral amyloid angiopathy, chronic subdural hematoma, Cushings disease, dementia, dementia due to metabolic causes, diabetes insipidus central, Huntington’s disease, hypopituitarism, melanoma of the eye, Menieres disease, metastatic brain tumor, multi-infarct dementia, multiple sclerosis, myelin, normal pressure hydrocephalus (NPH), optic glioma, partial (focal) seizure, petitmal seizure, pituitary tumor, prolactinoma, Reye syndrome, sinusitis, stroke, subdural hematoma, TMJ disorders, toxoplasmosis, Wernicke–Korsakoff syndrome, and Wilson’s disease [5].
Skull Stripping of MR Brain Images
The MRI system produces brain image as 3D volumetric data expressed as a stack of two-dimensional slices and it is necessary to use computer-aided tool to explore the information contained in these brain slices for various brain image applications such as volumetric analysis, study of anatomical structure, localization of pathology, diagnosis, treatment planning, surgical planning, computer-integrated surgery, construction of anatomical models, 3D visualization, and research.
Several image processing methods are required before the brain images can be explored. Image processing covers various techniques that are applicable to a wide range of applications, among which segmentation is an essential and important process in medical image processing and analysis [6]. There are number of algorithms being proposed in the field of medical image segmentation [7]. These techniques are broadly classified into four categories: methods based on gray level features, methods based on texture features, model-based segmentation methods, and atlas-based segmentation methods [8–13].
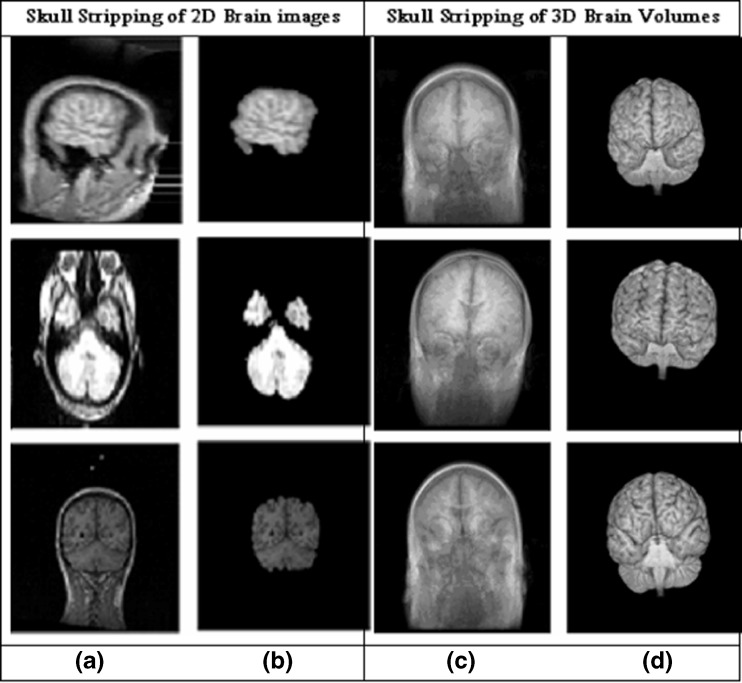
The quantitative morphometric studies of MR brain images often require a preliminary processing to isolate the brain from extra-cranial or non-brain tissues from MRI head scans, commonly referred to as skull stripping [14–17]. Because the brain images that have preprocessed with automatic skull stripping eventually lead to get better segmentation of different brain regions which results for accurate diagnosis of various brain-related diseases. The brain regions must be skull-stripped prior to the application of other image processing algorithms such as image registration and warping [18], brain volumetric measurement [19], inhomogeneity correction [20], tissue classification [21], analysis of cortical structure [22], cortical surface reconstruction [23], cortical thickness estimation [24], identification of brain parts [25], multiple sclerosis analysis [26], Alzheimer’s disease [27], schizophrenia [28], and monitoring the development or aging of the brain [29]. Some skull stripping results of 2D brain slices and 3D brain volumes are illustrated in Fig. 1.
Fig. 1.
Skull stripping results of 2D and 3D brain volume. a Original 2D brain slice. b Skull stripped 2D brain slice. c Original brain volume. d Skull stripped brain volume
Moreover, skull stripping being a preliminary step, designed to eliminate non-brain tissues from MR brain images for many clinical applications and analyses, its accuracy and speed are considered as the key factors in the brain image segmentation and analysis. However, the accurate and automated skull stripping methods help to improve the speed and accuracy of prognostic and diagnostic procedures in medical applications.
A number of automated skull stripping algorithms are available in the literature. Several comparative studies have also been carried out on the existing skull stripping methods to analyze their performance using the commonly available datasets. Each skull stripping method has their own merits and limitations. The objective of this paper is to present the current methods in MRI skull stripping, their scope and limitations. Remaining parts of the paper is organized as follows: in section 2, the classification and review on skull stripping methods and their challenges are given. The conclusion is given in section 3.
Skull Stripping Methods
Skull stripping methods which are available in the literature are broadly classified into five categories: mathematical morphology-based methods, intensity-based methods, deformable surface-based methods, atlas-based methods, and hybrid methods.
Morphology-Based Methods
Generally, these methods use the morphological erosion and dilation operations to separate the skull from the brain region. These methods require a combination of thresholding and edge detection methods to find the initial ROI (region of interest). The main rawbacks of these methods are that they often depend on many parameters such as size and shape of the structural element for morphological operation. These parameters are fixed by empirical experimentation; the value on these parameters directly influences the final output of these methods.
The method, automatic detection of brain contours in MRI datasets developed by Brummer et al. [30] is one of the first commonly used methods for skull stripping. It consists of histogram-based thresholding and morphological operations. Based on the brain anatomical knowledge, it discriminates between the desired and undesired structures. This method is implemented using a sequence of conventional and novel morphological operations, using 2D and 3D operations. As a final step, it performs overlap tests on candidates brain regions of interest in the neighboring slice images to propagate coherent 2D brain masks through the third dimension. However, existing methods that use mathematical morphology are sometimes sensitive to small data variations and it is difficult to find the optimum morphology size for separating the brain tissues from the non-brain tissues [31, 32]. A similar method proposed by Tsai et al. [33] is based on histogram analysis and morphological operations.
To detect anatomical brain boundaries, Sandor and Leahy [34] used 3D Marr–Hildreth edge detector and morphological operation as a preprocessing procedure to find and label the cortical surface in three-dimensional MR brain images. Exbrain [35] is a fully automatic algorithm that segments T1-weighted MR head scans. It uses 3D morphological operations and connected component analysis. Exbrain chooses a threshold and increments it by unit steps until there is a significant change in the volume found after a set of morphological and connected component operations. It works on normal as well as certain types of abnormal brain slices. It is fully 3D and therefore independent of scan orientation.
Brain surface extraction (BSE) for T1 and T2-weighted brain images proposed by Shattuck et al. [36] is an edge-based method that employs anisotropic diffusion filtering. Edge detection is implemented using a 2D Marr–Hildreth operator, employing low-pass filtering with a Gaussian kernel and localization of zero crossings in the Laplacian of the filtered image. BSE breaks connections between the brain and the other tissues in the head using a morphological erosion operation. After identifying the brain using a connected component operation, BSE applies a corresponding dilation operation to undo the effects of the erosion. As a final step, BSE applies a morphological closing operation that fills small pits and holes that may occur in the brain surface. BSE requires fixed parameters such as diffusion iteration, diffusion constant, edge constant, and erosion size. BSE is based on an edge detecting algorithm, sometimes it failed to work with poor contrast images.
A method based on seed growth and threshold techniques for automatic segmentation of brain MRI is employed by Shanthi and Sasikumar [37]. A method described by Mikheev et al. [38] is an automatic segmentation of brain from T1-weighted MR brain images. It uses an intensity threshold followed by removal of narrow connections using their Bridge Burner method, though, the Bridge Burner is not a skull stripping algorithm. However, the algorithm can be modified to produce an output similar to the other skull stripping methods by morphologically closing the output and then filling the holes in the mask.
Park and Lee [39] developed a skull stripping method for T1-weighted MR brain images based on 2D region growing method. It aims to automatically detect two seed regions of the brain and non-brain by using a mask produced by morphological operations. Then, the seed regions were expanded using 2D region growing algorithm, based on the general brain anatomy information.
Skull stripping MR brain images using anisotropic diffusion filtering and morphological processing is described by Gao and Xie [40]. Automatic skull stripping using image contour and a method to segment the brain from MRI human head scans were developed in [41, 42], which uses morphological operations and connected component analysis to identify the brain in T1-weighted MR brain images.
Brain extraction algorithm (BEA) [43] is a brain extraction method that uses diffusion, morphological operations and connected component analysis to extract the brain region in T2-weighted axial slices. Brain extraction method for T1-weighted MR brain images based on morphological operation and run-length scheme has also been proposed in [44].
The simple paradigm for extra-cerebral tissue removal (SPECTRE) is based on a watershed principle and it combines elastic registration, tissue segmentation, and morphological operators as described by Carass et al. [45], for T1-weighted brain images.
Intensity-Based Methods
Intensity-based methods use the intensity values of the image pixel to separate the brain and non-brain region. For example, histogram-based method, edge-based method, and region growing methods are intensity-based methods. These methods rely upon modeling the intensity distribution function to classify the brain and non-brain tissues in the brain images. The main limitation of these methods is they are sensitive to intensity bias due to various imperfection introduced in MRI head scan images such as low resolution, high level of noise, low contrast, and the presence of various imaging artifacts.
3dIntracranial [46, 47] is an automatic segmentation of intracranial regions in T1 and T2-weighted MRI brain images. In this, a down-hill simplex method is used to estimate means, standard deviations, and weights of presumed gray matter (GM), white matter (WM), and background compartments. From these estimated values, a probability density function (PDF) is derived to set upper and lower signal intensity bounds. These upper and lower bounds are set to exclude non-brain voxels. Then, the connected component analysis is carried out slice-by-slice to identify the brain, followed by a 3D envelope process over all the slices. Finally, a neighborhood analysis is performed on each voxel to include or exclude the misclassified voxels. In this technique, nine parameters are required to be estimated for each image. Poor results are obtained if the estimation and initialization are not done properly [31]. A connectivity-based threshold algorithm to extract the brain regions of 3D sagittal MR skull stripping was developed by [48].
Dawant et al. [49] developed an automatic method for 3D segmentation of internal structures of the head in MR images using a combination of similarity and free-form transformation. An adaptive fuzzy segmentation algorithm for 3D magnetic resonance image was employed in Pham and Prince [50].
The watershed algorithm (WAT) proposed by Hahn and Peitgen [51] is intensity-based approach for T1-weighted images, which relies on a 3D algorithm with pre-flooding performed on the intensity inverted image that operates under the assumption of white matter connectivity and segments the image into brain and non-brain components. But, it often produces over-segmentation and is sensitive to noise present in the image. It may fail to remove dura, skull, and various non-brain structures in the neck or eye area [52].
Statistical parameter mapping version 2 (SPM2) [53] does not explicitly generate a brain mask; however, it can be obtained from the sum of the GM and WM compartments after tissue segmentation process in T1-weighted brain images. The SPM5 [54], an enhanced version of SPM2 [53], like SPM2, does not explicitly generate a brain mask. It uses a probabilistic brain tissue segmentation method. This model combines image registration, tissue classification, and bias correction. The output images are probabilistic images per tissue class. The nonuniformity corrected T1-weighted image and the mask were given as inputs.
Zu et al. [55] proposed a skull stripping algorithm that consists of foreground and background thresholding, disconnection of the T1-weighted brain from the skull and head tissues by morphological operations, and removal of residue fragments for segmenting the brain region from MR head scans. Grau et al. [52] proposed a method which is an improvement over the WAT [51] which enables the use of different prior information based on the probability calculation instead of the usual gradient calculation and it combines the watershed transform and atlas registration using markers.
Graph cuts (GCUT) is a skull stripping method for T1-weighted images proposed by Sadananthan et al. [56] relies on graph-theoretic image segmentation techniques to position the cuts which serve to isolate and remove dura. First, it finds a threshold between the intensities of the GM and the CSF and uses it to generate a preliminary binary mask which ideally includes the brain, the skull, and some thin connections between them. Then, the graph cuts can be used to find a connected submask that minimizes the ratio between the cost of its boundary and its volume. This can be seen as a simple shape prior. This submask is post-processed to obtain the final segmentation. GCUT is usually quite accurate but sometimes makes large mistakes by following a wrong edge [43].
Somasundaram and Kalavathi [57] have developed a simple skull stripping method based on 2D region growing method. Segmentation in magnetic resonance human head scans using multi-seeded region growing method has been developed in [58]; it uses multiple seed points to extract the brain from T1, T2, and PD-weighted brain images. Brain asymmetry is computed on the segmented brain images in [59].
Deformable Surface-Based Method
Skull stripping methods based upon deformation models typically evolve and deform an active contour to fit the brain surface, which is identified using selected images characteristics. Active contour is a self-regulating dynamic curve that moves under the influence of energy functional towards the desired object boundaries. The basic idea of any active contour model starts with an initial closed curve which is iteratively shrunk or expanded with respect to the boundary of the object by satisfying some constraints associated with the image. The shrink/expand operations are referred to as curve evolution. These methods are dependent on the location of the initial curve and the image gradient to stop the evolving curve on the object boundary. The advantage of these methods is they can simultaneously detect both the interior and exterior boundaries of an object and however these methods are sensitive to noise. The active contour model uses the level set theory which provides more flexibility and convenience in its implementation. In general, deformable models have the potential to produce more robust and accurate skull stripping results than methods using edge detection and threshold classification.
Aboutanos et al. [60] evolved a 2D contour to find the brain border in T1-weighted image by maximizing its corresponding one-dimensional (1D) optimization problem, which was obtained via geometrical transformation from a 2D contour using dynamic programming techniques. The 1D optimization problem was described by a cost function that consists of intensity value, morphology, gradient, the moving speed of the contour, and the smoothness of the contour. Zeng et al. [61] proposed a system of two level set equations whose zero level curves represented their inner and outer boundaries of the gray matter of the cortex. Each level set equation was driven towards the inner or outer boundary by a force term determined by the intensity distribution of brain tissues (i.e., cerebrospinal fluid (CSF), WM, and GM). The two level set equations were further related to each other by constraining the distance between the inner and outer boundaries (i.e., the thickness of gray matter).
Suri [62] devised an active contour algorithm that uses the level set methods to evolve the active contour. It uses a fuzzy membership function to classify brain images into four components: WM, GM, CSF, and background, then used a gradient detector and a deformable model to evolve an active contour to fit the surface between the CSF and GM. Segmentation of brain from 3D MR images using level sets and dense registration proposed by Baillard et al. [63] integrates 3D segmentation and 3D registration processes. The segmentation process is based on the level set formation, in which the speed term was determined by the curvature of the evolving curve and by a sign function that indicates whether to include or exclude a pixel through which the curve passed.
Brain extraction tool (BET) developed by Smith [31] employs a deformable model that evolves to fit the brains surface by the application of a set of locally adaptive model forces. BET makes an intensity-based estimation of the brain and non-brain threshold, determines the center of gravity of the head, defines an initial sphere based on the center of gravity, and expands the tessellated sphere until it reaches the brain edge. It has two user-adjustable parameters, fractional intensity threshold and threshold gradient. BET produces the brain volume smoother than the other methods and often includes additional non-brain tissues. This algorithm was tested with T1 and T2-weighted images. However, BET has failed to extract the brain region in the bottom axial slices because the head scan included much neck portion, for these slices center of gravity of the volume was outside the brain, thus failed to extract the brain regions [64].
BET2 [65] is based on BET [31], which finds the brain boundary in the given MR brain image. BET attempts to find external skull surface voxels, but does not fit a surface to the brain boundary and the resulting crude skull image contains a relatively large number of false negatives and positives. BET2 uses high-resolution T1 and T2-weighted images, and it ideally requires a pair of T1 and T2-weighted images, preferably of 2 mm resolution. First, the brain surface in T1 is found using the original BET algorithm. Then T2 is registered to the T1 image.
3dSkullStrip [66], a part of the AFNI (analysis of functional neuro images) package, is a modified version of BET [31] for skull stripping the T1-weighted brain images based on the spherical surface expansion paradigm. It includes modifications for avoiding the eyes and ventricles. Statistical shape model for automatic skull stripping of T1-weighted brain images by Lao et al. [67] is a surface model of the brain boundary and is hierarchically represented by a set of overlapping surface patches, each of which has elastic properties and deformation range that is learned from a training set. The deformation of this model is hierarchical which adds robustness to local minima. Moreover, the deformation of the model is constrained and guided by global shape statistics. The model is deformed to the brain boundary by a procedure that matches the local image structure and evaluates the similarity in the whole patch rather than on a single vertex.
Model-based level set method (MLS) by Zhuang et al. [32] is based on active curve to remove the skull and intracranial tissues surrounding the brain in MR brain images. It was developed for controlling the evolution of the zero level curve that is implicitly embedded in the level set function. The evolution of the curve was controlled using two parameters in the level set equation, whose values represented the forces that determined the speed of the evolving curve. The first force was derived from the mean curvature of the curve and the second was designed to model the intensity characteristics of the cortex in MR images. The combination of these forces in a level set framework pushed or pulled the curve towards the brain surface. The MLS algorithm was tested with T1 and T2-weighted brain volumes. John et al. [68] also proposed a 3D skull stripping method based on mathematical morphological operations along with statistical techniques.
Yunjie et al. [69] developed a fast automatic skull stripping method based on an adaptive gauss mixture model and a 3D mathematical morphology method. The gauss mixture model is used to classify the brain tissues and to estimate the bias field in the brain tissues. The 3D mathematical morphology is used for skull stripping other tissues. A method based on an implicit deformable model which is described by radial basis functions is introduced by Liu et al. [70] for skull stripping.
A method that uses watershed segmentation, Gaussian mixture model clustering and a modification of BET is employed [71] to segment MR images of premature infant brains. Tao and Chang [72] developed a deformable surface-based algorithm that first analyzes the intensity of the entire image to find an approximate centroid of the brain and then it initializes an ellipsoidal surface around it. It uses tissue classification and bias field estimation to compute external force for surface deformation and relies on the internal force, derived from local surface patch to maintain the topology and smoothness of the surface. This algorithm was tested with T1 and T2-weighted brain images.
A skull stripping method using Chan–Vese active contour method has been developed in [73]. Hwang et al. [74] has introduced a skull stripping method using fast 3D level set method and a refinement process. This method uses a speedup operator on the conventional 3D level set method in order to accelerate the level set evolution and the accuracy of brain extraction is improved by adopting a refinement process.
An automated and simple method for brain MR image extraction proposed by Zhang et al. [75] uses an improved geometric active contour model to solve the boundary leakage problem in T1-weighted MR brain images. The method defines the initial function as a binary level set function to improve the computational efficiency. A novel skull stripping method for T1-weighted MRI human head scan images is employed by Somasundaram and Kalavathi [76]. Simplex mesh and histogram analysis skull stripping (SMHASS) described by Galdames et al. [77] is a brain extraction method for T1-weighted images based on deformable models and histogram analysis. In this method, a pre-segmentation step is used to find the optimal starting point for the deformation and is based on thresholds and morphological operators. Threshold values for this method are computed using comparisons with an atlas. The deformable model is based on a simplex mesh and its deformation is controlled by the image local gray levels and gray level statistical model constructed on the pre-segmentation. A contour-based brain segmentation method [78] uses two stage brain segmentation methods to segment the brain from T1, T2, and PD-weighted brain images.
Atlas/Template-Based Methods
Atlas/template-based method relies on fitting an atlas/template on the MRI brain image to separate the brain from the skull. It has an ability to separate brain and non-brain when no well-defined relation between regions and pixel intensities in the brain image. These methods vary in how many templates they use in distinguishing brain regions and also how they apply these atlases.
Dale et al. [79] described a skull stripping method as a preprocessing step for cortical surface reconstruction process. This procedure takes an intensity-normalized image and deforms a tessellated ellipsoidal template into the shape of the inner surface of the skull. The deformation process is driven by two kinds of forces: (i) an MRI-based force, designed to drive the template outward from the brain and (ii) a curvature reducing force, enforcing a smoothness constraint on the deformed template. This latter force can be seen as an encoding a priori knowledge about the smoothness of the inner surface of the skull. Wang et al. [80] study a method with initial skull stripping by co-registration of an atlas, followed by a refinement phase with a surface deformation scheme that is guided by prior information. Active shape model-based automated skull stripping method from infantile brain MR images has been described in Kobashi et al. [81]. Recently, Mahapatra [82] considered shaper prior information along with graph cuts for neonatal brain MRI.
The multi-atlas propagation and segmentation (MAPS) method presented by Leung et al. [83] generates brain segmentation by combining many segmentations performed by atlas registration.
BEaST is a brain extraction method based on nonlocal segmentation technique by Eskildsen et al. [84]. In this, a nonlocal segmentation is embedded in a multi-resolution framework. A library of 80 priors is semi-automatically constructed from the National Institutes of Health sponsored MRI study of normal brain development, the International Consortium for Brain Mapping, and the Alzheimer’s disease Neuroimaging Initiative databases.
Hybrid Methods
It combines more than one skull stripping results from different approaches in order to account for shortcomings of individual approaches. Many approaches that could be classified distinctly in one of the previous groups can be combined to integrate some feature for other method to produce accurate result.
Segmentation of brain tissue from magnetic resonance images developed by Kapur et al. [85] uses a combination of three existing techniques from the computer vision literature: expectation/maximization segmentation, binary mathematical morphology, and active contour models for segmenting the brain tissues.
A method by SFU (Simon Fraser University) is a fully automatic MRI brain segmentation algorithm developed by Atkins and Mackiewich [86]. It uses an integrated approach which employs image processing techniques based on anisotropic filters, snake contouring technique, and a priori knowledge, which are used to remove the eyes in MR brain images. It was originally created for PD/T2-weighted axially acquired multi-spectral datasets. Enhancements were made to the ImageJ [87] plugin version of this algorithm to handle coronal T1 datasets. This method is modeled for normal subjects and it failed to extract brain containing abnormal anatomic structures. It requires complex contouring algorithm to produce the results. The algorithm fails on the dataset with high density noise and poor contrast resolution [35].
Bauer et al. [88] used atlas-based geodesic active contour segmentation with level set based algorithm implementation in ITK for skull stripping in T1-weighted, T1-contrast, T2-weighted, T2-flair, and CT images.
McStrip (Minneapolis Consensus Stripping) for T1-weighted images developed by Rehm et al. [89] is an automatic hybrid algorithm implemented in Interactive Data Language (IDL) that incorporates BSE [36] and requires no user intervention; it relies on warping to a template, intensity thresholding, and edge detection procedures. McStrip is initialized with a warp mask using automated image registration (AIR) [90] and dilates the AIR mask to form a coarse mask. It then estimates the threshold for brain and non-brain tissues based on the intensity histogram and automatically adjusts this threshold to produce a threshold mask. The volume of tissues within the threshold mask determines the choice of the BSE mask from among a suite of 15 masks computed using parameter combinations spanning both smoothing and edge parameters. The final McStrip mask is a union of the threshold and BSE masks after void filling and smoothing.
Hybrid watershed algorithm (HWA) [91] is solely based on image intensity. It combines watershed algorithm [51] and deformable surface model [79]. This algorithm operates under the assumption of the WM connectivity. The algorithm first localizes a single WM voxel in a T1-weighted MR image and uses it to create a global minimum in the WM, before applying a watershed algorithm with a pre-flooding height. Then, the watershed algorithm builds an initial estimate of the brain volume, based on the 3D connectivity of the WM and segments the image into brain and non-brain components. A deformable surface model is then applied to locate the boundary of the brain in the image.
In order to overcome some of the weak points of the individual methods, Rex et al. [92] combined multiple results of various skull stripping techniques including BSE [36], BET [31], 3dintracranial [47], and MRI watershed techniques [79] to segment the brain region from T1-weighted image. A similar approach was undertaken in [93] to learn exemplars and combine with BSE [36], BET [31].
Huang et al. [94] proposed a method to extract brain from T1-weighted brain images. It is a hybrid method combined with the expectation maximization (EM) algorithm with a preprocessing and post-processing techniques. It is based on mathematical morphology and connected component analysis and finds the brain border using geodesic active contours.
Carass et al. [95] developed a skull stripping method that combines elastic registration, tissue segmentation, and morphological techniques into a fast hybrid method for extracting the brain in T1-weighted images. ROBEX [96] is a RObust, learning-based Brain EXtraction system. This method combines the discriminative and generative model to achieve the final results. The discriminative model is a random forest classifier, trained to detect the brain boundary and the generative model is a point distribution model that ensures that the result is plausible. When a new image is presented to the system, the generative model explores it, to find the contour with highest likelihood in accordance with the discriminative model. As the generic target shape is not perfectly represented by the generative model, the contour is refined using graph cuts to obtain the final segmentation.
Comparative Studies on Skull Stripping Methods
Several comparative studies [14, 97–100] have been carried out on some of the existing skull stripping methods. Lee et al. [97] compared the performance of the two automated methods (BET [31] and BSE [36]) and two semi-automated methods (ANALYZE 4.0 [101] and modified region growing (mRG) proposed by Yoon et al. [102]). Although a fully automated method can produce good results, it requires additional manual intervention either to adjust the initial parameters or to edit the final result. This nevertheless can be mitigated by fixing the parameters and post-processing depending on the dataset or imaging modality. In contrast, the semi-automated methods had produced accurate results, but they were time consuming and prone to operator bias. Therefore, Lee et al. [97] suggested that fully automated skull stripping method can be used as preprocessing method for various brain image segmentation and analysis methods as it takes less effort.
A study by Boesen et al. [98] compared the McStrip [89] method with SPM2 [53], BET [31], and BSE [36] using T1-weighted MR brain volumes. McStrip is a hybrid algorithm based on intensity thresholding, nonlinear warping, and edge detection. It consistently outperformed SPM2 [53], BET [31], and BSE, although BET [31] and BSE outperformed McStrip on the processing time. A comparative study on four skull stripping methods, BET [31], 3dIntracranial [46], HWA [91], and BSE [36] was carried out by Fennema-Notestine et al. [14] to investigate the effect of bias correction, type of image set, and local anatomy of brain slice and diagnosis group. Their findings suggested that bias correction through the use of nonparametric nonuniform intensity normalization (N3) [103] did not significantly improve the performance of the methods. HWA [91] may remove substantial non-brain tissue from the difficult face and neck regions, carefully preserving the brain, although the outcome often would benefit from further stripping of other non-brain regions; BSE [36] in contrast, more clearly reaches the surface of the brain, and but for few cases, some brain tissue may be removed. 3dIntracranial and BET [31] often left large non-brain regions and sometimes removed brain regions, particularly in the older populations.
Hartley et al. [99] compared two automated brain extraction methods BET [31] and BSE [36] to evaluate whether method accuracy is associated with the subject demographic and health characteristics. Both methods tend to produce under-segmentation and over-segmentation thereby producing both positive and negative errors. The study further showed that these methods are not entirely insensitive to subject characteristics.
Segmentation validation engine (SVE) [100] developed a web-based resource for evaluating the performance of skull stripping in T1-weighted MR brain images. The resource provides both the data to be segmented and an online application that performs a validation study on the data. It allows the users to download the test dataset which is segmented by an arbitrary method.
A comparative study among HWA [91], BET [31], and BSE [36] was performed by Shattuck et al. [100] to evaluate the performance of their developed framework. Their results showed that with proper parameter selection, all the three algorithms can achieve satisfactory skull stripping on the test dataset. A comparative study on various methods [14, 97–100] revealed that HWA [91] has the highest sensitivity in general but the lowest specificity. HWA [91] seems to be more robust to the change of parameters than other methods. BSE [36] had high specificity than the other methods, while BET [31] always under-segments by including more non-brain tissues and McStrip [89] out performs the other methods. However, most of the existing skull stripping methods are applicable to T1-weighted MR brain images. Moreover, none of these existing methods give satisfactory performance when evaluated with large-scale dataset of a wide range of scan types (T1, T2, and PD) and all types of scan orientations (axial, sagittal, and coronal). This is because of the complexity and variations in the human brain structures, presence of image noise, image contrast, and image artifacts [104–106]. The following table (Table 1) summarizes the techniques used in the existing skull stripping methods along with their input type and limitations.
Table 1.
Summary of the existing skull stripping methods
| Method | Techniques used | Input MR brain image type | Limitation |
|---|---|---|---|
| (i) Mathematical morphology-based methods | |||
| Brummer et al. [30] | Histogram-based thresholding and morphological operations. | T1-weighted coronal and sagittal brain images | Sensitive to small data variations and it is difficult to find the optimum morphology size for separating the brain tissues from the non-brain tissues. |
| Tsai et al. [33] | Histogram analysis and morphological operations. | T1-weighted images | Do not produce good skull stripping result when the image is affected with various image artifacts. |
| Exbrain [35] | 3D morphological operations and connected component analysis. | 3D T1-weighted images | Segmentation performance depends on the initial threshold value. |
| BSE [36] | Anisotropic diffusion filtering, edge detection using a 2D Marr-Hildreth operator. | T1 and T2-weighted images | Sometimes dura matter may also be included in the brain mask and therefore Marr–Hildreth edge detector cannot find a clear brain boundary. |
| Shanthi and Sasikumar [37] | Seed growth and threshold techniques. | T1-weighted images | Proper threshold estimation is required. |
| Mikheev et al. [38] | Intensity threshold, removal of narrow connections using Bridge Burner method. | T1-weighted images | Sometimes over-segmentation/under segmentation the brain images which are affected by intensity bias. |
| Park and Lee [39] | 2D region growing method. | T1-weighted images | Output depends on the proper selection of two seed points for brain and non-brain regions. |
| Gao and Xie [40] | Anisotropic diffusion filtering, morphological processing. | T1-weighted images | Output incorrect brain boundary when the image has high noise. |
| Somasundaram. and Kalavathi [41, 42] | Morphological operations and connected component analysis. | T1-weighted images | Results with over-segmentation/under segmentation for intensity imhomogeneity images. |
| BEA [44, 82] | Diffusion, morphological operations, and connected component analysis. | T1 and T2-weighted images | Morphological operation sometimes may fail to separate the brain and non-brain when the brain image has similar intensity profile. |
| SPECTRE [45] | Watershed principle and it combines elastic registration, tissue segmentation, and morphological operators. | T1-weighted images | This method always produces big masks which may include some non-brain tissues. |
| (ii) Intensity-based methods | |||
| 3dIntracranial [46, 47] | Down-hill simplex method, probability density function (PDF), connected component analysis and neighborhood analysis. | T1 and T2-weighted images | Requires accurate estimation and initialization of initial parameter for better result. |
| Huh et al. [48] | Anatomical information-based method and connectivity-based threshold algorithm. | 3D sagittal oriented images | This method is sensitive to image scanning parameters and image artifacts, such as noise and intensity imhomogeneity. |
| Dawant et al. [49] | Combination of a global similarity transformation and local free-form deformations. | T1-weighted images | It cannot be applied to pathological brain images such as tumors because the atlas-based method needs gross anatomical structure, whereas the tumors may dramatically alter the morphology of the brain. |
| Pham and Prince [50] | Adaptive fuzzy segmentation algorithm (AFCM). | 3D T2 and PD-weighted images | AFCM looks for cluster of the same shape and size and requires accurate initialization of some parameters. |
| WAT [51] | Intensity-based approach. | T1-weighted images | Does not work well for intensity-biased images. It produces better result when the intensity level of GM is as bright as CSF but not brighter than WM. |
| SPM2 [53] | Tissue segmentation using classification and the output is sum of the GM and WM compartments. | T1-weighted images | Failed to produce good segmentation on abnormal images. |
| SPM5, an enhanced version of SPM2 [54] | Image registration, tissue classification, and bias correction | T1-weighted images | Complex procedure is required to find the optimal initial parameters. |
| Zu et al. [55] | Foreground and background thresholding and morphological operations. | T1-weighted images | It may over-segment/under segment the brain due to morphological operations. |
| Grau et al. [52] | Improvement over the WAT [51], combines the watershed transform and atlas registration using markers. | T1 and T2-weighted images | The segmentation results influenced by the selection of markers image. |
| GCUT [56] | Graph-theoretic image segmentation techniques. | T1-weighted images | GCUT sometimes fail to produce accurate segmentation by following a wrong edge. |
| Somasundaram and Kalavathi [57–59] | 2D region growing and multi-seeded 2D region growing method. | T1, T2, and PD-weighted images | Do not work well for the brain images with large intensity bias. |
| ANALYZE 4.0 [101] & the latest version ANALYZE 12.0 [107] | Geometric operations, mathematical processing, histogram manipulation, image filtering and enhancement. Custom image filter creation FTT, convolution and deconvolution correction |
MRI and CT images | Semi-automatic tool |
| mRG [102] | Modified region growing | T1-weighted images | Semi-automatic method |
| (iii) Deformable surface-based method | |||
| Aboutanos et al. [60] | 2D contour geometrical transformation using dynamic programming techniques. | T1-weighted image | Failed to produce better result for pathological images. |
| Zeng et al. [61] | Used deformation model with two level set equations. | T1-weighted image | Requires more processing time to evolve the contours. |
| Suri [62] | Region-based geometric snake active contour algorithm and fuzzy membership function. | T1-weighted and synthetic brain images | Requires accurate setting of initial parameters. |
| Baillard et al. [63] | Level sets and dense 3D registration. | 3D T1-weighted images | Need more computation time, uses complex level set and registration process. |
| BET [31] | Set of locally adaptive model forces and thresholding. | T1-weighted images | Failed to extract the brain region in the bottom axial slices because when the head scan includes more neck portion, the center of gravity of the volume will be outside the brain, thus, the deformation covers the non-rain portions. |
| BET2 [65] | Intensity clamping, surface point detection, and mesh fitting. | T1 and T2-weighted images | It is necessary to give both T1 and T2-weighted images as input. |
| 3dSkullStrip [66] | Non-uniformity correction, 3D edge detection and surface deformation. | T1-weighted images | It is an interactive tool and requires to set many input parameters. |
| Lao et al. [67] | Deformation model and global shape statistics. | T1-weighted images | It needs more time to initialize attribute vector. |
| MLS [32] | Active curve model. Written in Java and is able to run on any java-enabled platform. | 2D or 3D T1 and T2-weighted images | It is slower than morphological-based methods. Failed to skull strip when the image has high noise or poor contrast. |
| John et al. [68] | 3D morphological operations and statistical techniques. | 3D T1-weighted images | Requires intensity inhomogeneity correction |
| Kobashi et al. [81] | Fuzzy rule and active shape model. | T1-weighted neonatal images | Do not extract brain completely in all slices. |
| Yunjie et al. [69] | Gauss mixture model and a 3D mathematical morphology method. | 3D T1-weighted images | Affected by intensity bias in the image. |
| Liu et al. [70] | Deformable model and radial basis functions. | T1-weighted images | Noise and intensity imhomogeneity influences the accuracy of threshold calculation. |
| Merisaari et al. [71] | Watershed segmentation, Gaussian mixture model clustering and a modification of BET [31] is employed | T1-weighted images | Requires corresponding T2-weighted brain image for accurate result. |
| Tao and Chang [72] | Thresholding, deformable surface-based algorithm and membership function | T1 and T2-weighted images | Difficult to skull strip when the brain image has poor contrast. |
| Somasundaram and Kalavathi [73, 76] | Chan–Vese active contour model. | T1, T2, and PD-weighted images | Requires more processing time to segment the brain. |
| Hwang et al. [74] | 3D level set method, speedup operator and a refinement process. | T1-weighted images | Requires accurate computation of speedup operator for curve refinement. |
| Zhang et al. [75] | Improved geometric active contour. | T1-weighted images | Devised to skull strip only on normal brain images. |
| SMHASS [77] | Deformable models, histogram analysis, and segmentation based on simplex meshes and pre-segmentation using statistical modeling | T1-weighted images | Requires complex thresholding calculation. |
| A contour-based brain segmentation method [78] | Contouring algorithm, morphological operation, and connected component analysis. | T1, T2, and PD-weighted images | For few slices, this method over-segment the brain due to inappropriate removal of brain tissues caused by morphological erosion process and under-segment the brain because of the strong intensity similarity between the brain and non-brain tissues |
| (iv) Atlas/template-based methods | |||
| Sandor and Leahy [34] | Pre-labeled brain atlas, Marr–Hildreth edge detector, morphological operation and deformable atlas matching. | 3D T1-weighted images | The Marr–Hildreth operator is highly dependent on information provided by a low-level processor, an error in this affects the accurate detection of brain region boundaries. |
| Dale et al. [79] | Intensity normalization and deformation process on tessellated ellipsoidal template. | T1-weighted images | Deformation process is driven by two kinds of forces and therefore requires more processing time. |
| Wang et al. [80] | Co-registration of an atlas and deformation scheme that is guided by prior information | T1-weighted images | Need manual extraction of atlas-based prior information to guide surface evolution and refinement. |
| Mahapatra [82] | Shaper prior information along with graph cuts. | T1-weighted neonatal images | Do not provide high segmentation accuracy due to poor contrast quality in neonatal bran images. |
| MAPS [83] | Atlas registration and segmentation. | T1-weighted images | Segmentation accuracy depends on the selection of best matched atlas. |
| BEaST [84] | Normalization, construction of brain atlas, patch-based segmentation and uses a library of 80 priors. | T1-weighted images | Segmented accuracy depends on the number priors used and selection of priors. Processing time is more. |
| (v) Hybrid methods | |||
| Kapur et al. [85] | Expectation/maximization segmentation, mathematical morphology, and active contour models. | T1-weighted images | Does not produce accurate segmentation result. |
| Atkins and Mackiewich [86] | Anisotropic filters, snake contouring technique, and a priori knowledge. | PD/T2-weighted axially acquired multi-spectral images | For few slices, the snake model requires manual initialization of the brain boundary. Topological mask of the brain is not related to the brain size. |
| ImageJ [87] | Complex contouring algorithm | Coronal T1-weighted images | It requires complex contouring algorithm to produce the results. This method is modeled for normal subjects and it failed to extract brain containing abnormal anatomic structures. |
| Bauer et al. [88] | Geodesic active contour segmentation with level set based algorithm using ITK. | T1, T1-contrast, T2, T2-flair and CT images | Sometimes the user needs to fine-tune the parameters for the registration or level-set segmentation according to their requirements. |
| McStrip [89] | Incorporates BSE [36], template warping, intensity thresholding, edge detection procedures and intensity histogram. | T1-weighted images | Requires the creation of good wrap masks from different models using automated image registration method. |
| HWA [91] | Combines watershed algorithm [51] and deformable surface model [79]. | T1-weighted images | It may include the non-brain portion in the segmented brain due to intensity bias in the input image. |
| BEMA [92] | Combines BSE [36], BET [31], 3DIntracranial [47], and watershed techniques [79] | T1-weighted images | Needs improvement in the atlas registration. Requires more processing time. Limitations on the combined methods also affect the performance of BEMA. |
| Huang et al. [94] | Expectation maximization (EM) mathematical morphology, connected component analysis and geodesic active contours | T1-weighted images | Pixel intensity-based segmentation is required and therefore output is influenced by the intensity profile of the image. |
| Carass et al. [95] | Combines elastic registration, tissue segmentation, and morphological techniques | T1-weighted images | Partial volume and image imhomogeneity present in the image affects the final result. |
| ROBEX [96] | Combines the discriminative and generative model | T1-weighted images | It over smooth the contour of the brain and there can leave out some gray matter, which can represent a problem if the next step in the image analysis pipeline is estimating the cortical thickness or measuring the gray matter volume. Image registration is not general for articulated or highly anatomically variable structures. |
Challenges in Skull Stripping Techniques
Skull stripping process is a sophisticated and challenging task due to the intrinsically imprecise nature of the brain images. Automated algorithms for skull stripping should be robust, efficient, reliable, and produce consistent and more accurate results on the large volume of datasets. However, the presence of noise and various imaging artifacts in MRI may introduce undesired distortions to the brain images which may substantially degrade their quality [93, 94]. Perusal of the literature reveals that the automatic skull stripping is still a persistent and challenging problem. Some of the challenges in the skull stripping techniques are as follows:
The brain images are obtained using different imaging parameters on different machines and for a given tissue type, they produce images with different contrast and scan quality.
The signal intensities for different brain structures often overlap; some non-brain tissues such as neck and scalp have the same intensities as brain tissues.
The echos can be seen in air/tissue borders in brain image.
The partial volume effect blurs the intensity distinction between tissue classes at the border of the two tissues.
The motion artifacts (blood vessels, muscles etc.,) cause noise or ringing around effect in the brain image.
Brain structures are not homogeneous and vary with individuals.
Not all anatomic borders are intensity-based borders and many edges are not sharp in the brain image.
Presence of imaging artifacts and various noises due to sensors and related electronic system may degrade the brain image quality and increase the difficulties in skull stripping process.
Another important problem which is gaining attention is skull stripping applied to brain MRI images with gross deformities such as glioblastoma [108, 109]. Standard skull stripping methods discussed so far fail in this case mostly due to additional difficulties in separating lesions which are located closer to the skull border. Thus, it requires further detection of features which can take into account shape deformities within skull stripping methods.
Conclusions
The skull stripping being a preliminary step, designed to eliminate non-brain tissues from MR brain images for many clinical applications and neuroimaging studies, its accuracy and speed are considered as the key factors. A number of techniques have been proposed, manual or semi-automated methods are labor-intensive, operator-dependent, time consuming and thus are not desirable in large-scale studies. The automated skull stripping methods help to improve the speed and accuracy of prognostic and diagnostic procedures in brain image segmentation and analysis. But, the majority of the skull stripping methods were devised only for T1-weighted brain images and majority of the existing methods cannot be applied for all brain image type and orientations. Because the appearance of the brain images may vary significantly between scans, which further complicates the task of devising an efficient skull stripping method that works across sequences and scanners. The existing skull stripping methods often need to be adapted specifically for a certain type of study or, in the best case, need to be tuned to work on a certain population. A method that works reliably and robustly on a variety of different brain morphologies and acquisition sequences without requiring adjustment of parameters would greatly reduce the need for manual intervention and exclusion of subjects in neuroimaging studies. Therefore, the development of novel, robust, and automated algorithm for MRI skull stripping that provides feasible solutions for all the challenges posed by skull stripping methods is still demanding area of research in field brain image processing and analysis.
Contributor Information
P. Kalavathi, Email: pkalavathi.gri@gmail.com
V. B. Surya Prasath, Email: prasaths@missouri.edu.
References
- 1.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic resonance imaging, physical principles and sequence design. New York: John Willey & Sons; 1999. [Google Scholar]
- 2.Quencer RM, Bradley WG. MR imaging of the brain: what constitutes the minimum acceptable capability? Am J Neuroradiol. 2001;22(8):1449–1450. [PMC free article] [PubMed] [Google Scholar]
- 3.Cheour M: Advantages of brain MRI, 2010, Available at: RadiologyInfo.org
- 4.Schmid P. Segmentation of digitized dermatoscopic images by Two-dimensional colour clustering. IEEE Trans Med Imaging. 1999;18(2):164–171. doi: 10.1109/42.759124. [DOI] [PubMed] [Google Scholar]
- 5.NLM-National Library of Medicine, (Rockville Pike, Bethesda U.S., 2011), Available online at: http://www.nlm.nih.gov
- 6.Gonzalez RC, Woods RE. Digital image processing. 3. New Delhi: Prentice Hall of India (P) Ltd; 2008. [Google Scholar]
- 7.Pham DL, Xu C, Prince JL. Current methods in medical image segmentation. Annu Rev Biomed Eng. 2000;2(1):315–338. doi: 10.1146/annurev.bioeng.2.1.315. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Aggarwal LM. Automated medical image segmentation techniques. J Med Phys. 2010;35(1):3–14. doi: 10.4103/0971-6203.58777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hizukuri A, Nakayama R, Nakako N, Kawanaka H, Takase H, Yamamoto K, Tsuruoka S. Computerized segmentation method for individual calcifications within clustered microcalcifications while maintaining their shapes on magnification mammograms. J Digit Imaging. 2012;25:377–386. doi: 10.1007/s10278-011-9420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younis A, Ibrahim M, Kabuka M, John N. An artificial immune-activated neural network applied to brain 3D MRI segmentation. J Digit Imaging. 2008;21(1):69–88. doi: 10.1007/s10278-007-9081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson BJ, Avula RTV. An algorithm for automatic segmentation and classification of magnetic resonance brain images. J Digit Imaging. 1998;11(2):74–82. doi: 10.1007/BF03168729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handels H, Tolxdorff T. A new segmentation algorithm for knowledge acquisition in tissue- characterizing magnetic resonance imaging. J Digit Imaging. 1990;3(2):89–94. doi: 10.1007/BF03170567. [DOI] [PubMed] [Google Scholar]
- 13.Hogan RE, Mark KE, Choudhuri I, Wang L, Joshi S, Miller MI, Bucholz RD. Magnetic resonance imaging deformation-based segmentation of the hippocampus in patients with mesial temporal sclerosis and temporal lobe epilepsy. J Digit Imaging. 2000;13(1):217–218. doi: 10.1007/BF03167670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fennema-Notestine C, Ozyurt IB, Clark CP, Morris S, Bischoff-Grethe A, Bondi MW, Jernigan TL, Fischl B, Segonne F, Shattuck DW, Leahy RM, Rex DE, Toga AW, Zou KH, Brain M, Brown GG. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: effects of diagnosis, bias correction and slice location. Hum Brain Mapp. 2006;27(2):99–113. doi: 10.1002/hbm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto S, Asato R, Konishi J. A fast Way to visualize the brain surface with volume rendering of MRI data. J Digit Imaging. 1999;12(4):185–190. doi: 10.1007/BF03168854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmood Q, Chodorowski A, Mehnert A, Gellermann J, Persson M, Unsupervised segmentation of head tissues from multi-modal MR images for EEG source localization J Digit Imaging, 2014 [DOI] [PMC free article] [PubMed]
- 17.Hata Y, Kobashi S, Kondo K, Kitamura YT, Yanagida T. Transcranial ultrasonography system for visualizing skull and brain surface aided by fuzzy expert system. IEEE Trans Syst Man Cybern. 2005;35(6):1360–1373. doi: 10.1109/TSMCB.2005.855593. [DOI] [PubMed] [Google Scholar]
- 18.Klein A, Ghosh SS, Avants B, Yeo B, Fischl B, Ardekani B, Gee JC, Mann J, Parsey RV. Evaluation of volume-based and surface-based brain image registration methods. NeuroImage. 2010;51(1):214–220. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalkers NF, Ameziane N, Bot JC, Minneboo A, Polman CH, Barkhof F. Longitudinal brain volume measurement in multiple sclerosis: rate of brain atrophy is independent of the disease subtype. Archit Neurol. 2002;58(10):1572–1576. doi: 10.1001/archneur.59.10.1572. [DOI] [PubMed] [Google Scholar]
- 20.Wels M, Zheng Y, Huber M, Hornegger J, Comaniciu D. A discriminative model-constrained EM approach to 3D MRI brain tissue classification and intensity Non-uniformity correction. Phys Med Biol. 2011;56(11):3269–3300. doi: 10.1088/0031-9155/56/11/007. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Chen Y, Pan X, Hong X, Xia D. Level set segmentation of brain magnetic resonance images based on local gaussian distribution fitting energy. J Neurosci Methods. 2010;188(2):316–325. doi: 10.1016/j.jneumeth.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PM, Mega MS, Woods RP, Zoumalan CI, Lindshield CJ, Blanton RE, Moussai J, Holmes CJ, Cummings JL, Toga AW. Cortical change in Alzheimer’s disease detected with a disease-specific population-based brain atlas. Cereb Cortex. 2001;11(1):1–16. doi: 10.1093/cercor/11.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Tosun D, Rettmann ME, Naiman DQ, Resnick SM, Kraut MA, Prince JL. Cortical reconstruction using implicit surface evolution: accuracy and precision analysis. NeuroImage. 2006;29(3):838–852. doi: 10.1016/j.neuroimage.2005.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;12(3):340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Ruotsalainen U, Hirvonen J, Hietala J, Tohka J. Automatic cerebral and cerebellar hemisphere segmentation in 3D MRI: adaptive disconnection algorithm. Med Image Anal. 2010;14(3):360–372. doi: 10.1016/j.media.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Zivadinov R, Bagnato F, Nasuelli D, Bastianello S, Bratina A, Locatelli L, Watts K, Finamore L, Grop A, Dwyer M, Catalan M, Clemenzi A, Millefiorini E, Bakshi R, Zorzon M. Short-term brain atrophy changes in relapsing-remitting multiple sclerosis. Neurol Sci. 2004;223(2):185–193. doi: 10.1016/j.jns.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Rusinek H, de Leon MJ, George AE, Stylopoulos LA, Chandra R, Smith G, Rand T, Mourino M, Kowalski H. Alzheimer disease: measuring loss of cerebral gray matter with MR imaging. Radiology. 1991;178(1):109–114. doi: 10.1148/radiology.178.1.1984287. [DOI] [PubMed] [Google Scholar]
- 28.Tanskanen P, Veijola JM, Piippo UK, Haapea M, Miettunen JA, Pyhtinen J, Bullmore ET, Jones PB, Isohanni MK. Hippocampus and amygdala volumes in schizophrenia and other psychoses in the northern Finland 1966 birth cohort. Schizophr Res. 2005;75(2-3):283–294. doi: 10.1016/j.schres.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Blanton RE, Levitt JG, Peterson JR, Fadale D, Sporty ML, Lee M, To D, Mormino EC, Thompson PM, McCracken JT, Toga AW. Gender differences in the left inferior frontal gyrus in normal children. NeuroImage. 2004;22(2):626–636. doi: 10.1016/j.neuroimage.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Brummer ME, Mersereau RM, Eisner RL, Lewine RRJ, Caeslles V, Kimmel R, Sapiro G. Automatic detection of brain contours in MRI datasets. IEEE Trans Image Process. 1993;12(2):153–166. doi: 10.1109/42.232244. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang AH, Valentino DJ, Toga AW. Skull stripping magnetic resonance images using a model-based level sets. NeuroImage. 2006;32(1):79–92. doi: 10.1016/j.neuroimage.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Tsai C, Manjunath BS, Jagadeesan R. Automated segmentation of brain MR images. Pattern Recogn. 1995;28(12):1825–1837. doi: 10.1016/0031-3203(95)00047-X. [DOI] [Google Scholar]
- 34.Sandor S, Leahy RM. Surface-based labeling of cortical anatomy using a deformable atlas. IEEE Trans Med Imaging. 1997;16(1):41–54. doi: 10.1109/42.552054. [DOI] [PubMed] [Google Scholar]
- 35.Lemieux G, Krakow KH, Woermann FG. Fast, automatic segmentation of the brain in T1-weighted volume magnetic resonance image data. Proc SPIE Med Imaging: Image Processing. 1999;3661:152–160. doi: 10.1117/12.348561. [DOI] [PubMed] [Google Scholar]
- 36.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. NeuroImage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 37.Shanthi KJ, Sasikumar M: Skull stripping and automatic segmentation of brain MRI using seed growth and threshold techniques, Proc. International Conference on Intelligent and Advanced Systems, Kuala Lumpur 1:422-426,2007
- 38.Mikheev B, Nevsky G, Govindan S, Grossman R, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using bridge burner algorithm. J Magn Reson Imaging. 2008;27(6):1235–1241. doi: 10.1002/jmri.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park GJ, Lee C. Skull stripping based on region growing for magnetic resonance images. NeuroImage. 2009;47(4):1394–1407. doi: 10.1016/j.neuroimage.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 40.Gao J, Xie M: Skull stripping MR brain images using anisotropic diffusion filtering and morphological processing, Proc. International Symposium on Computer Network and Multimedia Technology, Wuhan 1:1-4,2009
- 41.Somasundaram K, Kalavathi P. Automatic skull stripping of magnetic resonance images (MRI) of human head scans using image contour. New Delhi: Image Processing, Allied Publisher; 2010. pp. 147–151. [Google Scholar]
- 42.Somasundaram K, Kalavathi P: A hybrid method for automatic skull stripping of magnetic resonance images (MRI) of human head scans. Proc. International Conference on Computing Communication and Networking Technologies (ICCCNT), Karur, Tamilnadu, 1-5, 2010
- 43.Somasundaram K, Kalaiselvi T. Fully automatic brain extraction algorithm for axial T2- weighted magnetic resonance images. Comput Biol Med. 2010;40(10):811–822. doi: 10.1016/j.compbiomed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Somasundaram K, Kalaiselvi T: Automatic brain extraction methods for T1 magnetic resonance images using region labeling and morphological operations. Comput Biol Med 41(8):2011 [DOI] [PubMed]
- 45.Carass A, Cuzzocreo J, Wheeler MB, Bazin PL, Resnick SM, Prince JL. Simple paradigm for extra-cerebral tissue removal: algorithm and analysis. NeuroImage. 2011;56(4):1982–1992. doi: 10.1016/j.neuroimage.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance Neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 47.Ward BD. 3dIntracranial: automatic segmentation of intracranial region. UK: Technical Report, Biophysics Research Institute, Medical College of Wisconsin; 1999. [Google Scholar]
- 48.Huh S, Ketter TA, John KH, Lee C. Automated cerebrum segmentation from three- dimensional sagittal brain MR images. Comput Biol Med. 2002;32(5):311–328. doi: 10.1016/S0010-4825(02)00023-9. [DOI] [PubMed] [Google Scholar]
- 49.Dawant BM, Hartmann SL, Thirion JP, Maes F, Vandermeulen D, Demaerel P. Automatic 3-D segmentation of internal structures of the head in MR images using a combination of similarity and free-form transformations: part I. Methodology and validation on normal subjects. IEEE Trans Med Imaging. 1999;18(10):909–916. doi: 10.1109/42.811271. [DOI] [PubMed] [Google Scholar]
- 50.Pham DL, Prince JL. Adaptive fuzzy segmentation of magnetic resonance images. IEEE Trans Med Imaging. 1999;18(9):737–752. doi: 10.1109/42.802752. [DOI] [PubMed] [Google Scholar]
- 51.Hahn HK, Peitgen HO. The Skull Stripping Problem in MRI Solved by Single 3D Watershed Transform, Proc. Medical Image Computing and Computer Assisted Intervention (MICCAI) LNCS. 1935;2000:134–143. [Google Scholar]
- 52.Grau V, Mewes AUJ, Alcaiz M, Kikinis R, Warfield SK. Improved watershed transform for medical image segmentation using prior information. IEEE Trans Med Imaging. 2004;23(4):447–458. doi: 10.1109/TMI.2004.824224. [DOI] [PubMed] [Google Scholar]
- 53.Ashburner J, Friston KJ. Voxel based morphometry: the methods. NeuroImage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 54.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 55.Zu YS, Guang HY, Jing ZL. Automated histogram-based brain segmentation in T1- weighted three-dimensional magnetic resonance head images. NeuroImage. 2002;17(3):1587–1598. doi: 10.1006/nimg.2002.1287. [DOI] [PubMed] [Google Scholar]
- 56.Sadananthan S, Zheng W, Chee M, Zagorodnov V. Skull stripping using graph cuts. NeuroImage. 2010;49(1):225–239. doi: 10.1016/j.neuroimage.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 57.Somasundaram K, Kalavathi P. Skull stripping of MRI head scans based on 2D region growing, Proc. ICOM11. Tamil Nadu: Tiruchirappalli; 2011. pp. 18–23. [Google Scholar]
- 58.Somasundaram K, Kalavathi P. Brain segmentation in magnetic resonance human head scans using multi-seeded region growing. Imaging Sci J. 2014;62(5):273–284. doi: 10.1179/1743131X13Y.0000000068. [DOI] [Google Scholar]
- 59.Kalavathi P. Computation of brain asymmetry in 2D brain images. Int J Sci Eng Res. 2014;5(7):1167–1171. [Google Scholar]
- 60.Aboutanos GB, Nikanne J, Watkins N, Dawant BM. Model creation and deformation for the automatic segmentation of the brain in MR images. IEEE Trans Biomed Eng. 1999;46(11):1346–1356. doi: 10.1109/10.797995. [DOI] [PubMed] [Google Scholar]
- 61.Zeng X, Staib LH, Schultz RT, Duncan JS. Segmentation and measurement of the cortex from 3-D MR images using coupled-surfaces propagation. IEEE Trans Med Imaging. 1999;18(10):927–937. doi: 10.1109/42.811276. [DOI] [PubMed] [Google Scholar]
- 62.Suri JS. Two-dimensional fast magnetic resonance brain segmentation. IEEE Eng Med Biol. 2001;20(4):84–95. doi: 10.1109/51.940054. [DOI] [PubMed] [Google Scholar]
- 63.Baillard C, Hellier P, Barillot C. Segmentation of brain 3D MR images using level sets and dense registration. Med Image Anal. 2001;5(3):185–194. doi: 10.1016/S1361-8415(01)00039-1. [DOI] [PubMed] [Google Scholar]
- 64.Atkins MS, Siu K, Law B, Orchard JJ, Rosenbaum WL. Difficulties of T1 brain MRI segmentation techniques, medical imaging. Proc. SPIE. 2001;4684(1):1837–1844. [Google Scholar]
- 65.Jenkinson M, Pechaud M, Smith S. BET2 - MR-based estimation of brain, skull and scalp surfaces. Oxford: Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB); 2005. [Google Scholar]
- 66.3dSkullStrip, a part of the AFNI (Analysis of Functional Neuro Images) package. available at http://afni.nimh.nih.gov
- 67.Lao Z, Shen D, Davatzikas C. Statistical shape model for automatic skull-stripping of brain images. Washington, D.C: Proc. IEEE International Symposium on Biomedical Imaging; 2002. pp. 855–858. [Google Scholar]
- 68.John C, Kevin W, Emma L, Chao C, Barbara P, Declan J. Statistical morphological skull stripping of adult and infant MRI data. Comput Biol Med. 2007;37(3):342–357. doi: 10.1016/j.compbiomed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Yunjie C, Jianwei Z, Shunfeng W. A new fast brain skull stripping method, biomedical engineering and informatics. Tianjin: Proc. 2nd International Conference on Biomedical Engineering and Informatics, BMEI09; 2009. [Google Scholar]
- 70.Liu JX, Chen YS, Chen LF. Accurate and robust extraction of brain regions using a deformable model based on radial basis functions. J Neurosci Methods. 2009;183(2):255–266. doi: 10.1016/j.jneumeth.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Merisaari H, Parkkola R, Alhoniemia E, Teras M, Lehtonend L, Haataja L, Lapinleimu H, Nevalainen OS. Gaussian mixture model-based segmentation of MR images taken from premature infant brains. J Neurosci Methods. 2009;182(1):110–122. doi: 10.1016/j.jneumeth.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 72.Tao X, Chang MC. A skull stripping method using deformable surface and tissue classification, medical imaging. Proc. SPIE. 2010;7:623–630. [Google Scholar]
- 73.Somasundaram K, Kalavathi P. Skull stripping of MRI head scans based on chan-vese active contour model. Int J Knowl Manag e-learning. 2011;3(1):7–14. [Google Scholar]
- 74.Hwang J, Han Y, Park H. Skull-stripping method for brain MRI using a 3D level Set with a speedup operator. J Magn Reson Imaging. 2011;34(2):445–456. doi: 10.1002/jmri.22661. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Liu J, Zhu Z, et al: An automated and dimple method for brain MR image extraction, BioMed Eng OnLine 10(81),2011 [DOI] [PMC free article] [PubMed]
- 76.Somasundaram K, Kalavathi P: A novel skull stripping technique for T1-weighted MRI human head Scans, Proc. ICVGIP 1-8,2012
- 77.Galdames FJ, Jaillet F, Perez CA. An accurate skull stripping method based on simplex meshes and histogram analysis in magnetic resonance images. J Neurosci Methods. 2012;206(2):109–113. doi: 10.1016/j.jneumeth.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 78.Somasundaram K, Kalavathi P. Contour-based brain segmentation method for magnetic resonance imaging human head scans. J Comput Assist Tomogr. 2013;37(3):353–368. doi: 10.1097/RCT.0b013e3182888256. [DOI] [PubMed] [Google Scholar]
- 79.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Nie J, Yap P-T, Shi F, Guo L, Shen D. Robust deformable-surface-based skull- stripping for large-scale studies, Proc. medical image computing and computer assisted intervention (MICCAI) LNCS. 2011;6893:635–642. doi: 10.1007/978-3-642-23626-6_78. [DOI] [PubMed] [Google Scholar]
- 81.Kobashi S, Moto FY, Ogawa MD, Ando K, Ishikura R, Kando SH, Katy Y. Fuzzy- ASM based automated skull stripping method from infantile brain MR images. Proc. IEEE Int Conf Granular Comput San Jose California. 2007;1:632–635. [Google Scholar]
- 82.Mahapatra D. Skull stripping of neonatal brain MRI: using prior shape information with graph cuts. J Digit Imaging. 2012;25(6):802–814. doi: 10.1007/s10278-012-9460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung KK, Barnes J, Modat M, Ridgway GR, Bartlett JW, Fox NC, Ourselin S. Brain MAPS: an automated, accurate and robust brain extraction technique using a template library. NeuroImage. 2011;55(3):1091–1108. doi: 10.1016/j.neuroimage.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eskildsen SF, Coupe P, Fonov V, Manjon JV, Leung KK, Guizard N, Wassef SN, Ostergaard LR, Collins DL. BEaST: brain extraction based on Non-local segmentation technique. NeuroImage. 2012;59(3):2362–2373. doi: 10.1016/j.neuroimage.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Kapur T, Grimson WEL, Wells WM, III, Kikinis R. Segmentation of brain tissue from magnetic resonance images. Med Image Anal. 1996;1(2):109–127. doi: 10.1016/S1361-8415(96)80008-9. [DOI] [PubMed] [Google Scholar]
- 86.Atkins MS, Mackiewich B. Fully automatic segmentation of the brain in MRI. IEEE Transactions Med Imaging. 1998;17(1):98–107. doi: 10.1109/42.668699. [DOI] [PubMed] [Google Scholar]
- 87.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with image. J Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 88.Bauer S, Fejes T, Reyes M: A skull-stripping filter for ITK, Insight Journal, 2012. http://hdl.handle.net/10380/3353
- 89.Rehm K, Schaper K, Anderson J, Woods R, Stoltzner S, Rottenberg D. Putting our heads together: a consensus approach to brain/Non-brain segmentation in T1-weighted MR volumes. NeuroImage. 2004;22(3):1262–1270. doi: 10.1016/j.neuroimage.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC. Automated image registration: II intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 91.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 92.Rex DE, Shattuck DW, Woods RP, Narr KL, Luders E, Rehm K, Stolzner SE, Rotten-berg DA, Toga AW. A meta-algorithm for brain extraction in MRI. NeuroImage. 2004;23(2):625–637. doi: 10.1016/j.neuroimage.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 93.Shi F, Wang L, Gilmore JH, Lin W, Shen D. Learning-based meta-algorithm for MRI brain extraction, Proc. medical image computing and computer assisted intervention (MICCAI) LNCS. 2011;6893:313–321. doi: 10.1007/978-3-642-23626-6_39. [DOI] [PubMed] [Google Scholar]
- 94.Huang A, Abugharbieh R, Tam R, Traboulsee A. MRI brain extraction with combined expectation maximization and geodesic active contours. Proc. IEEE Int Symp Signal Proc Inf Technol. 2006;107(1):107–111. [Google Scholar]
- 95.Carass A, Cuzzocreo J, Wheeler MB, et al: A joint registration and segmentation approach to skull stripping, Proc. IEEE Symposium on Biomedical Imaging. 655-659,2007
- 96.Iglesias JE, Liu CY, Thompson PM, Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans Med Imaging. 2011;30(9):1617–1634. doi: 10.1109/TMI.2011.2138152. [DOI] [PubMed] [Google Scholar]
- 97.Lee JM, Yoon U, Nam SM, Kim JH, Kim IY, Kim SI. Evaluation of automated and semi-automated skull stripping algorithms using similarity index and segmentation error. Comput Biol Med. 2003;33(6):495–507. doi: 10.1016/S0010-4825(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 98.Boesen K, Rehm L, Schaper K, Stoltzner S, Woods R, Luders E, Rottenberg D. Quan- titative comparison of four brain extraction algorithms. NeuroImage. 2004;22(3):1255–1261. doi: 10.1016/j.neuroimage.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 99.Hartley SW, Scher AI, Korf ESC, White LR, Launer LJ. Analysis and validation of automated skull stripping tools: a validation study based on 296 MR images from Honolulu Asia aging study. NeuroImage. 2006;30(4):1179–1186. doi: 10.1016/j.neuroimage.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 100.Shattuck DW, Prasad G, Mirza M, Narr KL, Toga AW. Online resource for validation of brain segmentation methods. NeuroImage. 2009;45(2):431–439. doi: 10.1016/j.neuroimage.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Richard A. Biomedical imaging, visualization and analysis. New York, USA: John Wiley & Sons Inc; 2000. [Google Scholar]
- 102.Yoon UC, Kim JS, Kim IY, Kim SI. Adaptive fuzzy C-means for improved classification as a preprocessing procedure of brain parcellation. J Digit Imaging. 2001;14(2):238–240. doi: 10.1007/BF03190353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 104.Somasundaram K, Kalavathi P. Medical image denoising using Non-linear spatial mean filters for edge detection. New Delhi: Proc. Signal and Image Processing; 2012. pp. 149–154. [Google Scholar]
- 105.Somasundaram K, Kalavathi P. Analysis of imaging artifacts in MR brain images. Oriental J Comput Sci Technol. 2012;5(1):135–141. [Google Scholar]
- 106.Somasundaram K, Kalavathi P. Medical image contrast enhancement based on gamma correction. Int J Knowl Manag e-learning. 2011;3(1):15–18. [Google Scholar]
- 107.ANALYZE 12.0 – Visualization and analysis software for medical imaging. Available at : http://analyzedirect.com/analyze-12-0/
- 108.Speier W, Iglesias JE, El-Kara L, Tu Z, Arnold C. Robust skull stripping of clinical glioblastoma multiforme data, Proc. medical image computing and computer assisted intervention (MICCAI) LNCS. 2011;6893:659–666. doi: 10.1007/978-3-642-23626-6_81. [DOI] [PubMed] [Google Scholar]
- 109.Bauer S, Nolte L-P, Reyes M: Skull-stripping for tumor-bearing brain images. In Philippe Buchler and Stephen Ferguson, editors, Annual Meeting of the Swiss Society for Biomedical Engineering, page 2, Bern, April 2011. SSBE