Abstract
Objective
To identify the gestational age of planned delivery in pregnancies complicated by chronic hypertension that minimizes the risk of perinatal death and severe adverse events.
Methods
This was a retrospective cohort study of all singletons complicated by hypertension. Detailed patient-level information was collected by chart review, including indication for delivery. Planned delivery at 36–36.6, 37–37.6, 38–38.6, and 39–39.6 weeks was compared to expectant management beyond each respective gestational age. Patients were excluded for fetal anomalies, inaccurate dating, and major medical problems other than hypertension, diabetes or renal disease. The primary outcome was a composite of stillbirth, neonatal death, assisted ventilation, cord pH<7.0, 5-minute Apgar ≤3, and neonatal seizures. Secondary outcomes were preeclampsia, severe preeclampsia, primary cesarean, and infant length of stay >5 days. Groups were compared using Student’s t-test and χ2 tests.
Results
Six hundred eighty three women with hypertension reached 36 weeks. Patients with planned delivery <39 weeks were more likely to have baseline renal disease. Prior to 37 weeks, planned delivery was associated with a statistically significant increase in the primary composite adverse neonatal outcome (10.0% versus 2.6%, p=0.04); after 38 weeks, expectant management was associated with a non-statistically significant increase in the primary composite outcome (0% versus 2.3%, p=0.40). Expectant management beyond 39 weeks was associated with a statistically significant increase in severe preeclampsia (0 versus 10.3%, p=0.001).
Conclusion
Expectant management beyond 39.0 weeks was associated with increasing incidence of severe preeclampsia; planned delivery prior to 37 weeks was associated with an increase in adverse neonatal outcomes. Further, well-powered studies are needed to delineate the optimal gestational age of delivery.
Chronic hypertension complicates up to 5% of pregnancies in the United States; this number is expected to increase both due to the trend of increasing age at childbearing as well as increasing obesity in women of reproductive age.(1) Pregnancies complicated by chronic hypertension are at a twofold to threefold increased risk of stillbirth compared to pregnancies not complicated by hypertesnion; at term the risk of stillbirth in pregnancies complicated by chronic hypertension is 2–6 fetal deaths per 1,000 live births.(2–5) Concerns regarding the risk of stillbirth at term must be balanced regarding the risk of neonatal and infant death and morbidities with delivery prior to 39 weeks.(6, 7)
The American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development provide a broad range of gestational ages at which it might be appropriate to deliver pregnancies complicated by chronic hypertension, anywhere from 36–39 weeks of gestation.(8, 9) These recommendations are based on limited or inconsistent evidence. We sought to define an optimal gestational age of delivery to minimize perinatal morbidity and mortality.
Materials and Methods
We performed a retrospective cohort study of all singleton pregnancies delivered at the University of Alabama at Birmingham with a diagnosis of chronic hypertension from January 1, 2000-June 1, 2014. Institutional review board approval was obtained.
Patients were identified by a diagnosis of chronic hypertension as listed as a diagnosis in the electronic prenatal records. A diagnosis of chronic hypertension was confirmed by chart review with a self-reported history of chronic hypertension (based on patient report of current diagnosis of chronic hypertension), use of antihypertensives, or documented blood pressures ≥140 mm Hg systolic or ≥90 mm Hg diastolic on at least 2 occasions (separate days) prior to 20 weeks of gestation. Standardized chart abstraction forms were used to abstract data from the medical charts by physicians and medical students trained in chart abstraction. Data collected included detailed information on maternal demographics, medical and obstetrical history, prenatal blood pressures and urinalysis, baseline laboratory information, medication use, labor and delivery events, and neonatal outcomes. All women were managed by institutional protocol under the supervision of maternal-fetal medicine specialists, with target blood pressures <150/90 mm Hg. Serial fetal growth was followed after 28 weeks gestation, and at least weekly antenatal testing was performed with either contraction stress tests or biophysical profiles after 32–34 weeks. More frequent testing (twice weekly) was left to the discretion of the provider based on unique clinical scenarios.
Women with a live singleton gestation reaching 36 weeks and the diagnosis of chronic hypertension were included in the study. Women were excluded for major maternal medical illnesses other than hypertension, diabetes, or renal disease. Women with diabetes were included as this is a frequent co-morbidity of chronic hypertension and the clinical scenario of both chronic hypertension and diabetes is encountered frequently in clinical practice. Baseline renal disease was defined as urinary excretion of ≥300 mg of protein in a 24-hour period or a serum creatinine ≥1.2 mg/dL documented prior to 20 weeks gestation. Women were excluded if the pregnancies were complicated by fetal anomalies or if prenatal care was initiated after 20 completed weeks gestation. Only the first pregnancy meeting inclusion criteria during the study period was included in the analysis.
The primary outcome of the study was a composite adverse perinatal outcome of stillbirth, neonatal death, assisted ventilation (defined as receiving continuous positive airway pressure or mechanical ventilation), umbilical cord pH < 7.0, 5-minute Apgar ≤ 3, and neonatal seizures. Secondary outcomes considered were preeclampsia, preeclampsia with severe features, primary cesarean delivery, and infant length of stay >5 days. Preeclampsia diagnosis was based on objective information obtained from the chart review and was defined as blood pressures ≥140/90 mm Hg with either documented proteinuria or serum laboratory abnormalities. Proteinuria was based on a urine protein to creatinine ratio ≥0.3or urinary excretion of ≥300 mg of protein in 24-hours. Serum laboratory abnormalities were defined as platelets <100,000/mL, AST>80 mU/mL (twice the reference laboratory value), or creatinine ≥1.2 mg/dL. Preeclampsia with severe features was defined as blood pressure ≥160/110 mm Hg or the presence of serum laboratory abnormalities. As patient symptoms were not consistently documented over the study period, these could not be considered in the definition.
In order to determine the gestational age of delivery that minimized perinatal morbidity, planned delivery was compared to expectant management at 360/7-366/7, 370/7-376/7, 380/7-386/7, 390/7-396/7 weeks. Planned delivery was defined as either induction or scheduled cesarean for the indications of chronic hypertension, documented fetal lung maturity, prior stillbirth, gestational age, or elective. Expectant management was defined as delivery outside of the gestational age window or medically or obstetrically indicated delivery in the gestational age window. Medically or obstetrically indicated delivery included spontaneous labor, spontaneous rupture of membranes, vaginal bleeding, uncontrolled hypertension without proteinuria, preeclampsia or eclampsia, stillbirth, non-reassuring fetal testing, or oligohydramnios. Indicated deliveries were included in the expectant management group because development of a complication (such as abruption, non-reassuring fetal testing, preeclampsia) or labor is a consequence of expectant management. A secondary analysis was performed comparing outcomes with any planned delivery prior to 39 weeks and any planned delivery at or after 39 weeks.
Baseline characteristics of the planned delivery group and expectant management groups were compared with descriptive and univariable statistics using Student’s t-test and chi-squared tests, as appropriate. Normal distribution of continuous variables was assessed visually with histograms and with the Kolmogorov-Smirnov test. The incidence of outcomes between the exposed and unexposed groups was compared using the chi-squared test or Fisher’s exact test as appropriate. All analyses were performed using Stata SE, version 13 (College Station, TX). Statistical significance was considered to be a p<0.05; no adjustments were made for multiple comparisons.
Results
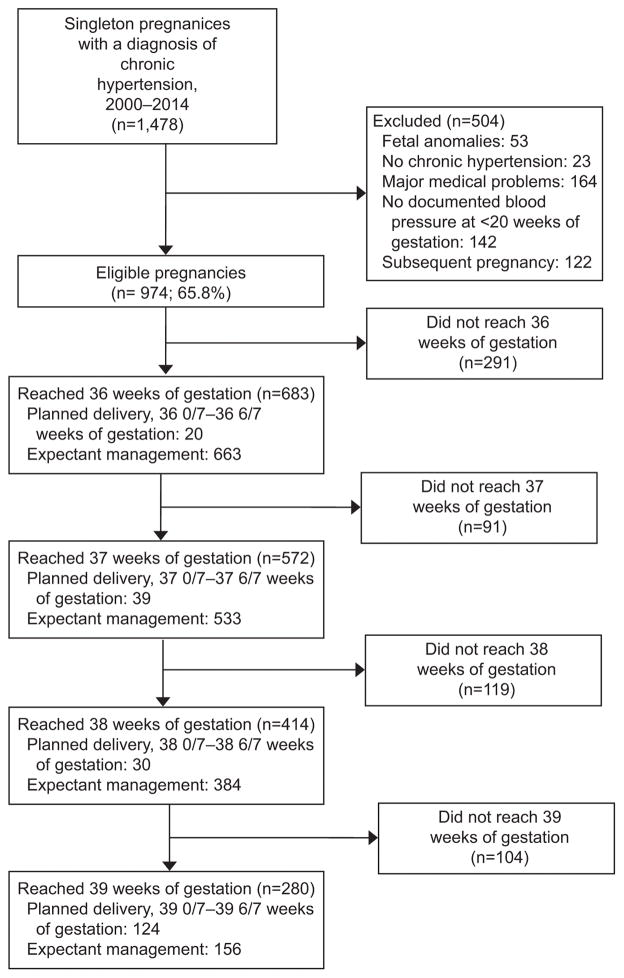
Of 1,478 pregnancies complicated by chronic hypertension identified over the study period, 504 were excluded (Figure 1). Of the remaining 974 women, 683 (70.1%) with chronic hypertension reached 36 weeks.
Figure 1.
Derivation of cohort
Of these 683 women, 20 (2.9%) underwent a planned delivery between 360/7-366/7 weeks (Table 1). Compared to the women expectantly managed beyond 36 weeks, women undergoing planned delivery at 36 weeks were more likely to have baseline renal disease. Women undergoing planned delivery at 36 weeks were also more likely to have pregestational diabetes. Medication use and average systolic and diastolic blood pressures in the first and second trimesters did not differ significantly between the two groups (Table 2). The average systolic and diastolic blood pressures from 28–32 weeks and 32–36 weeks were significantly higher in the planned delivery at 36 weeks group, although both averages were <140/90 mm Hg. The primary neonatal composite outcome occurred in 2 (10.0%) of women undergoing planned delivery between 360/7-366/7 weeks compared to 17 (2.6%) of those receiving expectant management (Table 3, p=0.04, relative risk 3.9, 95% CI 1.0–15.8). In the planned delivery group, the two adverse neonatal composite outcomes consisted of a neonatal death secondary to cardiorespiratory failure and one infant placed on the ventilator for 7 days. In the expectant management group the 17 neonatal composite outcomes consisted of four stillbirths, six cord gases with a pH ≤7.0, six infants placed on the ventilator, and one infant requiring continuous positive airway pressure. The minimum amount of time spent on the ventilator was 1 day.
Table 1.
Baseline characteristics of cohort
| 36 wks | 37 wks | 38 wks | 39 wks | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Delivery at 36–36.6 |
Expectant Mgmt >=36.0 |
Delivery at 37–37.6 |
Expectant Mgmt >=37.0 |
Delivery at 38–38.6 |
Expectant Mgmt >=38.0 |
Delivery at 39–39.6 |
Expectant Mgmt >=39.0 |
| n | 20 | 663 | 39 | 533 | 30 | 384 | 124 | 156 |
| Age (years) | 29.4 ± 5.8 | 30.3 ± 6.0 | 30.1 ± 5.6 | 30.4 ± 6.0 | 30.6 ± 5.0 | 30.1 ± 6.2 | 30.0 ± 6.3 | 29.6 ± 6.2 |
| Race | ||||||||
| White | 7 (35.0%) | 147 (22.2%) | 10 (25.6%) | 119 (22.3%) | 8 (26.7%) | 84 (21.9%) | 28 (22.6%) | 38 (24.4%) |
| Black | 13 (65%) | 486 (73.3%) | 28 (71.8%) | 391 (73.4%) | 22 (73.3%) | 278 (72.4%) | 92 (74.2%) | 108 (69.2%) |
| Hispanic | 0 | 17 (2.6%) | 1 (2.6%) | 13 (2.4%) | 0 | 13 (3.4%) | 3 (2.4%) | 3 (1.9%) |
| Other | 0 | 13 (1.9%) | 0 | 10 (1.9%) | 0 | 9 (2.3%) | 1 (0.8%) | 7 (4.5%) |
| Nulliparous | 7 (35.0%) | 185 (27.9%) | 12 (30.8%) | 147 (27.6%) | 7 (23.3%) | 117 (30.5%) | 43 (34.7%) | 44 (28.2%) |
| Government Insurance | 13 (65.0%) | 477 (72.0%) | 22 (56.4%) | 389 (73.0%) | 24 (80.0%) | 288 (75.0%) | 94 (75.8%) | 126 (80.8%) |
| Midtrimester Body Mass Index (kg/m2) | 39.2 ± 6.8 | 39.6 ± 11.1 | 40.0 ± 13.1 | 39.9 ± 10.9 | 44.7 ± 11.0† | 39.8 ± 10.5† | 41.6 ± 11.2 | 39.4 ± 9.9 |
| Tobacco use | 15.0% | 19.9% | 69 (15.6%) | 103 (19.3%) | 5 (16.7%) | 73 (19.0%) | 18 (14.5%) | 34 (21.8%) |
| Pregestational Diabetes | 9 (45.0%)* | 136 (20.5%)* | 19 (48.7%) | 92 (17.3)%* | 24 (19.4%)† | 54 (14.1%)† | 24 (19.4)%* | 8 (5.1%)* |
| Gestational Diabetes | 1 (5.0%) | 83 (12.5%) | 5 (12.8%) | 67 (12.5%) | 5 (16.7%) | 48 (12.5%) | 13 (10.5%) | 26 (16.7%) |
| Baseline Renal Disease | 4 (20.0%*) | 42 (6.3%*) | 5 (12.8%)† | 26 (4.9%)† | 4 (10.0%) | 14 (3.7%) | 2 (1.6%) | 4 (2.6%) |
| Preeclampsia in a Prior Pregnancy | 5 (25.0%) | 206 (31.1%) | 12 (30.8%) | 163 (30.6%) | 10 (33.3%) | 108 (28.1%) | 23 (18.6%)* | 54 (34.6%)* |
| Aspirin Use | 4 (20.0%)† | 47 (7.1%)† | 3 (7.7%) | 38 (7.1%) | 2 (6.7%) | 25 (6.5%) | 6 (4.8%) | 7 (4.5%) |
| Antihypertensives Used Prior to Pregnancy | 11 (57.9%) | 364 (54.9%) | 26 (66.7%) | 280 (52.5%) | 18 (60.0%)* | 197 (51.3%)* | 74 (59.7%) | 67 (43.0%) |
| Medications Used During Pregnancy | 17 (85.0%) | 497 (75.0%) | 29 (74.4%) | 401 (75.3%) | 21 (70.0%) | 291 (75.8%) | 97 (78.2%) | 111 (71.2%) |
Data presented as mean ± standard deviation or as %
Comparisons being made between gestational age groups (i.e. Planned delivery at 36-366/7 weeks compared to expectant management ≥36.0 weeks)
p<0.01
p<0.05
Table 2.
Pregnancy Characteristics of Cohort
| 36 wks | 37 wks | 38 wks | 39 wks | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Delivery at 36–36.6 |
Expectant Mgmt >=36.0 |
Delivery at 37–37.6 |
Expectant Mgmt >=37.0 |
Delivery at 38–38.6 |
Expectant Mgmt >=38.0 |
Delivery at 39–39.6 |
Expectant Mgmt >=39.0 |
| n | 20 | 663 | 39 | 533 | 30 | 384 | 124 | 156 |
| Gestational Age at Delivery (weeks)* | 36.5 ± 0.3 | 38.4 ± 1.2 | 37.4 ± 0.3 | 38.8 ± 0..9 | 38.4 ± 0.3 | 39.2 ± 0.7 | 39.2 ± 0.2 | 39.7 ± 0.6 |
| Blood Pressures (mm Hg) | ||||||||
| Average BP <20 Weeks EGA | 130/75 ± 10/8 | 126/76 ± 11/8 | 125/74 ± 11/8 | 126/76 ± 11/8 | 125/75 ± 8/8 | 126/76 ± 10/8 | 125/75 ± 10/8 | 127/76 ± 11/8 |
| Average BP 24–28 Weeks EGA | 127/74 ± 11/7 | 123/72 ± 11/9 | 122/71 ± 13/7 | 123/72 ± 11/9 | 124/74 ± 11/10 | 122/71 ± 11/9 | 122/71 ± 10/8 | 122/72 ± 12/9 |
| Average BP 28–32 Weeks EGA | 130/75 ± 10/9* | 123/72 ± 11/8* | 122/71 ± 13/8 | 123/72 ± 11/8 | 124/72 ± 10/9 | 122/72 ± 11/8 | 122/72 ± 11/8 | 123/72 ± 12/9 |
| Average BP 32–36 Weeks EGA | 131/79 ± 15/12* | 125/75 ± 11/8* | 126/76 ± 17/10 | 125/74 ± 11/8 | 126/77 ± 15/7 | 124/73 ± 10/8 | 121/72 ± 9/7 | 124/73 ± 11/7 |
Data presented as mean ± standard deviation
BP: Blood Pressure, EGA: Estimated Gestational age.
Blood pressures were compared as systolic versus systolic, diastolic versus diastolic.
p<0.01
Table 3.
Outcomes of Planned Delivery versus Expectant Management at 36, 37, 38, and 39 weeks Gestation
| 36 wks | 37 wks | 38 wks | 39 wks | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Delivery at 36–36.6 |
Expectant Mgmt >=36.0 |
Delivery at 37–37.6 |
Expectant Mgmt >=37.0 |
Delivery at 38–38.6 |
Expectant Mgmt >=38.0 |
Delivery at 39–39.6 |
Expectant Mgmt >=39.0 |
| n | 20 | 663 | 39 | 533 | 30 | 384 | 124 | 156 |
| Primary Neonatal Composite Outcome | 2 (10.0%) | 17 (2.6%)‡ | 2 (5.1%) | 10 (1.9%) | 0 | 9 (2.3%) | 1 (0.8%) | 5 (3.2%) |
| Stillbirth | 0 | 4 (0.6%) | 0 | 2 (0.4%) | 0 | 2 (0.5%) | 0 | 2 (1.3%) |
| Neonatal Death | 1 (0.2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe Preeclampsia/Eclampsia | 1 (5.0%) | 61 (9.2%) | 0 | 42 (7.9%) | 0 | 29 (7.6%) | 0 | 16 (10.3%)* |
| Preeclampsia (any) | 2 (10.0%) | 113 (17.0%) | 2 (5.1%) | 79 (14.8%) | 0 | 51 (13.3%)† | 3 (2.4%)* | 24 (15.4%)* |
| Primary Cesarean in Labor | 2/7 (28.6%) | 126/487 (25.9%) | 7/20 (35.0%) | 107/401 (26.7%) | 8/22 (36.4%) | 77/297 (25.9%) | 34/88 (38.6%)* | 25/125 (20.0%)* |
| Infant Length of Stay >5 days§ | 2/19 (10.5%) | 52/659 (7.9%) | 4 /39 (10.3%) | 35/531 (6.6%) | 2/30 (6.7%) | 25/382 (6.5%) | 9/124 (7.3%) | 4/154 (2.6%) |
p<0.01
p=0.03
p=0.05
Infant length of stay not calculated for stillbirths or neonatal deaths
Mgmt=Management
Bold face print indicates a p-value <0.05
Of the 572 women reaching 370/7 weeks, 39 (6.8%) underwent planned delivery between 370/7-376/7 weeks. Women undergoing planned delivery were more likely to have diabetes and baseline renal disease. Use of antihypertensive medication and average blood pressures throughout pregnancy were not different between groups. The primary neonatal composite outcome occurred more frequently in the planned delivery at 370/7-376/7 weeks, although this was not statistically significant (5.1% versus 1.9%, p=0.17). No perinatal deaths occurred in the planned delivery group while 2 (0.4%) occurred in the expectant management group, both of which were stillbirths. In the planned delivery at 37 weeks group, the two adverse neonatal outcomes were ventilator use and an arterial pH ≤7.0. In the expectant management group, the adverse composite neonatal outcomes consisted of two stillbirths, four arterial pH ≤7.0, 3 infants requiring ventilation for >1 day, and one infant requiring continuous positive airway pressure ventilation.
Of the 414 women reaching 380/7 weeks, 30 (7.2%) underwent planned delivery between 380/7-386/7 weeks. Women undergoing planned delivery were more likely to have diabetes. Women undergoing planned delivery were also more likely to have baseline renal disease although this difference did not reach statistical significance. The primary neonatal outcome did not occur in any women undergoing planned delivery between 380/7-386/7 weeks and in 9 (2.3%) of women expectantly managed after 38 weeks. No perinatal deaths occurred in the planned delivery group while 2 (0.5%) occurred in the expectant management group; both perinatal deaths were stillbirths. The other primary neonatal outcome events in the expectant management group were four infants with an arterial pH≤7, two infants requiring ventilation, one infant requiring continuous positive airway pressure ventilation.
Of the 280 women reaching 390/7 weeks, 124 (44.3%) underwent planned delivery between 390/7-396/7 weeks. Women undergoing planned delivery were similar to those receiving expectant management with respect to diabetes and baseline renal disease; women undergoing planned delivery were more likely to have had preeclampsia in a prior pregnancy. The use of antihypertensive medication and average blood pressures prior to delivery were not significantly different. The primary composite neonatal outcome occurred in only 1 (0.8%) woman undergoing planned delivery between 390/7-396/7 weeks compared to 5 (3.2%) in women receiving expectant management (p=0.17). No perinatal deaths occurred in the planned delivery group; two stillbirths occurred in the expectant management group. The adverse neonatal event in the planned delivery group was an arterial cord pH of 6.96. Of the remaining three adverse neonatal events in the expectant management group, two were arterial pH ≤7 and one was ventilator use for one day.
Preeclampsia, with or without severe features, was more likely to occur in the expectant management group, although these differences were not statistically significant until 38 weeks. Planned delivery was associated with an increased risk of primary cesarean delivery after a trial of labor only after 39 weeks (38.6% versus 20% at 39 weeks, relative risk 1.9, 95% CI 1.2–3.0). The incidence of an infant length of stay longer than 5 days was higher with planned delivery at every gestational age except for at 380/7-386/7 weeks, although this was not statistically significant at any gestational age.
As a secondary analysis, we compared outcomes with a planned delivery between 360/7-386/7 weeks with planned delivery at 390/7 weeks and beyond (Table 4, Table 5). The planned delivery beyond 390/7 weeks include those with an indicated or spontaneous delivery between 360/7-386/7 weeks, as these are consequences of planned delivery beyond 39 weeks. Women with a planned delivery 360/7-386/7 weeks weeks were more likely to have pregestational diabetes or baseline renal disease and use antihypertensives prior to pregnancy than those undergoing a planned delivery at 390/7 weeks and beyond (Table 4). The primary composite neonatal outcome was not significantly different between groups, nor was the incidence of perinatal death, primary cesarean in labor, or infant length of stay (Table 5). The incidence of severe preeclampsia and any preeclampsia was higher in the planned delivery beyond 39 weeks. Details of the four stillbirths and one neonatal death occurring after 36 weeks gestation are presented in Table 6.
Table 4.
Baseline Demographics and Pregnancy Characteristics, Deliveries Planned 36–386/7 Weeks Compared to Planned ≥39 Weeks
| Characteristic | Planned Delivery 36–386/7 Weeks | Planned Delivery ≥39 Weeks | p |
|---|---|---|---|
| n | 89 | 594 | |
| Age (years) | 30.2 ± 5.5 | 30.3 ± 6.0 | 0.88 |
| Race | 0.52 | ||
| White | 25 (28.1%) | 129 (21.7%) | |
| Black | 63 (70.8%) | 436 (73.4%) | |
| Hispanic | 1 (1.1%) | 16 (2.7%) | |
| Other | 0 (0%) | 13 (2.2%) | |
| Nulliparous | 26 (29.2%) | 166 (28.0%) | 0.80 |
| Government Insurance | 59 (66.3%) | 431 (72.6%) | 0.04 |
| Midtrimester Body Mass Index (kg/m2) | 41.5 ± 11.5 | 39.3 ± 10.9 | 0.12 |
| Tobacco use | 14 (15.7%) | 121 (20.4%) | 0.59 |
| Pregestational Diabetes | 36 (40.5%) | 109 (18.4%) | <0.01 |
| Gestational Diabetes | 11 (12.4%) | 73 (12.3%) | 0.28 |
| Baseline Renal Disease | 13 (14.6%) | 33 (5.6%) | <0.01 |
| Preeclampsia in a Prior Pregnancy | 27 (30.3%) | 184 (31.0%) | 0.44 |
| Aspirin Use | 9 (10.1%) | 42 (7.1%) | 0.05 |
| Antihypertensives Used | 55 (62.5%) | 320 (53.9%) | <0.01 |
| Prior to Pregnancy | |||
| Medications Used During Pregnancy | 67 (75.3%) | 447 (75.3%) | 0.99 |
| Blood Pressures (mm Hg) | |||
| Average BP <20 Weeks EGA | 126/74 ± 10/8 | 126/76 ± 11/8 | 0.79, 0.24* |
| Average BP 24–28Weeks EGA | 123/72 ± 12/8 | 123/72 ± 11/9 | 0.76, 0.53* |
| Average BP 28–32Weeks EGA | 124/72 ± 12/8 | 123/72 ± 11/8 | 0.38, 0.94* |
| Average BP 32–36 Weeks EGA | 127/77 ± 16/10 | 125/74 ± 11/9 | 0.14, 0.01* |
P-value for blood pressures: The first p-value represents the comparison of average systolic, the second p-value represents the comparison of the average diastolic. BP=blood pressure
Table 5.
Outcomes of Deliveries Planned 36–386/7 Weeks Compared to Planned ≥39 Weeks
| Outcome | Planned Delivery 36–386/7 Weeks | Planned Delivery ≥39 Weeks | p | AOR (95% CI) |
|---|---|---|---|---|
| n | 89 | 594 | ||
| Primary Neonatal Composite Outcome | 4 (4.5%) | 15 (2.5%) | 0.29 | 1.9* (0.6–5.9) |
| Perinatal Death | 1 (1.1%) | 4 (0.7%) | 0.50 | - |
| Severe Preeclampsia/Eclampsia | 1 (1.1%) | 61 (10.3%) | <0.01 | 0.07† (0.01–0.5) |
| Preeclampsia (any) | 4 (4.5%) | 111 (18.7%) | <0.01 | 0.1‡ (0.04–0.35) |
| Primary Cesarean in Labor | 17 (34.7%) | 111 (24.9%) | 0.14 | 1.6§ (0.8–3.0) |
| Infant Length of Stay>5 days | 8 (9.1%) | 46 (7.8%) | 0.67 | 1.0¶ (0.5–2.3) |
| Gestational Age at Delivery (weeks) | 37.6 ± 0.8 | 38.4 ± 1.2 | <0.01 | - |
Adjusted for nulliparity, tobacco use
Adjusted for baseline renal disease
Adjusted for baseline renal disease, aspirin use
Adjusted for nulliparity, body mass index
Adjusted for nulliparity, diabetes
-Not performed due to either small numbers or continuous variable
Table 6.
Characteristics of Stillbirths after 36 weeks
| Characteristic | Stillbirth #1 | Stillbirth#2 | Stillbirth#3 | Stillbirth#4 | Neonatal Death |
|---|---|---|---|---|---|
| Gestational Age at Delivery | 36.7 | 36.8 | 39.2 | 40 | 36.8 |
| Maternal Age | 23 | 17 | 24 | 30 | 29 |
| Race | Black | Black | White | White | Black |
| Nulliparous | No | Yes | Yes | Yes | Yes |
| Prior Cesarean | No | No | No | No | No |
| Tobacco Use in Pregnancy | Yes | No | Yes | No | No |
| Diabetes | No | Pregestational | No | Pregestational | No |
| Renal Disease | No | No | No | No | No |
| Years with Chronic Hypertension | 1 | 1 | <1 | 3 | 3 |
| Medications Prior to Pregnancy | No | Yes | No | No | Yes |
| Medications During Pregnancy | Yes Methyldopa | Yes Methyldopa | No | Yes Methyldopa | No |
| Gender | Male | Male | Male | Male | Female |
| Birth Weight | 3230 | 1485 | 3750 | 3725 | 2790 |
| Average Blood Pressures | |||||
| <20 weeks | 136/90 | 125/77 | 135/72 | 119/77 | 114/69 |
| 24–28 weeks | 132/83 | 130/92 | 136/63 | 131/81 | 120/73 |
| 28–32 weeks | 126/75 | 129/70 | 140/67 | 125/91 | 116/69 |
| 32–36 weeks | 129/73 | 126/76 | 124/74 | 124/85 | 120/71 |
| Last Prenatal Care Visit | |||||
| Gestational Age | 35.7 wks | 36.6 | 38.8 | 38.9 | 36.8 |
| Blood Pressure | 140/90 | 132/84 | 152/88 | 120/80 | 120/70 |
| Urinalysis for Protein | Not Documented | Not Documented | +4 | Not Documented | None Detected |
| Antenatal Testing | Yes, CST | No | Yes, CST | Yes, CST | Yes, CST |
CST=Contraction Stress Test
Discussion
In this cohort, expectant management of pregnancies complicated by chronic hypertension beyond 390/7 weeks was associated with increasing preeclampsia with severe features. Planned delivery at 36 weeks was associated with a significantly increased risk of the primary neonatal composite outcome, and delivery at 37 weeks with a non-significant increase in the risk of the primary neonatal composite outcome. These findings suggest that planned deliveries for uncomplicated chronic hypertension should occur after 38 weeks, although the optimal gestational age of delivery remains uncertain.
Our data are consistent with those of Hutcheon et al, who investigated the optimal gestational age of delivery in pregnancies with pre-existing hypertension in a large period-linked birth-infant death and stillbirth database.(10) In this study the risk of serious neonatal morbidity was significantly reduced by delaying delivery until after 38 weeks without significant increases in stillbirth risk if delivery was accomplished by 40 weeks. Unfortunately, as their database did not distinguish between pre-existing hypertension and super-imposed preeclampsia, they were unable to distinguish between indicated and non-medically indicated late preterm or early term deliveries. Consequently, the risk of ongoing pregnancy in the absence of super-imposed preeclampsia may be overstated.
Searching ClinicalTrials.gov and PubMed using the search terms “chronic hypertension,” “pregnancy,” and “induction of labor,” we identified a single randomized control trial investigating the gestational age of delivery in women with chronic hypertension (11) where 74 women were randomized to elective delivery at 37 weeks versus expectant management until 41 weeks. No difference was found in the risk of severe preeclampsia between groups; however, as the average gestational age at delivery in the planned delivery was 35 weeks, the lack of difference may be due to management choices and the risk of preeclampsia between recruitment and delivery, rather than lack of benefit to delivery at 37 weeks.
The main limitation of this study is the sample size—a limited number of patients were electively delivered prior to 39 weeks. As a result, we had limited power to detect a difference between groups. To detect the difference of 1.9% in the primary neonatal composite with expectant management compared to 5.1% with delivery, 578 patients would have been required for 80% power. The limited numbers of patients in the planned delivery group at 37 and 38 weeks therefore prevent us from making definitive recommendations. Additionally, intent was assessed retrospectively. For example, a patient presenting with spontaneous labor, stillbirth or with a new diagnosis of preeclampsia at 374/7 week may have had a planned delivery within 370/7-376/7 weeks that was not captured in data collection. This could have resulted in a misclassification bias. However, this is unlikely to have had a major influence on the results as we reviewed both the last chart note and the admission note to assess the intended gestational age of delivery. We also confirmed with chart review of the four late-preterm and term stillbirths that none were scheduled for elective delivery prior to diagnosis of the stillbirth, thus eliminating any misclassification bias for this crucial outcome.
Nonetheless, we feel that our results add to the literature due to the detailed, patient-level information that is available to us. In particular, we abstracted the indications for delivery, making it possible to determine whether the patient was electively delivered or if the delivery was indicated due to obstetrical or medical complications. We also considered the impact of the entire expectant management period on outcomes by comparing planned delivery at a given gestational age range (370/7 – 376/7 weeks) to expectant management at ≥370/7 weeks, which is the most accurate reflection of clinical management and more inclusive of the risks that a patient occurs. For example, if a clinician sees a patient at 370/7 weeks and elects to expectantly manage her until her next clinic visit at 38 weeks, she is incurring risk not just at ≥38 weeks, but also from 370/7 to 376/7. Although studies using large data sets have compared delivery at a given gestational age range to expectant management beyond that range (i.e. delivery at 370/7-376/7 weeks compared to delivery at ≥380/7 weeks), we were able to distinguish between presentation for a scheduled delivery versus and an unscheduled delivery. Additionally, we had detailed information regarding maternal co-morbidities and blood pressure throughout pregnancy. Finally, we used strict ACOG definitions of preeclampsia and severe preeclampsia with either documented proteinuria or laboratory abnormalities required for the diagnosis in addition to blood pressures.
In conclusion, expectant management beyond 390/7 weeks was associated with increasing incidence of severe preeclampsia and planned delivery prior to 37 weeks was associated with increased adverse neonatal outcomes. This suggests that the optimal gestational age of delivery for chronic hypertension may be at 38–39 weeks. Further well-powered studies are needed to confirm the optimal gestational age of delivery in this growing population of women with pregnancies complicated by chronic hypertension.
Acknowledgments
Dr. Harper is supported by K12HD001258-13, PI WW Andrews, which partially supports this work.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Presented as a poster at the Society for Maternal Fetal Medicine Annual Meeting, Atlanta Georgia, February 1–6, 2016.
References
- 1.Sibai BM. Chronic hypertension in pregnancy. Obstetrics and gynecology. 2002 Aug;100(2):369–77. doi: 10.1016/s0029-7844(02)02128-2. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad AS, Samuelsen SO. Hypertensive disorders in pregnancy and fetal death at different gestational lengths: a population study of 2 121 371 pregnancies. BJOG : an international journal of obstetrics and gynaecology. 2012 Nov;119(12):1521–8. doi: 10.1111/j.1471-0528.2012.03460.x. [DOI] [PubMed] [Google Scholar]
- 3.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. American journal of obstetrics and gynecology. 2012 Feb;206(2):134, e1–8. doi: 10.1016/j.ajog.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy UM, Laughon SK, Sun L, Troendle J, Willinger M, Zhang J. Prepregnancy risk factors for antepartum stillbirth in the United States. Obstetrics and gynecology. 2010 Nov;116(5):1119–26. doi: 10.1097/AOG.0b013e3181f903f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanit KE, Snowden JM, Cheng YW, Caughey AB. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. American journal of obstetrics and gynecology. 2012 Oct;207(4):333, e1–6. doi: 10.1016/j.ajog.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tita AT, Lai Y, Landon MB, Spong CY, Leveno KJ, Varner MW, et al. Timing of elective repeat cesarean delivery at term and maternal perioperative outcomes. Obstetrics and gynecology. 2011 Feb;117(2 Pt 1):280–6. doi: 10.1097/AOG.0b013e3182078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstetrics and gynecology. 2011 Jun;117(6):1279–87. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spong CY, Mercer BM, D'Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstetrics and gynecology. 2011 Aug;118(2 Pt 1):323–33. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of O, Gynecologists. ACOG committee opinion no. 560: Medically indicated late-preterm and early-term deliveries. Obstetrics and gynecology. 2013 Apr;121(4):908–10. doi: 10.1097/01.AOG.0000428648.75548.00. [DOI] [PubMed] [Google Scholar]
- 10.Hutcheon JA, Lisonkova S, Magee LA, Von Dadelszen P, Woo HL, Liu S, et al. Optimal timing of delivery in pregnancies with pre-existing hypertension. BJOG : an international journal of obstetrics and gynaecology. 2011 Jan;118(1):49–54. doi: 10.1111/j.1471-0528.2010.02754.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamed HO, Alsheeha MA, Abu-Elhasan AM, Abd Elmoniem AE, Kamal MM. Pregnancy outcomes of expectant management of stable mild to moderate chronic hypertension as compared with planned delivery. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2014 Oct;127(1):15–20. doi: 10.1016/j.ijgo.2014.04.010. [DOI] [PubMed] [Google Scholar]