Abstract
Background and aims
Examine age, sex, race, and socioeconomic status as modifiers of the association between carotid intimal medial thickness (IMT) and neurocognitive performance in a socioeconomically diverse, biracial, urban, adult population.
Methods
Participants were 1,712 community-dwelling adults (45% men, 56% African-American, 38% below poverty threshold, aged 30-64 years) enrolled in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Participants underwent initial carotid ultrasonography followed by cognitive testing on up to two occasions over 4 years. Mixed-effects regression analyses were adjusted for demographic, behavioral, and biomedical covariates.
Results
Significant cross-sectional IMT × race × poverty interactions were identified for measures of delayed recall memory, auditory-verbal attention, and working memory. An IMT × race interaction also appeared for auditory-verbal learning. Higher IMT was generally associated with worse cognitive performance, but the disadvantage was most pronounced among those with higher socioeconomic status and white participants. No longitudinal associations were identified.
Conclusions
Carotid IMT-cognition associations differed as a function of race and socioeconomic status and were most compelling for measures of attention, executive function, and memory. These findings highlight the possibility that subclinical atherosclerosis may be differentially informative as a predictor of cognitive performance among varied demographic subgroups.
Keywords: carotid intimal medial thickness, subclinical cardiovascular disease, cognitive function
Introduction
Subclinical carotid atherosclerosis has been linked with future cardiovascular events1, 2 and all-cause mortality3. A growing literature further demonstrates relations of subclinical atherosclerosis, estimated by carotid intimal medial thickness (IMT)4, to brain health outcomes and cognitive functioning5. In cross-sectional studies, carotid IMT has been associated with lower levels of cognitive functioning among multiple populations, including community-dwelling adults6, patients with cardiovascular disease7, and survivors of stroke8. Individuals with amnestic mild cognitive impairment9, Alzheimer’s disease10, and vascular dementia11 also have been found to have thicker IMTs. Longitudinally, our group has shown carotid IMT and/or plaque to predict cognitive decline among adults without clinical vascular disease12, as well as portend dementia diagnosis above and beyond the presence of other cardiovascular risk factors and diseases13. Other studies have identified similar longitudinal findings involving varied populations, including community-dwelling adults14, cognitively normal elderly15, and individuals with type 2 diabetes16.
Although the bulk of the evidence supports an association between subclinical atherosclerosis and cognitive functioning, three limitations of the current literature bear mention. First, much of the research has focused on screening measures such as the Mini-Mental State Examination17, 18, rather than a full neurocognitive battery designed to provide domain-specific information. Second, null findings have been identified19, suggesting that further research is necessary to identify the reasons for these inconsistencies. Third, very few, if any, studies have comprehensively addressed demographic modification of associations (e.g., age, sex, race, socioeconomic status), raising the possibility that subgroup-specific findings have been overlooked, particularly among vulnerable groups. For example, examination of socioeconomic status (SES) as a moderator has revealed carotid IMT-cognition associations to be most pronounced among lower SES participants in the Whitehall II study20. Young adults, women, whites, and individuals of higher SES generally have lower IMTs and slower IMT progression over time21-23, although higher SES African-Americans may be uniquely susceptible to faster IMT progression24. Although these studies have documented sex-, race-, and SES-related differences in carotid IMT and IMT progression, to our knowledge, none have directly examined these differences (or interactions of these differences) in relation to cognition.
In the present study, we addressed these limitations using data from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study, which is uniquely designed to evaluate multiple demographic modifiers of health associations simultaneously because of its biracial, socioeconomically diverse sample of 30-64-year-old men and women. We used mixed-effects regression models to examine age, sex, race, and SES as effect modifiers of IMT in the prediction of neurocognitive performance. Because of the novelty of this study and associated lack of precedent in the literature, these analyses were exploratory, but we posited that select subgroups may show relative vulnerability to the effect of carotid IMT on cognition. We expected the domains of memory, attention, and executive function to be most affected, based on prior literature.12, 25
Materials and methods
Participants
Participants were recruited via field interview and enrolled in the baseline (August 2004 – March 2009) and first examination follow-up (June 2009 – July 2013) waves of the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. HANDLS is a prospective, population-based, longitudinal study examining the influences and interaction of race and SES on the development of health disparities. Comprehensive information regarding study design and procedures has been published elsewhere26. Briefly, a fixed cohort of community-dwelling adults was recruited from an area probability sample of 13 Baltimore City neighborhoods chosen to span diverse levels of income and socioeconomic status. Approximately equal numbers of participants were recruited from separate clusters of contiguous census tracts – neighborhoods – containing sufficient numbers of residents to fill a factorial cross of sex, race (African-American or white), 5-year age groups (ranging from 30-64 years), and poverty status (above or below 125% of the federal poverty lines). Households were selected randomly for potential participation, as were individuals within households. Participants were eligible if they self-identified as either Black/African-American or White/Caucasian. Recruiters visited 32,959 dwellings in which they found 14,799 potentially eligible individuals in 9,904 households among whom 8,150 individuals actually met initial screening criteria. Of these potentially eligible individuals, 3,720 participants met all study inclusion criteria and none of the exclusion criteria listed below. Study inclusion criteria at baseline were 1) age of 30-64 years, 2) able to give informed consent, 3) able to perform at least five data measures on the medical research vehicle (MRV), and 4) able to provide valid picture identification. Study exclusion criteria at baseline were 1) pregnancy and 2) being within 6 months of receiving chemotherapy, radiation, or biological treatments for cancer. If the participant was too ill to participate due to AIDS or blood pressure >160/100 at the first MRV visit, the visit was delayed until their health improved. Seventy-eight percent of all eligible and non-excluded individuals agreed to participate in the first wave of the HANDLS study. HANDLS was approved by the MedStar Institutional Review Board and the National Institute of Environmental Health Science, NIH. All participants provided informed consent.
Among individuals who completed both phases of the HANDLS protocol (n=2,707, described below), 1,993 participants completed carotid ultrasonography. Exclusions for carotid ultrasonography included 1) elevated blood pressure at time of ultrasound (>200/100), 2) presence of carotid bruit, 3) weight exceeding or equal to 295 pounds, and 4) inability to lie in a completely supine position for 15 minutes. For the present analysis, we additionally excluded individuals with stroke (n=32), dementia (n=2), ongoing dialysis treatment (n=1), history of carotid endarterectomy (n=2), heart failure (n=36), HIV/AIDS (n=45), epilepsy (n=63), Parkinson’s disease (n=1), multiple sclerosis (n=7), schizophrenia (n=17), bipolar disorder (n=74), or missing data on all cognitive measures (n=1). The final sample thus included 1,712 participants, 1,258 of whom participated at both Waves 1 and 3.
General procedures
The HANDLS protocol was administered in two phases during the first wave. Phase I was conducted in participants’ homes and involved screening, recruitment, informed consent, and administration of an interview regarding sociodemographic characteristics, neighborhood characteristics, and similar information. Phase II, conducted at Waves 1 and 3, took place in mobile MRVs that visited each neighborhood. Data collected in the MRVs included medical history, physical examination, laboratory measurements, cognitive testing, and other physiological diagnostic procedures.
Carotid IMT assessment
High resolution B-mode ultrasonography of the left common carotid artery was performed with a standard transducer (5.OL45) and equipment (Acuson CV 70, Siemens) at the Wave 1 MRV visit. A region 1.5 cm proximal to the carotid bifurcation was identified, and the IMT of the far arterial wall was evaluated as the distance between the intimal-luminal interface and the medial-adventitial interface. Specific care was taken to measure IMT in areas devoid of plaque. IMT was measured on a frozen-frame image, magnified to achieve higher resolution of detail. The IMT measurement was obtained from five contiguous sites at approximate 1-mm intervals; the mean of these values was used in statistical analyses. Measurements were performed by a single sonographer. Intraobserver correlation between repeated carotid IMT measurements on 10 participants was 0.96 (p <0.001).27
Neurocognitive assessment
During both Waves 1 and 3, cognitive measures were administered by highly trained psychometrists. The numbers that follow each test indicate respective sample sizes because of test-specific missing data. The Mini-Mental State Examination (MMSE; n=1,696) is a 30-item cognitive screening measure28. The Benton Visual Retention Test (BVRT, 5th edition, form D, administration A; n=1,710) evaluated immediate visuospatial memory29. Total number of errors served as the outcome measure. A modified version of the California Verbal Learning Test (CVLT; n=1,707) assessed auditory-verbal learning and memory30. Three learning trials were administered instead of the standard five trials. Outcome measures included total correct for List A trials 1-3, short-delay free recall, and long-delay free recall. A 60-second animal fluency trial (n=1,686) measured language and semantic association fluency. The Numbers trial of the Brief Test of Attention (n=1,428) assessed auditory divided attention31, 32. The forward and backward trials of the Digit Span subtest of the Wechsler Adult Intelligence Scale-Revised (n=1,707) measured attention and working memory33. Lastly, parts A and B of the Trail Making Test (TMT; n=1,683) assessed attention, visual scanning, psychomotor speed, and mental flexibility34.
Demographic characteristics and covariates
Demographic characteristics included age (in years), sex (0=female, 1=male), self-identified race (0=White, 1=African-American), education (in years), and poverty status (0=above 125% of the poverty threshold, 1=at or below 125% of the poverty threshold). Poverty status was based on household size and reported family income relative to the 2004 federal poverty threshold (e.g., $18,850 per year for a family of 4) published by the Department of Health and Human Services (DHHS) based on national averages. In Baltimore, 125% of the poverty threshold better approximates economic hardship due to the city’s higher cost of living relative to the national average.
During the MRV visit, a HANDLS physician or nurse practitioner conducted a comprehensive physical examination and medical history. The assessor recorded any diagnosable medical conditions and use of medications as carefully as possible. Antihypertensive and lipid-lowering medication use were both coded dichotomously (0=not currently taking, 1=currently taking). In the present analyses, a cardiovascular disease cluster variable was created based on diagnoses of coronary artery disease, myocardial infarction, peripheral artery disease, atrial fibrillation, angioplasty, and coronary artery bypass surgery. Each medical condition was coded dichotomously (0=not present, 1=present), and a summation score was created to represent the cluster variable. Diabetes mellitus diagnosis was coded as its own variable (0=no diabetes, 1=diabetes). Body mass index (BMI) was calculated as the ratio of weight (kg) to height (m) squared, both measured with calibrated equipment. Resting brachial systolic blood pressure (SBP) and diastolic blood pressure were measured in both arms in the seated position with an aneroid manometer and stethoscope. Left and right arm SBPs were averaged for analyses. Fasting venous blood specimens for total cholesterol assay were collected on the mobile MRV and analyzed at the NIA Clinical Research Branch Core Laboratory (Baltimore, MD, USA) and Quest Diagnostics Inc. (Baltimore, MD and Chantilly, VA, USA) using a spectrophotometer (AU5400 Immuno Chemistry Analyzer; Olympus, Center Valley, PA, USA). Self-reported alcohol, cigarette smoking, and illicit drug (marijuana, cocaine, opiates) status were each coded dichotomously (0=never used, 1=ever used). The 20-item Center for Epidemiological Studies-Depression scale35 assessed depressive symptoms.
Statistical analyses
All statistical analyses were performed using SAS version 9.3 (Cary, NC). Mixed-effects regression analyses were conducted to examine longitudinal relations of baseline continuous carotid IMT to cognitive performance across both waves. Each cognitive measure was entered as a single outcome variable in separate sequential models. Covariates included age, sex, race, poverty status, education, alcohol status, cigarette status, illicit drug status, depressive symptoms, SBP, total cholesterol, BMI, antihypertensive use, lipid-lowering medication use, cardiovascular disease cluster, and diabetes mellitus. Height and weight were also considered in lieu of BMI but were found not to affect the analyses meaningfully, so BMI alone was retained for the sake of parsimony. Except for age, demographic characteristics and other covariates were coded in a time-independent fashion (i.e., based on Wave 1 data only). Age was centered and transformed linearly to reflect decade (i.e., divided by 10) for model estimation purposes. Age was also modeled as a random effect to index time. Neither Part A nor Part B of the Trail Making Test was normally distributed, so these measures were natural log transformed prior to analysis.
In accordance with the factorial design of HANDLS, initial mixed-effects models examined all possible 3-way interactions involving carotid IMT and the demographic characteristics of age, sex, race, and poverty status. Backward elimination of non-significant higher-order terms was then employed until a final model was specified. All significant interactions were probed for simple effects using SAS PROC GLM. For the purpose of simple effects testing only, IMT was dichotomized according to a median split (</≥0.7 mm). Coefficients with p <0.05 were considered statistically significant.
Because of an unusually high number of participants scoring zero on the CVLT List A total score measure (n=252), a sensitivity analysis was performed to examine any changes in results associated with listwise exclusion of these participants. These floor scores occurred in the context of broadly intact performance on other memory measures and absence of diagnosed dementia, which raised suspicion that the challenging and repetitive nature of the task may have contributed to suboptimal effort in a subset of participants.
Results
Sample characteristics
Table 1 presents baseline descriptive statistics, both for the full sample and stratified by race and poverty status. Overall, participants were 45% men, 56% African-American, and ranged in age from 30 to 64 years (mean=47 years). Approximately 38% of participants reported having a household income below the 125% federal poverty threshold.
Table 1.
Baseline descriptive statistics, full sample and stratified by race/poverty.
| Full sample (n=1,712) |
African-American below poverty (n=428) |
African-American above poverty (n=523) |
White below poverty (n=226) |
White above poverty (n=535) |
|
|---|---|---|---|---|---|
|
| |||||
| Variable | Mean(SD)/% | Mean(SD)/% | Mean(SD)/% | Mean/SD/% | Mean(SD)/% |
| Age (years) | 46.9 (9.3) | 45.8 (9.0) | 47.5 (9.4) | 46.1 (9.2) | 47.6 (9.4) |
| Male (%) | 44.9 | 44.9 | 48.6 | 34.5 | 45.8 |
| African-American (%) | 55.6 | N/A | N/A | N/A | N/A |
| Below Poverty Line (%) | 38.2 | N/A | N/A | N/A | N/A |
| Education (years) | 12.7 (3.1) | 11.7 (2.3) | 13.0 (2.7) | 11.6 (3.0) | 13.7 (3.6) |
| Cigarette use (% ever) | 72.5 | 73.1 | 72.5 | 75.7 | 70.7 |
| Alcohol use (% ever) | 77.2 | 68.5 | 80.3 | 79.2 | 80.2 |
| Illicit drug use (% ever) | 45.4 | 50.7 | 49.3 | 40.3 | 39.4 |
| CES-D (total score) | 14.0 (10.8) | 16.4 (11.1) | 12.2 (9.8) | 17.4 (11.7) | 12.5 (10.4) |
| Cardiovascular disease (%) | 3.2 | 3.5 | 3.1 | 4.0 | 2.8 |
| Diabetes mellitus (%) | 9.9 | 7.7 | 13.0 | 13.3 | 7.1 |
| Systolic blood pressure (mm Hg) | 119.5 (17.2) | 120.9 (17.8) | 120.6 (16.4) | 118.2 (18.0) | 117.8 (16.9) |
| Total cholesterol (mg/dl) | 186.9 (40.7) | 182.5 (41.6) | 184.2 (38.9) | 187.0 (42.5) | 192.9 (40.4) |
| Body mass index (kg/m2) | 29.4 (7.4) | 28.3 (7.4) | 29.8 (7.0) | 30.7 (8.6) | 29.4 (7.0) |
| Antihypertensive use (%) | 25.3 | 24.3 | 29.3 | 19.0 | 24.9 |
| Lipid-lowering medication use (%) | 11.0 | 6.8 | 10.7 | 10.6 | 14.8 |
| Intimal medial thickness (mm) | 0.69 (0.13) | 0.69 (0.14) | 0.72 (0.12) | 0.67 (0.13) | 0.67 (0.13) |
CESD, Center for Epidemiological Studies-Depression scale.
Mixed-effects models
Fully specified and adjusted mixed-effects models included all possible 3-way interactions involving carotid IMT and the demographic characteristics of age, sex, race, and poverty status (IMT × race × poverty, IMT × race × sex, IMT × race × age, IMT × poverty × sex, IMT × poverty × age, IMT × sex × age). Longitudinal effects, which would have been indicated by significant age-related interaction terms, were not identified (all ps >0.05). Significant interactions appeared only for IMT × race × poverty, so all other 3-way interactions (and associated lower-order 2-way interactions) were eliminated to produce final models. These models thus included terms for age, sex, race, poverty status, IMT, IMT × race, IMT × poverty, race × poverty, IMT × race × poverty, and each aforementioned covariate. Results of the final models are presented in Table 2.
Table 2.
Results from mixed-effects regression models predicting neurocognitive test performance from carotid IMT, interactive effects, and covariates1.
| Test | IMT | IMT × Race |
IMT × Poverty |
IMT × Race × Poverty |
|---|---|---|---|---|
|
| ||||
| b (SE) | b (SE) | b (SE) | b (SE) | |
| Mini-mental state examination (total score) | −0.30(0.65) | 0.50(0.93) | 1.36(1.15) | −2.30(1.48) |
| Benton Visual Retention Test (total errors) | −0.11(1.48) | −3.44(2.10)† | −0.97(2.61) | 2.47(3.35) |
| California Verbal Learning Test (List A Total) | −2.93(2.55) | 8.19(3.69)* | 7.68(3.69)† | −10.3(5.90)† |
| California Verbal Learning Test (Free Recall-Short) | −0.83(0.97) | 2.46(1.39)† | 2.67(1.72) | −3.80(2.21)† |
| California Verbal Learning Test (Free Recall-Long) | −1.15(0.96) | 3.08(1.38)* | 3.38(1.71)* | −5.64(2.20)* |
| Animal fluency (total score) | −2.71(1.60)† | 3.22(2.31) | 1.33(2.87) | 0.16(3.69) |
| Brief Test of Attention (total score) | −0.47(0.69) | 0.67(.97) | 1.03(1.21) | −2.30(1.55) |
| Digit Span Forward (raw score) | −1.26(0.73)† | 1.79(1.05)† | 4.26(1.30)** | −3.51(1.67)* |
| Digit Span Backward (raw score) | −0.05(0.68) | 1.46(0.98) | 2.36(1.22)† | −4.26(1.57)** |
| Trail Making Test, Part A (seconds) | −16.4(14.2) | 29.4(19.9) | 6.55(24.2) | −18.6(31.2) |
| Trail Making Test, Part B (seconds) | −7.92(48.0) | −15.2(67.7) | −90.0(85.3) | 135.8(108.6) |
p <0.05,
p <0.01,
p <0.10.
Models adjusted for age, sex, race, poverty status, education, substance use, depressive symptoms, systolic blood pressure, total cholesterol, body mass index, antihypertensive use, lipid-lowering medication use, cardiovascular disease, and diabetes. Race × poverty coefficients not shown for brevity. IMT, intimal medial thickness.
IMT × race
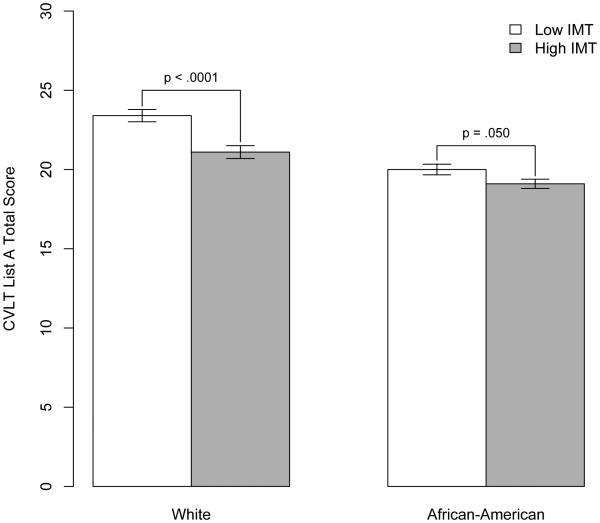
A significant IMT × race effect appeared for CVLT List A total score (b=8.19, SE=3.69, p=0.027). Shown in Figure 1, individuals with lower IMT learned more words on this measure, but the discrepancy was significant only among whites (p < .0001) and not African-Americans (p=0.050).
Fig. 1. Mean cognitive performance by carotid IMT and race for California Verbal Learning Test (CVLT) List A total score.
IMT was dichotomized according to median split (</≥0.7 mm) only for the purposes of simple effects testing and graphical representation.
IMT × race × poverty
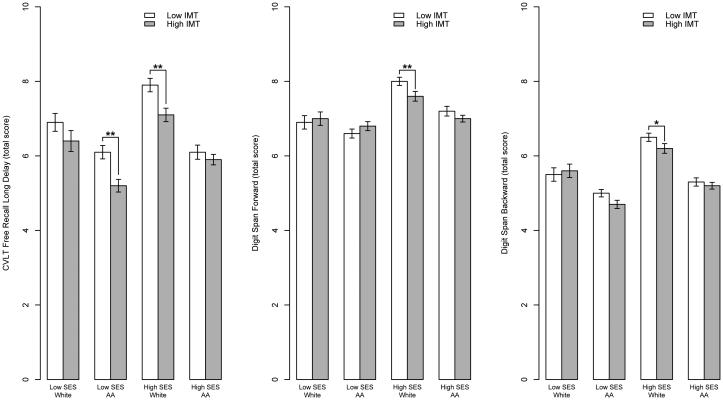
Significant IMT × race × poverty interactions appeared for the long-delay free recall trial of the CVLT (b=-5.64, SE=2.20, p=0.010), Digit Span Forward (b=− 3.51, SE=1.67, p=0.036), and Digit Span Backward (b=−4.26, SE=1.57, p=0.007). Figure 2 illustrates these findings.
Fig. 2. Mean cognitive performance by carotid IMT, race, and poverty status.
(A) California Verbal Learning Test (CVLT) long delayed recall; (B) Digit Span Forward; (C) Digit Span Backward. IMT was dichotomized according to median split (</≥0.7 mm) only for the purposes of simple effects testing and graphical representation. *p <0.05 **p <0.01
Simple effects testing revealed group-specific differences in IMT effects. For the long- delay free recall trial of the CVLT, high SES white participants with lower IMT recalled significantly more words than high SES white participants with higher IMT (p=0.0004), but among high SES African-American participants, the performance difference across IMT groups was nonsignificant (p=0.469). Conversely, among low SES participants, African-Americans with lower IMT performed significantly better than those with higher IMT (p=0.0003), but there was no significant difference among whites (p=0.145). For Digit Span Forward, there was a significant performance difference across IMT groups only for high SES white participants (p=0.006). The same pattern appeared for Digit Span Backward, such that high SES white participants with low IMT performed better than their counterparts with high IMT (p=0.049).
Sensitivity analysis
As described earlier, a sensitivity analysis was performed with listwise removal of participants with scores of zero on CVLT List A total score (n=252). Results of this analysis are shown in the supplementary table online. Significance of results remained identical for all but three cognitive measures. First, an additional three-way IMT × race × poverty effect appeared for CVLT List A total (b=−9.54, SE=4.73, p=0.044). Simple effects testing revealed that high SES whites with low IMT recalled significantly more words than high SES whites with higher IMT (p <0.0001). Second, the previously significant IMT × race × poverty term for Digit Span Forward became non-significant, leaving a significant IMT × poverty term instead (b=3.48, SE=1.29, p=0.007). High SES participants with low IMT recalled significantly more digits than high SES participants with high IMT (p=0.0004); there was no significant difference across IMT groups among low SES participants (p=0.12). Lastly, an IMT main effect appeared for animal fluency (b=−3.73, SE=1.63, p=0.023), such that individuals with low IMT produced significantly more words (M=19.5, SD=5.6) than individuals with high IMT (M=18.9, SD=5.3), regardless of race or poverty status.
Discussion
In this prospective, population-based, longitudinal study of a biracial, socioeconomically diverse sample of 30-64-year-old adults, we have novelly extended the current IMT-cognition literature by examining patterns of demographic variability within these associations, which suggest that subgroup-specific findings may account for inconsistencies that have appeared in the literature. To our knowledge, no prior studies have simultaneously considered race and SES as moderators of IMT-cognition associations. In the present study, higher IMT was generally associated with worse cognitive performance, but the disadvantage was most pronounced among those with higher SES and white participants. Carotid IMT-cognition associations were most compelling for measures of attention, executive function, and memory. These findings highlight the possibility that subclinical atherosclerosis may be differentially informative as a predictor of cognitive performance among varied demographic subgroups.
Our results are consistent with an increasingly established literature connecting carotid IMT with cross-sectional cognitive performance5, 6 We have additionally identified significant cross-sectional interactions among IMT, race, and poverty status for multiple cognitive tests, including measures of delayed recall memory (CVLT delayed recall), auditory-verbal attention (Digit Span Forward), and working memory (Digit Span Backward). An interaction of IMT and race was also noted for auditory-verbal learning (CVLT List A Total). Higher IMT was generally associated with worse cognitive performance. However, the IMT-related cognitive disadvantage was most pronounced among certain subgroups, most commonly higher SES and white participants, although the pattern also appeared in lower SES African-American participants in one instance. A sensitivity analysis using a slightly curtailed sample revealed similar results.
One prior study examined socioeconomic status as an individual moderator of IMT- cognition associations20. In that study, Singh-Manoux and colleagues found carotid IMT to be associated with six measures of cognitive function (MMSE, immediate verbal memory, inductive reasoning, vocabulary, semantic fluency, phonemic fluency) among only low SES participants enrolled in Phase 7 of the Whitehall II study (n=3,896, ages 50-74 years). Socioeconomic status was based on British civil service grade of employment and defined as high (administrative), intermediate (professional or executive), or low (clerical or support). This operationalization is important, as it differs substantially from the definition used in the present study of above/below the 125% U.S. federal poverty line. Many HANDLS participants in our “low SES” group were unemployed or employed in lower-income occupations (e.g., unskilled labor) than that of a typical “low SES” Whitehall II study participant, who most often held a white-collar position. Relatedly, our “high SES” participants should not be considered representative of the wealthy elite that “high SES” commonly connotes. Rather, many “high SES” HANDLS participants were employed in occupations commensurate with all three of the British civil service grades defined as low, intermediate, or high in the Whitehall II study. Substantial space is dedicated to this distinction here to emphasize that although our findings ostensibly appear to conflict with Singh- Manoux et al.’s results, the reality may be quite different given important differences in the sample demographics, definitions of SES, and variable consideration of race. The Whitehall II study presumably was not properly equipped to study racial differences, as the vast majority of participants were white (>90%, based on other studies using similar data36).
SES definitional issues aside, we speculate that carotid IMT-cognition associations were most striking among higher SES whites in HANDLS because other more potent cardiovascular risk factors/diseases were either excluded or covaried in the analyses. That is, lower SES and/or African-American individuals, who are known to be more vulnerable groups, may not robustly demonstrate IMT-cognition associations because a subclinical disease is insufficiently informative after accounting for other risk factors and conditions, which are known to be more prevalent in these groups. With respect to cognition in a relatively “young” group (i.e., non- elderly), we wonder whether subclinical disease may be less revealing among vulnerable populations than among less vulnerable populations.
A variety of cognitive domains have been linked with carotid IMT5. However, in the more extensive cardiovascular risk factor/disease literature, attention and executive functioning have been highlighted as especially susceptible domains 25. This conclusion is consistent with our findings relevant to Digit Span Forward and Digit Span Backward. In this context, it is surprising that the Trail Making Test (particularly Part B) would not demonstrate significance as well. It is possible that the processing speed component of this test was less sensitive in this non- elderly sample, but this factor alone seems insufficient to explain its non-significance. Memory has also received recent attention as a vulnerable domain relevant to clinical and subclinical cardiovascular disease37 because of vulnerability of the mesial temporal lobes to hypoperfusion and increasing recognition of frequently “mixed” (i.e., vascular and Alzheimer’s) dementia pathology. Indeed, our group previously identified robust carotid IMT-memory associations among adults without clinical vascular or brain disease12. The pervasive CVLT findings in the present study are thus consistent with current literature.
No longitudinal findings were identified in the present study, which is not necessarily unexpected in the context of a relatively short test-retest interval for young and middle-aged adults. That is, although slight decrements in cognitive performance can be identified over 5 years in this age group, these changes may not have been substantial enough for significant covariation with carotid IMT. We would hypothesize that future analyses of HANDLS or similar samples would detect longitudinal effects over longer follow-up of similar-aged individuals.
In our sample, both low SES whites and low SES African-Americans had fewer years of education than their high SES counterparts. Although including education as a primary term of interest was beyond the scope of our analyses, and we are thus limited in our ability to draw conclusions regarding its role in our results, we hope future papers address education specifically. Years of education does not always equivalently index true educational status across races due to variable quality of education,38 so future studies should also consider alternative measures such as literacy or educational quality measures when available, particularly when considering cognitive function outcomes.
Numerous mechanisms may link carotid IMT with cognition, including shared risk factors, common genetic vulnerability, chronic cerebral hypoperfusion, and silent cerebrovascular disease39-41. IMT-dependent regional cerebral blood flow (rCBF) differences have been identified42, and neuronal viability suffers with presence of subclinical atherosclerosis43. Carotid IMT has also been increasingly linked with future dementia, both due to Alzheimer’s disease and vascular causes 13, 44, 45, and IMT has been shown to predict response to cholinesterase inhibitors in Alzheimer’s disease46. While there is no shortage of explanatory hypotheses, the specific interrelations and pathways are yet to be understood, especially in relation to demographic subgroups. Regardless, findings of the present study suggest that future mechanistic work would benefit from consideration of race and SES as moderating influences.
The major strength of this investigation was its use of a socioeconomically diverse, biracial, population-based sample for the purpose of a thorough examination of effect modification of carotid IMT-cognition associations. Our use of a comprehensive neurocognitive test battery also extends an existing literature that is seriously limited by over-reliance on cognitive screening measures such as the MMSE. Several limitations also warrant mention. Carotid IMT was measured only once, which precluded examination of longitudinal covariation of carotid IMT and cognitive changes. Additionally, only a sub-sample of HANDLS participants underwent carotid ultrasonography due to necessary additional exclusion criteria and scheduling demands, which raises the possibility of selection bias (e.g., our sample may be healthier overall than the larger HANDLS cohort, or more willing/available to undergo an additional test on the mobile MRV). Our longitudinal cognitive follow-up was also limited to a single follow-up visit, although data collection is ongoing and will allow for more extended tracking in the future. Lastly, the HANDLS design did not allow for a more nuanced examination of different operationalized definitions of SES, such as continuous income level or occupational status.
In summary, IMT-cognition associations differed as a function of race and SES among participants in the HANDLS study. Memory, attention, and executive function were most affected, and associations were typically strongest among higher SES white participants. While the clinical significance of these findings may be small on an individual level, aggregate effects on a population level have important public health implications (e.g., population-specific prevention efforts, identification and use of appropriate risk markers in appropriate groups). At a minimum, the presence of interactive associations suggests that future studies would benefit from consideration of demographic moderators. Carotid IMT and other markers of subclinical cardiovascular disease may be more or less informative risk indicators in certain groups. Inclusion of comprehensive neurocognitive testing, rather than reliance on cognitive screening, also seems critical to avoid inadvertent misinterpretations and conclusions (e.g., Type II errors due to neglect of domain-specific effects), particularly among non-elderly samples.
Supplementary Material
Greater carotid IMT was generally associated with worse cognitive performance.
The domains of attention, executive function, and memory were most affected.
IMT-cognition associations differed as a function of race and socioeconomic status.
Cognitive disadvantage was most pronounced among higher SES and white individuals.
Acknowledgments
Financial support
This work was supported by the National Institute on Aging Intramural Research Program of the National Institutes of Health (ZIA-AG000195) and a National Institutes of Health grant (RO1 AG034161). We thank the HANDLS Mobile Medical Research Vehicle Staff with special recognition to the psychometrists and the ultrasonographer, Mary Lassiter, for their careful evaluation of HANDLS participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- [1].Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- [2].O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. New England Journal of Medicine. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- [3].Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- [4].Grobbee DE, Bots ML. Carotid artery intima-media thickness as an indicator of generalized atherosclerosis. J Intern Med. 1994;236:567–573. doi: 10.1111/j.1365-2796.1994.tb00847.x. [DOI] [PubMed] [Google Scholar]
- [5].Wendell CR, Waldstein SR. Subclinical cardiovascular disease and neurocognition. In: Waldstein SR, Elias MF, editors. Neuropsychology of Cardiovascular Disease. Taylor & Francis; New York: 2015. pp. 319–342. [Google Scholar]
- [6].Zhong W, Cruickshanks KJ, Huang GH, et al. Carotid atherosclerosis and cognitive function in midlife: the Beaver Dam Offspring Study. Atherosclerosis. 2011;219:330–333. doi: 10.1016/j.atherosclerosis.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haley AP, Forman DE, Poppas A, et al. Carotid artery intima-media thickness and cognition in cardiovascular disease. International Journal of Cardiology. 2007;121:148–154. doi: 10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Talelli P, Ellul J, Terzis G, et al. Common carotid artery intima media thickness and post-stroke cognitive impairment. Journal of the Neurological Sciences. 2004;223:129–134. doi: 10.1016/j.jns.2004.05.013. [DOI] [PubMed] [Google Scholar]
- [9].Casado Naranjo I, Portilla Cuenca JC, Duque de San Juan B, et al. Association of vascular factors and amnestic mild cognitive impairment: a comprehensive approach. J Alzheimers Dis. 2015;44:695–704. doi: 10.3233/JAD-141770. [DOI] [PubMed] [Google Scholar]
- [10].Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- [11].Ban Y, Watanabe T, Miyazaki A, et al. Impact of increased plasma serotonin levels and carotid atherosclerosis on vascular dementia. Atherosclerosis. 2007;195:153–159. doi: 10.1016/j.atherosclerosis.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [12].Wendell CR, Zonderman AB, Metter EJ, et al. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke. 2009;40:3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wendell CR, Waldstein SR, Ferrucci L, et al. Carotid atherosclerosis and prospective risk of dementia. Stroke. 2012;43:3319–3324. doi: 10.1161/STROKEAHA.112.672527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arntzen KA, Schirmer H, Johnsen SH, et al. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects - the Tromso study. Cerebrovasc Dis. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- [15].Sander K, Bickel H, Forstl H, et al. Carotid- intima media thickness is independently associated with cognitive decline. The INVADE study. Int J Geriatr Psychiatry. 2010;25:389–394. doi: 10.1002/gps.2351. [DOI] [PubMed] [Google Scholar]
- [16].Feinkohl I, Keller M, Robertson CM, et al. Clinical and subclinical macrovascular disease as predictors of cognitive decline in older patients with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2013;36:2779–2786. doi: 10.2337/dc12-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnston SC, O'Meara ES, Manolio TA, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Annals of Internal Medicine. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- [18].Rossetti HC, Weiner M, Hynan LS, et al. Subclinical atherosclerosis and subsequent cognitive function. Atherosclerosis. 2015;241:36–41. doi: 10.1016/j.atherosclerosis.2015.04.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- [20].Singh-Manoux A, Britton A, Kivimaki M, et al. Socioeconomic status moderates the association between carotid intima-media thickness and cognition in midlife: Evidence from the Whitehall II study. Atherosclerosis. 2008;197:541–548. doi: 10.1016/j.atherosclerosis.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Diez-Roux AV, Nieto FJ, Tyroler HA, et al. Social inequalities and atherosclerosis. The atherosclerosis risk in communities study. Am J Epidemiol. 1995;141:960–972. doi: 10.1093/oxfordjournals.aje.a117363. [DOI] [PubMed] [Google Scholar]
- [22].Folsom AR, Eckfeldt JH, Weitzman S, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- [23].Lynch J, Kaplan GA, Salonen R, et al. Socioeconomic status and progression of carotid atherosclerosis. Prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol. 1997;17:513–519. doi: 10.1161/01.atv.17.3.513. [DOI] [PubMed] [Google Scholar]
- [24].Ranjit N, Diez-Roux AV, Chambless L, et al. Socioeconomic differences in progression of carotid intima-media thickness in the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2006;26:411–416. doi: 10.1161/01.ATV.0000198245.16342.3d. [DOI] [PubMed] [Google Scholar]
- [25].O'Brien JT. Vascular cognitive impairment. American Journal of Geriatric Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- [26].Evans MK, Lepkowski JM, Powe NR, et al. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- [27].Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–1509. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- [28].Folstein MF, Folstein SE, McHugh PR. "Mini-mental state." A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [29].Sivan AB. Benton Visual Retention Test. The Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- [30].Delis DC, Kramer J, Kaplan E, et al. California Verbal Learning Test: Adult version. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- [31].Schretlen D. The Brief Test of Attention. Psychological Assessment Resources; Lutz, FL: 1989. [Google Scholar]
- [32].Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the Brief Test of Attention. The Clinical Neuropsychologist. 1996;10:80–89. [Google Scholar]
- [33].Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- [34].Reitan R. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychological Laboratory; Tucson, AZ: 1992. [Google Scholar]
- [35].Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- [36].Forde I, Chandola T, Raine R, et al. Socioeconomic and ethnic differences in use of lipid-lowering drugs after deregulation of simvastatin in the UK: the Whitehall II prospective cohort study. Atherosclerosis. 2011;215:223–228. doi: 10.1016/j.atherosclerosis.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Waldstein SR, Wendell CR. Neurocognitive function and cardiovascular disease. J Alzheimers Dis. 2010;20:833–842. doi: 10.3233/JAD-2010-091591. [DOI] [PubMed] [Google Scholar]
- [38].Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care. 2006;44:S10–16. doi: 10.1097/01.mlr.0000245427.22788.be. [DOI] [PubMed] [Google Scholar]
- [39].Appelman AP, van der Graaf Y, Vincken KL, et al. Combined effect of cerebral hypoperfusion and white matter lesions on executive functioning - The SMART-MR study. Dement Geriatr Cogn Disord. 2010;29:240–247. doi: 10.1159/000289813. [DOI] [PubMed] [Google Scholar]
- [40].de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer's disease? Neurol Res. 2006;28:637–644. doi: 10.1179/016164106X130362. [DOI] [PubMed] [Google Scholar]
- [41].Haan MN, Shemanski L, Jagust WJ, et al. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Journal of the American Medical Association. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- [42].Sojkova J, Najjar SS, Beason-Held LL, et al. Intima-media thickness and regional cerebral blood flow in older adults. Stroke. 2010;41:273–279. doi: 10.1161/STROKEAHA.109.566810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Haley AP, Tarumi T, Gonzales MM, et al. Subclinical atherosclerosis is related to lower neuronal viability in middle-aged adults: a 1H MRS study. Brain Res. 2010;1344:54–61. doi: 10.1016/j.brainres.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- [45].van Oijen M, de Jong FJ, Witteman JC, et al. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- [46].Modrego PJ, Rios C, Perez Trullen JM, et al. Carotid intima-media thickness as a predictor of response to cholinesterase inhibitors in Alzheimer's disease: an open-label trial. CNS Drugs. 2009;23:253–260. doi: 10.2165/00023210-200923030-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.