Abstract
Objective
To investigate whether women who deliver preterm have excess risk for metabolic dysregulation independent of prepregnancy factors.
Methods
We conducted a a multi-center, longitudinal, observational study of 1,205 women (50% Black) in the Coronary Artery Risk Development in Young Adults (CARDIA) study with at least one birth between baseline (1985–1986) and year 25, and no metabolic syndrome or diabetes before pregnancy. Cardiometabolic factors were measured prepregnancy and at up to five subsequent examinations. We estimated the relative hazards (RH) of incident metabolic syndrome in women with 1 or more preterm births (<37 weeks, n=295) versus only term births (≥37 weeks, n=910). Self-reported gestational diabetes mellitus (GDM), hypertension during pregnancy, and time-dependent weight gain were also considered as covariates.
Results
Of 315 metabolic syndrome cases in 17,717 person-years of follow-up, the incidence rate was higher among women with preterm compared to term births (22.0 vs. 16.4 per 1,000 person-years; RH 2.91 [95% CI 2.75. 3.09]). After adjustment for prepregnancy cardiometabolic factors and covariates, the RH (95%CI) for metabolic syndrome was 1.52 (1.22, 1.88) for women with preterm compared with term births. Gestational diabetes mellitus, hypertension during pregnancy, and weight gain only modestly attenuated this association. Elevated blood pressure (36.3% vs. 26.7%, p=0.002) and central adiposity (51.5% vs. 44.0%, p=0.02) were the individual metabolic syndrome components that were different in women with preterm compared with term births.
Conclusion
Women with a history of preterm birth have increased risk of incident metabolic syndrome compared to those with term births, independent of the prepregnancy metabolic status and pregnancy complications.
Introduction
Women who deliver preterm have excess risk for diabetes and cardiovascular disease, although mechanisms linking these are not well understood.1–5 Length of gestation is inversely related to blood pressure, insulin resistance, and low grade inflammation in mothers several years after delivery.6–8 These data suggest that dysregulated metabolic factors may link preterm delivery to later life maternal disease.
The metabolic syndrome is a cluster of cardiometabolic risk factors including hyperglycemia, hypertension, hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-C), and central adiposity. The presence of metabolic syndrome is associated with a 5-fold increased risk of diabetes and a 2-fold increased risk for CVD over 5–10 years compared to absence of the syndrome.9
We have previously reported in a cross sectional study that 8 years after delivery, women with preterm compared to term births have excess metabolic syndrome, adjusted for demographic, smoking and body size factors.10 An outstanding question, however, is whether a preterm delivery induces lasting metabolic aberrations or whether these might have been present prior to the pregnancy. We sought to evaluate the association between preterm birth and development of the metabolic syndrome utilizing a longitudinal study design to account for the accumulation of risk factors across the reproductive years. This is essential in order to disentangle the interplay between aging, pre-pregnancy and pregnancy-induced metabolic aberrations. We also sought to evaluate whether the preterm birth-metabolic syndrome association is independent of hypertension or diabetes during pregnancy. We hypothesized that women with a preterm birth would have excess metabolic syndrome across 25 years of follow up after accounting for pre-pregnancy metabolic factors, and weight gain.
Materials and Methods
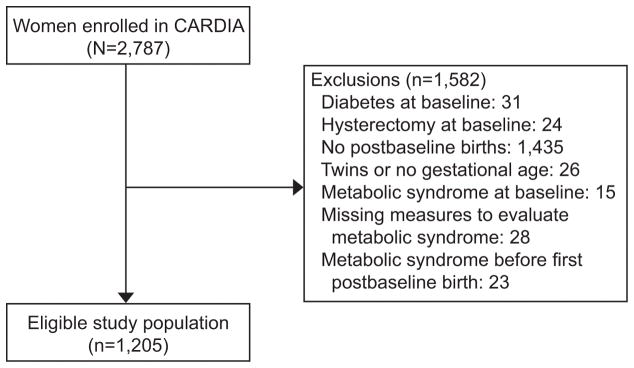
Coronary Artery Risk Development in Young Adults (CARDIA) is a multi-center, longitudinal, observational study designed to describe the development of risk factors for coronary heart disease in young black and white men and women.11, 12 Participants were recruited from four geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. From 1985 to 1986, 5,115 participants (2,787 women; 52% black) aged 18 to 30 years were enrolled and provided written informed consent. A variety of self-reported measures were obtained as well as physiologic measures and blood specimens. Retention rates ranged from 72 to 81 percent of the surviving cohort at years 7, 10, 15, 20, and 25 after baseline. Of note, retention of CARDIA participants alive at year 25 was higher for women compared to men (69% vs. 62% for Black women compared to men; 78% vs. 77% for White women compared to men). Of the 2,787 women enrolled in CARDIA, 1,205 women are included in this analysis (Figure 1). This study was approved by the University of Pittsburgh Institutional Review Board (#PRO12060448)
Figure 1.
Study population, Coronary Artery Risk Development in Young Adults (CARDIA) study.
Women were categorized as those who were nulliparous (no births prior to baseline) vs. parous at baseline. All births that occurred after enrollment in CARDIA are included in this analysis. For each of these births women reported the gestational age at delivery (weeks) and birth weight. Preterm births were those delivered <37 completed weeks. A validation study compared maternal report of gestational age at delivery to medical record abstractions (n=211). The sensitivity for maternal report of ever delivering preterm (<37 weeks) was 84% (16/19), and the specificity was 89% (170/192).
Women also reported whether each birth was complicated by gestational diabetes (GDM) or hypertensive disorders of pregnancy. Self-report of GDM history was validated by comparison to abstraction of glucose tolerance test results from prenatal records (n=200 births; 100% sensitivity and 92% specificity).13 When compared to the medical record, self-reported hypertensive disorders of pregnancy were over-reported (sensitivity was 40%) but the negative predictive value of no self-report of preeclampsia or gestational hypertension was 90%. Thus, women with no reported hypertension during pregnancy were largely normotensive during pregnancy.14
At each examination, venous blood samples were drawn in the morning after an overnight fast of ≥ 8 hours, and we did not include the few examinations in which women were pregnant. Procedures for the collection and storage of plasma samples, as well as laboratory quality-control procedures and methodology to assess plasma triglycerides, HDL-C, LDL-C and glucose have all been reported.15 Three resting blood pressure measurements were obtained with a random-zero sphygmomanometer through year 15 and with Omron (Omron Corp, Schaumburg, IL) HEM907XL oscillometer at years 20 and 25; the mean of the second and third readings was used for this report. Omron results were calibrated to be consistent with the random-zero results.14 Waist circumference was measured as the abdominal girth midway between the iliac crest and the bottom of the ribcage. Weight and height were assessed according to a standardized protocol. Metabolic syndrome was identified at each examination according to the National Cholesterol Education Program Adult Treatment Panel III criteria for women.16 Incident cases were identified when three out of the following five factors were met: waist circumference > 88 cm; fasting triglycerides >= 150 mg/dL; HDL-C < 50 mg/dL; systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg and/or on antihypertensive medication; fasting glucose ≥ 100 mg/dL and /or treatment with diabetes medication.
Information on demographic characteristics (age, sex, and race) was obtained at baseline; educational achievement was self-reported on standardized questionnaires during each examination. Smoking was measured as self-reported smoking status (nonsmoker, ex-smoker, and current smoker) at each examination. Habitual physical activity was measured at each examination by use of the CARDIA Physical Activity History, a simplified version of the Minnesota Leisure Time Physical Activity Questionnaire.17 CARDIA did not query duration of bouts of physical activity; therefore, physical activity is characterized as exercise units, which increase with frequency and intensity of performance.
We evaluated differences according to preterm birth status in baseline characteristics using t-tests and chi-squared tests for continuous and categorical variables, respectively. We obtained relative hazard ratios for incident metabolic syndrome in women with preterm birth (vs. term birth) using a complimentary log-log model built for interval-censored time-to-event data.18 We identified incident cases of metabolic syndrome at five longitudinal intervals. Women entered the analysis at baseline (pre-pregnancy)and were classified into interim birth groups (preterm birth vs. term births) following each examination as they transitioned over time and accumulated births; therefore, a woman could contribute time at risk for the “term birth” group until she had a preterm birth, at which time she was reclassified into the “preterm birth” category. To account for the documented associated between parity and metabolic syndrome we modeled additional births as a time-varying covariate.19 Covariates known to be related to metabolic syndrome risk were specified a priori and considered in the modeling as follows. Our first model adjusted baseline metabolic syndrome components. We then sequentially adjusted for race, baseline age, education, nulliparity at baseline, smoking status, BMI and time-varying parity. We then additionally adjusted for other pregnancy complications known to be associated with metabolic syndrome (self-reported gestational diabetes or hypertension in pregnancy). A final model then additionally added time-varying weight gain. Effect modification (p<0.1) by race, baseline obesity and baseline parity was evaluated, given the relevance of each of these factors to PTB risk and metabolic syndrome. We also further examined components of metabolic syndrome in women with and without preterm births by comparing crude changes in measured values from pre-pregnancy to diagnosis of metabolic syndrome or the final CARDIA examination, and by evaluating the proportion of women who met individual metabolic syndrome thresholds prior to pregnancies accrued in CARDIA, and at censoring or the final CARDIA examination.
Results
Of the 1,205 women in the study population, 295 (24.5%) had at least one preterm birth and 910 (75.5%) had only term births (Table 1). Women with at least one PTB were more likely to be Black, to have less than high school education at the time of CARDIA enrollment, and were more likely to report pregnancies complicated by hypertension. Women that went on to have at least one PTB did not have a significantly worse risk profile for any of the metabolic syndrome components at baseline compared to women with only term births; women with term births had higher triglycerides at baseline. In contrast, those with incident metabolic syndrome had higher measures of each metabolic syndrome component at baseline and were also more likely to be black, have less than a high school education, be parous, and to smoke (Appendix 1, available online at http://links.lww.com/xxx).
Table 1.
Maternal characteristics among women with one or more births during CARDIA follow up, by post-baseline preterm birth statusa
| Term (n=910) | Preterm (n=295) | p-valueb | |
|---|---|---|---|
| Age at baseline, yearsc | 24 (21–27) | 23 (20–26) | 0.032 |
| Black race (n, %) | 402 (44.2%) | 195 (66.1%) | <.001 |
| High school education or less (n, %) | 290 (31.9%) | 140 (47.4%) | <.001 |
| Parous at baseline (n, %) | 273 (30.0%) | 106 (35.9%) | 0.062 |
| Baseline BMI, kg/m2 | 22.3 ± 4.3 | 22.6 ± 4.1 | 0.403 |
| Smoking at baseline (n, %) | 220 (24.3%) | 79 (26.8%) | 0.615 |
| Physical Activity scored | 359 ± 257 | 320 ± 246 | 0.022 |
| Post-Baseline Birthsc | 1 (1–2) | 1 (1–2) | 0.116 |
| Hypertension (n, %) | |||
| During pregnancy (ever) | 91 (10.0%) | 46 (15.6%) | 0.009 |
| Before index pregnancy | 20 (2.2%) | 6 (2.0%) | 0.866 |
| After index pregnancy | 270 (29.7%) | 120 (40.7%) | <.001 |
| Gestational diabetes (n, %) | 100 (11.0%) | 34 (11.5%) | 0.799 |
| Age at first delivery, years | 30.2 ± 4.7 | 28.7 ± 5.1 | <.001 |
| Years from baseline to first delivery | 6.4 ± 4.1 | 5.4 ± 4.2 | <.001 |
| Years from last birth to last CARDIA visit | 14.0 ± 6.7 | 15.0 ± 6.9 | 0.021 |
| Metabolic syndrome components (Measured at baseline before index pregnancies) | |||
| Waist circumference (cm) | 71.8 ± 9.5 | 72.1 ± 9.7 | 0.718 |
| Blood pressure (mmHg) | |||
| Systolic | 105.0 ± 8.9 | 106.0 ± 8.7 | 0.071 |
| Diastolic | 65.6 ± 8.5 | 65.5 ± 8.6 | 0.917 |
| Triglycerides (mg/dl)e | 65.2 ± 30.3 | 60.9 ± 30.0 | 0.032 |
| HDL-cholesterol (mg/dl) e | 55.8 ± 12.2 | 57.2 ± 13.6 | 0.115 |
| Glucose (mg/dl) e | 79.1 ± 7.5 | 79.4 ± 7.8 | 0.554 |
| HOMA-IR at baseline e | 2.1 ± 1.6 | 2.2 ± 1.4 | 0.346 |
Continuous variables displayed as mean ± SD; categorical variables shown as n (%)
P-value from t-test (continuous) or chi squared (categorical)
Median, and interquartile range
Physical activity score increases with frequency and intensity of performance
Measured in samples collected after ≥ 8 hours fasting
Based on 315 incident metabolic syndrome cases in 17,717 person-years, the crude incidence rate was higher among women with preterm birth (22.0 per 1,000 person-years, 95% CI [17.5, 26.4]) than among those with only term births (16.4 per 1,000 person-years, 95% CI [15.1, 17.6]). The crude hazard ratio of metabolic syndrome was 2.91 (2.75, 3.09) among women with at least one PTB compared to women with term births (Table 2). This was attenuated to 1.52 (1.22, 1.88) after accounting for pre-pregnancy metabolic syndrome components. Adjustment for race and baseline characteristics (age, nulliparity, education, smoking, BMI) and time-varying parity had little influence. The association was only modestly affected after additional adjustments for lifetime exposure to GDM and hypertensive disorders of pregnancy (HR 1.47 [1.19, 1.84]) and time-dependent weight gain (HR 1.41 [1.13, 1.77]). In addition, results were unchanged when women with self-reported hypertension in pregnancy, the leading cause of medically indicated preterm births, were excluded (Table 3). We explored race-specific associations in stratified models (Appendixes 2 and 3, available online at http://links.lww.com/xxx). The association between preterm birth and metabolic syndrome was similar in both Black and White women (p for interaction, 0.66), Further, this association did not vary according to pre-pregnancy BMI (p=0.65) or baseline nulliparity (p=0.13).
Table 2.
Relative hazard ratios (95% CI) of incident metabolic syndrome according to preterm birth status (n=1,205)
| Model | Preterm Hazard ratio (HR)a | 95% CI | |
|---|---|---|---|
| 1 | Unadjusted | 2.91 | 2.75, 3.09 |
| 2 | Adjusted for baseline metabolic syndrome componentsb | 1.53 | 1.28, 1.82 |
| 3 | Model 2 + age, race, and education | 1.29 | 1.08, 1.55 |
| 4 | Model 3 + baseline BMI | 1.29 | 1.07, 1.55 |
| 5 | Model 4 + parous at baseline, smoking at baseline, and time varying parity | 1.52 | 1.22, 1.88 |
| 6 | Model 5 + time varying exposure to GDM or hypertensive disorders of pregnancy | 1.47 | 1.19, 1.84 |
| 7 | Model 6 + time varying weight gain | 1.41 | 1.13, 1.77 |
Referent for all models is women with term births.
Baseline metabolic syndrome components include blood pressure, waist circumference, triglycerides, glucose, HDL-cholesterol
Table 3.
Relative hazard ratios (95% CI) of incident metabolic syndrome according to preterm birth status, restricted to women with no reported hypertension during pregnancy (n=1,068)
| Model | Preterm Hazard ratio (HR)a | 95% CI | |
|---|---|---|---|
| 1 | Unadjusted | 3.09 | 2.91, 3.30 |
| 2 | Adjusted for baseline metabolic syndrome componentsb | 1.56 | 1.28, 1.92 |
| 3 | Model 2 + age, race, and education | 1.28 | 1.03, 1.58 |
| 4 | Model 3 + baseline BMI | 1.28 | 1.03, 1.58 |
| 5 | Model 4 + parous at baseline, smoking at baseline, and time varying parity | 1.53 | 1..18, 1.97 |
| 6 | Model 5 + time varying exposure to GDM or hypertensive disorders of pregnancy | 1.56 | 1.21, 2.02 |
| 7 | Model 6 + time varying weight gain | 1.50 | 1.16, 1.96 |
Referent for all models is women with term births.
Baseline metabolic syndrome components include blood pressure, waist circumference, triglycerides, glucose, HDL-cholesterol
We then explored how individual metabolic syndrome components and markers of insulin resistance changed from pre-pregnancy to diagnosis of metabolic syndrome or the final CARDIA examination in women with and without preterm births (Table 4). Blood pressure increased over time among all women, but the change was significantly larger among women with preterm compared to term births 11.2 [SD 15.8] vs. 8.2 [15.3] mmHg systolic; 8.9 [12.2] vs. 6.3 [12.4] mmHg diastolic, p<0.01 for both). Similarly, women with preterm births were more likely to start blood pressure-lowering medications in the years after delivery compared to women with term births (17.6% vs 12.2%, p=0.017). HDL-cholesterol increased in all women over time, but the increase was more modest in women with preterm compared to term births (2.4 [14.6] vs 5.1 [16.2], p=0.013). Triglycerides and fasting glucose concentrations increased non-differentially. Notably, women with preterm vs. term births gained significantly more weight from baseline to follow up (p=0.009). They were also more likely to achieve a pre-diabetes status (fasting glucose between 100 and 125 mg/dl; 18.0% vs. 13.3%, p-0.047), although rates of overt diabetes were not different and HOMA-IR increased from baseline non-differentially among women with preterm vs. term births.
Table 4.
Change in Metabolic Syndrome Components and Related Markers of Insulin Resistance From Prepregnancy (Baseline) to Diagnosis of Metabolic Syndrome or Final Coronary Artery Risk Development in Young Adults Study Examination, According to Preterm Birth Statusa
| Term (N=910) | Preterm (N=295) | p-valueb | |
|---|---|---|---|
| Change in Waist Circumference (cm) | 15.5 ± 11.0 | 16.7 ± 10.4 | 0.093 |
| Change in Triglycerides (mg/dl) | 33.4 ± 61.8 | 35.2 ± 78.1 | 0.672 |
| Change in HDL Cholesterol (mg/dl) | 5.1 ± 16.2 | 2.4 ± 14.6 | 0.013 |
| Change in Systolic Blood Pressure (mmHg) | 8.2 ± 15.3 | 11.2 ± 15.8 | 0.003 |
| Change in Diastolic Blood Pressure (mmHg) | 6.3 ± 12.4 | 8.9 ± 12.2 | 0.002 |
| Added Blood Pressure Meds, % | 111 (12.2%) | 52 (17.6%) | 0.017 |
| Change in Glucose (mg/dl) | 12.6 ± 16.8 | 14.0 ± 19.2 | 0.255 |
| Added Diabetes Meds, % | 17 (1.9%) | 6 (2.0%) | 0.858 |
| Weight gain (kg) | 15.8 ± 14.0 | 18.2 ± 13.4 | 0.009 |
| Incident pre-diabetes statusc | 121 (13.3%) | 53 (18.0%) | 0.047 |
| Change in HOMA-IRd | 3.3 ± 4.4 | 4.1 ± 4.3 | 0.180 |
Continuous variables displayed as mean ± SD; categorical variables shown as n (%); all changes are positive
P-value from t-test (continuous) or chi squared (categorical)
Pre-diabetes status (fasting glucose between 100–125 mg/dl) achieved between baseline and metabolic syndrome diagnosis or final visit
HOMA-IR, homeostatis model assessment measure of insulin resistance
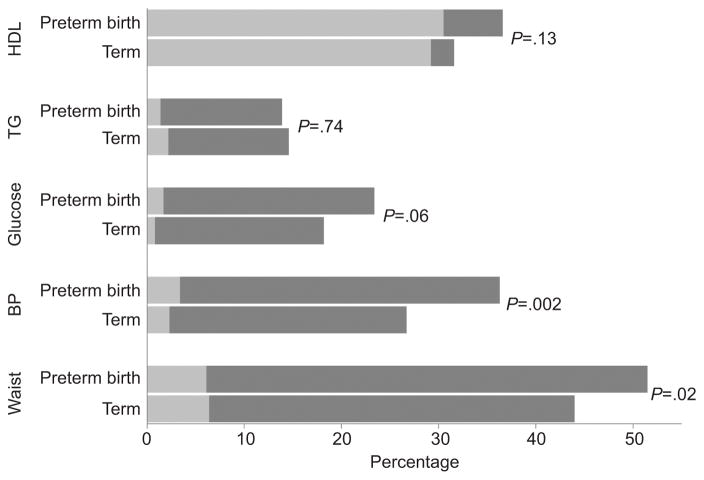
Viewed according to metabolic syndrome thresholds, women with preterm births were more likely to meet the blood pressure (≥ 130/85 mmHg or begin anti-hypertensive medication) and waist circumference (>88 cm) cutpoints compared to women with term births (36.3% vs. 26.7%, p=0.002 for BP; 51.5% vs. 44.0%, p=0.02 for waist circumference, Figure 2). Women with preterm vs. term births crossed the glucose threshold at a higher rate, but not significantly. The proportion of women with low HDL-C did not vary according to preterm birth history.
Figure 2.
Proportion of women meeting metabolic syndrome thresholds at the Coronary Artery Risk Development in Young Adults (CARDIA) study baseline (light gray bars) and at either diagnosis of metabolic syndrome or final visit (dark gray bars). P values based on chi-square tests comparing the proportion meeting each metabolic syndrome component threshold at censoring. HDL, high-density lipoprotein cholesterol <50 mg/dL); TG, triglycerides ≥150 mg/dL; glucose, fasting glucose >100 mg/dL or treatment with diabetes medication; BP, blood pressure ≥130/85 mm Hg or anti-hypertension medication use; waist, waist circumference >88 cm.
Discussion
Our results reveal that women with a history of preterm birth have excess risk for metabolic syndrome in the decades after delivery compared to women with term births. Of note, this risk was independent of metabolic syndrome components and BMI measured before the index pregnancy. Our findings suggest that while a portion of the incident metabolic syndrome risk is attenuated by the pre-pregnancy profile, risk accumulates after delivery in women with preterm births independent of these factors and independent of weight gain.
The metabolic syndrome is a composite index of cardiometabolic risk factors that are related to a five-fold risk of diabetes and a two-fold excess risk of CVD. These metabolic abnormalities may be more importantly related to disease risk in women compared to men, and pregnancy is a unique biologic exposure that may affect this risk. Indeed, childbearing itself has been linked in CARDIA to excess metabolic syndrome risk,19 as has a history of GDM.19 Our findings extend these results to indicate that preterm birth is an independent risk factor for incident metabolic syndrome. While the magnitude of the risk is more modest for preterm birth compared to GDM (HR 1.52 vs. 2.43, respectively), the high prevalence of preterm birth which affects 10% of U.S. deliveries suggests that the absolute risk of metabolic syndrome associated with a history of preterm birth is quite high. Indeed, prior to accounting for cardiometabolic factors measured before the index pregnancy, women with preterm birth had a3-fold higher incident rate compared to women with term births.
Our results are consistent with the few studies relating metabolic syndrome characteristics to preterm birth. There is evidence that elevated lipids, modestly elevated blood pressure and pro-inflammatory markers are higher in gestation among women destined to deliver preterm.20–23 Chatzi, et al, have reported that as early as 12 weeks of gestation, women with metabolic syndrome features during pregnancy had a threefold excess risk for preterm birth. The risk was higher for indicated preterm births but also detectable for women with spontaneous preterm births.24 We have reported in a cross-sectional study that women with preterm births have excess metabolic syndrome measured, on average, 8 years after delivery at a magnitude that was remarkably consistent with the results of our current longitudinal analysis.10
Our results raise the possibility that preterm birth may mark women at elevated diabetes and CVD risk. The relative risk of CVD related to metabolic syndrome is generally higher in women, especially young women, compared to men.25, 26 Whether the metabolic syndrome confers higher CVD risk independent of individual risk factors is debated, however, there are no composite screening tests to evaluate long term disease risk in reproductive age women. We considered that the metabolic syndrome may be an important intermediate marker of elevated disease risk in women. Metabolic syndrome is more prevalent in women, with the fastest growing group being young women ages 20–39 in whom prevalence rates have increased more than 50% since 1980.27 Additionally, aligned with our findings, abdominal obesity is the dominant metabolic syndrome feature in women, whereas risk factor combinations are more heterogeneous in men.28 Until risk algorithms such as the Framingham or Reynolds scores are adapted for women younger than age 50, metabolic syndrome may be useful to identify preclinical risk for both diabetes and heart disease.
Our study must be considered in the context of limitations. Preterm births were self-reported and we could not reliably distinguish medically indicated from spontaneous subtypes. Although we and others have demonstrated good recall of gestational age at delivery, our results should be replicated in cohorts with pregnancy recruitment and clinical characteristics of preterm birth including clinical subtypes derived from the medical record. In addition, the number of women with recurrent PTB was too small to evaluate separately, but this should be considered in larger studies. Strengths of our study include the measurement of metabolic syndrome factors before and after pregnancy, and the ability to study women across the span of the reproductive years as they accrued multiple pregnancy exposures.
Our findings reveal that preterm birth marks women at excess risk of the metabolic syndrome that is independent of pre-pregnancy cardiometabolic factors, other pregnancy features, and weight gain across the life course. Elevated blood pressure and central adiposity were the factors that increased the most, pointing to the importance of vascular and visceral adiposity factors among women with preterm births. In addition, our longitudinal results suggest that the pre-pregnancy cardiometabolic profile and weight gain over time mediates a portion but not all of this risk.
Supplementary Material
Acknowledgments
Sources of Funding: The Coronary Artery Risk Development in Young Adults (CARDIA) study is conducted and supported by the National Heart, Lung and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). The analyses were supported by NIH grants from K12 HD043441 (Catov, PI) and R01 DK090047 (Gunderson, PI). This manuscript was reviewed by CARDIA for scientific content.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented at the American Heart Association Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism 2014 Scientific Sessions, San Francisco, CA, March 18–21, 2014.
References
- 1.Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20:604–9. doi: 10.1016/j.annepidem.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James-Todd TM, Karumanchi SA, Hibert EL, Mason SM, Vadnais MA, Hu FB. Gestational Age, Infant Birth Weight, and Subsequent Risk of Type 2 Diabetes in Mothers: Nurses' Health Study II. Preventing Chronic Disease. 2013;10:E156. doi: 10.5888/pcd10.120336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GD, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. The Lancet. 2000;356:2066–2067. doi: 10.1016/S0140-6736(00)03406-1. [DOI] [PubMed] [Google Scholar]
- 4.James-Todd T, Wise L, Boggs D, Rich-Edwards J, Rosenberg L, Palmer J. Preterm Birth and Subsequent Risk of Type 2 Diabetes in Black Women. Epidemiology. 2014;25:805–810. doi: 10.1097/EDE.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG. 2010;117:274–81. doi: 10.1111/j.1471-0528.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- 6.Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm Birth and Future Maternal Blood Pressure, Inflammation, and Intimal-medial Thickness: The CARDIA Study. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.111.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastie CE, Smith GCS, Mackay DF, Pell JP. Association between preterm delivery and subsequent C-reactive protein: a retrospective cohort study. Am J Obstet Gynecol. 2011;205:556e1–4. doi: 10.1016/j.ajog.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 8.Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Ann Epidemiol. 2014;25:40–45. doi: 10.1016/j.annepidem.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC., Jr Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 10.Catov JM, Dodge R, Yamal JM, Roberts JM, Piller LB, Ness RB. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstet Gynecol. 2011;117:225–32. doi: 10.1097/AOG.0b013e3182075626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutter G, Burke G, Dyer A, Friedman G, Hilner J, Huges G, Hulley S, Jacobs D, Lie K, Manolio T. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. Cardia: study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 13.Gunderson EP, Lewis CE, Tsai AL, Chiang V, Carnethon M, Quesenberry CP, Jr, Sidney S. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;56:2990–6. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunderson EP, Chiang V, Lewis CE, Catov J, Quesenberry CP, Jr, Sidney S, Wei GS, Ness R. Long-Term Blood Pressure Changes Measured From Before to After Pregnancy Relative to Nonparous Women. Obstet Gynecol. 2008;112:1294–1302. doi: 10.1097/AOG.0b013e31818da09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Jacobs DR, Jr, Liu K, Williams OD, Hilner JE, Perkins LL, Marcovina SM, Hulley SB. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young Adults: the CARDIA Study. Annals of Epidemiology. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 16.Grundy S, JIC, Daniels S. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–7. [PubMed] [Google Scholar]
- 17.Jacobs D, Hahn L, Haskell W, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentice R, Gloeckler L. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 19.Gunderson EP, Jacobs DR, Chiang V, Lewis CE, Tsai A, Quesenberry CP, Sidney S. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201:177e1–9. doi: 10.1016/j.ajog.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and Dyslipidemia Related to Risk of Spontaneous Preterm Birth. American Journal of Epidemiology. 2007;166:1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 21.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, Muenke M. Adverse Birth Outcome Among Mothers With Low Serum Cholesterol. Pediatrics. 2007;120:723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 22.Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2012;91:726–35. doi: 10.1111/j.1600-0412.2012.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Villar J, Sun W, Merialdi M, Abdel-Aleem H, Mathai M, Ali M, Yu KF, Zavaleta N, Purwar M, Nhu Ngoc NT, Campodonico L, Landoulsi S, Lindheimer M, Carroli G. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:162e1–6. doi: 10.1016/j.ajog.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, Kafatos A, Koutis A, Kogevinas M. Metabolic Syndrome in Early Pregnancy and Risk of Preterm Birth. Am J Epidem. 2009;170:829–836. doi: 10.1093/aje/kwp211. [DOI] [PubMed] [Google Scholar]
- 25.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan A. Sex differences in the metabolic syndrome: Implicaitons for cardiovascular health in women. Clin Chem. 2014;60:44–52. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- 27.Mozumdar A, Ligouri G. Persistent Increase of Prevalence of Metabolic Syndrome Among U.S. Adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34:216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuk JL, Ardern C. Age and sex differences in the clustering of metabolic syndrome factors. Diabetes Care. 2010;33:2457–2461. doi: 10.2337/dc10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.