Summary
Searching for a neurobiological understanding of human intellectual capabilities has long occupied those very capabilities. Brain gyrification, or folding of the cortex, is as highly-evolved and variable a characteristic in humans as is intelligence. Indeed, gyrification scales with brain size, and relationships between brain size and intelligence have been demonstrated in humans [1-3]. However, gyrification shows a large degree of variability that is independent from brain size [4-6], suggesting that the former may independently contribute to cognitive abilities, and thus supporting a direct investigation of this parameter in the context of intelligence. Moreover, uncovering the regional pattern of such an association could offer insights into evolutionary and neural mechanisms. We tested for this brain-behavior relationship in two separate, independently-collected, large cohorts: 440 healthy adults and 662 healthy children, using high-resolution structural neuroimaging and comprehensive neuropsychometric batteries. In both samples, general cognitive ability was significantly associated (pfdr<0.01) with increasing gyrification in a network of neocortical regions, including large portions of the prefrontal cortex, inferior parietal lobule, and temporoparietal junction, as well as the insula, cingulate cortex, and fusiform gyrus, a regional distribution that was nearly identical in both samples (Dice similarity coefficient=0.80). This neuroanatomical pattern is consistent with an existing, well-known proposal, the Parieto-Frontal Integration Theory of Intelligence [7], and is also consistent with research in comparative evolutionary biology showing rapid neocortical expansion of these regions in humans relative to other species. These data provide a framework for understanding the neurobiology of human cognitive abilities, and suggest a potential neurocellular association.
Results
The overall degree of cortical folding, or gyrification, in the brain has been associated with cognitive ability across species in a general sense, with putatively more intelligent species such as primates, cetaceans, and pachyderms exhibiting greater brain gyrification [6]. However, even among these species, the regional pattern of gyrification differs; for example, humans have evolved a unique pattern of relatively larger, more developed frontal lobes [8, 9]. This observation suggests that increased gyrification in specific brain regions may contribute to differences in cognitive ability in humans. The few studies testing for such associations in people have provided decidedly mixed results. The only prior investigation of regional associations between gyrification and cognition used a proxy measure of gyrification, cortical curvature, in a small sample and found only a restricted region of the medial temporo-occipital junction to be associated with IQ [10]. In another recent study, average gyrification across the whole brain was associated with cognitive ability in an aging sample, though the effect was mainly driven by brain size and regional associations were not examined [11].
Various measures have been used to quantify brain gyrification over the past century [12, 13]. The current gold-standard metric, gyrification index (GI), was originally described on 2-dimentional coronal slices [5] and quantifies as the ratio of pial surface area to the surface area of the cortical hull, or outer contour of the brain. GI can also be calculated in 3-dimensions as an average global whole-brain measure (GGI), or regionally as a local gyrification index (LGI). The analytic approach employed here calculates the LGI of each of 198,812 nodes per hemisphere of a standardized mesh representation of the pial brain surface [14], as well as GGI. The regionally-specific nature of LGI allows for neuroanatomically-specific delineation of associations of brain gyrification with variables of interest. In this study, we sought to determine whether variations in human brain gyrification are associated with general cognitive ability and, especially, to define the regional pattern of any such associations.
Structural MRI and comprehensive batteries of neuropsychological data from two independent samples were analyzed. The first sample consisted of 440 healthy adult participants (aged 31.3+/−9.4 years, 250 females) studied at the National Institute of Mental Health (NIMH) Intramural Research Program [15], and the second sample included 662 typically developing children (aged 14.7+/−3.3 years, 378 females) assessed as part of the Philadelphia Neurodevelopmental Cohort (PNC) [16]. As estimates of general cognitive ability (g) [17] from different, reasonably comprehensive neuropsychological batteries have been shown to be comparable [18, 19], we used parallel methodology to calculate estimates of g for each participant in both samples (see Supplemental Experimental Procedures and Tables S1 and S2).
High quality structural MRIs were obtained for participants in both samples using 3T scanners and similar acquisition methodologies. MRI scans for each participant were processed with the widely used Freesurfer software package [20] to create representations of each individual’s brain surface and to measure LGI for each node on each brain’s surface to test our primary hypothesis about regionally-specific relationships with g, as well as GGI for ancillary analyses. Correlations between LGI and g were computed in each sample separately, while controlling for effects due to age and sex.
Regional Associations between Gyrification and Intelligence
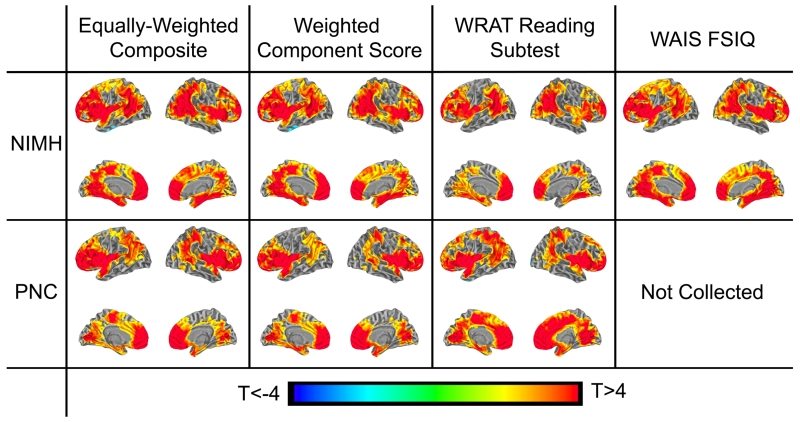
Consistent with the hypothesis that gyrification of specific brain regions contributes to general cognitive ability, associations were observed between g and LGI in specific brain regions in both independent samples (p<0.01, FDR-corrected for multiple comparisons). LGI values extracted and averaged from the significant nodes in these regions accounted for 11.5% of the variance (R2) of g (derived from the equally-weighted composite estimate) in the NIMH sample and 5.2% in the PNC sample (both p’s<1×10−6). Moreover, there was remarkable between-sample correspondence of the neuroanatomical pattern of these associations (R2=0.53, Dice similarity coefficient (DSC)=0.80 comparing the two samples’ statistical maps), encompassing large areas of the prefrontal cortex, inferior parietal lobule, temporo-parietal junction, insula, fusiform gyrus, cingulate cortex and medial temporo-occipital junction (Figure 1, and see Supplemental Experimental Procedures for calculation of R2 and DSC measures). This pattern of LGI-g associations remained unchanged, whether we calculated g using an equally-weighted composite score (Table S1), a component-loading-weighted index (the first component from a principal components analysis, Table S2), the WRAT reading subtest estimate of IQ, or a short-form full-scale IQ estimate of the WAIS-R (only available in the NIMH sample), suggesting that the method of deriving g does not significantly affect the results (see Table S3 for R2 and DSC values). Correlations between LGI and g were positive, and no negative relationships were observed in either sample.
Figure 1. LGI-g associations in both NIMH and PNC samples controlling for age and sex.
Figure shows statistical maps for each sample (NIMH and PNC), calculated from different estimates of general cognitive ability (g): an equally-weighted composite score, the first principal component from a component–weighted factor analysis of all data, FSIQ estimate from the WRAT reading subtest, and a validated 4-subtest short-form of the WAIS-R to estimate FSIQ (the latter only available in the NIMH data). Statistical maps are thresholded at an FDR-corrected p<0.01. Note the high spatial correspondence with all methods of estimating g and across samples. See also Figure S1 and Tables S1, S2 and S3.
Effects of Total Brain Volume on the Association between Cognition and Regional Gyrification
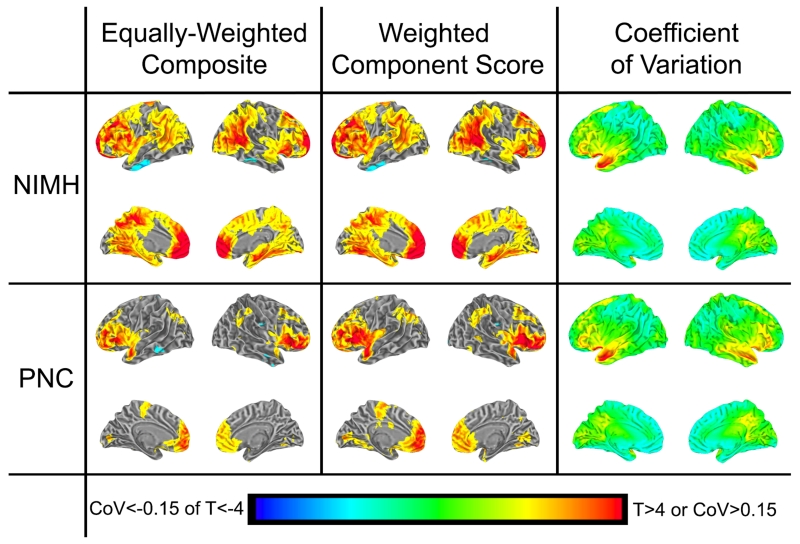
Since global brain gyrification has been shown to be significantly correlated with total brain volume (TBV)[2, 3], and prior analyses have suggested that correlations between GI and g may be driven by TBV [11], we examined the influence of TBV on the regional pattern of associations between LGI and g by repeating the analysis while adding TBV to the control variables age and sex used above (Figure 2). In the NIMH sample of healthy adults, the regional pattern of associations remained relatively unchanged (R2=0.85, DSC=0.73 comparing statistical maps using the equally-weighted composite after controlling for age and sex; or for age, sex, and TBV). After accounting for effects of TBV in the PNC sample of healthy children, the associations of g with gyrification of frontal lobe and anterior insula remained significant at the p<0.01 FDR-corrected level, however the associations with parietal and medial brain gyrification were only significant at the p<0.05 level. TBV explained 6.2% of the variance of g in the NIMH sample and 5.8% of the variance in the PNC sample (both p’s<1×10−6) after accounting for age and sex effects with linear regression using SPSS. Adding the average LGI of the significant regions into this regression analysis accounted for an additional 6.4% (p<2×10−6) of the variance of g in the NIMH sample and 1.5% (p<2×10−4) in the PNC sample.
Figure 2. LGI-g associations in both NIMH and PNC samples controlling for age, sex, and total brain volume; and LGI Coefficient of Variation.
Figure shows statistical maps of the LGI-g relationship for each sample (NIMH and PNC) after controlling for age, sex and TBV, using two estimates of g (Left and Middle), thresholded at FDR-corrected p<0.05. Right shows the variability of LGI (Coefficient of Variation) in each sample. Note that the pattern of the LGI-g relationship was similar in both samples and with different estimates of g, whereas the pattern of LGI variability was quite different. See also Tables S1 and S2.
Associations of Age and Sex with Gyrification
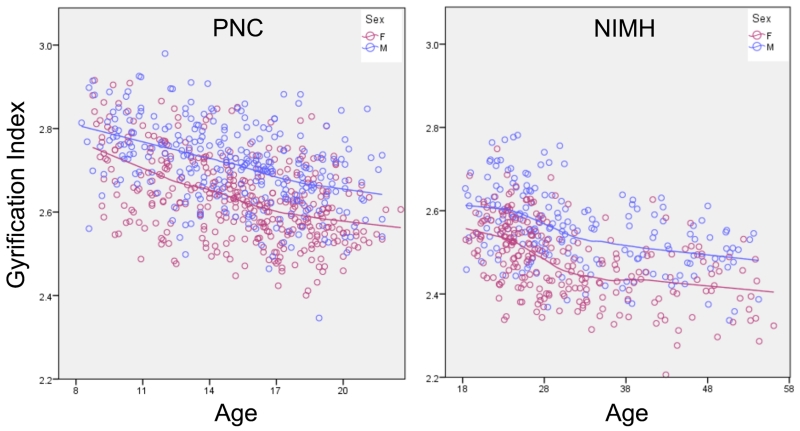
To ensure that the analyses were not biased by the statistical controls employed for age and sex, we tested for regional effects of these demographic variables on LGI. In both samples, age was negatively correlated with LGI and males exhibited increased LGI compared to females. These relationships were of similar magnitude throughout the entire brain, suggesting that controlling for age and sex was not responsible for the regional pattern of the LGI-g association. As expected from the across-brain consistency of the age and sex correlations, age correlated negatively with the global measure of gyrification, GGI (PNC: R2=0.192, NIMH: R2=0.135; both p’s<1×10−6), and GGI was greater in males (PNC: t(660)=9.81, NIMH: t(438)=8.26; both p’s<1×10−6) (Figure 3).
Figure 3. Plot of age versus global gyrification index in each sample.
Left shows the PNC sample of children aged 8-21 and Right shows the NIMH sample of adults aged 18-56. Note the negative association between age and GI in both samples. Blue dots and line represent males, pink represent females. See also Figure S2.
To further examine the effects of age and sex, we also tested the LGI-g relationship separately in males and females, and in separate groups of participants binned by age across the lifespan. Neuroanatomical patterns of LGI-g association in the two sexes were similar, did not differ statistically in any region, and were consistent with the pattern observed in the entire group (Figure S1). To characterize the LGI-g relationship throughout the lifespan, the PNC and NIMH samples were divided into five non-overlapping age groups and the LGI-g relationship was mapped across the brain. In each age group, the regional pattern of the LGI-g relationship was stable and similar to that found in the entire group (Figure S2).
Regional LGI Variance
One potential explanation for the neuroanatomical pattern of the relationship between LGI and g could be that the identified brain areas are simply those having increased variance in the LGI measure and that this provides statistical power to enable detection of the brain-behavior relationship. To investigate this possibility, we examined the regional pattern of the coefficient of variation of LGI (defined for each node as the standard deviation divided by the mean value) and found it to be anatomically distinct from the pattern of the LGI-g relationship (R2=0.005 in the NIMH sample and R2=0.003 in the PNC sample); in contrast to the LGI-g correlation, LGI variation showed higher variability in Broca’s area and superior temporal sulcus, and lower variability in the inferior parietal lobule, temporo-parietal junction and medial frontal lobe, suggesting that the degree to which gyrification varies locally does not account for the very different regional LGI-g association pattern (Figure 2).
Discussion
The present work identifies a network of brain regions in which the degree of regional brain gyrification was associated with general cognitive ability. These observations were made in two large cohorts of individuals who, together, span a large portion of the human lifespan. The neuroanatomical patterns of the association – encompassing frontal, parietal, temporal and cingulate cortical regions – were highly consistent across the two independent cohorts, across sexes, across methods of estimating general intelligence, and across the age span studied. The degree to which the brain is gyrified in general has long been hypothesized to be associated with general cognitive ability, both across species and within humans, and some previous work has demonstrated an association at a global level [11]. However, regionally specific brain associations between cognition and gyrification have not been carefully examined. This work not only supports the link between cognitive ability and gyrification, but also shows that the pattern of associations is congruent with one prominent formulation regarding the neural basis of intelligence, the Parieto-Frontal Integration Theory (P-FIT) [7].
The P-FIT model, originally based on a review of structural and functional neuroimaging studies and now further supported by additional structural, task-related, and resting-state MRI studies [21-23], postulates that individual differences in intelligence are manifestations of differences in the interactions between parietal and frontal lobes. It further posits that unimodal, primary sensory regions gather salient information about the environment and then feed this information to the parietal lobe for abstraction, symbolism and elaboration. Parietal and frontal regions interact to hypothesis-test and resolve this information. After a solution is obtained, the cingulate cortex functions to constrain response selection [7]. The neuroanatomical pattern of LGI-g relationship found in the present work fits well within this framework.
It is noteworthy that all brain areas in which we found a relationship between gyrification and g are multimodal association regions, which incorporate information from multiple sensory modalities, and that no significant results were observed in primary, unimodal regions [24], whose primary function in P-FIT is to gather information from the environment rather than to perform cognitive manipulations. This selectivity suggests that the neural correlates of increased cognitive abilities in humans are represented, at least in part, in the degree of folding of these multimodal cortical regions. Though it is not possible to determine cytoarchitechture from neuroimaging of living participants, it is interesting that nearly all cortical regions identified here are part of the neocortex, which is comprised of six cortical layers and thought to have undergone rapid expansion in humans relative to other species. In contrast, unimodal cortical regions, such as primary sensory and motor cortices, which were not identified here as associated with intelligence, are cytoarchitecturally distinct from multimodal regions, with one or two layers that are restricted or absent [25]. For example, primary motor cortex is characterized by its agranular nature, with cortical layer 4 being restricted or absent, likely representing fewer afferent connections in this region, as its major function is to provide efferent fibers involved in movement. The fact that regions of strong association between gyrification with intelligence localized only to cortical regions composed of six layers suggests that cytoarchitecture may be a critical element to the neurobiological foundation for higher cognition.
It should be noted that in these analyses, LGI accounted for 12% of the variation of g in the NIMH sample and 6% in the PNC sample, and although these findings are of similar magnitude to prior reports of associations of other brain measures with intelligence [3], a considerable proportion of the variance of g remains unaccounted for. There are likely many factors contributing to individual cognitive ability, including genetic predisposition, education, early-life environment, prenatal nutrition, and others. Many of these factors likely impact g independently from brain gyrification, contributing to the unexplained variance of these analyses. Moreover, it should also be noted that the results reported here are correlational and do not speak to the directionality of the relationship. While it may be inferred from our results that increased gyrification within the identified brain regions provides a neural substrate that allows for increased cognitive ability, or even for cognitive reserve in conditions like dementia, it is important to note that environmental factors, such as those listed above, are known to lead to increased cognitive ability, which in turn, may be associated with increased brain gyrification.
In this study, we chose to focus on brain gyrification as opposed to brain size, which has previously been associated with intelligence scores. Though relationships with brain size have been shown in the past, this is a global measure and not able to reveal regional associations. Further, across species, larger-brained animals are not necessarily smarter. For example, capybaras have brains that are 183-fold larger than mice, with a 22-fold increase in neuron number; however there is not a commensurate increase in cognitive ability [26]. Instead, GI appears to perform better than brain size at grouping species of similar cognitive ability together, as the mouse and capybara have similar, relatively low GI’s, 1.03 and 1.30 respectively [6], lending credence to our choice of this metric.
Given the known associations between brain volume and intelligence, it is not surprising that controlling for TBV would decrease the power of the association between gyrification and g. In the NIMH adult sample, the regional pattern of these associations, though of lower significance, remained consistent when TBV was also included as an additional covariate; however, in the PNC sample of healthy children, this pattern was altered, particularly in parietal brain regions. One explanation for this change may be related to the co-occurring changes in gyrification and brain volume during adolescence. In fact, a significant, negative relationship was observed between age and brain gyrification, which is consistent with prior reports [27, 28]. The slope of the age-related decline in GI appears to change in the third decade of life, suggesting distinct mechanisms at different life-stages. Age-related cortical volume loss is one potential mechanism, though such a mechanism would likely play a greater role in these associations later in life and not during adolescence. Instead, processes of cortical pruning and/or white matter maturation may better account for the changes in GI occurring during this time period [28, 29].
In sum, we describe areas of associations between regional brain gyrification and general cognitive ability in separate data sets comprised of two large samples that were analyzed using parallel methodologies. The pattern of association was remarkably consistent between the samples and largely unaffected by obvious demographic differences or method of estimating cognitive ability. This regional pattern is congruent with leading proposals concerning the neural basis of cognitive ability, is consistent with comparative biological research, and may provide insight into the cytoarchitectural specialization of the human brain.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD. The data for the NIMH cohort were obtained under protocol 00-M-0085/NCT00004571. The data for the PNC cohort were obtained from dbGAP (accession number phs000607) and collection of the data was supported by grants RC2MH089983 awarded to Raquel Gur and RC2MH089924 awarded to Hakon Hakonarson. Some of this work utilized the computational resources of the NIH HPC Biowulf cluster. (http://hpc.nih.gov).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Conceptualization, Methodology and Investigation: MDG, JSK, JC, DD, VSM, DRW and KFB; Resources: KFB and DRW; Writing: MDG, DD and KFB; Supervision: DRW and KFB; Funding Acquisition: DRW and KFB.
All authors report no conflicts of interest with regard to this manuscript.
References
- 1.Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- 2.McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- 3.Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: How strong are they and what do they mean? Neuroscience and biobehavioral reviews. 2015;57:411–432. doi: 10.1016/j.neubiorev.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Rogers J, Kochunov P, Zilles K, Shelledy W, Lancaster J, Thompson P, Duggirala R, Blangero J, Fox PT, Glahn DC. On the genetic architecture of cortical folding and brain volume in primates. NeuroImage. 2010;53:1103–1108. doi: 10.1016/j.neuroimage.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anatomy and embryology. 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- 6.Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends in neurosciences. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. The Behavioral and brain sciences. 2007;30:135–154. doi: 10.1017/S0140525X07001185. discussion 154-187. [DOI] [PubMed] [Google Scholar]
- 8.Shoshani J, Kupsky WJ, Marchant GH. Elephant brain. Part I: gross morphology, functions, comparative anatomy, and evolution. Brain research bulletin. 2006;70:124–157. doi: 10.1016/j.brainresbull.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Manger PR. An examination of cetacean brain structure with a novel hypothesis correlating thermogenesis to the evolution of a big brain. Biological reviews of the Cambridge Philosophical Society. 2006;81:293–338. doi: 10.1017/S1464793106007019. [DOI] [PubMed] [Google Scholar]
- 10.Luders E, Narr KL, Bilder RM, Szeszko PR, Gurbani MN, Hamilton L, Toga AW, Gaser C. Mapping the relationship between cortical convolution and intelligence: effects of gender. Cerebral cortex. 2008;18:2019–2026. doi: 10.1093/cercor/bhm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty AR, Hagler DJ, Jr., Panizzon MS, Neale MC, Eyler LT, Fennema-Notestine C, Franz CE, Jak A, Lyons MJ, Rinker DA, et al. Does degree of gyrification underlie the phenotypic and genetic associations between cortical surface area and cognitive ability? NeuroImage. 2015;106:154–160. doi: 10.1016/j.neuroimage.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Bonin G. Side Lights on Cerebral Evolution: Brain Size of Lower Vertebrates and Degree of Cortical Folding. The Journal of General Psychology. 1941;25:273–282. [Google Scholar]
- 13.Cunningham DJ. Contributions to the Surface Anatomy of the Cerebral Hemispheres. Vol. 7. Royal Irish Academy Press; Dublin, Ireland: 1892. [Google Scholar]
- 14.Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE transactions on medical imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- 15.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. The American journal of psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 16.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spearman C. “General intelligence ” objectively determined and measured. Am J Psychol. 1904;15:201–292. [Google Scholar]
- 18.Johnson W, Bouchard TJ, Krueger RF, McGue M, Gottesman II. Just one g: Consistent results from three test batteries. Intelligence. 2004;32:95–107. [Google Scholar]
- 19.Johnson W, te Nijenhuis J, Bouchard TJ. Still just 1 g: Consistent results from five test batteries. Intelligence. 2008;36:81–95. [Google Scholar]
- 20.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 21.Colom R, Burgaleta M, Roman FJ, Karama S, Alvarez-Linera J, Abad FJ, Martinez K, Quiroga MA, Haier RJ. Neuroanatomic overlap between intelligence and cognitive factors: morphometry methods provide support for the key role of the frontal lobes. NeuroImage. 2013;72:143–152. doi: 10.1016/j.neuroimage.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T. Brain spontaneous functional connectivity and intelligence. NeuroImage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Vakhtin AA, Ryman SG, Flores RA, Jung RE. Functional brain networks contributing to the Parieto-Frontal Integration Theory of Intelligence. NeuroImage. 2014;103:349–354. doi: 10.1016/j.neuroimage.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 24.Mesulam MM. From sensation to cognition. Brain: a journal of neurology. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 25.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth; Leipzig: 1909. [Google Scholar]
- 26.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cerebral cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- 28.White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain and cognition. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and biobehavioral reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.