Abstract
Background
Although metabolic reprogramming is critical in the pathogenesis of heart failure, studies to date have focused principally on fatty acid and glucose metabolism. Contribution of amino acid metabolic regulation in the disease remains understudied.
Methods and Results
Transcriptomic and metabolomic analyses were performed in mouse failing heart induced by pressure-overload. Suppression of branched-chain amino acids (BCAAs) catabolic gene expression along with concomitant tissue accumulation of branched-chain α-keto acids (BCKAs) was identified as a significant signature of metabolic reprogramming in mouse failing hearts, and validated to be shared in human cardiomyopathy hearts. Molecular and genetic evidence identified the transcription factor KLF15 as a key upstream regulator of the BCAA catabolic regulation in the heart. Studies using a genetic mouse model revealed that BCAA catabolic defect promoted heart failure associated with induced oxidative stress and metabolic disturbance in response to mechanical overload. Mechanistically, elevated BCKA directly suppressed respiration and induced superoxide production in isolated mitochondria. Finally, pharmacological enhancement of branched-chain α-keto acid dehydrogenase activity significantly blunted cardiac dysfunction following pressure-overload.
Conclusions
BCAA catabolic defect is a metabolic hallmark of failing heart resulted from KLF15 mediated transcriptional reprogramming. BCAA catabolic defect imposes a previously unappreciated significant contribution to heart failure.
Keywords: Branched-Chain Amino Acid, Branched-Chain Keto Acid, Heart Failure, Catabolic Remodeling, Mitochondrial Defect
Alterations in cardiac metabolism are hallmarks of the pathological changes in the failing heart1 with studies over the past several decades centered on fatty acid and glucose utilization. Suppression of oxidative phosphorylation with reduced utilization of fatty acid in conjunction with increased glucose consumption is a common feature of heart failure2–5. However, little is known about the metabolic changes of amino acid and their functional relevance in the pathogenesis of heart failure.
Amino acids serve as building blocks for protein synthesis as well as energy-providing substrates, although the relative importance of a bioenergetic contribution by amino acids in the heart remains unclear under either physiological or pathological conditions6. In addition, derivatives of amino acids, like taurine, creatine, carnitine, and glutathione are critical to bioenergenesis and cellular function in the heart7. An early study by Peterson et al. suggested that total free amino acid concentrations were increased in failing right ventricle8. In patients with mitral valve disease, higher glutamine and glutamate concentrations were detected in the dilated left ventricle when compared to the right ventricle9. A metabolomic study has also demonstrated that intra-tissue concentrations of several amino acids were changed significantly in the failing rat heart10. More recently, two reports using multi-systems analysis in hypertrophied and early stage failing mouse hearts following pressure-overload or myocardial infarction also revealed profound metabolic derangement, including amino acid metabolism, associated with pathological remodeling11,12. These observations indicate that amino acid homeostasis is perturbed in diseased heart tissue.
In this study, we found BCAA catabolic pathway was the most significantly altered metabolic change in the mouse failing heart and this coordinated suppression of BCAA catabolic pathway was regulated by Krüppel-like factor 15 (KLF15)13,14, 15. Furthermore, we found the loss of BCAA catabolic gene expression and the resulting accumulation of intra-myocardial levels of BCAA catabolic mediators, such as BCKAs, were conserved metabolic signatures in human failing hearts. Impairment of BCAA catabolic pathway impaired heart function and promoted pressure-overload induced heart failure, associated with elevated superoxide production, oxidative injury, and profound metabolic changes in the heart. Finally, pharmacological enhancement of BCAA catabolic activity significantly preserved cardiac function following pressure-overload. These findings established that defect of BCAA catabolism is an underappreciated integral part of the metabolic re-programming in stressed hearts, and amino acid metabolism has a significant contribution to the progression of heart failure and BCAA catabolic pathway can serve as a potential therapeutic target for the disease.
METHODS
Animals and Human Cohorts
PP2Cm germ-line knockout mice (PP2Cm KO) were generated as previously described16. Human cohorts of dilated cardiomyopathy and controls were obtained from Columbia and Duke with institutional IRB approval. All animal procedures were carried out in accordance with the guidelines and protocols approved by the University of California at Los Angeles Institutional Animal Care and Use Committee (IACUC). Trans-Aortic Constriction (TAC) and cardiac echocardiography were performed as reported earlier17 on mice from different genotypes between 14–1 6 weeks of age. Compound BT2 (3,6-dichlorobenzo[b]thiophene-2-carboxylic acid) was purchased from Sigma-Aldrich and administrated by oral gavage at 40 mg/kg/day as previously described18.
Molecular Methods and Reagents
The details of expression vectors, transfection methods, cell culture, immunoblotting, RT-PCR and chromatin immunoprecipitation methods were provided in On-line Supplemental Method and Material. Superoxide measurement was performed by electron spin resonance method and BCKA and BCAA measurements from tissue or plasma were performed following the method published by Olson, et al19 with modifications with more details described in On-line Supplemental Method and Material. The global metabolomic analysis was carried out by Metabolon, Inc. (Durham, NC) using heart tissues from PP2Cm KO and wildtype male mice at 14–16 weeks of age with detailed description of analysis shown in On-line Supplemental Method and Material.
Statistics
Unless otherwise specified, statistical analyses to compare two groups were performed with either the Student’s t-test or Wilcoxon Rank Sum test (when n < 5 or in which the variances distributions differed based on Bartlett test). When more than two groups were analyzed, standard ANOVA followed by Newman-Keuls test was performed when n > 5 for all groups and passed by the Bartlett test of homogeneity of variances. Otherwise, Kruskal-Wallis test followed by Dunn’s multiple comparison test was performed. Presented values are mean with standard deviation or SEM (standard error of the mean). Linear Mixed Effect Model test was performed for repeated measurements over time. A p value of less than 0.05 was considered statistically significant.
RESULTS
BCAA catabolic gene regulation in developing and pathologically stressed hearts
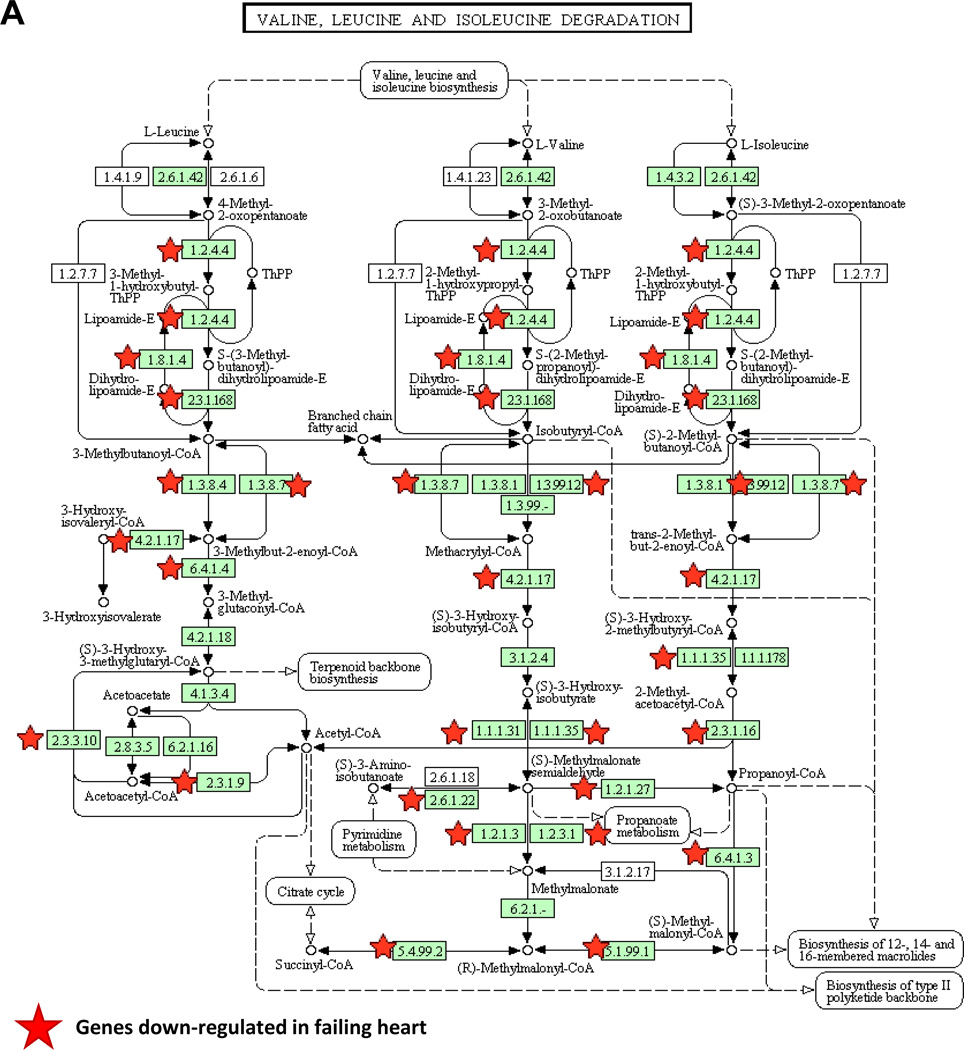
It has been well established that postnatal maturation of developing heart results in dynamic shifts from glucose to fatty acid utilization, a phenotype that is reversed in the diseased heart. These changes are orchestrated, at least in part, at the transcriptional level as part of the so called "fetal-like" gene expression re-programming20–22. From cardiac transcriptome in pressure-overload induced failing mouse hearts23, we performed Functional Annotation Analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID, http://david.abcc.ncifcrf.gov) to identify Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways significantly overrepresented in differentially expressed genes24. The analysis of down-regulated genes in the failing heart revealed more than 20 specific metabolic pathways that were significantly enriched (Table S1). Unexpectedly, among them, valine, leucine and isoleucine (or branched-chain amino acids) catabolic pathway demonstrated the most significant changes associated with heart failure (Figure 1A).
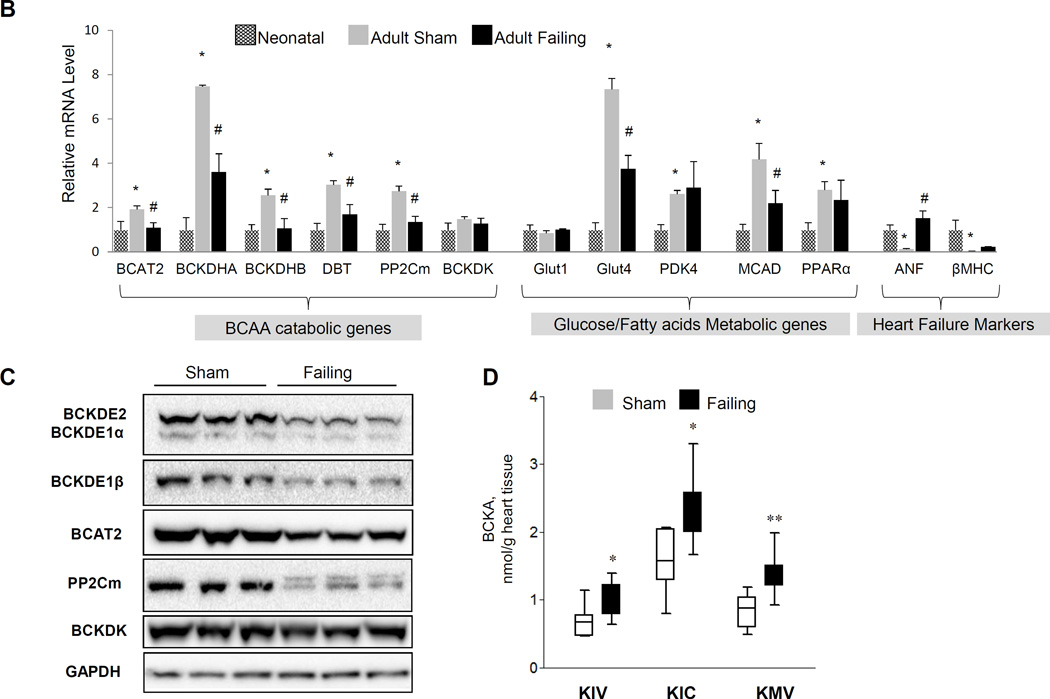
Figure 1.
Remodeling of BCAA catabolism in murine failing heart. A, The down-regulated genes in failing heart were mapped into BCAA catabolism pathway by KEGG. B, Real-time RT-PCR result of specific genes using mRNA from myocardium of Neonatal (n=3), normal (Adult Sham, n=3) and failing (Adult Failing, n=3) mouse hearts. Y axis represents relative mRNA level. ANOVA followed by Newman-Keul test was performed*, p<0.05 compared to Neonatal; #, p<0.05 compared to Adult Sham. C, Western blotting result of proteins involved in BCAA catabolism (GAPDH as loading control) using tissue lysates from three individual normal (Sham) or failing mouse hearts (n=3). D, Individual BCKA concentration in tissues from normal (Sham, n=9) and failing (n=7) mouse hearts. Error bars represent standard deviation (B) or SEM (D). *, p<0.05, **, p<0.01.
A total of 25 out of 46 genes in the KEGG BCAA catabolic pathway showed reduced expression in the failing heart compared to the sham controls (Figure 1A and Table S2). The reduced expression of these key BCAA catabolic genes, including BCAT2, BCKD subunits E1αE1β and E2, as well as the BCKD phosphatase PP2Cm, was verified at both the mRNA and/or protein levels (Figure 1B–C). In contrast, no reduction was seen in the expression of the BCKDK (Figure 1B–C, Figure S1A). In contrast, we observed a coordinated induction of the same set of genes during post-natal maturation from neonatal to adult. This dynamic expression pattern is comparable to what is observed for glucose and fatty acid metabolic genes including Glut4 (Glucose Transporter 4) and Mcad (medium-chain acyl-CoA dehydrogenase), along with other well established "fetal-like" marker genes including Nppa and Myh7 (Figure 1B). Therefore, the rate-limiting and downstream steps of the BCAA catabolic pathway are coordinately down-regulated as part of the "fetal-like" transcriptome remodeling in failing heart.
A significant reduction in PP2Cm expression along with an unchanged expression of BCKDK led to enhanced phosphorylation of BCKD regulatory subunit E1α in the failing hearts comparing to the controls (Figure S1B). Phosphorylation level of BCKD E1α subunit inversely correlates with the BCKD enzymatic activity, consequently, the levels of the intra-myocardial BCKA were significantly increased in the mouse failing hearts (Figure 1D) while the total BCAA levels remained unchanged (Figure S2).
Defect of BCAA catabolism in human failing hearts
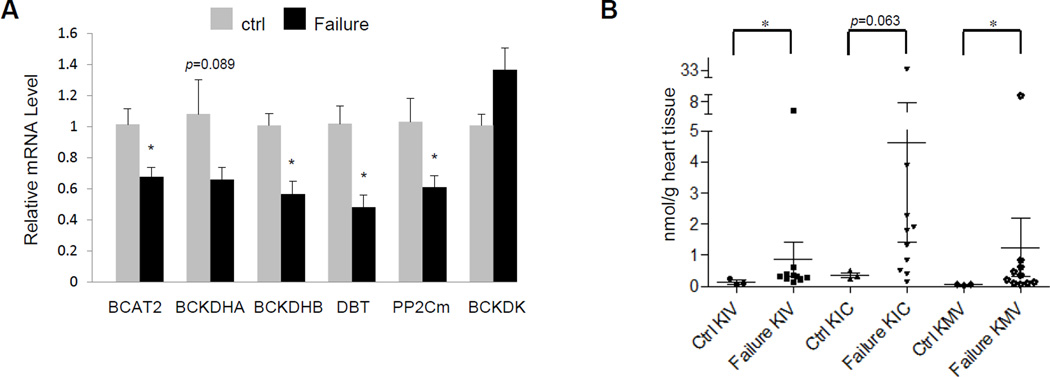
Human cardiomyopathy hearts demonstrated a striking parallel to the observations in rodents with coordinated reduction of all key BCAA catabolic gene products including BCAT2, BCKD subunits, and PP2Cm while BCKDK expression was slightly increased (Figure 2A). Importantly, intra-myocardial levels of BCKA were also significantly increased in human cardiomyopathy hearts (Figure 2B). A significantly higher level of KMV, but not KIC or KIV, was also observed in plasma from humans with heart failure (Figure S3). In contrast, intra-myocardial BCAA levels were not significantly altered (Figure S4). Therefore, impairment of BCAA catabolic activity and intra-myocardial accumulation of BCKA metabolites are conserved metabolic alterations in mouse and human failing hearts.
Figure 2.
Impaired BCAA catabolism in human failing heart. A, Real-time RT-PCR result of specific genes using mRNA from myocardium of control (Ctrl, n=4) and failing (Failure, n=11–15) human hearts. Y axis: relative mRNA level. B, Individual BCKA concentration in tissues from control (n=3) and failing (n=10) human hearts. Error bars represent SEM. *, p<0.05-.
KLF15 regulates cardiac BCAA catabolic gene expression
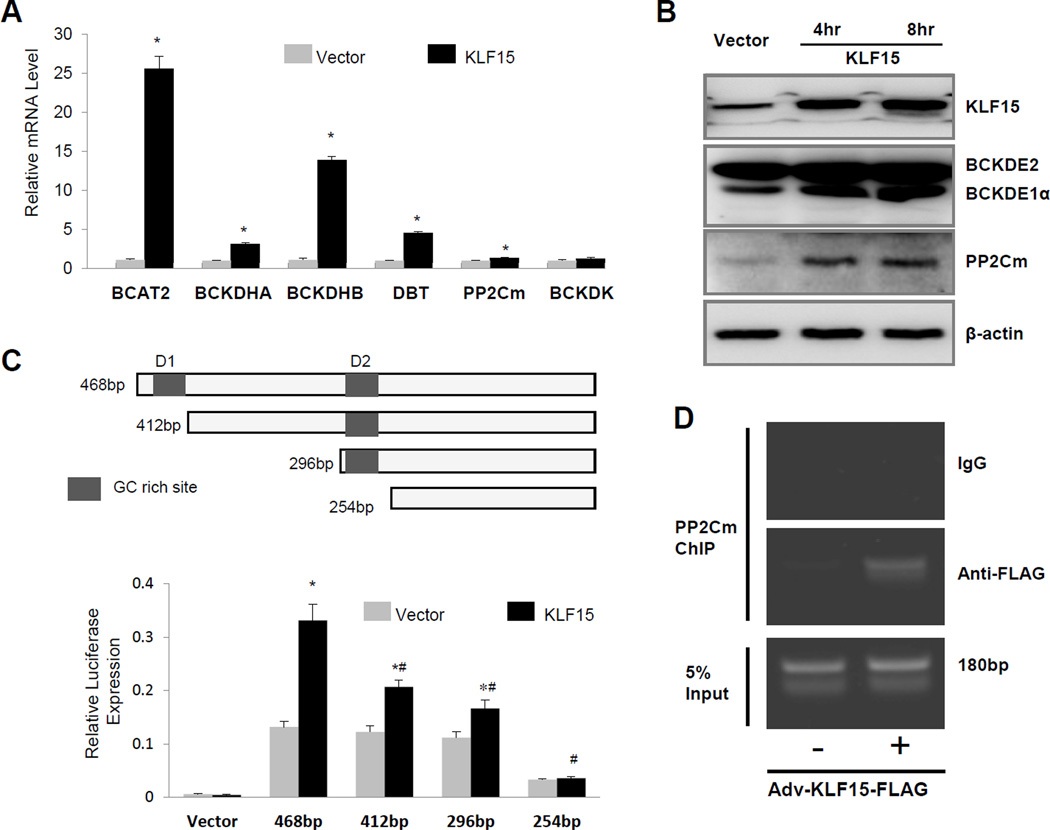
Coordinated regulation of BCAA gene products suggests a shared regulatory mechanism at the transcriptional level. We performed Upstream Regulator Analysis using Ingenuity Pathway Analysis (IPA) Software (http://www.ingenuity.com) for the genes showing altered expression in the mouse failing heart23. The analysis of down-regulated genes in the failing heart predicted numerous factors involved in their regulation (Table S3). The top three candidates were MAP4K4, Krüppel-like factor 15 (KLF15), and PPARA. KLF15 was reported to be a direct transcriptional activator of BCAT225, 26,27. In cultured cardiomyocytes, overexpression of KLF15 significantly induced the mRNA expression of BCAT2, BCKD subunits, and PP2Cm, with a notable exception of BCKDK (Figure 3A). Ectopic expression of KLF15 also induced the expression of these targets in non-myocytes (Figure 3B, Figure S5A). Using a PP2Cm promoter luciferase reporter, we showed KLF15 directly induced the transcriptional activity of the PP2Cm promoter containing putative KLF15 binding motifs (Figure 3C, Figure S5B). Finally, chromatin immunoprecipitation analysis revealed a significant accumulation of KLF15 binding to the endogenous PP2Cm promoter in cardiomyocytes (Figure 3D). Taken together, these data support a previously unidentified broad regulatory role for KLF15 in myocardial BCAA catabolic gene expression.
Figure 3.
KLF15 regulates BCAA catabolic gene expression. A, Real-time RT-PCR result of specific genes using mRNA from neonatal rat ventricular myocytes with (KLF15) or without (Vector) KLF15 overexpression. (n=6) *, p <0.05 compared to vector control. B, Western blotting result of proteins involved in BCAA catabolism (GAPDH as loading control) using cellular lysates from KLF15 overexpressed Hela cells. C, Illustration of partial mouse PP2Cm promoter fragments with two GC rich sites and Luciferase assay result of PP2Cm promoter-luciferase in HeLa cells co-transfected with either KLF15 or corresponding empty vector. The data represented the average values with standard deviation of triplicate samples from one experiment representative of three independent experiments. *, p <0.05 compared to same promoter without KLF15 overexpression. #, p<0.05 compared to 468bp promoter with KLF15 overexpression (n=3). D, The representative result of chromatin immunoprecipitation-PCR validation for KLF15 binging to PP2Cm gene’s promoter in neonatal rat ventricular myocytes after KLF15 overexpression. The experiment has been repeated twice with similar result.
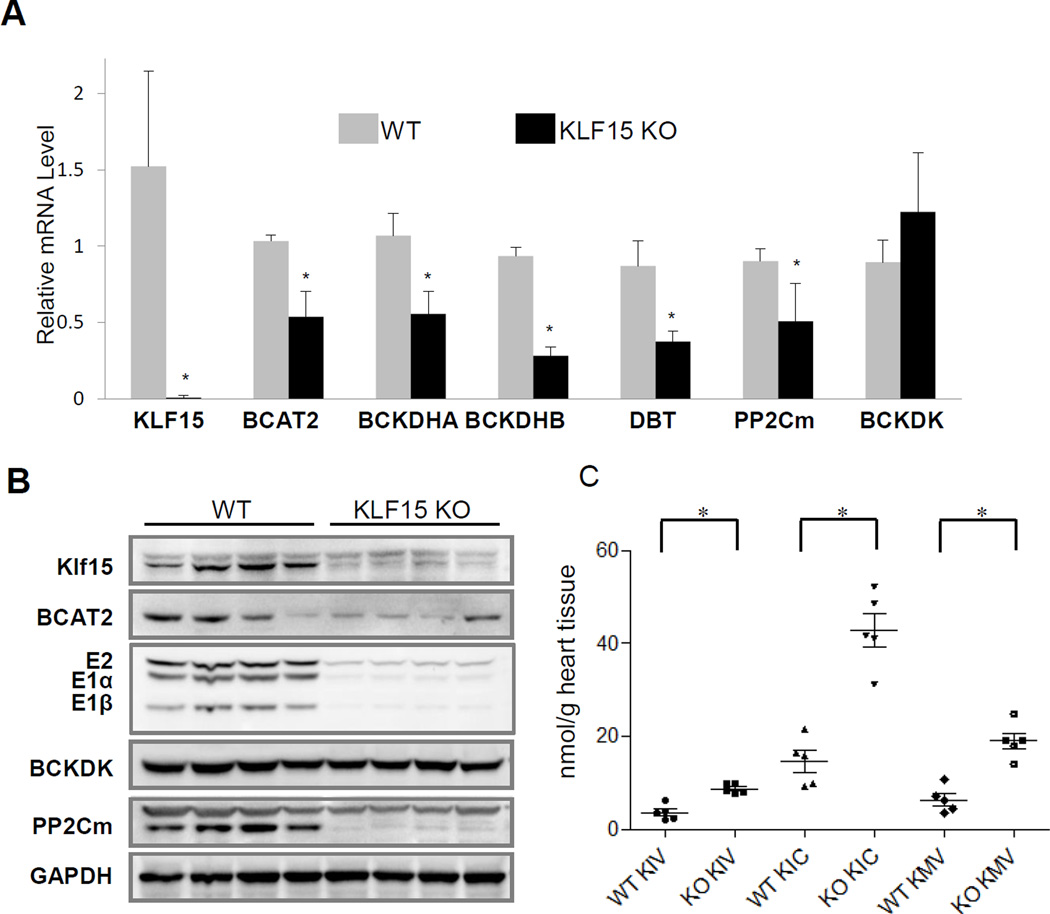
We examined the abundance of BCAA catabolic genes in Klf15 null hearts (KLF15-KO). Consistent with in vitro observations, the KLF15 deficient hearts displayed reduced expression of BCAT2, BCKD (E1a, E1b, E2), and PP2Cm, again with notable exception of BCKDK at both mRNA and protein levels (Figure 4A–B and Figure S6A), phenocopying what were observed in diseased mouse and human hearts (Figures 1 and 2). Also similar to what we observed in failing human and mouse heart samples, elevated intra-myocardial BCKA levels were identified in the KLF15-KO hearts (Figure 4C). Moreover, KLF15 expression was reduced in pressure overloaded murine hearts (Figure S6B) as well as in human cardiomyopathy as previously demonstrated14, 15. Therefore, our data identify KLF15 as a central transcriptional regulator of the BCAA catabolic pathway and that loss of KLF15 is a potential molecular mechanism underlying stress-induced BCAA catabolic defects in the diseased heart.
Figure 4.
Ablation of cardiac KLF15 down-regulates BCAA catabolism. A and B, Real-time RT-PCR (A) and Western blotting (B) result of specific genes in wildtype (WT, n=4) and KLF15 deficient (KLF15 KO, n=4) hearts. C. Level of BCKAs in wildtype (WT, n=4) and KLF15 deficient (KLF15 KO, n=5) heart. Error bars represent standard deviation (A) or SEM (C). *, p <0.05 compared to wildtype.
BCAA catabolic defect impaired cardiac contractile function
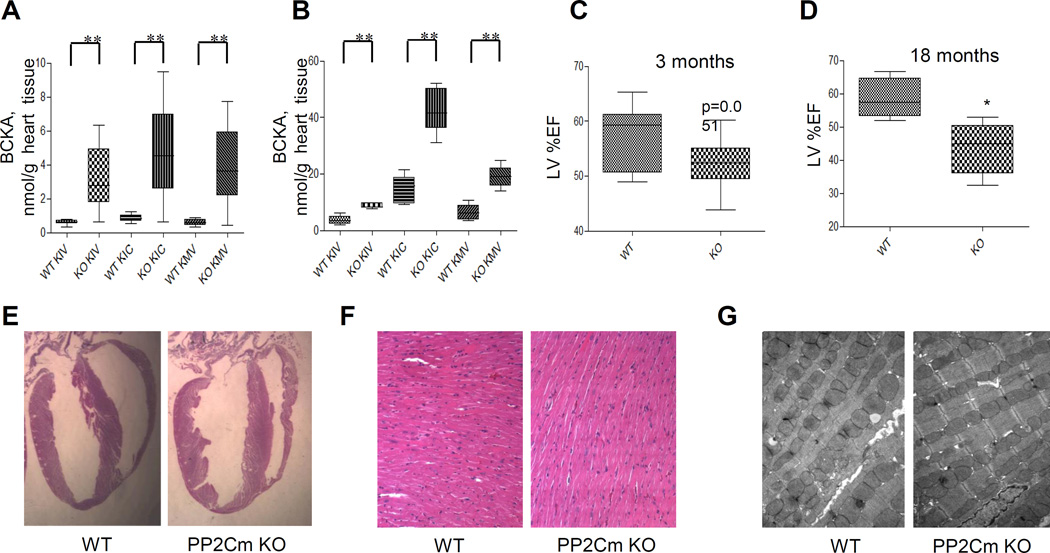
In order to directly assess the effect of BCAA catabolic defect on heart function, we utilized a mouse model carrying genetically inactivated PP2Cm coding gene ppm1k (PP2Cm-KO) where BCKD activity is significantly inhibited due to constantly elevated E1α phosphorylation16, 28. Indeed, compared to wildtype controls, intra-myocardial BCKA and BCAA levels were significantly increased in the PP2Cm deficient hearts from mice fasted for 6 hours (Figure 5A) at comparable levels (< 5 nM/g) to what were observed in mouse and human failing hearts (Figure 1D and 2B). However, cardiac BCKA concentrations became much higher (15–45 nM/g) in the PP2Cm KO heart under feeding condition (Figure 5B, Figure S7A), highlighting the potential dietary influence on BCKA accumulation in the diseased heart when BCAA catabolic activity is compromised. Echocardiogram measurements showed a modest but statistically significant reduction in cardiac systolic function in the PP2Cm deficient mice at 3 months of age (Figure 5C). By 18 months of age, their cardiac function was further reduced compared to the age-matched wildtype controls (Figure 5D). However, young PP2Cm deficient mice exhibited no major changes in cardiac morphology, histology, and ultrastructure, as well as molecular markers of myocardial remodeling (Figure 5E–G, Figure S7B). Therefore, abnormal BCAA catabolism is sufficient to promote contractile dysfunction over time in the absence of any external pathological stressor.
Figure 5.
BCAA catabolic defect impairs cardiac function but not structure. A and B, Individual BCKA concentrations in cardiac tissue of PP2Cm knockout (KO) and wildtype (WT) mice. A, Mice on a normal chow (20% protein) were fasted for 6 hours (WT, n=9; KO, n=10). B, Mice were fasted overnight and fed with a high protein diet (40% protein) for 2 hours (WT, n=5; KO, n=5). Error bars represent SEM. **, p<0.01 compared to WT. C and D, Left ventricular ejection fraction (LV%EF) from WT and PP2Cm KO mice at 3 (C, WT n=12 and KO n=15) or 18 (D, n=5 in each group) months of age. E, Morphology of hearts from wild-type (WT) and PP2Cm deficient (PP2Cm KO) mice. F, Longitudinally sectioned heart was stained with hematoxylin and eosin. Magnification, 200×. G, Transmission electron microscopy was performed in hearts from PP2Cm knockout (KO) and wildtype (WT) mice. Magnification, 7400×.
BCAA catabolic defect enhances susceptibility to heart failure in response to pathological stress
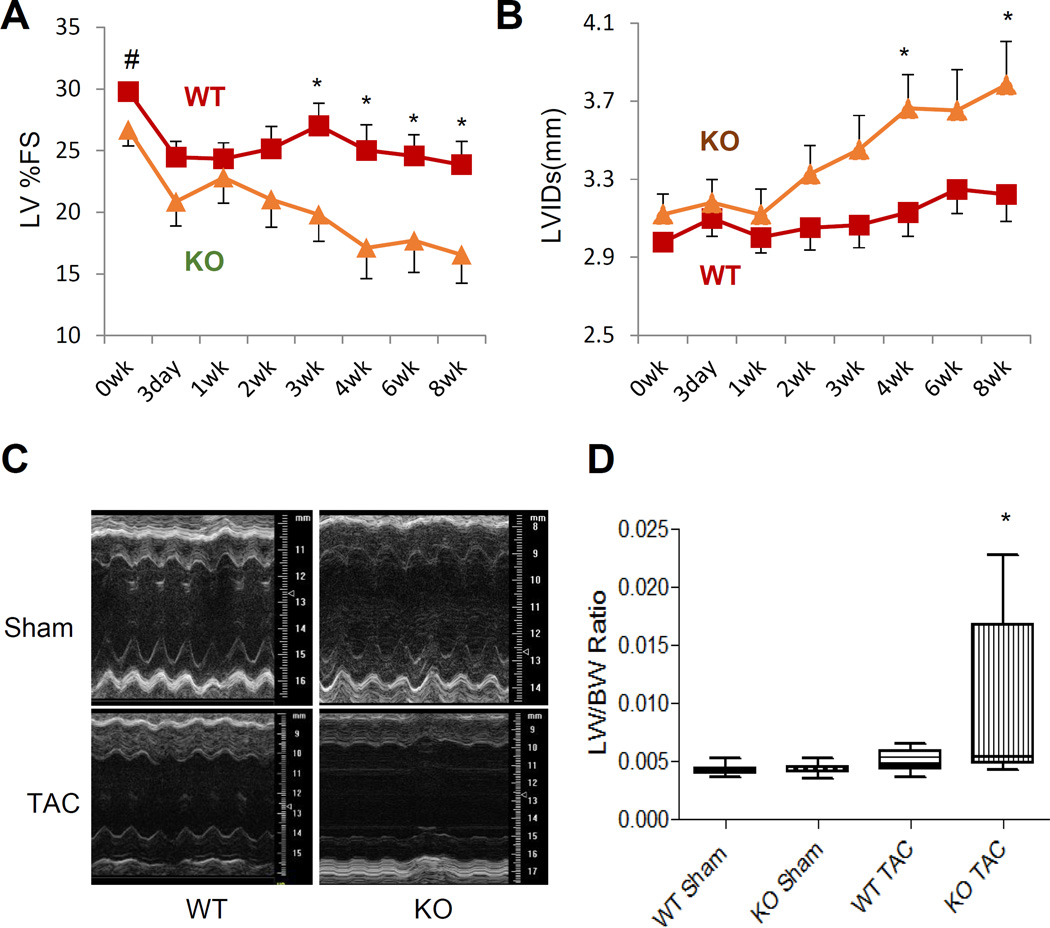
We then subjected wildtype and PP2Cm-KO mice (3–4 months of age) to pressure-overload. From the second week of transverse aortic constriction (TAC), PP2Cm deficient mice exhibited a marked reduction in contractile function (Figure 6A, Figure S8). A repeated measures linear model analysis demonstrated that changes in cardiac echocardiogram parameters such as Left Ventricular Internal Dimension in Systole (LVIDs) for group*day (p=3.54e-6) as well as Left Ventricular Fraction Shortening (LV%FS) (p=1.27e-3) were significant between the PP2Cm deficient and wildtype mice. At 8 weeks post-TAC, PP2Cm deficient mice displayed signs of heart failure as evidenced by significantly reduced LV ejection fraction, chamber dilation, and elevated wet lung weights, an indicator of severe pulmonary congestion due to heart failure (Figure 6). Collectively, these data indicate that deficient BCAA catabolism can directly impair cardiac function and accelerates pressure overload-induced cardiomyopathy.
Figure 6.
BCAA catabolic defect promotes heart failure progression. A and B, Time course for left ventricular fractional shortening (%FS, A) and left ventricular internal dimension at systole (LVIDs, mm, B) from WT (n=11–15) and PP2Cm KO mice (n=13–19) with TAC surgery. The X-axis shows the time in weeks after surgery. C and D, Representative M-mode echocardiographs (C) or ratio of lung weight (LW) to body weight (BW) (D, WT Sham n=9; KO Sham n=10; WT TAC n=8; KO Sham n=8) from WT and PP2Cm KO mice at 8 weeks after surgery. Error bars represent SEM. Statistical analyses were performed with Student’s t-test (A–B) to compare the values of WT and PP2Cm KO at the same time point (#, p=0.05, *, p<0.05) or Kruskal-Wallis test followed by Dunn’s multiple comparison was performed (D) (*, p<0.05 compared to KO Sham). A repeated measures linear model was also fitted for LVIDs (A) and %FS (B).
Impaired metabolic and redox homeostasis by BCAA catabolic deficiency in heart
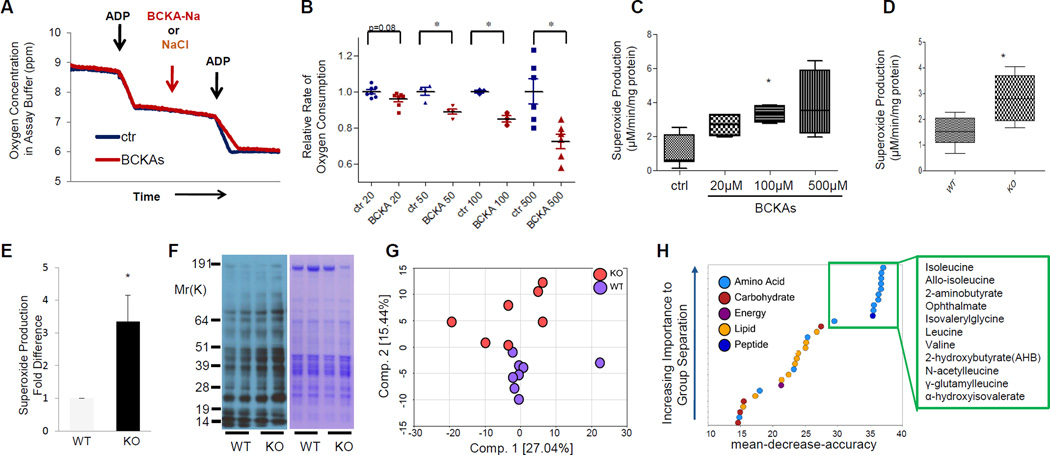
The impact of BCAA catabolic defects on cardiac function is consistently correlated with elevated BCKA metabolites in the diseased heart tissue. We found that, in isolated cardiac mitochondria, BCKAs directly inhibited Complex I but not Complex II mediated respiration (Figure 7A and Figure S9A–B). The inhibition was dose-dependent with significant marked decrease observed at concentrations as low as 20μM BCKAs (Figure 7B). In the meantime, BCKAs also promoted superoxide production in isolated cardiac mitochondria in a dose-dependent manner (Figure 7C). A significant increase in superoxide production was detected from the PP2Cm-deficient mitochondria (Figure 7D) and myocardium (Figure 7E), associated with the enhanced oxidative injury to cardiac proteins (Figure 7F). These data suggest that accumulated BCKAs due to BCKD inactivation may directly impact cardiac mitochondrial activity and redox homeostasis.
Figure 7.
Disturbed metabolic and redox homeostasis by BCKAs. A, Oxygen consumption in mitochondria isolated from wildtype hearts in absence or presence of 500μM BCKAs. B, Relative oxygen consumption rate in the absence or presence of BCKAs at different concentrations (n=3–8 in each group; *, p<0.05 vs control). C, Superoxide production in isolated cardiac mitochondria (n=4–7 in each group; *, p<0.05, vs control). D and E, Superoxide production in isolated mitochondria (D, n=5–6 in each group) and myocardium (E, n=3 in each group) from wildtype and PP2Cm deficient mice. F, Immunoblotting of total protein oxidation detected by carbonyl groups (left) from tissue lysates of wildtype (WT) and PP2Cm deficient (KO) mouse hearts. G, Principal component analysis (PCA) of metabolomic profiles revealed a distinct genotype-based separation for the heart samples (WT, n=8; KO, n=7). H, List of the top 30 biochemicals that separated different genotypes based on their importance. Error bars represent standard deviation SEM (B C, D, E).
We performed additional targeted metabolomic analysis on more than 300 metabolic intermediates in hearts from wildtype and PP2Cm-KO mice. Principal component analysis (PCA) revealed a divergent separation between wildtype and PP2Cm-KO hearts (Figure 7G), suggesting global metabolic changes associated with BCKD inhibition. When Random Forest analysis was applied to cluster all samples, changes in intra-cardiac metabolites separated the wildtype and PP2Cm-KO mice with 100% predictive accuracy (Figure S10A). In addition to BCAA and their metabolites, the top 30 most significantly changed metabolites in the PP2Cm-KO hearts include lipids and carbohydrates (Figure 7H). Specifically, the levels of glucose, glycolytic intermediates, and glucose-derived sugars such as fructose and mannose 6-phosphate were markedly elevated in the PP2Cm deficient heart. These results support the notion that BCAA catabolic deficiency and elevated BCKA can result in impaired mitochondrial function, ROS induction and global perturbations in the myocardial metabolic profile.
Inhibition of BCKDK promoted BCKA degradation and preserved heart function
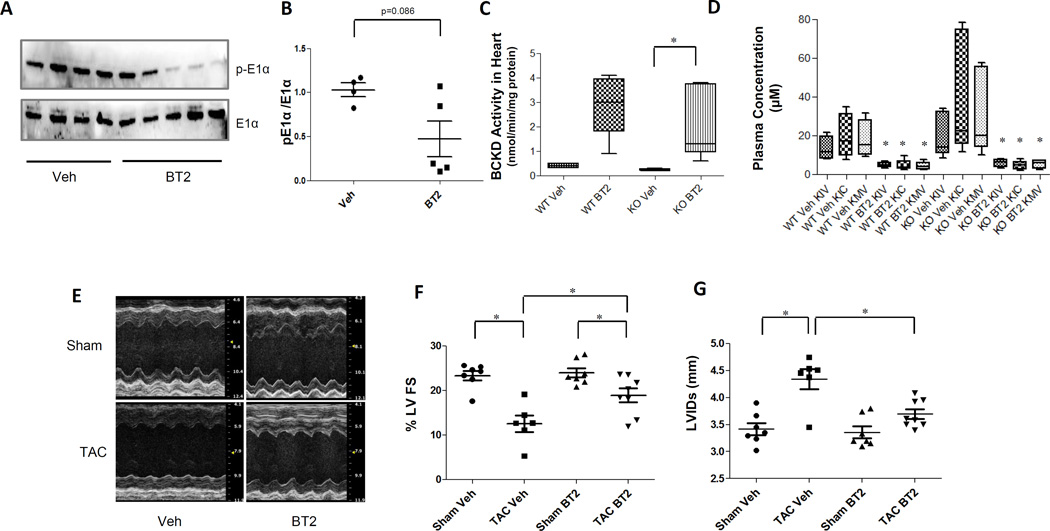
Given the significant contribution of BCAA catabolic defect to cardiac dysfunction, we investigated the impact of enhancing BCAA catabolic activity on pressure-overload induced heart failure in mice. 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2) is a highly specific and potent inhibitor of BCKD kinase18. Administration of BT2 in mice significantly reduced the phosphorylation of BCKD subunit E1α in heart (Figure 8 A and B) and dramatically enhanced cardiac BCKD activity in both wildtype (~7 folds) and the PP2Cm-KO mice (~9 folds) (Figure 8C). Consequently, the plasma BCKA level in both wildtype and PP2Cm-KO mice was markedly reduced (Figure 8D), but with modest impact on plasma BCAA level (Figure S11). More importantly, at 4 weeks post-TAC, BT2-treated mice displayed significantly preserved LV ejection fraction and reduced chamber dilation (Figure 8 D–F and Figure S12). These results suggested that enhancing BCKA degradation by targeted inhibition of BCKDK significantly preserved cardiac function in response to pathological stress.
Figure 8.
Inhibition of BCKDK by BT2 promotes BCKA degradation and preserves cardiac function in pressure-overloaded heart. A, Immunoblot for total and phosphorylated BCKD subunit E1α in heart from wildtype mice treated with vehicle (veh, n=4) or with BT2 (n=5). B, The average phosphorylation level of E1α vs. total E1α are presented with SEM. Error bars represent SEM, *, p < 0.05 between Veh and BT2 treated samples. C, BCKD activity in cardiac tissues from wildtype or PP2Cm-KO mice treated with vehicle or BT2 (n=4–5 in each group). *, p < 0.05 between Veh and BT2 treated groups. D, Individual BCKA concentration in plasma from wildtype and PP2Cm-KO (n=4–6) mice treated with vehicle (veh group) or with BT2 (BT2 group).*, p<0.05 between Veh and BT2 treated groups of same genotype. E, Representative M-mode echocardiographs of mouse hearts following sham or post-TAC treated with vehicle (Veh) or BT2. F, Left ventricular Ejection Fraction (%LVEF, n=6–8), and G, left ventricular internal dimension at systole (LVIDs, n=6–8) from mice with sham or TAC surgery for 4 weeks, treated with or without BT2 as indicated. Error bars represent SEM., *, p< 0.05 between designated groups.
DISCUSSION
In the present study, we reveal that BCAA catabolic gene expression are coordinately suppressed in both murine and human failing hearts as part of the fetal-like gene expression and metabolic re-programming. KLF15-mediated transcriptional regulation is central for this coordinated reduction of BCAA catabolism. Genetic and cellular analyses suggest that BCAA catabolic defects and the resulting accumulation of BCKA metabolites cause cardiac ROS injury and global metabolic alteration and significantly contribute to the progression of heart failure.
Amino acids serve as both important nutrients and potent signaling molecules29. However, compared to the extensive knowledge of fatty acid and glucose metabolism, current understanding regarding amino acid metabolic regulation under normal development or pathological conditions is very limited. BCAAs, including leucine, isoleucine and valine, are essential amino acids with a shared catabolic pathway. In addition to participating in de novo protein synthesis, BCAA also function as potent nutrient signal molecules for cellular metabolism and growth. Through mTOR pathway, BCAA (particularly leucine) can regulate vital cellular processes including protein translation, autophagy and insulin signaling30, affecting glucose and fatty acid metabolism31, muscle anabolism32, and life span33. Genetic defect of BCAA catabolism leads to Maple Syrup Urine Disease (MSUD)34. Recently, abnormal plasma BCAA levels have been associated with neurological, cardiovascular, metabolic diseases, and cancer in numerous studies35–40. These findings highlight the importance of BCAA metabolism in normal physiology and a broad spectrum of human diseases. Suppressed BCAA catabolic activity appears to be a common feature in the stressed heart. Earlier reports by Kato et al. using Dahl Salt-Sensitive rats demonstrate cardiac valine, isoleucine, and leucine levels are elevated following feeding of a high salt diet10. Several other studies, including our current study in both rodent and human have now linked high levels of BCAA with cardiac diseases10, 36, 41–43. Therefore, BCAA catabolic defect is another metabolic hallmark of heart diseases that may be exploited as additional metabolic biomarkers for cardiac pathology.
The coordinated loss of BCAA catabolic gene expression suggests a common regulatory machinery for the pathway. This notion is consistent with two recent studies reported in hypertrophied and early stage failing heart11,12. From both bioinformatic and genetic approaches, we identify KLF15 as a "master" transcription factor responsible for BCAA catabolic gene expression in heart. The functional role of KLF15 is well documented in cardiac hypertrophy13, 44, heart failure14, and cardiac fibrosis45. In addition to hypertrophic genes, KLF15 also serves as a key regulator of glucose, fatty acid and amino acid metabolism25, 26, 46–51. KLF15 is reported to directly modulate the expression of BCAT2 as a mechanism to modulate mTOR signaling in skeletal muscle25, 26. KLF15 has previously been shown to be regulated by diverse pathologic stimuli. Human and murine forms of pressure overload cardiomyopathy have been shown to reduce KLF15 levels; a result in humans that is reversed by mechanical unloading15, 47, 52, 53. Moreover, hypertrophic stimuli (including AngII, PE, and ET-1) have been shown to reduce KLF15 levels both in vivo and in vitro52, 53. Our data further enforce the notion that KLF15 is an important regulator for metabolic reprogramming in heart by modulating several important branches of macronutrient metabolism including fatty acid, glucose and amino acids.
It is intriguing that metabolic profiling in hypertrophic (1 week post-TAC) or post-MI hearts revealed elevated BCAA concentrations11, 12 in cardiac tissue in contrast to what we observed in both end-stage human cardiomypathy hearts and 8 week post-TAC mouse failing hearts. It is plausible to speculate that BCAA catabolic reprogramming is a compensatory mechanism at least at the initial stage of the myocardium’s response to stress given that BCAA preservation would redirect amino acids from catabolic consumption to protein synthesis and cell growth during cardiac hypertrophy. Perturbation of BCAA catabolic activity may have significant impact on mTOR signaling, leading to potential changes in cardiac growth, metabolism and survival. However, defective BCKD activity also causes accumulation of BCKA in hearts, which may lead to a detrimental effect due to cytotoxic effects on mitochondrial function and ROS homeostasis16, 54. Indeed, a direct and dose-dependent impact of BCKA treatment on mitochondrial function and ROS production as demonstrated in this study highlights the potential contribution of BCKA as the true pathogenic culprit underlying BCAA catabolic defects in the progression of heart failure. BCKA and BCKA mediated mitochondrial and cellular defects should be further explored as both metabolic biomarkers and therapeutic targets for heart failure..
Our genetic data from this report clearly implicates BCAA catabolic defect as a significant contributor to the pathogenesis of heart failure. The results from our study using BCKDK inhibitor (Figure 8) clearly demonstrated the translational value of targeting BCAA catabolism as a therapy for heart failure17, 55. It also raised the question about the potential impact of dietary influence on disease progression. Tanada et al showed BCAA supplement ameliorated the progression of heart failure in rats56 associated with preserved skeletal muscle weight and mitochondrial function. It is not clear if the benefit of BCAA supplement at early stage of heart failure is derived from enhanced BCAA flux and preserved BCAA catabolic activities. Clearly more pre-clinical and clinical studies are needed to fully establish the therapeutic window and approaches to manipulate BCAA catabolism in heart failure. In conclusion, we have elucidated a previously unappreciated role for BCAA catabolism in the cardiac metabolic adaptation to stress. This insight can be further exploited for future diagnostic and therapeutic development.
Supplementary Material
Clinical Perspectives.
Heart failure is a leading cause of mortality and hospitalization, and effective therapies remain elusive. The present study uncovers that branched-chain amino acids (BCAA, including leucine, isoleucine and valine) catabolic defect is a hallmark of metabolic changes in murine failing heart and human dilated cardiomyopathy hearts. Accumulation of branched-chain alpha-keto acids (BCKA) resulted from BCAA catabolic defect directly impairs mitochondrial activity, induces oxidative stress, and promotes cardiac dysfunction. More importantly, restoration of BCAA catabolism by pharmacological agent blunts disease progression in pressure-overload induced heart failure. Therefore, defects in BCAA/BCKA catabolism is not only a new metabolic biomarker for heart failure but also a significant contributor to heart failure. Promoting BCAA catabolic activity by utilizing pharmacological agent can be a potentially effective therapeutic strategy to ameliorate the pathogenic progression of heart failure. Finally, considering the dietary source of BCAA, the present study indicates a role of protein intake in the disease progression of heart failure and thus serves as a preclinical basis to develop a more appropriate nutritional intervention approach for this disease.
Acknowledgments
Funding Sources: This work is supported in part by Ministry of Science and Technology of China Grant 2012BAI02B05 and 2013YQ030923; National Institute of Health grants HL108186, HL103205, HL098954, HL080111 (to Y. Wang), HL075427, HL076754, HL084154, HL086548, and HL097593 (M. Jain), DK58398 (to C.B. Newgard), DK053843 and DK062880 (to C.J. Lynch), DK62306 and the Welch Foundation Grant I-1286 (to DTC) T32HL105338 and F32HL110538 (to D.A. Prosdocimo); R01HG006264 (to X. Xiao), HL119968 (to HC), the Laubisch Fund (UCLA); American Heart Association (AHA) Western States Affiliate Post-doctoral Fellowship Award and AHA Science Development Grant (to H. Sun); AHA Established Investigator Award (Y. Wang and M. Jain). This work is also supported by National Natural Science Foundation of China (NSFC30971094 and NSFC81270317), Science and Technology Commission of Shanghai Municipality (13ZR1423300, 11410709000 and 12PJ1405800), and Shanghai Jiaotong University K.C. Wong Medical Fellowship Fund.
Footnotes
Disclosures: None.
References
- 1.Gropler RJ, Beanlands RSB, Dilsizian V, Lewandowski ED, Villanueva F, Ziadi MC. Imaging Myocardial Metabolic Remodeling. J Nucl Med. 2010;51:88S–101S. doi: 10.2967/jnumed.109.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Bilsen M, Smeets PJH, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226. doi: 10.1016/j.cardiores.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 3.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 4.Tuunanen H, Knuuti J. Metabolic remodelling in human heart failure. Cardiovasc Res. 2011;90:251–257. doi: 10.1093/cvr/cvr052. [DOI] [PubMed] [Google Scholar]
- 5.Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res. 2011;90:210–219. doi: 10.1093/cvr/cvr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taegtmeyer H, Harinstein ME, Gheorghiade M. More Than Bricks and Mortar: Comments on Protein and Amino Acid Metabolism in the Heart. Am J Cardiol. 2008;101:S3–S7. doi: 10.1016/j.amjcard.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 7.Soukoulis V, Dihu JB, Sole M, Anker SD, Cleland J, Fonarow GC, Metra M, Pasini E, Strzelczyk T, Taegtmeyer H, Gheorghiade M. Micronutrient Deficiencies: An Unmet Need in Heart Failure. J Am Coll Cardiol. 2009;54:1660–1673. doi: 10.1016/j.jacc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Peterson MB, Mead RJ, Welty JD. Free amino acids in congestive heart failure. J Mol Cell Cardiol. 1973;5:139–147. doi: 10.1016/0022-2828(73)90047-3. [DOI] [PubMed] [Google Scholar]
- 9.Venturini A, Ascione R, Lin H, Polesel E, Angelini G, Suleiman MS. The importance of myocardial amino acids during ischemia and reperfusion in dilated left ventricle of patients with degenerative mitral valve disease. Mol Cell Biochem. 2009;330:63–70. doi: 10.1007/s11010-009-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Cir Heart Fail. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 11.Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, Kapoor K, Koves TR, Stevens R, Ilkayeva OR, Vega RB, Attie AD, Muoio DM, Kelly DP. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail. 2014;7:1022–1031. doi: 10.1161/CIRCHEARTFAILURE.114.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail. 2014;7:634–642. doi: 10.1161/CIRCHEARTFAILURE.114.001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto YM, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:7074–7079. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haldar SM, Lu Y, Jeyaraj D, Kawanami D, Cui Y, Eapen SJ, Hao C, Li Y, Doughman YQ, Watanabe M, Shimizu K, Kuivaniemi H, Sadoshima J, Margulies KB, Cappola TP, Jain MK. Klf15 deficiency is a molecular link between heart failure and aortic aneurysm formation. Sci Transl Med. 2010;2:26ra26. doi: 10.1126/scitranslmed.3000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prosdocimo DA, Anand P, Liao X, Zhu H, Shelkay S, Artero-Calderon P, Zhang L, Kirsh J, Moore D, Rosca MG, Vazquez E, Kerner J, Akat KM, Williams Z, Zhao J, Fujioka H, Tuschl T, Bai X, Schulze PC, Hoppel CL, Jain MK, Haldar SM. Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism. J Biol Chem. 2014;289:5914–5924. doi: 10.1074/jbc.M113.531384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, Wang Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–1687. doi: 10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao C, Ren S, Lee JH, Qiu J, Chapski DJ, Rau CD, Zhou Y, Abdellatif M, Nakano A, Vondriska TM, Xiao X, Fu XD, Chen JN, Wang Y. RBFox1-mediated RNA splicing regulates cardiac hypertrophy and heart failure. J Clin Invest. 2016;126:195–206. doi: 10.1172/JCI84015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tso S-C, Gui W-J, Wu C-Y, Chuang JL, Qi X, Skvorak KJ, Dorko K, Wallace AL, Morlock LK, Lee BH, Hutson SM, Strom SC, Williams NS, Tambar UK, Wynn RM, Chuang DT. Benzothiophene Carboxylate Derivatives as Novel Allosteric Inhibitors of Branched-chain α-Ketoacid Dehydrogenase Kinase. J Biol Chem. 2014;289:20583–20593. doi: 10.1074/jbc.M114.569251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson KC, Chen G, Lynch CJ. Quantification of branched-chain keto acids in tissue by ultra fast liquid chromatography-mass spectrometry. Anal Biochem. 2013;439:116–122. doi: 10.1016/j.ab.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. In: Molecular Changes in Fatty Acid Oxidation in the Failing Heart Molecular Defects in Cardiovascular Disease. Dhalla NS, Nagano M, Ostadal B, editors. New York: Springer; 2011. pp. 153–175. [Google Scholar]
- 21.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program. Ann N Y Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolwicz SC, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011;90:194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J-H, Gao C, Peng G, Greer C, Ren S, Wang Y, Xiao X. Analysis of Transcriptome Complexity Through RNA Sequencing in Normal and Failing Murine Hearts / Novelty and Significance. Circ Res. 2011;109:1332–1341. doi: 10.1161/CIRCRESAHA.111.249433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S-i, Nakae J, Tagata Y, Nishitani S, Takehana K, Sano M, Fukuda K, Suematsu M, Morimoto C, Tanaka H. Crosstalk between Glucocorticoid Receptor and Nutritional Sensor mTOR in Skeletal Muscle. Cell Metab. 2011;13:170–182. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Gray S, Wang B, Orihuela Y, Hong E-G, Fisch S, Haldar S, Cline GW, Kim JK, Peroni OD, Kahn BB, Jain MK. Regulation of Gluconeogenesis by Krüppel-like Factor 15. Cell Metab. 2007;5:305–312. doi: 10.1016/j.cmet.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol. 2013;115:1065–1074. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M, Lu G, Gao C, Wang Y, Sun H. Tissue-specific and Nutrient Regulation of the Branched-chain α-Keto Acid Dehydrogenase Phosphatase, Protein Phosphatase 2Cm (PP2Cm) J Biol Chem. 2012;287:23397–23406. doi: 10.1074/jbc.M112.351031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells. 2013;35:463–473. doi: 10.1007/s10059-013-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab. 2012;302:E1329–E1342. doi: 10.1152/ajpendo.00525.2011. [DOI] [PubMed] [Google Scholar]
- 31.Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes. 2012;3:38–53. doi: 10.4239/wjd.v3.i3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vary TC, Lynch CJ. Nutrient Signaling Components Controlling Protein Synthesis in Striated Muscle. J Nutr. 2007;137:1835–1843. doi: 10.1093/jn/137.8.1835. [DOI] [PubMed] [Google Scholar]
- 33.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Barschak AG, Marchesan C, Sitta A, Deon M, Giugliani R, Wajner M, Vargas CR. Maple syrup urine disease in treated patients: biochemical and oxidative stress profiles. Clin Biochem. 2008;41:317–324. doi: 10.1016/j.clinbiochem.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Mochel F, Charles P, Seguin Fo, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A. Early Energy Deficit in Huntington Disease: Identification of a Plasma Biomarker Traceable during Disease Progression. PLoS ONE. 2007;2:e647. doi: 10.1371/journal.pone.0000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a Peripheral Blood Metabolic Profile With Coronary Artery Disease and Risk of Subsequent Cardiovascular Events. Circ Cardiovasc Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 37.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, Magnusson M, Fox CS, O'Donnell CJ, Vasan RS, Melander O, Clish CB, Gerszten RE, Wang TJ. Metabolite Profiling Identifies Pathways Associated With Metabolic Risk in Humans. Circulation. 2012;125:2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, Davidson SM, Papagiannakopoulos T, Yang A, Dayton TL, Ogino S, Stampfer MJ, Giovannucci EL, Qian ZR, Rubinson DA, Ma J, Sesso HD, Gaziano JM, Cochrane BB, Liu S, Wactawski-Wende J, Manson JE, Pollak MN, Kimmelman AC, Souza A, Pierce K, Wang TJ, Gerszten RE, Fuchs CS, Vander Heiden MG, Wolpin BM. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novarino G, El-Fishawy P, Kayserili H, Meguid NA, Scott EM, Schroth J, Silhavy JL, Kara M, Khalil RO, Ben-Omran T, Ercan-Sencicek AG, Hashish AF, Sanders SJ, Gupta AR, Hashem HS, Matern D, Gabriel S, Sweetman L, Rahimi Y, Harris RA, State MW, Gleeson JG. Mutations in BCKD-kinase Lead to a Potentially Treatable Form of Autism with Epilepsy. Science. 2012;338:394–397. doi: 10.1126/science.1224631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, Schwinn DA, Glower DD, Newgard CB, Podgoreanu MV. Metabolomic Profiling Reveals Distinct Patterns of Myocardial Substrate Use in Humans With Coronary Artery Disease or Left Ventricular Dysfunction During Surgical Ischemia/Reperfusion. Circulation. 2009;119:1736–1746. doi: 10.1161/CIRCULATIONAHA.108.816116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah SH, Hauser ER, Bain JR, Muehlbauer MJ, Haynes C, Stevens RD, Wenner BR, Dowdy ZE, Granger CB, Ginsburg GS, Newgard CB, Kraus WE. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol. 2009;5:258. doi: 10.1038/msb.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Héliès-Toussaint C, Moinard C, Rasmusen C, Tabbi-Anneni I, Cynober L, Grynberg A. Aortic banding in rat as a model to investigate malnutrition associated with heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1325–R1331. doi: 10.1152/ajpregu.00320.2004. [DOI] [PubMed] [Google Scholar]
- 44.Leenders JJ, Wijnen WJ, Hiller M, van der Made I, Lentink V, van Leeuwen REW, Herias V, Pokharel S, Heymans S, de Windt LJ, Høydal MA, Pinto YM, Creemers EE. Regulation of Cardiac Gene Expression by KLF15, a Repressor of Myocardin Activity. J Biol Chem. 2010;285:27449–27456. doi: 10.1074/jbc.M110.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Haldar SM, Lu Y, Ibrahim OA, Fisch S, Gray S, Leask A, Jain MK. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol. 2008;45:193–197. doi: 10.1016/j.yjmcc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horie T, Ono K, Nishi H, Iwanaga Y, Nagao K, Kinoshita M, Kuwabara Y, Takanabe R, Hasegawa K, Kita T, Kimura T. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem Biophys Res Commun. 2009;389:315–320. doi: 10.1016/j.bbrc.2009.08.136. [DOI] [PubMed] [Google Scholar]
- 47.Gray S, Feinberg MW, Hull S, Kuo CT, Watanabe M, Sen-Banerjee S, DePina A, Haspel R, Jain MK. The Krüppel-like Factor KLF15 Regulates the Insulin-sensitive Glucose Transporter GLUT4. J Biol Chem. 2002;277:34322–34328. doi: 10.1074/jbc.M201304200. [DOI] [PubMed] [Google Scholar]
- 48.Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M. Role of Krüppel-like Factor 15 (KLF15) in Transcriptional Regulation of Adipogenesis. J Biol Chem. 2005;280:12867–12875. doi: 10.1074/jbc.M410515200. [DOI] [PubMed] [Google Scholar]
- 49.Nagare T, Sakaue H, Matsumoto M, Cao Y, Inagaki K, Sakai M, Takashima Y, Nakamura K, Mori T, Okada Y, Matsuki Y, Watanabe E, Ikeda K, Taguchi R, Kamimura N, Ohta S, Hiramatsu R, Kasuga M. Overexpression of KLF15 Transcription Factor in Adipocytes of Mice Results in Down-regulation of SCD1 Protein Expression in Adipocytes and Consequent Enhancement of Glucose-induced Insulin Secretion. J Biol Chem. 2011;286:37458–37469. doi: 10.1074/jbc.M111.242651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeyaraj D, Scheer FA, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA, Eapen SJ, Eapen BL, Cui Y, Mahabeleshwar GH, Lee HG, Smith MA, Casadesus G, Mintz EM, Sun H, Wang Y, Ramsey KM, Bass J, Shea SA, Albrecht U, Jain MK. Klf15 Orchestrates Circadian Nitrogen Homeostasis. Cell Metab. 2012;15:311–323. doi: 10.1016/j.cmet.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haldar SM, Jeyaraj D, Anand P, Zhu H, Lu Y, Prosdocimo DA, Eapen B, Kawanami D, Okutsu M, Brotto L, Fujioka H, Kerner J, Rosca MG, McGuinness OP, Snow RJ, Russell AP, Gerber AN, Bai X, Yan Z, Nosek TM, Brotto M, Hoppel CL, Jain MK. Kruppel-like factor 15 regulates skeletal muscle lipid flux and exercise adaptation. Proc Natl Acad Sci U S A. 2012;109:6739–6744. doi: 10.1073/pnas.1121060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haldar SM, Lu Y, Jeyaraj D, Kawanami D, Cui Y, Eapen SJ, Hao C, Li Y, Doughman Y-Q, Watanabe M, Shimizu K, Kuivaniemi H, Sadoshima J, Margulies KB, Cappola TP, Jain MK. Klf15 Deficiency Is a Molecular Link Between Heart Failure and Aortic Aneurysm Formation. Sci Transl Med. 2010;2:26ra26. doi: 10.1126/scitranslmed.3000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto Y, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:7074–7079. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tso SC, Qi X, Gui WJ, Chuang JL, Morlock LK, Wallace AL, Ahmed K, Laxman S, Campeau PM, Lee BH, Hutson SM, Tu BP, Williams NS, Tambar UK, Wynn RM, Chuang DT. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain alpha-ketoacid dehydrogenase kinase. Proc Natl Acad Sci U S A. 2013;110:9728–9733. doi: 10.1073/pnas.1303220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanada Y, Shioi T, Kato T, Kawamoto A, Okuda J, Kimura T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci. 2015;137:20–27. doi: 10.1016/j.lfs.2015.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.