Abstract
Objectives
Although systemic lupus erythematosus (SLE) most commonly occurs in reproductive-age women, some are diagnosed after age 50. Recognizing that greater than one third of SLE criteria are cutaneous, we undertook a systematic review and meta-analysis to evaluate differences in cutaneous manifestations in early and late-onset SLE patients.
Methods
We searched the literature using PubMed, CINAHL, Web of Science and Cochrane Library. We excluded studies that did not include ACR SLE classification criteria, early-onset controls, that defined late-onset SLE as <50 years of age, or were not written in English. Two authors rated study quality using the Newcastle Ottawa Quality Scale. We used Forest plots to compare odds ratios (95% confidence intervals) of cutaneous manifestations by age. Study heterogeneity was assessed using I2.
Results
Thirty five studies, representing 11,189 early-onset and 1,727 late-onset patients with SLE, met eligibility criteria. The female: male ratio was lower in the late-onset group (5:1 versus 8:1). Most cutaneous manifestations were less prevalent in the late-onset group. In particular, malar rash (OR 0.43 (0.35, 0.52)), photosensitivity (OR 0.72 (0.59, 0.88)) and livedo reticularis (OR 0.33 (0.17, 0.64)) were less common in late-onset patients. In contrast, sicca symptoms were more common (OR 2.45 (1.91, 3.14)). The mean Newcastle Ottawa Quality Scale score was 6.3 ±0.5 (scale 0–9) with high inter-rater reliability for the score (0.96).
Conclusions
Overall, cutaneous manifestations are less common in late-onset SLE patients, except sicca symptoms. Future studies should investigate etiologies for this phenomenon including roles of immune senescence, environment, gender and immunogenetics.
Keywords: Systemic Lupus Erythematosus, Cutaneous, Late-Onset, Sicca, Malar Rash, Photosensitivity, Alopecia, Raynaud
Introduction
Systemic lupus erythematosus (SLE) most often occurs in women of reproductive age. SLE onset in adults ≥ 50 years old is referred to as “late-onset SLE.” Previous studies report that late-onset SLE patients are more likely to include men and have a more insidious onset of disease [1–7]. Over one third of the ACR SLE classification criteria reflect cutaneous manifestations so it is not surprising that arthritis and cutaneous findings remain the most common presenting symptoms in both late-onset and early-onset SLE. Yet, previous literature suggested that these are less common in late-onset disease [3, 8–12]. Overall, the proportion of late-onset SLE among all SLE cases is relatively low, ranging from 4% to 20% [1, 3, 4, 8, 10, 13, 14]. However, due to a higher life expectancy and increasing awareness of the disease, the prevalence of late-onset SLE is expected to rise. Therefore, identifying the unique characteristics of this patient population is important. Conclusions drawn from previous studies including a 1989 meta-analysis of nine studies with 170 late-onset SLE patients [15] were limited by small sizes and heterogeneity of patient groups. To gain additional insight into the cutaneous manifestations of late-onset SLE, we conducted a systematic review and meta-analysis of published literature. We compared cutaneous manifestations in patients with early and late-onset SLE.
Methods
We performed a systematic review of the literature to identify articles comparing the cutaneous manifestations of patients with late versus early-onset lupus. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) consensus was followed in the completion of this systematic review and meta-analysis [16]. With assistance from a professional medical librarian we electronically searched the literature in PubMed, CINAHL, Web of Science and Cochrane Library with MESH and keyword subject headings “systemic lupus erythematosus,” “cutaneous lupus erythematosus,” “SLE,” or “late-onset SLE,” AND “age factors,” “age of onset,” “late-onset,” “older-onset,” “over 50,” “older adults,” and “geriatrics,” for entries published from databases’ inception through August 2013. Potential articles were reviewed first by title and abstract only, next by full text, and lastly by analyzing eligible studies in detail. A second reviewer scrutinized a random 10% of all potential titles and abstracts. The reviewers demonstrated 100% agreement in articles included and excluded. Bibliographies of all included articles were reviewed to identify additional citations.
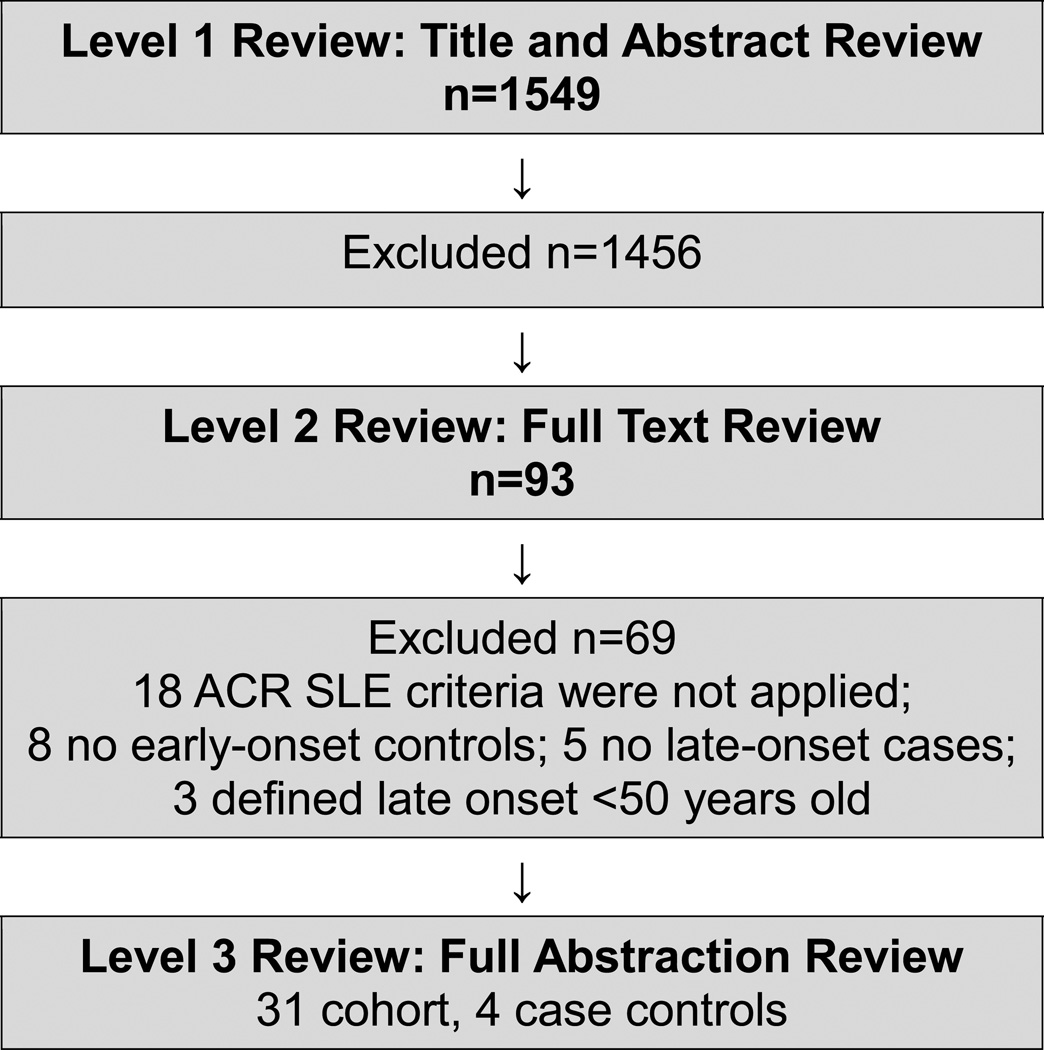
Studies with the following criteria were included: (A) confirmed SLE using American College of Rheumatology (ACR) criteria [17] and (B) data on cutaneous findings of late-onset SLE defined as ≥ 50 years of age. We excluded studies that did not require SLE patients to meet ACR classification criteria, did not include early-onset controls, defined late-onset SLE as <50 years of age, or were written in a language other than English (Figure 1).
Figure 1.
Study selection process with description of study inclusion and exclusions during the three level review for the systematic review and meta-analysis.
Methodological quality of eligible studies and risk of bias were evaluated using the Newcastle Ottawa Quality Assessment Scale for cohort and case control studies [18]. The scale assesses cohort selection and comparisons between groups (cases and controls), outcomes, and adequacy of follow-up. Two reviewers rated each study, assigning a score out of 9 possible points. Discrepancies in scores were resolved by consensus with a third MD reviewer. Inter-rater reliability of two reviewers was calculated.
Data was extracted by two authors including date of publication, study location (country and population vs hospital or clinic based), study type (cohort vs case study), follow-up period, late-onset age definition, and clinical manifestations. The numbers of late-onset patients who exhibited SLE cutaneous manifestations including malar rash, discoid rash, photosensitivity, mucosal ulceration, alopecia, sicca symptoms, Raynaud’s phenomenon, cutaneous vasculitis, livedo reticularis, and subacute cutaneous lupus (SCLE) were recorded and compared to numbers of these manifestations in early-onset patients.
Statistical Analysis
We created Forest plots to summarize composite data, generating odds ratios and corresponding 95% confidence intervals for each cutaneous manifestation. Heterogeneity between studies was evaluated using the I2 statistic with 25%, 50% and 75% indicating low, moderate, and high heterogeneity, respectively. Funnel plots were reviewed to detect publication bias. We performed additional sub-group analyses for Forest plots demonstrating >30% heterogeneity, excluding case-control studies and determining the relative risk of the cutaneous manifestation. All analyses were performed using R software version 3.1.2 and the package “meta.”
Results
Literature searches yielded 1,549 potential articles. After screening titles and abstracts, 95 full articles were retrieved for full-text evaluation. After application of exclusion criteria, 35 articles met criteria for final inclusion and level 3 review (Figure 1), including 31 cohort studies and 4 case control studies [1–14, 19–39].
The 35 studies included in the systematic review and meta-analysis are summarized in Table 1. Studies reflected a geographically and ethnically diverse population. Overall, 27 studies used the classic inclusion of age ≥50 years old, while the remaining eight had definitions ranging from >50 to >65 years old. Of note, 24 of the 35 studies also included individuals < 18 years of age in the control group. The mean ± standard deviation Newcastle Ottawa Quality Scale score of the 35 included articles was 6.3 ±0.45 with a maximum possible score of 9 points. Inter-rater reliability for these quality scores was k=0.96 with two independent MD reviewers.
Table 1.
Descriptions of studies included in meta-analysis of Late vs. Early-Onset SLE
| Author and Publication Year |
Setting (I)npatient (O)utpatient (P)opulation |
Study type |
Years | Early - onset SLE (n) |
Late - onset SLE (n) |
Late - onset Age Def. |
Newcastle Ottawa Score |
|---|---|---|---|---|---|---|---|
| Alonso 201219 | Spain; I | cohort | 1987–2006 | 91 | 59 | ≥50* | 6 |
| Antolin 19958 | Spain; I | cohort | 1980–1992 | 134 | 29 | >50 | 7 |
| Appenzeller 200820 | Brazil; I | c-c | 1974–2005 | 60 | 16 | ≥50* | 7 |
| Boddaert 20041 | France; O | cohort | 1980–2000 | 114 | 47 | ≥50* | 6 |
| Cartella 201321 | Italy; O | cohort | 1976–2008 | 495 | 40 | ≥50 | 6 |
| Cervera 19939 | Europe; P | cohort | 1980–1990 | 910 | 90 | >50* | 6 |
| Chen 200922 | Taiwan; I/O | cohort | 1998–2008 | 50 | 19 | ≥60* | 6 |
| Cooper 200223 | USA; P | c-c | 1997–1999 | 195 | 61 | ≥50 | 6 |
| Costallat 199413 | Brazil; O | cohort | 1973–1992 | 223 | 10 | ≥50* | 6 |
| Dimant 19792 | USA: I/O | cohort | 1966–1976 | 218 | 16 | >50* | 6 |
| Domenech 199210 | England; I/O | cohort | 1985–1991 | 232 | 15 | ≥50* | 6 |
| Feng 201024 | China; I | cohort | unknown | 1550 | 131 | ≥50* | 5 |
| Font 19913 | Spain; I/O | cohort | 1980–1988 | 210 | 40 | ≥50* | 6 |
| Gomez 200625 | Spain; P | cohort | 1992-? | 259 | 91 | ≥50* | 6 |
| Hashimoto 19874 | Japan; O | cohort | 1955–1985 | 501 | 21 | ≥50* | 6 |
| Ho 199811 | China; O | cohort | 1971–1997 | 100 | 25 | >50* | 7 |
| Jacobsen 199814 | Denmark; P | cohort | 1975–1995 | 354 | 103 | ≥50 | 6 |
| Janwityanujit 199512 | Thailand; I | cohort | 1990–1992 | 308 | 27 | ≥50* | 7 |
| Karoubi Nordon 20075 | France; O | c-c | 1995–2003 | 11 | 11 | ≥50 | 8 |
| Lalani 201026 | Canada; P | cohort | 2005-? | 1367 | 161 | ≥50* | 6 |
| Liu 198827 | Taiwan; I/O | cohort | 1977–1986 | 207 | 11 | ≥50* | 7 |
| Maddison 198728 | England; O | cohort | unknown | 93 | 19 | >60* | 6 |
| Mak 199829 | China; I | cohort | 1985–1995 | 89 | 13 | >50* | 6 |
| Mok 200530 | China; I/O | cohort | 1991–2003 | 213 | 22 | >50* | 8 |
| Pu 200031 | Taiwan; I | cohort | 1988–1998 | 152 | 42 | ≥50 | 6 |
| Sayarlioglu 200432 | Turkey; O | cohort | 1978–2001 | 100 | 20 | ≥50* | 6 |
| Shaikh 19956 | Malaysia; O | cohort | 1976–1992 | 52 | 17 | >50 | 6 |
| Stefanidou 201333 | Greece; P | cohort | 1989–2007 | 430 | 121 | ≥50* | 6 |
| Tang 201134 | China; O | cohort | 1986–2008 | 100 | 35 | ≥50 | 6 |
| Tomic-Lucic 201335 | Serbia; O | c-c | unknown | 30 | 30 | ≥50 | 7 |
| Voulgari 200236 | Greece; O | cohort | 1981–2000 | 398 | 90 | ≥55* | 7 |
| Wang 20077 | China; I/O | cohort | 1999–2005 | 615 | 80 | ≥50* | 6 |
| Webb 201137 | USA; P | cohort | unknown | 1038 | 168 | ≥50 | 5 |
| Wilson 198138 | USA; O | cohort | 1970–1978 | 49 | 17 | ≥50* | 7 |
| Xu 201139 | China; I | cohort | 2000–2008 | 241 | 30 | ≥50 | 6 |
| TOTAL | 11189 | 1727 |
Abbreviations: c-c=case control;
indicates SLE patients < 18 years old included in analysis.
Our pooled cohorts included 1,727 patients with late-onset SLE and 11,189 early-onset controls. Female predominance was greater in the early-onset group compared to the late-onset group (89% vs. 83%, p<0.001).
Meta-analysis Results
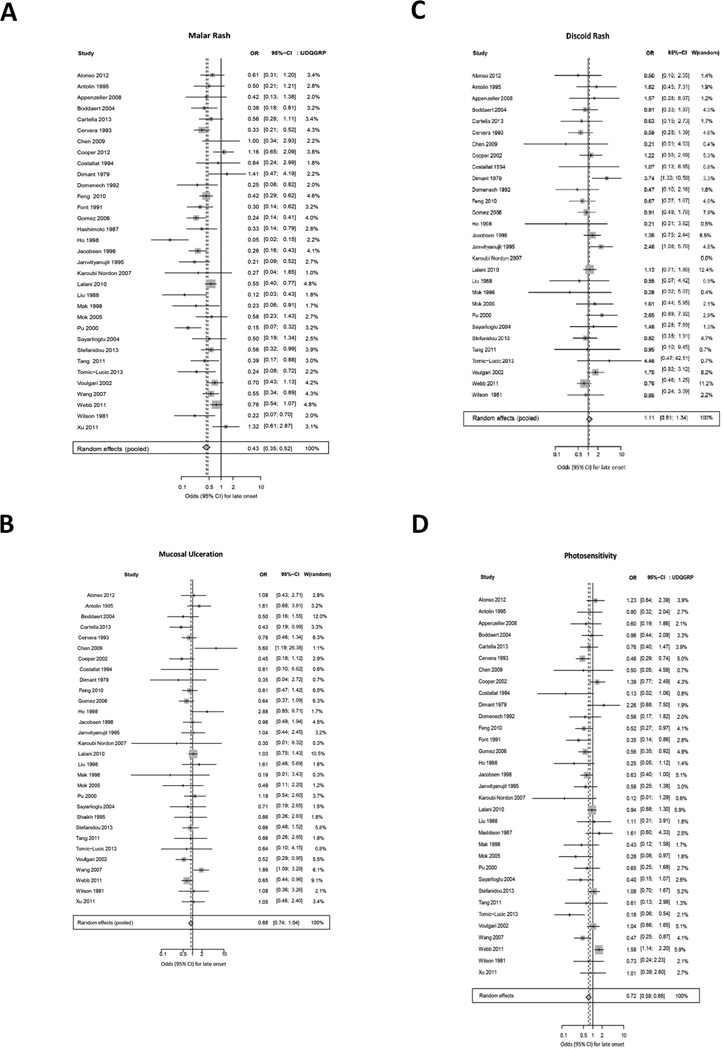
Random effects models were performed for each cutaneous manifestations to compare prevalence in late versus early-onset SLE (Table 2). First, we examined results of Forest plots for the manifestations included as ACR classification criteria as shown in Figure 2. In the random effects model, malar rash was significantly less common in late-onset, compared to early-onset, SLE patients (OR 0.43 (0.35, 0.52)). Due to study heterogeneity (I2 64%, p<0.0001), we performed sensitivity analysis by omitting the case-control studies. The subsequent relative risk of malar rash was similar (RR 0.65 (0.57, 0.73)).
Table 2.
Meta-analysis summary statistics for cutaneous manifestations in Late vs Early-onset SLE
| Cutaneous Manifestation |
Total Cases n= 12,916 |
Late- onset n= 1,727 |
Early- onset n= 11,189 |
OR (95% CI) |
Heterogeneity I2 (%), p |
|---|---|---|---|---|---|
| Malar rash | 12,731 | 1,691 | 11,040 |
0.43 (0.35,0.52) |
64, <0.001 |
| Mucosal ulcerations | 11,697 | 1,616 | 10,081 | 0.88 (0.74, 1.04) |
22, 0.14 |
| Discoid | 10,997 | 1,520 | 9,477 | 1.11 (0.91, 1.34) |
9, 0.33 |
| Photosensitivity | 12,318 | 1,698 | 10,620 |
0.72 (0.59, 0.88) |
54, <0.001 |
| Sicca | 5,489 | 833 | 4,656 |
2.45 (1.91, 3.14) |
13, 0.31 |
| Raynaud | 8,515 | 1,194 | 7,321 |
0.84 (0.71, 0.99) |
14, 0.26 |
| Alopecia | 7,290 | 992 | 6,298 |
0.63 (0.48, 0.82) |
36, 0.06 |
| Cutaneous vasculitis | 3,711 | 480 | 3,231 | 0.78 (0.49, 1.26) |
37, 0.11 |
| Livedo reticularis | 1,619 | 197 | 1,422 | 0.63 (0.17, 2.31) |
62, 0.03 |
| Subacute cutaneous lupus | 1,060 | 120 | 940 | 0.92 (0.43, 1.98) |
0, 0.80 |
I2 interpretation: low heterogeneity ≤25%, moderate 50%, and high >75%
Figure 2.
Meta-analysis results of weighted forest plots comparing prevalence of ACR cutaneous manifestations in late-onset versus early-onset SLE patients using random effects model ORs.
Photosensitivity was also significantly less common in late-onset SLE patients (Figure 2, OR 0.72 (0.59, 0.88)). Again, due to observed heterogeneity (I2 53.5%, p<0.0002), sensitivity analysis was performed and when excluding case control studies, the relative risk of photosensitivity was nearly identical to the OR derived from inclusion of all studies (RR 0.85 (0.75, 0.96)). Odds of mucosal ulceration was similar in young and late-onset SLE (OR 0.88 (0.74, 1.04)) with low heterogeneity between studies (I2 22.2%, p=0.14). The composite OR for discoid rash was similar in early and late-onset SLE patients (OR 1.11 (0.91, 1.34)) with low study heterogeneity (I2 9.2%, p=0.33).
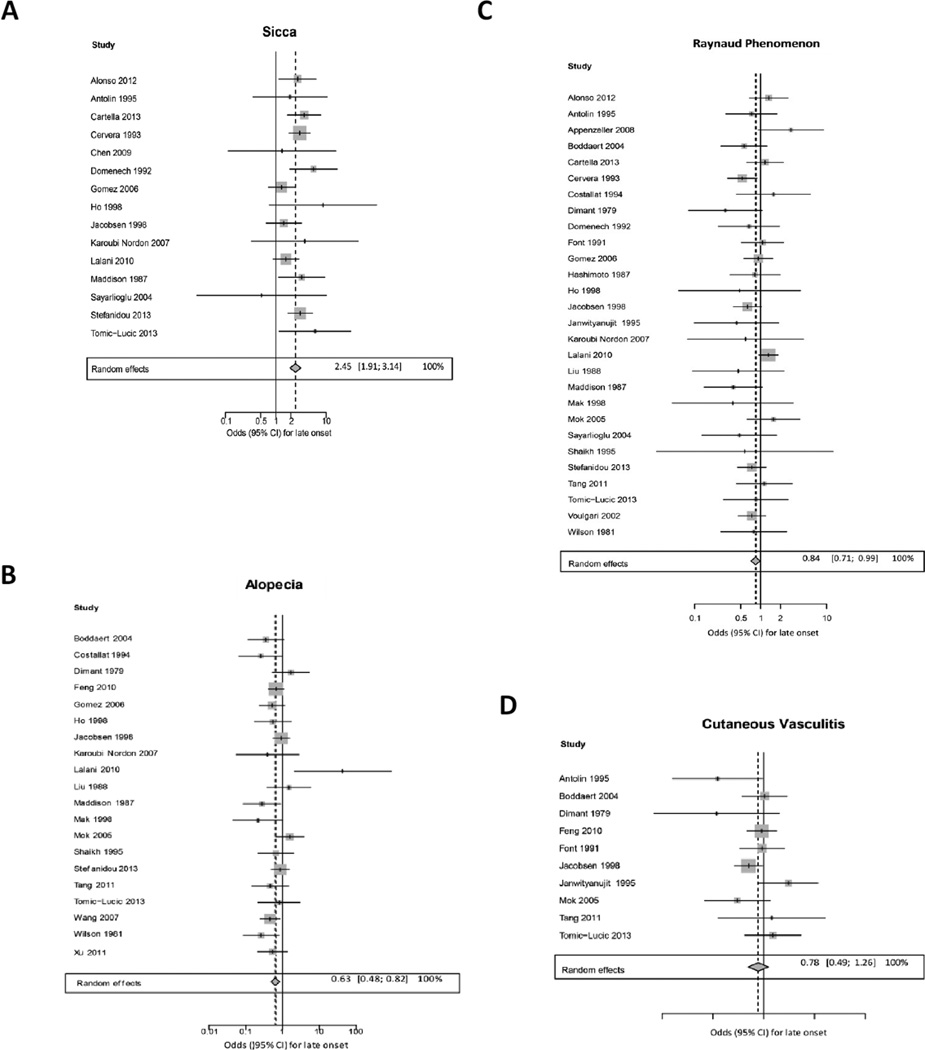
We next compared the age-related prevalence of cutaneous manifestations that are not part of the ACR diagnostic criteria for SLE. The composite OR for sicca symptoms was significantly higher in late-onset SLE patients (OR 2.45 (1.91, 3.14)) with low heterogeneity (I2 13.1%, p=0.31) (Figure 3). Raynaud’s phenomenon and alopecia were significantly less likely in late-onset SLE patients (OR 0.84 (0.71, 0.99) and OR 0.63 (0.48, 0.82)) respectively (Figure 3) both with low heterogeneity. The odds ratios for cutaneous vasculitis, livedo reticularis and SCLE were similar in early and late onset patients (Figure 3).
Figure 3.
Meta-analysis results of weighted forest plots comparing prevalence of non-ACR cutaneous manifestations in late-onset versus early-onset SLE patients using random effects model ORs.
Discussion
Our systematic review and meta-analysis of cutaneous manifestations in late onset-SLE shows that while cutaneous findings are still common, most cutaneous manifestations are less common in late compared to early-onset SLE (Table 2). In our pooled analysis of 1,727 patients with late-onset SLE, malar rash, photosensitivity, alopecia, and Raynaud phenomenon occurred less frequently in late than in early-onset SLE patients. In more conservative random effects models for the meta-analysis of ACR classification cutaneous manifestations, findings showed significantly lower odds of malar rash and photosensitivity. Our findings agree with several individual cohort studies, reporting fewer cutaneous disease in older adults [3, 8–10, 24, 25]. In our pooled analysis and the meta-analysis, sicca symptoms were more common in late versus early onset lupus patients, consistent with prior reports on this subject [9, 10, 15, 19, 21, 33, 40].
Late-onset lupus patients might have fewer cutaneous manifestations due to immune senescence and gender. Senescence of the immune system with aging is felt to contribute to the differences in disease manifestations and the generally milder disease course observed in late-onset SLE patients [4, 9]. Several age-related changes in the immune system contribute to decreased cutaneous immune responsiveness including decreased production of keratinocyte immune cytokines, decreased density of Langerhans cells, and decreased T cell production resulting in less B cell activation [41]. Some studies also found that men experience fewer cutaneous manifestations including malar rash, mucosal ulceration, and alopecia [24, 30]. Such observations might contribute to lower odds of cutaneous manifestations in our study, since men were more often represented in the late-onset SLE group as reported by others [31].
The association between sicca symptoms and late-onset SLE was also reported in a 2012 meta-analysis comparing patients with lone SLE to those with SLE-Sjogren’s overlap syndrome [40]. Those authors postulated that patients with late-onset SLE and sicca symptoms may have a lupus-SS overlap disease with its own defining characteristics and milder SLE [40]. This idea is supported by the similar immunogenic profiles and enlargement of salivary glands in patients with primary Sjogren’s syndrome and late-onset SLE with sicca features, in direct contrast to rheumatoid arthritis with secondary SS [42]. In that study patients with primary SS and those with SLE and sicca symptoms both demonstrated increased frequency of HLA DRB1*0301, whereas those with SLE without sicca symptoms had increased frequency of DRB1*1501 and DQB1*0602. Other studies likewise report an increased prevalence of HLA DR3 in late-onset SLE patients with sicca symptoms [28, 43, 44]. It is notable that sicca symptoms are more common in older adults, so perhaps the higher prevalence is also age or medication-related rather than a SLE specific manifestation, as in one study that found 27% of older subjects had sicca symptoms [45].
Our systematic review and meta-analysis overall reveals less prevalence of cutaneous manifestations in late-onset SLE patients compared to their early-onset SLE peers, with the exception of sicca symptoms. We analyzed 35 studies encompassing 1727 late onset patients to update the last meta-analysis of nine studies (n=170 late-onset patients) that evaluated clinical manifestations, including cutaneous features, in late versus early-onset SLE patients [15]. Clinicians must be able to recognize and diagnose SLE in older patients and understanding the phenotype of fewer external cutaneous manifestations and more sicca symptoms for instance may be helpful. Our study highlights sicca as a potential clue to SLE requiring vigilance beyond Sjogren’s diagnosis, particularly when additional SLE features are present such as arthritis, serositis, and lymphadenopathy.
Strengths of this study are the inclusion of a large-pooled multinational cohort and use of rigorous meta-analysis methods. The quality of studies was good, with a mean rating of 6.2 ± 0.45 using the Newcastle Ottawa Scale. As with any study, one must also consider limitations, including those related to the methodological qualities of the primary studies. First, a majority of the cohort studies were retrospective, which might under-report mild features such as cutaneous manifestations or features that are not included as lupus classification criteria such as sicca, vasculitis, livedo, and SCLE. In addition, the relatively small sample size of those evaluated for SCLE limits our ability to draw firm conclusions on the comparative prevalence of this manifestation between early and late onset SLE. Information bias is also possible; shorter lengths of follow-up in one SLE group might reduce the observed frequency of a cutaneous manifestation [15]. Competing medical problems or explanations in older adults might also impact lower disease manifestation rates if alopecia for instance were deemed age versus disease related in older SLE patients. Likewise, a recent Olmstead County cohort showed increased incidence of isolated cutaneous lupus in older males [46] although such patients would have been excluded from this analysis restricted to those meeting full lupus criteria. As with all meta-analyses, there is always potential for publication bias as well as uncontrolled confounding variables. Finally since non-English studies were excluded, language bias is possible.
Conclusion
Our pooled analysis demonstrates that when SLE is diagnosed in older adults, most cutaneous manifestations are significantly less common. By contrast, sicca was significantly more prevalent in late-onset individuals. Future studies should examine differences in SLE manifestations in older versus younger-onset disease including investigating the roles of immune senescence in the skin and impact of gender and gene-environment interactions.
Acknowledgments
Authors would like to thank reference librarians at UW-School of Medicine and Public Health, Courtney Maxcy, Sarah Loring, and Becky Burton for help with manuscript preparation.
Funding Information: Bartels receives support from National Institutes of Health (NIH) National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) (K23 #AR062381).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no direct financial, consultant, or institutional conflict of interest pertaining to this article.
Contributor Information
Jennifer L. Medlin, University of Wisconsin Hospital and Clinics.
Karen E. Hansen, Department of Medicine, Rheumatology Division, University of Wisconsin School of Medicine and Public Health.
Sara R. Fitz, Department of Dermatology, Medical Associates Clinic, Dubuque, IA.
Christie M. Bartels, Department of Medicine, Rheumatology Division, University of Wisconsin School of Medicine and Public Health.
References
- 1.Boddaert J, et al. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 2004;83(6):348–359. doi: 10.1097/01.md.0000147737.57861.7c. [DOI] [PubMed] [Google Scholar]
- 2.Dimant J, et al. Systemic lupus erythematosus in the older age group: computer analysis. J Am Geriatr Soc. 1979;27(2):58–61. doi: 10.1111/j.1532-5415.1979.tb03342.x. [DOI] [PubMed] [Google Scholar]
- 3.Font J, et al. Systemic lupus erythematosus in the elderly: clinical and immunological characteristics. Ann Rheum Dis. 1991;50(10):702–705. doi: 10.1136/ard.50.10.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto H, et al. Differences in clinical and immunological findings of systemic lupus erythematosus related to age. J Rheumatol. 1987;14(3):497–501. [PubMed] [Google Scholar]
- 5.Karoubi Nordon E, et al. Late onset systemic lupus erythematosus: a new approach. Lupus. 2007;16(12):1011–1014. doi: 10.1177/0961203307077148. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh SK, Wang F. Late-onset systemic lupus erythematosus: clinical and immunological characteristics. Med J Malaysia. 1995;50(1):25–31. [PubMed] [Google Scholar]
- 7.Wang J, et al. Systemic lupus erythematosus: a genetic epidemiology study of 695 patients from China. Arch Dermatol Res. 2007;298(10):485–491. doi: 10.1007/s00403-006-0719-4. [DOI] [PubMed] [Google Scholar]
- 8.Antolin J, et al. Systemic lupus erythematosus: clinical manifestations and immunological parameters in 194 patients. Subgroup classification of SLE. Clin Rheumatol. 1995;14(6):678–685. doi: 10.1007/BF02207936. [DOI] [PubMed] [Google Scholar]
- 9.Cervera R, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1993;72(2):113–124. [PubMed] [Google Scholar]
- 10.Domenech I, et al. Systemic lupus erythematosus in 50 year olds. Postgrad Med J. 1992;68(800):440–444. doi: 10.1136/pgmj.68.800.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho CT, et al. Late onset systemic lupus erythematosus in southern Chinese. Ann Rheum Dis. 1998;57(7):437–440. doi: 10.1136/ard.57.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janwityanujit S, et al. Age-related differences on clinical and immunological manifestations of SLE. Asian Pac J Allergy Immunol. 1995;13(2):145–149. [PubMed] [Google Scholar]
- 13.Costallat LT, Coimbra AM. Systemic lupus erythematosus: clinical and laboratory aspects related to age at disease onset. Clin Exp Rheumatol. 1994;12(6):603–607. [PubMed] [Google Scholar]
- 14.Jacobsen S, et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus. I. Disease manifestations and analyses of clinical subsets. Clin Rheumatol. 1998;17(6):468–477. doi: 10.1007/BF01451282. [DOI] [PubMed] [Google Scholar]
- 15.Ward MM, Polisson RP. A meta-analysis of the clinical manifestations of older-onset systemic lupus erythematosus. Arthritis Rheum. 1989;32(10):1226–1232. doi: 10.1002/anr.1780321007. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2011 [cited 2011; Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 19.Alonso MD, et al. Late-onset systemic lupus erythematosus in Northwestern Spain: differences with early-onset systemic lupus erythematosus and literature review. Lupus. 2012;21(10):1135–1148. doi: 10.1177/0961203312450087. [DOI] [PubMed] [Google Scholar]
- 20.Appenzeller S, Pereira DA, Costallat LT. Greater accrual damage in late-onset systemic lupus erythematosus: a long-term follow-up study. Lupus. 2008;17(11):1023–1028. doi: 10.1177/0961203308089695. [DOI] [PubMed] [Google Scholar]
- 21.Cartella S, et al. Evaluation of mortality, disease activity, treatment, clinical and immunological features of adult and late onset systemic Lupus erythematosus. Autoimmunity. 2013;46(6):363–368. doi: 10.3109/08916934.2013.794793. [DOI] [PubMed] [Google Scholar]
- 22.Chen T-L, et al. Systemic Lupus Erythematosus in the Elderly. International Journal of Gerontology. 3(2):108–113. [Google Scholar]
- 23.Cooper GS, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus. 2002;11(3):161–167. doi: 10.1191/0961203302lu161oa. [DOI] [PubMed] [Google Scholar]
- 24.Feng JB, et al. Gender and age influence on clinical and laboratory features in Chinese patients with systemic lupus erythematosus: 1,790 cases. Rheumatol Int. 2010;30(8):1017–1023. doi: 10.1007/s00296-009-1087-0. [DOI] [PubMed] [Google Scholar]
- 25.Gomez J, et al. Systemic lupus erythematosus in Asturias, Spain: clinical and serologic features. Medicine (Baltimore) 2006;85(3):157–168. doi: 10.1097/01.md.0000224711.54886.b1. [DOI] [PubMed] [Google Scholar]
- 26.Lalani S, et al. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37(1):38–44. doi: 10.3899/jrheum.080957. [DOI] [PubMed] [Google Scholar]
- 27.Liu H-W, et al. Subset of Systemic Lupus Erythematosus with Late Onset. The Kaohsiung Journal of Medical Sciences. 1988;4(10):547–552. [PubMed] [Google Scholar]
- 28.Maddison PJ. Systemic lupus erythematosus in the elderly. J Rheumatol Suppl. 1987;14(Suppl 13):182–187. [PubMed] [Google Scholar]
- 29.Mak SK, Lam EK, Wong AK. Clinical profile of patients with late-onset SLE: not a benign subgroup. Lupus. 1998;7(1):23–28. doi: 10.1191/096120398678919723. [DOI] [PubMed] [Google Scholar]
- 30.Mok CC, et al. Long-term survival of southern Chinese patients with systemic lupus erythematosus: a prospective study of all age-groups. Medicine (Baltimore) 2005;84(4):218–224. doi: 10.1097/01.md.0000170022.44998.d1. [DOI] [PubMed] [Google Scholar]
- 31.Pu SJ, et al. The clinical features and prognosis of lupus with disease onset at age 65 and older. Lupus. 2000;9(2):96–100. doi: 10.1191/096120300678828109. [DOI] [PubMed] [Google Scholar]
- 32.Sayarlioglu M, et al. Characteristics of patients with late onset systemic lupus erythematosus in Turkey. Int J Clin Pract. 2005;59(2):183–187. doi: 10.1111/j.1742-1241.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- 33.Stefanidou S, et al. Clinical expression and course in patients with late onset systemic lupus erythematosus. Hippokratia. 2013;17(2):153–156. [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Z, et al. Late onset lupus nephritis: analysis of clinical manifestations and renal pathological features in Chinese patients. Rheumatol Int. 2011;31(12):1625–1629. doi: 10.1007/s00296-010-1536-9. [DOI] [PubMed] [Google Scholar]
- 35.Tomic-Lucic A, et al. Late-onset systemic lupus erythematosus: clinical features, course, and prognosis. Clin Rheumatol. 2013;32(7):1053–1058. doi: 10.1007/s10067-013-2238-y. [DOI] [PubMed] [Google Scholar]
- 36.Voulgari PV, et al. Gender and age differences in systemic lupus erythematosus. A study of 489 Greek patients with a review of the literature. Lupus. 2002;11(11):722–729. doi: 10.1191/0961203302lu253oa. [DOI] [PubMed] [Google Scholar]
- 37.Webb R, et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann Rheum Dis. 2011;70(1):151–156. doi: 10.1136/ard.2010.141697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson HA, et al. Age influences the clinical and serologic expression of systemic lupus erythematosus. Arthritis Rheum. 1981;24(10):1230–1235. doi: 10.1002/art.1780241002. [DOI] [PubMed] [Google Scholar]
- 39.Xu YX, et al. Late onset lupus nephritis in Chinese patients: classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Lupus. 2011;20(8):801–808. doi: 10.1177/0961203310397563. [DOI] [PubMed] [Google Scholar]
- 40.Yao Q, Altman RD, Wang X. Systemic lupus erythematosus with Sjogren syndrome compared to systemic lupus erythematosus alone: a meta-analysis. J Clin Rheumatol. 2012;18(1):28–32. doi: 10.1097/RHU.0b013e31823ecbdf. [DOI] [PubMed] [Google Scholar]
- 41.Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Investig Dermatol Symp Proc. 2002;7(1):51–58. doi: 10.1046/j.1523-1747.2002.19636.x. [DOI] [PubMed] [Google Scholar]
- 42.Manoussakis MN, et al. Sjogren's syndrome associated with systemic lupus erythematosus: clinical and laboratory profiles and comparison with primary Sjogren's syndrome. Arthritis Rheum. 2004;50(3):882–891. doi: 10.1002/art.20093. [DOI] [PubMed] [Google Scholar]
- 43.Hochberg MC, et al. Systemic lupus erythematosus: a review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine (Baltimore) 1985;64(5):285–295. [PubMed] [Google Scholar]
- 44.Bell DA, et al. HLA antigens in systemic lupus erythematosus: relationship to disease severity, age at onset, and sex. The Journal of rheumatology. 1984;11(4):475–479. [PubMed] [Google Scholar]
- 45.Schein OD, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999;159(12):1359–1363. doi: 10.1001/archinte.159.12.1359. [DOI] [PubMed] [Google Scholar]
- 46.Jarukitsopa S, et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken) 2015;67(6):817–828. doi: 10.1002/acr.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]