Abstract
Background and purpose
Acute leukocytosis is a well-established response to intracerebral hemorrhage (ICH). Leukocytes, because of their interaction with platelets and coagulation factors, may in turn play a role in hemostasis. We investigated whether admission leukocytosis was associated with reduced bleeding following acute ICH.
Methods
Consecutive patients with primary ICH were prospectively collected from 1994 to 2015 and retrospectively analyzed. We included subjects with a follow-up CT scan available and automated complete white blood cell (WBC) count performed within 48 h from onset. Baseline and follow-up hematoma volumes were calculated with semi-automated software and hematoma expansion was defined as volume increase > 30% or 6 mL. The association between WBC count and ICH expansion was investigated with multivariate logistic regression.
Results
1302 subjects met eligibility criteria (median age 75 years, 55.8 % males), of whom 207 (15.9 %) experienced hematoma expansion. Higher leukocyte count on admission was associated with reduced risk of hematoma expansion (Odds Ratio for 1000 cells increase [OR] 0.91, 95 % Confidence Interval [CI] 0.86–0.96, p=0.001). The risk of hematoma expansion was inversely associated with neutrophil count (OR 0.90, 95 % CI 0.85–0.96, p=0.001) and directly associated with monocyte count (OR 2.71, 95 % CI 1.08–6.83, p=0.034). There was no association between lymphocyte count and ICH expansion (OR 0.96, 95 % CI 0.79–1.17, p=0.718).
Conclusions
Higher admission WBC count is associated with lower risk of hematoma expansion. This highlights a potential role of the inflammatory response in modulating the coagulation cascade following acute ICH.
Keywords: intracerebral hemorrhage, hematoma expansion, inflammation, leukocytes, neutrophils, monocytes
Introduction
Intracerebral hemorrage (ICH) is still the deadliest type of stroke and accounts for 10 to 20% of all cerebrovascular events (1,2). Hematoma expansion occurs in about one third of patients and is strongly associated with poor outcome (3). As a potentially modifiable determinant of ICH prognosis, hematoma expansion represents an appealing target for acute ICH treatment (4).
The relationship between acute inflammation, white blood cell (WBC) count and ICH pathophysiology is complex. ICH is associated with leukocytosis, but the inflammatory activation represented by leukocytosis may, in turn, play a role in ICH severity. The results of multiple studies suggest that higher WBC counts accompany more severe ICH as measured by reduced consciousness, higher baseline hematoma volume and intraventricular hemorrhage (IVH) presence (5,6). Leukocyte count has also been associated with higher risk of early neurological deterioration, increased long term mortality and poor functional outcome (7–9). However, Di Napoli et al. found that leukocytosis does not independently predict poor ICH prognosis when controlling for other outcome determinants including age, baseline hematoma volume and admission Glasgow Coma Scale (10).
Leukocytes interact with platelets, endothelium and coagulation factors, and have been widely recognized as important contributors to facilitating hemostasis in physiologic and pathologic conditions (11–13). Acute leukocytosis shifts the hemostatic balance in favor of coagulation and may therefore play an important role in the arrest of bleeding after an ICH occurs. In order to test the hypothesis that acute leukocytosis limits the extent of bleeding following acute ICH, we investigated whether acute leukocytosis reduced the risk of subsequent hematoma expansion.
Methods
Patient selection
Institutional Review Board approval was received for all the procedures of this research. Subjects for the present analysis were selected from an ongoing prospectively collected cohort of patients with primary ICH, admitted to our Institution from 1994 to 2015 (14,15). Patients were included if they presented with 1) diagnosis of spontaneous ICH on noncontrast CT scan (NCCT), obtained within 48 hours from onset 2) available follow-up NCCT 3) complete WBC performed within 48 hours from onset. Exclusion criteria were: 1) evidence of traumatic intracranial bleeding, 2) neoplastic or vascular lesion presumed to be the underlying cause of the hemorrhage 3) intracranial bleeding secondary to hemorrhagic transformation of ischemic stroke, 4) primary intraventricular hemorrhage (IVH), 5) missing clinical and demographic information, 6) surgical evacuation of the hematoma performed before the follow-up NCCT scan. All the subjects eligible for the study were stratified in quartiles according to the median and interquartile values of WBC count. Admission leukocytosis was defined as total WBC count > 11.000 cells/uL (6).
Image acquisition and analysis
NCCT scans were obtained using an axial technique, with the following acquisition parameters: 120 – 140 kVp, 100 – 500 mA and 5-mm slice thickness reconstruction. Baseline and follow-up ICH volumes were obtained analyzing NCCT images with a semi-automated computer assisted technique (Analyze Direct 11.0 software). Hematoma expansion was defined as an absolute volume increase > 6 mL or a relative volume increase > 30 % from baseline ICH volume (16).
Clinical Variables
Clinical and demographic data was acquired through patient or family members’ interviews or with retrospective review of hospital charts. We collected the following information: medical history of hypertension, diabetes mellitus, hypercholesterolemia, antiplatelet therapy, oral anticoagulant treatment (OAT). Time from symptom onset to NCCT and WBC blood samples, admission platelet (PLT) count, activated partial thromboplastin time (aPTT) and international normalized ratio (INR) were also measured.
Statistical Analyses
Continuous variables were expressed as median (interquartile range, IQR) and categorical variables as count (percentage). Differences in continuous variables and categorical variables across WBC quartiles were examined with Kruskal-Wallis test and χ2 test respectively. The association between total and differential WBC count and the risk of hematoma expansion was investigated with a multivariable binary logistic regression model, adjusted for previously identified predictors of hematoma expansion (17) and variables with a p value < 0.1 in the univariate analysis comparing WBC quartiles.
Single subjects’ predicted probability of hematoma expansion was derived from individual data and from the binary logistic regression model estimates and expressed as a continuous variable ranging from 0 to 1. P values < 0.05 were considered statistically significant and all the analyses were performed using SPSS version 21, 2012 (www.spss.com).
Results
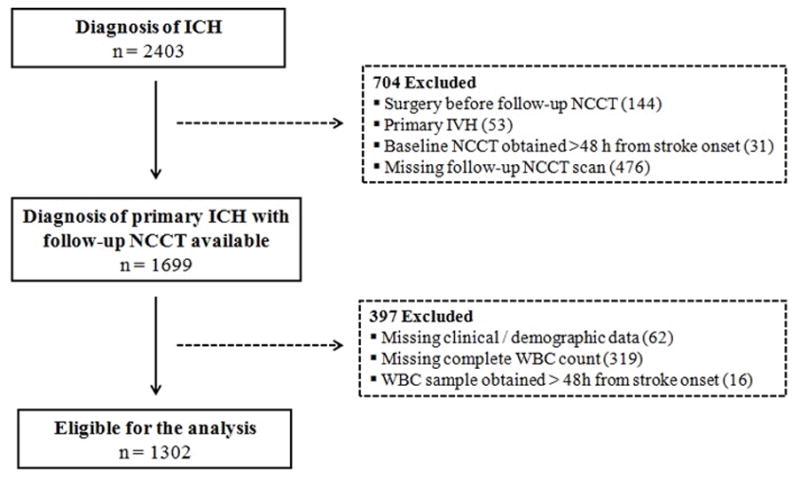
After application of the inclusion and exclusion criteria 1302 patients (median age 75, IQR 64–82, 55.8 % males) were included in the analysis (Figure 1). Table 1 shows the demographic and clinical characteristics of the study population. The median WBC count on admission was 9200 cells/uL (IQR 7100 – 11700) and 413 (31.7 %) patients had admission leukocytosis. A total of 207 patients (15.9 %) experienced hematoma expansion. Excluded patients were more likely to have infratentorial hematomas, were more often females and on antiplatelet medications and were less likely to have a positive medical history of hypertension and hypercholesterolemia (P<0.05). In addition, the excluded patients had larger baseline hematoma volume and higher admission WBC count (all P values <0.05).
Figure 1.
Cohort selection process.
ICH indicates intracerebral hemorrhage; NCCT, non-contrast computed tomography; IVH, intraventricular hemorrhage; WBC, white blood cells.
Table 1.
Baseline characteristics of the study population (n = 1302)
| Age, median (IQR), y | 75 (64 – 82) |
| Sex, male, n (%) | 727 (55.8) |
| History of hypertension, n (%) | 1041 (80.0) |
| History of diabetes, n (%) | 296 (22.6) |
| History of hypercholesterolemia, n (%) | 542 (41.6) |
| Antiplatelet treatment, n (%) | 614 (47.2) |
| Anticoagulant treatment, n (%) | 283 (21.7) |
| Time from symptom onset to NCCT, median (IQR), h | 4.4 (1.9 – 8.0) |
| Time from onset to blood sample, median (IQR), h | 3.4 (1.6 – 6.5) |
| WBC count, median (IQR), 1000 cells/uL | 9.2 (7.1 – 11.7) |
| Admission Leukocytosis, n (%) | 413 (31.7) |
| Baseline ICH volume, median (IQR), mL | 14 (5 – 34) |
| Presence of IVH, n (%) | 572 (43.9) |
| Hematoma Expansion, n (%) | 207 (15.9) |
IQR indicates interquartile range; NCCT non-contrast computed tomography; WBC, white blood cells; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage.
Individuals with the highest WBC had higher platelet counts, and a trend toward shorter aPTT. In addition, they had significantly larger baseline hematoma volume and longer time from symptom onset to NCCT and WBC blood sample (Table 2). Hematoma expansion rate was significantly higher in those individuals with the lowest WBC and progressively decreased with increasing WBC quartiles (P=0.027).
Table 2.
Comparison of demographic and clinical characteristics between WBC quartiles (n = 1302)
| WBC quartiles, 1000 cells/uL
|
p value | ||||
|---|---|---|---|---|---|
| Q1, < 7.10 (n = 326) | Q2, 7.10 – 9.19 (n = 319) | Q3, 9.20 – 11.70 (n = 328) | Q4, > 11.70 (n = 329) | ||
| Age, median (IQR), y | 75 (65 – 81) | 75 (65 – 82) | 76 (65 – 83) | 74 (62 – 81) | 0.138 |
| Sex, male, n (%) | 193 (59.2) | 187 (58.6) | 175 (53.4) | 172 (52.3) | 0.171 |
| History of hypertension, n (%) | 256 (78.5) | 249 (78.1) | 267 (81.4) | 269 (81.8) | 0.526 |
| History of diabetes, n (%) | 83 (25.5) | 65 (20.4) | 66 (20.1) | 82 (24.9) | 0.207 |
| History of hypercholesterolemia, n (%) | 148 (45.4) | 138 (43.3) | 135 (41.2) | 121 (36.8) | 0.140 |
| Antiplatelet treatment, n (%) | 153 (46.9) | 150 (47.0) | 150 (45.7) | 161 (48.9) | 0.875 |
| Anticoagulant treatment, n (%) | 76 (23.3) | 71 (22.3) | 67 (20.4) | 69 (21.0) | 0.808 |
| Time from symptom onset to NCCT, median (IQR), h | 3.1 (1.6 – 6.8) | 4.0 (1.9 – 9.9) | 4.6 (1.9 – 8.4) | 5.1 (3.0 – 7.9) | 0.002 |
| Time from onset to blood sample, median (IQR), h | 2.4 (1.2 – 5.0) | 3.9 (1.8 – 8.3) | 3.5 (2.0 – 6.5) | 4.1 (2.3 – 6.5) | <0.001 |
| PLT count, median (IQR), 1000 cells/uL | 197 (154 – 240) | 221 (178 – 258) | 230 (191 – 277) | 255 (209 – 325) | <0.001 |
| aPTT, median (IQR), s | 26.3 (23.8 – 30.3) | 26.2 (23.7 – 29.1) | 26.1 (23.6 – 29.4) | 25.3 (23.1 – 29.0) | 0.091 |
| INR, median (IQR) | 1.1 (1.0 – 1.3) | 1.1 (1.0 – 1.3) | 1.1 (1.0 – 1.2) | 1.1 (1.0 – 1.2) | 0.620 |
| Baseline ICH volume, median (IQR), mL | 10 (4 – 26) | 11 (4 – 28) | 16 (6 – 36) | 22 (7 – 48) | <0.001 |
| Presence of IVH, n (%) | 122 (37.4) | 113 (35.4) | 152 (46.3) | 185 (56.2) | <0.001 |
| Hematoma Expansion, n (%) | 69 (21.2) | 47 (14.7) | 46 (14.0) | 45 (13.7) | 0.027 |
WBC indicates white blood cells; IQR, interquartile range; NCCT, computed tomography; PLT, platelet; aPTT, activated partial thromboplastin time; INR, international normalized ratio; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage.
Hematoma expansion analysis
After adjustment for established predictors of hematoma expansion, higher admission WBC count was independently associated with reduced risk of hematoma expansion (OR for 1000 cells increase 0.91, 95 % CI 0.86 – 0.96, P=0.001). In order to determine whether the effect was driven by a particular subset of WBC, we analyzed neutrophil, lymphocyte and monocyte count separately. Increased neutrophil count was associated with lower risk of hematoma expansion (OR for 1000 cells increase 0.90, 95 % CI 0.85 – 0.96, P=0.001). Conversely higher admission monocyte count was an independent predictor of hematoma expansion (OR for 1000 cells increase, 2.71, 95 % CI 1.08–6.83, P=0.034). There was no detectable association between lymphocyte count and hematoma expansion (OR for 1000 cells increase 0.96, 95 % CI 0.79 – 1.17, P=0.718). Table 3 shows the results of the multivariate regression model and all results were unchanged when admission PLT count, aPTT, time from onset to WBC blood sample and the presence of IVH were also included in the multivariate analysis.
Table 3.
Multivariable Analysis of Predictors of hematoma expansion
| OR (95% Wald CI) | P value | |
|---|---|---|
|
|
||
| Baseline ICH volume > 30 mL | 4.69 (3.12 – 7.06) | < 0.001 |
| Time from onset to baseline NCCT < 6 h | 5.51 (3.15 – 9.66) | < 0.001 |
| Anticoagulant treatment | 4.40 (2.84 – 6.82) | < 0.001 |
| Total WBC count * | 0.91 (0.86 – 0.96) | 0.001 |
| Neutrophil count | 0.90 (0.85 – 0.96) | 0.001 |
| Lymphocyte count | 0.96 (0.79 – 1.17) | 0.718 |
| Monocyte count | 2.71 (1.08 – 6.83) | 0.034 |
ICH, intracerebral hemorrhage. NCCT, non-contrast computed tomography. WBC, white blood cells.
OR for 1000 cells increase
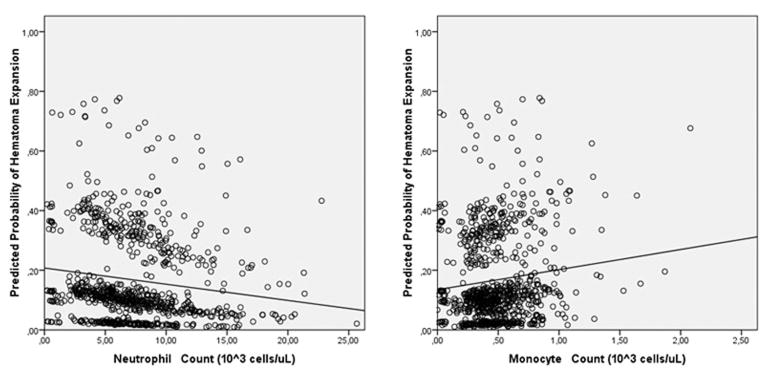
The predicted probability of hematoma expansion was inversely correlated with neutrophil count, and directly correlated with monocyte count, in a linear, dose-dependent relationship (figure 2).
Figure 2.
Association between the predicted probability of intracerebral hemorrhage expansion, neutrophil and monocyte count.
Discussion
The different effect of neutrophils and monocytes on hematoma expansion is the most striking result of our study. Higher neutrophil count is associated with reduced risk of hematoma expansion, as opposed to monocyte count that correlates with higher risk.
From a pathophysiological standpoint, our results provide further insights into the complex relationship between acute inflammation and ICH, raising the intriguing hypothesis that the inflammatory response in the hyperacute phase of the disease is not only a non-specific stress-related reaction but may also be beneficial for ICH patients, improving the coagulation response and limiting hematoma expansion.
Leukocytes interact with platelets, endothelium and coagulation factors (18) and therefore may play an important role in hematoma expansion pathophysiology, through modulation of the coagulation system (19). This hypothesis is indirectly supported by our observation of a progressively higher PLT count and shorter aPTT with increasing WBC quartiles.
Neutrophils are the first inflammatory cells to invade the central nervous system when an ICH occurs (20). Beyond the well known inflammatory and antimicrobial actions, neutrophils have been increasingly recognized as important contributors to several physiologic processes. In particular activated neutrophils exhibit significant pro-coagulant properties (21–23). First, neutrophils shift the coagulation balance in favor of thrombus formation through significant expression and release of tissue factor (TF) (24). Second, neutrophils indirectly increase the amount of active TF downregulating the TF pathway inhibitor (25). Third, neutrophil extracellular traps can activate platelets, factor X and factor XII, enhance thrombin generation and contribute to stabilization of the fibrin clot as well (25–27). Neutrophils’ activation in the hyperacute ICH phase may therefore promote a procoagulant state that limits hematoma expansion.
Conversely, the monocyte surface is rich with strong physiologic anticoagulants like thrombomodulin (TM) and TF pathway inhibitor (28–30). Therefore it is biologically plausible that monocyte activation may interfere with clot formation and fibrin stabilization and facilitate further bleeding.
In addition, monocyte activation in animal models and absolute monocyte count in humans were associated with poor outcome after ICH (31–33). A previous report hypothesized that hematoma expansion may be the link between elevated monocyte count and poor ICH outcome, although this association was not statistically significant (33). Our results confirm this hypothesis, showing that monocyte count is associated with increased odds of hematoma expansion. Further evidence supporting the association between monocytes and hematoma expansion comes from preclinical data obtained in a mouse model of ICH. Yao et al. found that a deficiency of the CCL2-CCR2 monocyte chemoattractant system was associated with decreased extent of bleeding after intracranial injection of bacterial collagenase (34).
We observed that subjects with the highest leukocyte count had longer time from onset to baseline NCCT and this may have influenced our analyses, because of the known, inverse relationship between time from symptom onset to baseline imaging and the risk of hematoma expansion (17). However, the association between leukocyte count and the risk of hematoma expansion remained significant after adjusting for time from onset to NCCT in the multivariable regression model.
Neuroinflammation and leukocyte infiltration of the hematoma have been the main targets of several neuroprotective strategies in ICH patients (35–37). However, a distinction between acute inflammatory response and chronic inflammation should be made. While the latter is a proven determinant of secondary damage in ICH (20,38), acute inflammation and ICH invasion by leukocytes may be beneficial preventing further hematoma expansion. Furthermore, the divergent effect of neutrophils and monocytes on hematoma expansion highlights the complexity of the acute inflammatory response following an ICH and suggests that future therapeutic strategies should focus on fine modulation of the immune response rather than broad, non-selective anti-inflammatory treatment.
Our results have important implications for future studies and open new areas of investigation in the field of inflammation in ICH. First, in order to define possible therapeutic targets, further studies are needed to characterize the biological mechanisms underlying the relationships between neutrophils, monocytes, and hematoma expansion. Second, microglia and astrocytes are key players in central nervous system (CNS) inflammation and little is known about the interaction between peripheral leukocytes and CNS immune cells (31,35). Third, several promising imaging techniques can potentially provide in vivo images of CNS inflammation and may improve our understanding of the inflammatory response following ICH (39).
Finally, from a clinical perspective, hematoma expansion is a potentially modifiable determinant of ICH outcome (3,4) and limiting further bleeding is an appealing therapeutic strategy (40). Hematoma expansion occurs in the first hours following an ICH and therefore early identification of patients with the highest risk of expansion is necessary. Current hematoma expansion predictive models are mainly based on clinical and imaging markers (17,41,42). Neutrophil and monocyte counts are cheap, fast and widely available biomarkers that might improve our ability to stratify the risk of hematoma expansion in clinical practice.
Some limits of the present study should be considered. First, our results are derived from a retrospective, single-center analysis. Second, the subjects screened and included were enrolled over a long time course and changes in ICH management in this period may have influenced our results. Third, a significant proportion of the screened subjects were excluded because of missing follow-up NCCT or missing admission complete WBC count, introducing a potential bias. Finally, although unlikely, the observed elevation in WBC count may be the consequence of chronic inflammatory disorders or acute infections and we were not able to control for these potential confounders in our analysis.
Conclusion
Monocyte count, as opposed to neutrophil count, is associated with higher risk of hematoma expansion. Our findings highlight the role of inflammation and leukocyte activation in coagulation following acute ICH and may offer an opportunity for early identification and treatment of patients at high risk of hematoma expansion in clinical practice.
Acknowledgments
Sources of fundings
This study was supported by the following awards from the NINDS: 5R01NS073344, K23AG02872605, K23 NS086873, R01NS059727.
None of the funding entities had any involvement in study design; data collection, analysis, and interpretation; writing of the manuscript; or decision to submit the study for publication.
Footnotes
Disclosures
Dr. Goldstein received research and consulting fees from CSL Behring and consulting fees from Bristol Myers Squibb.
Contributor Information
Andrea Morotti, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Italy. Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston. Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Chia-Ling Phuah, Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston. Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Christopher D. Anderson, Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston. Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Michael J. Jessel, Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston. Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Kristin Schwab, Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Alison M. Ayres, Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Alessandro Pezzini, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Italy.
Alessandro Padovani, Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Italy.
M. Edip Gurol, Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Anand Viswanathan, Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Steven M. Greenberg, Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston.
Joshua N. Goldstein, Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston. Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston. Department of Emergency Medicine, Massachusetts General Hospital, Boston.
Jonathan Rosand, Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston. Hemorrhagic Stroke Research Center, Massachusetts General Hospital, Boston
References
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14:300–6. doi: 10.1007/s11883-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology. 2011;76:1238–44. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35:195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki S, Kelley RE, Dandapani BK, Reyes-Iglesias Y, Dietrich WD, Duncan RC. Acute leukocyte and temperature response in hypertensive intracerebral hemorrhage. Stroke. 1995;26:1020–3. doi: 10.1161/01.str.26.6.1020. [DOI] [PubMed] [Google Scholar]
- 6.Behrouz R, Hafeez S, Miller CM. Admission Leukocytosis in Intracerebral Hemorrhage: Associated Factors and Prognostic Implications. Neurocrit Care. 2015;23:370–3. doi: 10.1007/s12028-015-0128-7. [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Peacock A, Becker J, Phillips-Bute B, Laskowitz DT, James ML. Correlation of leukocytosis with early neurological deterioration following supratentorial intracerebral hemorrhage. J Clin Neurosci. 2012;19:1096–100. doi: 10.1016/j.jocn.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leira R, Dávalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–7. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri S, Czap A, Staff I, Fortunato G, McCullough LD. Peripheral leukocyte counts and outcomes after intracerebral hemorrhage. J Neuroinflammation. 2011;8:160. doi: 10.1186/1742-2094-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Napoli M, Godoy DA, Campi V, Del Valle M, Piñero G, Mirofsky M, et al. C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke. 2011;42:1230–6. doi: 10.1161/STROKEAHA.110.604983. [DOI] [PubMed] [Google Scholar]
- 11.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–56. [PubMed] [Google Scholar]
- 12.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–30. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard BA, Tracy PB. Platelets, leukocytes, and coagulation. Curr Opin Hematol. 2001;8:263–9. doi: 10.1097/00062752-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Biffi A, Cortellini L, Nearnberg CM, Ayres AM, Schwab K, Gilson AJ, et al. Body mass index and etiology of intracerebral hemorrhage. Stroke. 2011;42:2526–30. doi: 10.1161/STROKEAHA.111.617225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, Schwab K, et al. CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care. 2012;17:421–8. doi: 10.1007/s12028-012-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–62. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 17.Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TWK, et al. Predicting Hematoma Expansion After Primary Intracerebral Hemorrhage. JAMA Neurol. 2014;71:158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 19.Afshar-Kharghan V, Thiagarajan P. Leukocyte adhesion and thrombosis. Curr Opin Hematol. 2006;13:34–9. doi: 10.1097/01.moh.0000190107.54790.de. [DOI] [PubMed] [Google Scholar]
- 20.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillis S, Furie BC, Furie B. Interactions of neutrophils and coagulation proteins. Semin Hematol. 1997;34:336–42. [PubMed] [Google Scholar]
- 22.Ruf W, Ruggeri ZM. Neutrophils release brakes of coagulation. Nat Med. 2010;16:851–2. doi: 10.1038/nm0810-851. [DOI] [PubMed] [Google Scholar]
- 23.Steppich BA, Seitz I, Busch G, Stein A, Ott I. Modulation of tissue factor and tissue factor pathway inhibitor-1 by neutrophil proteases. Thromb Haemost. 2008;100:1068–75. [PubMed] [Google Scholar]
- 24.Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, et al. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4:1323–30. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 25.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–96. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 27.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SHC, Weitz JI, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–84. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 28.Hwang SM, Kim J-E, Han K-S, Kim HK. Thrombomodulin phenotype of a distinct monocyte subtype is an independent prognostic marker for disseminated intravascular coagulation. Crit Care. 2011;15:R113. doi: 10.1186/cc10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCachren SS, Diggs J, Weinberg JB, Dittman WA. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood. 1991;78:3128–32. [PubMed] [Google Scholar]
- 30.Pou J, Rebollo A, Piera L, Merlos M, Roglans N, Laguna JC, et al. Tissue factor pathway inhibitor 2 is induced by thrombin in human macrophages. Biochim Biophys Acta. 2011;1813:1254–60. doi: 10.1016/j.bbamcr.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh KB, Padmini S, Langefeld CD, Moomaw CJ, Elkind MSV, Boehme AK, et al. Monocyte Count and 30-Day Case Fatality in Intracerebral Hemorrhage. Stroke. 2015:2302–5. doi: 10.1161/STROKEAHA.115.009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adeoye O, Walsh K, Woo JG, Haverbusch M, Moomaw CJ, Broderick JP, et al. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:e107–11. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao Y, Tsirka SE. The CCL2-CCR2 system affects the progression and clearance of intracerebral hemorrhage. Glia. 2012;60:908–18. doi: 10.1002/glia.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 36.Kellner CP, Connolly ES. Neuroprotective strategies for intracerebral hemorrhage: trials and translation. Stroke. 2010;41:S99–102. doi: 10.1161/STROKEAHA.110.597476. [DOI] [PubMed] [Google Scholar]
- 37.Hwang BY, Appelboom G, Ayer A, Kellner CP, Kotchetkov IS, Gigante PR, et al. Advances in neuroprotective strategies: Potential therapies for intracerebral hemorrhage. Cerebrovasc Dis. 2011;31:211–22. doi: 10.1159/000321870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang Q-W. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs AH, Tavitian B. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32:1393–415. doi: 10.1038/jcbfm.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke. 2010;41:402–9. doi: 10.1161/STROKEAHA.109.552919. [DOI] [PubMed] [Google Scholar]
- 41.Yao X, Xu Y, Siwila-Sackman E, Wu B, Selim M. The HEP Score: A Nomogram-Derived Hematoma Expansion Prediction Scale. Neurocrit Care. 2015;32:179–87. doi: 10.1007/s12028-015-0147-4. [DOI] [PubMed] [Google Scholar]
- 42.Huynh TJ, Aviv RI, Dowlatshahi D, Gladstone DJ, Laupacis A, Kiss A, et al. Validation of the 9-Point and 24-Point Hematoma Expansion Prediction Scores and Derivation of the PREDICT A/B Scores. Stroke. 2015;46:3105–10. doi: 10.1161/STROKEAHA.115.009893. [DOI] [PubMed] [Google Scholar]