Abstract
Objective
To examine utilization and efficacy of chemotherapy for stage I ovarian cancer.
Methods
We conducted a retrospective cohort study using the National Cancer Data Base (NCDB) to identify women with stage I ovarian cancer treated from 1998–2012. Patients were classified into 3 groups based on grade and stage: stage IA or IB, grade 1 (low risk); stage IA or IB, grade 2 (intermediate risk); stage IA or IB, grade 3 or any stage IC (high risk). Multivariable models were developed to examine predictors of chemotherapy use and survival.
Results
We identified 21,758 patients including 4,196 (19.3%) low-risk, 3,777 (17.4%) intermediate-risk, and 13,785 (63.4%) high-risk women. The median follow-up time of the cohort was 63.9 months. Use of chemotherapy within the groups was 15.5%, 39.5%, and 69.8%, respectively (P<0.001). Among low-risk patients, chemotherapy was not associated with a change in survival (aHR=1.10; 95% CI, 0.85–1.42), while chemotherapy was associated with reduced mortality for high-risk patients (aHR=0.78; 95% CI, 0.71–0.85). For intermediate-risk patients (stage IA/IB, grade 2), chemotherapy was associated with a 26% reduction in mortality (aHR=0.74; 95% CI, 0.62–0.89). The association between chemotherapy and improved survival among intermediate-risk patients remained significant when limited to patients who underwent staging lymphadenectomy (aHR=0.77; 95% CI, 0.62–0.97).
Conclusions
There is widespread variation in the patterns of care for early-stage ovarian cancer. Chemotherapy was associated with improved survival for stage IA/IB, grade 2 patients.
Introduction
While survival is improving for ovarian cancer, the majority of women present with advanced stage disease that is associated with a poor prognosis.1 For women with early-stage, ovarian confined tumors, outcomes are more favorable, with survival of greater than 90% in some subsets.2 The treatment of early-stage ovarian cancer is typically oophorectomy with comprehensive surgical staging. Prior studies have shown that up to 30% of women have occult metastases at the time of surgery.3–5
For women with stage I ovarian cancer, adjuvant chemotherapy is tailored based upon pathologic risk factors.6,7 Studies by the Gynecologic Oncology Group (GOG) suggested that chemotherapy was not beneficial in women with stage IA and IB, grade 1–2 tumors (low-risk).8 However, these studies contained a relatively small number of women with grade 2 tumors and treatment of this subset of patients remains controversial.6,7 Consensus guidelines suggest that either observation or chemotherapy are appropriate for stage IA/IB, grade 2 neoplasms.7 In contrast, women with stage IA or IB, grade 3 tumors and stage IC neoplasms are at higher risk for recurrence and are generally treated with chemotherapy.4,9,10
Despite consensus recommendations, prior studies have shown widespread variation in the patterns of care for early-stage ovarian cancer.11–18 Given the uncertainty in the treatment of early-stage ovarian cancer, we explored the utilization of chemotherapy and outcomes for stage I ovarian cancer. Specifically, we examined the use of chemotherapy and its association with survival in subgroups of women in which the potential benefits of chemotherapy are unknown (stage IA/IB, grade 2).
Materials and Methods
A retrospective cohort study using the National Cancer Data Base (NCDB) was performed to analyze treatment-based outcomes in women with early stage ovarian cancer.19,20 NCDB is a nationwide registry jointly developed by the American College of Surgeons and the American Cancer Society that captures approximately 80% of newly diagnosed cancers from 1500 Commission on Cancer (CoC) affiliated hospitals throughout the United States. NCDB contains approximately 30 million historical records. The NCDB includes data on patient demographic factors, tumor characteristics and treatment data, staging, and survival.19–21 The study used de-identified data and was deemed exempt by the Columbia University Institutional Review Board.
Women with stage I epithelial ovarian cancer who underwent cancer-directed surgery from 1998–2012 were included in the analysis. Only women with data on use of chemotherapy were included for analysis. Patients were classified into 3 groups based on grade and stage: stage IA or IB, grade 1 (low risk); stage IA or IB, grade 2 (intermediate risk); stage IA or IB, grade 3 or stage IC with any tumor grade (high risk). Patients with clear cell histology were classified as high risk. Use of chemotherapy was considered to have occurred if a patient received chemotherapy at any facility after the surgery. Data on the specific agents and number of cycles is not recorded within NCDB.
Demographic data analyzed included age (<40, 40–49, 50–59, 60–69, ≥ 70 years), race (white, black, Hispanic, other/unknown), insurance status (private, Medicaid, Medicare, uninsured, other governmental/unknown). Comorbidity was measured using the Deyo classification of the Charlson comorbidity score (unknown, 0,1, ≥ 2).22 Tumor stage, grade, histology (serous, mucinous, endometrioid, clear cell, transitional cell, or other), lymph node examination (yes, none, unknown), and year of diagnosis were recorded.
Hospital characteristics including facility region (eastern, south, Midwest, west) and location (metropolitan, urban, rural) were analyzed. Facility type (academic/research, comprehensive community cancer center, or community cancer center) was defined by the American Cancer Society’s Commission on Cancer as: academic/research hospitals (institutions affiliated with university medical schools or those designated as National Cancer Institute Comprehensive Cancer programs), community cancer centers (institutions that diagnose or treat 100–649 cancer cases annually) and comprehensive community centers (institutions that diagnose or treat ≥ 650 cancer cases annually).20,23
Frequency distributions between categorical variables were compared using χ2 tests and trends analyzed using Cochran-Armitage tests. To examine predictors of chemotherapy use we fit log-linear regression models (with Binomial errors and log-link function) based on the methods of generalized estimating equations to account for facility-level clustering. These models included clinical and demographic characteristics potentially predictive of chemotherapy use. Estimates are reported as adjusted risk ratios (aRR) with 95% confidence intervals (CI). Separate models are reported for low, intermediate, and high-risk groups.
Overall survival was examined using marginal Cox proportional hazards models. These models account for clinical and demographic characteristics as well as facility-level clustering. Survival was estimated from the time of diagnosis until death or last follow-up. Kaplan-Meier curves were developed to compare survival based on receipt of adjuvant chemotherapy stratified by risk cohort. Survival was compared using log-rank tests. All hypothesis testing was two-sided and a P-value of <0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
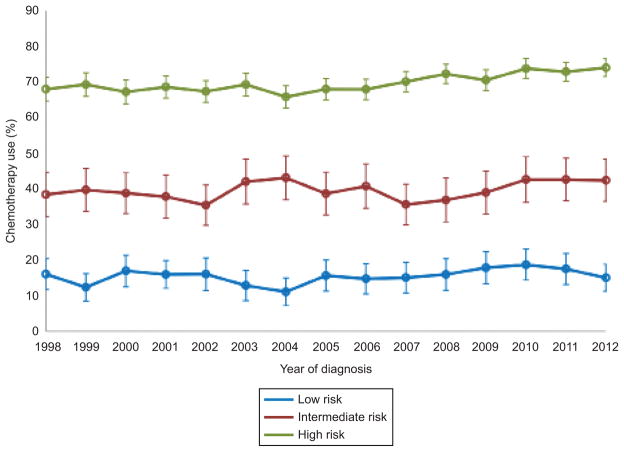
A total of 21,758 patients were identified, including 4,196 (19.3%) low-risk, 3,777 (17.4%) intermediate-risk, and 13,785 (63.4%) high-risk patients. Use of chemotherapy within the groups was 15.5%, 39.5%, and 69.8%, respectively (Table 1). Use of chemotherapy remained relatively constant over time for women with low and intermediate risk tumors (P=0.19 and P=0.24, respectively) but increased for patients with high-risk cancers from 68.0% in 1998 to 74.0% in 2012 (P<0.001) (Figure 1).
Table 1.
Clinical and demographic characteristics of the cohort.
| Low Risk (N=4,196) | Intermediate Risk (N=3,777) | High Risk (N=13,785) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Chemotherapy | P-value | Chemotherapy | P-value | Chemotherapy | P-value | |||
| N | % | N | % | N | % | ||||
| 652 | (15.5) | 1,493 | (39.5) | 9,628 | (69.8) | ||||
| Age | <0.001 | <0.001 | <0.001 | ||||||
| <40 | 102 | (13.9) | 166 | (37.8) | 802 | (68.0) | |||
| 40–49 | 182 | (17.3) | 359 | (42.8) | 2,131 | (70.1) | |||
| 50–59 | 205 | (19.0) | 488 | (46.1) | 3,371 | (74.3) | |||
| 60–69 | 113 | (16.1) | 283 | (39.1) | 2,034 | (73.4) | |||
| ≥70 | 50 | (8.0) | 197 | (27.5) | 1,290 | (57.2) | |||
| Race | 0.03 | 0.67 | <0.001 | ||||||
| White | 571 | (16.2) | 1,258 | (39.7) | 8,158 | (70.4) | |||
| Black | 29 | (11.7) | 72 | (38.1) | 369 | (62.7) | |||
| Hispanic | 22 | (10.2) | 78 | (36.3) | 473 | (68.8) | |||
| Other/unknown | 30 | (14.8) | 85 | (41.7) | 628 | (68.6) | |||
| Insurance | 0.01 | <0.001 | <0.001 | ||||||
| Private | 450 | (17.2) | 987 | (43.3) | 6,368 | (72.1) | |||
| Medicaid | 34 | (14.7) | 81 | (44.0) | 426 | (72.1) | |||
| Medicare | 108 | (11.6) | 300 | (30.8) | 2,024 | (62.8) | |||
| Uninsured | 39 | (14.9) | 77 | (36.7) | 449 | (72.2) | |||
| Other government/unknown | 21 | (13.8) | 48 | (37.2) | 361 | (69.3) | |||
| Comorbidity | 0.99 | 0.17 | <0.001 | ||||||
| 0 | 358 | (15.5) | 818 | (39.9) | 5,800 | (70.8) | |||
| 1 | 60 | (15.7) | 157 | (44.0) | 909 | (71.0) | |||
| 2 | 15 | (16.7) | 28 | (35.0) | 166 | (61.7) | |||
| Unknown | 219 | (15.5) | 490 | (38.0) | 2,753 | (68.0) | |||
| Facility location | 0.10 | 0.01 | <0.001 | ||||||
| Eastern | 159 | (17.9) | 331 | (41.9) | 2,167 | (74.3) | |||
| Midwest | 223 | (15.4) | 543 | (41.1) | 3,358 | (71.8) | |||
| South | 180 | (15.2) | 414 | (39.0) | 2,451 | (67.7) | |||
| West | 90 | (13.4) | 205 | (34.1) | 1,652 | (64.3) | |||
| Facility Type | 0.75 | 0.02 | <0.001 | ||||||
| Community cancer | 40 | (13.9) | 90 | (35.9) | 579 | (68.0) | |||
| Comprehensive community cancer | 340 | (15.7) | 774 | (38.6) | 4,657 | (66.3) | |||
| Academic/research | 272 | (15.7) | 616 | (41.1) | 4,383 | (74.4) | |||
| Other | - | - | 13 | (68.4) | * | * | |||
| Urban/rural | 0.67 | 0.69 | 0.31 | ||||||
| Metropolitan | 542 | (15.8) | 1,209 | (39.5) | 7,868 | (69.7) | |||
| Urban | 79 | (14.4) | 197 | (40.6) | 1,198 | (71.5) | |||
| Rural | * | * | 21 | (32.8) | 141 | (70.9) | |||
| Unknown | 23 | (14.4) | 66 | (40.0) | 421 | (67.8) | |||
| Histology | <0.001 | <0.001 | <0.001 | ||||||
| Serous | 65 | (12.9) | 265 | (40.9) | 2,850 | (69.6) | |||
| Mucinous | 153 | (9.8) | 313 | (29.4) | 1,105 | (61.8) | |||
| Endometrioid | 397 | (20.9) | 826 | (45.3) | 2,625 | (71.1) | |||
| Clear cell | - | - | - | - | 2,466 | (74.3) | |||
| Transitional cell | * | * | * | * | 58 | (69.1) | |||
| Epithelial tumor NOS | 37 | (16.7) | 84 | (35.4) | 524 | (64.5) | |||
| Grade | - | - | <0.001 | ||||||
| 1 | 652 | (15.5) | - | - | 1,262 | (63.8) | |||
| 2 | - | - | 1,493 | (39.5) | 2,182 | (72.7) | |||
| 3 | - | - | - | - | 4,986 | (71.4) | |||
| Unknown | - | - | - | - | 1,198 | (65.6) | |||
| Stage | <0.001 | <0.001 | <0.001 | ||||||
| IA | 552 | (14.1) | 1,295 | (37.8) | 2,279 | (65.8) | |||
| IB | 100 | (37.0) | 198 | (56.3) | 355 | (68.3) | |||
| IC | - | - | - | - | 6,994 | (71.4) | |||
| Lymph nodes examined | 0.24 | 0.02 | <0.001 | ||||||
| Yes | 502 | (16.1) | 1,205 | (40.7) | 7,713 | (71.6) | |||
| No | 144 | (13.9) | 278 | (35.5) | 1,806 | (63.1) | |||
| Unknown | * | * | * | * | 109 | (72.7) | |||
Values censored due to small sample size
Figure 1.
Chemotherapy use stratified by risk group and year of diagnosis: low risk (P=.19), intermediate risk (P=.24), high risk (P<.001 for trend). Error bars indicate 95% confidence intervals.
The clinical and demographic characteristics of the cohort are displayed in Table 1. Of note, 74.4% of low-risk, 78.4% of intermediate risk, and 78.2% of high-risk patients underwent lymph node sampling. Chemotherapy was administered to 47.6% of patients who did not undergo lymph node sampling, compared to 55.8% of patients who underwent lymph node sampling (P=0.001).
In a series of multivariable models, advanced age, mucinous histology and treatment in the western U.S. were associated with a lower likelihood of receipt of chemotherapy, while higher stage and grade were associated with an increased likelihood of receipt of chemotherapy (P<0.05 for all) (Table 2). In the high-risk group, black race was associated with decreased use of chemotherapy (aRR=0.88; 95% CI, 0.83–0.94). Among women with high-risk tumors, those with mucinous neoplasms were less likely to undergo chemotherapy, while patients with clear cell tumors more commonly received chemotherapy. Similarly, high-risk women who did not undergo lymphadenectomy were less likely to receive chemotherapy.
Table 2.
Multivariable models of chemotherapy use.
| Covariate | Low Risk 1 | Intermediate Risk | High Risk |
|---|---|---|---|
| Age | |||
| <40 | Referent | Referent | Referent |
| 40–49 | 1.12 (0.92–1.38) | 1.03 (0.89–1.20) | 0.99 (0.95–1.04) |
| 50–59 | 1.22 (1.00–1.49) | 1.10 (0.96–1.27) | 1.04 (1.00–1.09) |
| 60–69 | 1.05 (0.81–1.36) | 0.97 (0.83–1.14) | 1.05 (1.00–1.10) |
| ≥70 | 0.56 (0.39–0.79)* | 0.74 (0.61–0.89)* | 0.84 (0.79–0.90)* |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 0.74 (0.52–1.04) | 0.97 (0.80–1.17) | 0.88 (0.83–0.94)* |
| Hispanic | 0.63 (0.41–0.97)* | 0.95 (0.80–1.13) | 0.98 (0.93–1.04) |
| Other/unknown | 0.94 (0.67–1.32) | 1.10 (0.90–1.34) | 0.96 (0.91–1.02) |
| Insurance | |||
| Private | Referent | Referent | Referent |
| Medicaid | 1.06 (0.77–1.48) | 1.04 (0.86–1.25) | 1.03 (0.97–1.09) |
| Medicare | 0.99 (0.78–1.27) | 0.88 (0.77–1.01) | 0.96 (0.93–1.00) |
| Uninsured | 1.01 (0.75–1.35) | 0.87 (0.73–1.04) | 1.02 (0.97–1.07) |
| Other government/unknown | 1.00 (0.68–1.47) | 0.92 (0.74–1.16) | 0.98 (0.93–1.04) |
| Facility Location | |||
| Eastern | Referent | Referent | Referent |
| Midwest | 0.90 (0.73–1.12) | 1.00 (0.86–1.17) | 0.97 (0.93–1.02) |
| South | 0.95 (0.77–1.18) | 0.97 (0.83–1.14) | 0.94 (0.89–0.99)* |
| West | 0.75 (0.59–0.96)* | 0.84 (0.69–1.02) | 0.88 (0.82–0.94)* |
| Facility Type | |||
| Community cancer | - | Referent | Referent |
| Academic/research | - | 1.11 (0.91–1.35) | 1.06 (0.99–1.12) |
| Comprehensive community cancer | - | 1.06 (0.87–1.29) | 0.96 (0.90–1.03) |
| Other | - | 1.81 (1.49–2.21)* | 0.75 (0.70–0.80)* |
| Urban/Rural | |||
| Metropolitan | Referent | Referent | Referent |
| Urban | 0.91 (0.73–1.13) | 1.05 (0.92–1.20) | 1.03 (0.99–1.06) |
| Rural | 0.79 (0.42–1.49) | 0.85 (0.60–1.19) | 1.04 (0.94–1.14) |
| Unknown | 0.89 (0.63–1.26) | 1.05 (0.87–1.27) | 0.98 (0.93–1.04) |
| Histology | |||
| Serous | Referent | Referent | Referent |
| Mucinous | 0.85 (0.65–1.12) | 0.72 (0.63–0.83)* | 0.90 (0.86–0.94)* |
| Endometrioid | 1.69 (1.33–2.16)* | 1.09 (0.99–1.21) | 1.01 (0.98–1.05) |
| Clear cell | – | – | 1.07 (1.03–1.10)* |
| Transitional cell | – | 2.15 (1.45–3.17)* | 1.00 (0.87–1.14) |
| Epithelial tumor NOS | 1.37 (0.93–2.01) | 0.87 (0.71–1.06) | 0.95 (0.89–1.00) |
| Grade | |||
| 1 | – | – | Referent |
| 2 | – | – | 1.14 (1.09–1.19)* |
| 3 | – | – | 1.18 (1.13–1.23)* |
| Unknown | – | – | 1.00 (0.95–1.06) |
| Stage | |||
| IA | Referent | Referent | Referent |
| IB | 2.43 (2.02–2.91)* | 1.37 (1.23–1.51)* | 1.06 (0.99–1.13) |
| IC | – | – | 1.17 (1.14–1.21)* |
| Lymph nodes examined | |||
| Yes | Referent | Referent | Referent |
| No | 0.98 (0.81–1.17) | 0.95 (0.85–1.06) | 0.93 (0.90–0.96)* |
| Unknown | 1.03 (0.49–2.14) | 0.88 (0.55–1.41) | 1.05 (0.94–1.17) |
| Year of Diagnosis | |||
| 1998 | Referent | Referent | Referent |
| 1999 | 0.75 (0.51–1.10) | 1.00 (0.81–1.24) | 1.02 (0.95–1.09) |
| 2000 | 0.91 (0.64–1.30) | 1.00 (0.80–1.24) | 0.98 (0.91–1.05) |
| 2001 | 0.90 (0.63–1.28) | 0.97 (0.77–1.22) | 1.01 (0.94–1.08) |
| 2002 | 0.88 (0.59–1.29) | 0.90 (0.72–1.13) | 0.99 (0.92–1.06) |
| 2003 | 0.75 (0.49–1.12) | 1.03 (0.84–1.26) | 1.01 (0.94–1.08) |
| 2004 | 0.62 (0.39–0.96)* | 1.07 (0.86–1.33) | 0.95 (0.88–1.02) |
| 2005 | 0.88 (0.60–1.29) | 0.99 (0.80–1.23) | 0.99 (0.92–1.06) |
| 2006 | 0.83 (0.58–1.18) | 1.02 (0.81–1.28) | 0.98 (0.92–1.05) |
| 2007 | 0.83 (0.56–1.23) | 0.90 (0.72–1.14) | 1.02 (0.95–1.09) |
| 2008 | 0.91 (0.63–1.32) | 0.98 (0.78–1.24) | 1.05 (0.99–1.12) |
| 2009 | 0.91 (0.63–1.31) | 0.97 (0.77–1.22) | 1.02 (0.96–1.09) |
| 2010 | 1.06 (0.74–1.52) | 1.08 (0.87–1.33) | 1.06 (1.00–1.14) |
| 2011 | 1.02 (0.72–1.45) | 1.11 (0.90–1.36) | 1.05 (0.98–1.12) |
| 2012 | 0.87 (0.60–1.25) | 1.10 (0.89–1.36) | 1.06 (0.99–1.13) |
Adjusted risk ratio (95% confidence interval).38 patients missing facility identifier were excluded; 4,188, 3,771, and 13,759 patients were analyzed in low, intermediate, and high-risk groups.
P<0.05
Two transitional cell patients were excluded
Generalized estimating equations were fit accounting for facility-level clustering. The covariates included were: low-risk: age, race, insurance status, facility region, facility location, histology, stage, lymph node examination, and year of diagnosis; intermediate-risk: age, race, insurance status, facility region, facility type, facility location, histology, stage, lymph node examination, and year of diagnosis; high-risk: age, race, insurance status, facility region, facility type, facility location, histology, stage, grade, lymph node examination, and year of diagnosis.
In a multivariable model of survival, chemotherapy was not associated with survival among women with low-risk tumors (aHR=1.10; 95% CI, 0.85–1.42) (Table 3). In contrast, among women with high-risk cancers, chemotherapy was associated with decreased mortality (aHR=0.78; 95% CI, 0.71–0.85). For women with intermediate-risk cancers, use of chemotherapy was associated with a 26% decrease in mortality (aHR=0.74; 95% CI, 0.62–0.89). For women with intermediate risk tumors, advanced age, Medicare or Medicaid insurance coverage, more advanced stage (stage IB), and failure to perform lymphadenectomy were associated with decreased survival.
Table 3.
Multivariable models of survival stratified by low, intermediate, and high-risk classification.
| Covariate | Low Risk | Intermediate Risk | High Risk |
|---|---|---|---|
| Follow-up months | |||
| Median (range) | 65.12 (0.00–188.52) | 66.30 (0.03–187.33) | 62.88 (0.00–190.26) |
| Chemotherapy | |||
| No | Referent | Referent | Referent |
| Yes | 1.10 (0.85–1.42) | 0.74 (0.62–0.89)* | 0.78 (0.71–0.85)* |
| Age | |||
| <40 | Referent | Referent | Referent |
| 40–49 | 1.52 (0.90–2.57) | 1.17 (0.79–1.74) | 1.16 (0.96–1.42) |
| 50–59 | 3.89 (2.46–6.15)* | 1.44 (0.96–2.16) | 1.38 (1.14–1.66)* |
| 60–69 | 4.79 (2.93–7.85)* | 2.01 (1.36–2.97)* | 1.64 (1.34–2.00)* |
| ≥70 | 9.86 (5.85–16.63)* | 3.63 (2.43–5.44)* | 3.45 (2.81–4.25)* |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 0.86 (0.55–1.34) | 1.31 (0.91–1.88) | 1.50 (1.26–1.78)* |
| Hispanic | 0.91 (0.55–1.49) | 0.56 (0.32–0.98)* | 1.01 (0.83–1.23) |
| Other/unknown | 0.80 (0.46–1.39) | 1.04 (0.70–1.55) | 1.02 (0.85–1.23) |
| Insurance | |||
| Private | Referent | Referent | Referent |
| Medicaid | 2.68 (1.76–4.07)* | 1.82 (1.17–2.83)* | 1.48 (1.20–1.82)* |
| Medicare | 1.66 (1.22–2.27)* | 1.48 (1.16–1.90)* | 1.22 (1.08–1.38)* |
| Uninsured | 2.23 (1.42–3.48)* | 1.47 (0.94–2.29) | 1.16 (0.93–1.45) |
| Other government/unknown | 1.52 (0.97–2.38) | 1.49 (1.01–2.20)* | 1.25 (0.99–1.57) |
| Facility Location | |||
| Eastern | Referent | Referent | Referent |
| Midwest | 1.22 (0.95–1.57) | 1.32 (1.03–1.68)* | 1.05 (0.93–1.19) |
| South | 1.19 (0.91–1.56) | 1.26 (0.98–1.60) | 1.11 (0.98–1.26) |
| West | 1.27 (0.93–1.72) | 0.89 (0.65–1.20) | 0.99 (0.87–1.14) |
| Facility Type | |||
| Community cancer | Referent | Referent | Referent |
| Academic/research | 1.53 (1.07–2.20)* | 1.03 (0.74–1.43) | 1.06 (0.90–1.24) |
| Comprehensive community cancer | 1.45 (1.02–2.05)* | 0.98 (0.71–1.36) | 1.04 (0.89–1.22) |
| Other | – ╪ | 2.73 (1.87–3.98)* | 1.06 (0.88–1.29) |
| Urban/Rural | |||
| Metropolitan | Referent | Referent | Referent |
| Urban | 1.08 (0.82–1.42) | 1.03 (0.82–1.30) | 1.04 (0.92–1.17) |
| Rural | 0.88 (0.45–1.71) | 0.59 (0.27–1.29) | 1.37 (1.04–1.80)* |
| Unknown | 1.06 (0.65–1.74) | 1.76 (1.27–2.45)* | 1.57 (1.33–1.85)* |
| Histology | |||
| Serous | Referent | Referent | Referent |
| Mucinous | 1.32 (1.00–1.75) | 1.13 (0.89–1.45) | 1.14 (1.00–1.30) |
| Endometrioid | 0.85 (0.64–1.14) | 0.80 (0.64–1.00) | 0.73 (0.66–0.82)* |
| Clear cell | - | - | 1.07 (0.95–1.20) |
| Transitional cell | - ╪ | - ╪ | 0.99 (0.61–1.59) |
| Epithelial tumor NOS | 1.50 (1.01–2.21)* | 0.81 (0.57–1.16) | 1.04 (0.90–1.21) |
| Grade | |||
| 1 | - | - | Referent |
| 2 | - | - | 1.52 (1.30–1.77)* |
| 3 | - | - | 1.96 (1.69–2.28)* |
| Unknown | - | - | 1.52 (1.29–1.79)* |
| Stage | |||
| 1A | Referent | Referent | Referent |
| 1B | 1.20 (0.86–1.69) | 1.61 (1.23–2.12)* | 1.37 (1.14–1.64)* |
| 1C | - | - | 1.53 (1.37–1.71)* |
| Lymph nodes examined | |||
| Yes | Referent | Referent | Referent |
| No | 1.54 (1.27–1.87)* | 1.45 (1.20–1.73)* | 1.62 (1.49–1.76)* |
| Unknown | 1.56 (0.63–3.85) | 1.27 (0.56–2.88) | 1.47 (1.04–2.08)* |
| Year of Diagnosis | |||
| 1998 | Referent | Referent | Referent |
| 1999 | 1.37 (0.95–1.96) | 1.19 (0.80–1.76) | 0.85 (0.71–1.01) |
| 2000 | 1.04 (0.69–1.55) | 1.42 (0.99–2.05) | 0.72 (0.59–0.87)* |
| 2001 | 1.18 (0.79–1.76) | 1.11 (0.75–1.65) | 0.89 (0.76–1.05) |
| 2002 | 1.38 (0.90–2.11) | 1.14 (0.78–1.67) | 0.92 (0.77–1.10) |
| 2003 | 1.52 (0.99–2.33) | 0.97 (0.63–1.51) | 0.97 (0.81–1.16) |
| 2004 | 1.57 (1.04–2.37)* | 1.33 (0.85–2.08) | 0.85 (0.71–1.03) |
| 2005 | 1.38 (0.90–2.11) | 1.43 (0.95–2.14) | 0.87 (0.72–1.04) |
| 2006 | 1.52 (0.95–2.43) | 0.96 (0.59–1.58) | 0.95 (0.77–1.15) |
| 2007 | 1.30 (0.77–2.22) | 1.08 (0.69–1.70) | 0.99 (0.81–1.20) |
| 2008 | 1.00 (0.56–1.79) | 1.04 (0.61–1.77) | 0.90 (0.74–1.08) |
| 2009 | 1.17 (0.62–2.24) | 1.05 (0.61–1.81) | 0.93 (0.73–1.18) |
| 2010 | 0.91 (0.44–1.87) | 1.00 (0.49–2.04) | 1.03 (0.79–1.34) |
| 2011 | 0.87 (0.37–2.04) | 1.06 (0.51–2.18) | 1.13 (0.86–1.50) |
Adjusted hazard ratio (95% confidence interval).
Age, race, insurance status, facility region, facility type, facility location, histology, stage, lymph node examination, and year of diagnosis were included in the marginal Cox proportional hazard models accounting for facility-level clustering. Grade was further adjusted for in the high-risk group.
Non-estimable.
1,833 patients missing follow-up time, vital status, or facility identifier were excluded; 3,851, 3,502, and 12,572 patients were modeled in the low, intermediate, and high-risk groups.
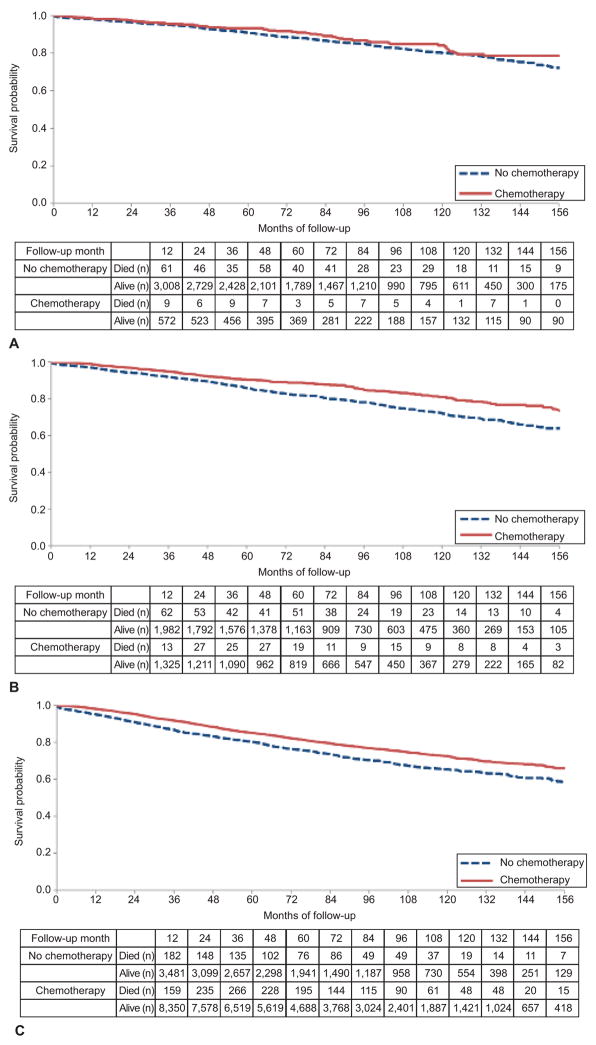
When the intermediate risk patients were stratified by performance of lymphadenectomy, chemotherapy was associated with decreased mortality among women who underwent lymph node sampling (aHR=0.77; 95% CI, 0.62–0.97) but was not associated with survival among those women who did not undergo lymphadenectomy (aHR=0.73; 95% CI 0.52–1.03) (Table 4). In Kaplan-Meier analyses, there was no difference in survival for low-risk patients based on receipt of chemotherapy (P=0.17), but was survival was improved in the intermediate (P<0.001) and high-risk (P<0.001) patients treated with chemotherapy (Figure 2).
Table 4.
Multivariable estimates of survival for women with intermediate risk tumors stratified by performance of lymphadenectomy.
| Covariate | Lymph Node Dissection Performed | Lymph Node Dissection Not Performed |
|---|---|---|
| Follow-up months | ||
| Median (range) | 66.40 (0.03–187.33) | 65.88 (0.03–186.41) |
| Chemotherapy | ||
| No | Referent | Referent |
| Yes | 0.77 (0.62–0.97)* | 0.73 (0.52–1.03) |
| Age | ||
| <40 | Referent | Referent |
| 40–49 | 0.94 (0.61–1.45) | 3.84 (1.06–13.88)* |
| 50–59 | 1.25 (0.80–1.95) | 3.75 (1.06–13.29)* |
| 60–69 | 1.67 (1.07–2.60)* | 6.42 (1.86–22.22)* |
| ≥70 | 3.22 (1.98–5.22)* | 9.44 (2.69–33.14)* |
| Race | ||
| White | Referent | Referent |
| Black | 1.14 (0.69–1.88) | 1.71 (0.97–3.04) |
| Hispanic | 0.62 (0.34–1.15) | 0.41 (0.09–1.92) |
| Other/unknown | 1.07 (0.70–1.65) | 1.29 (0.63–2.63) |
| Insurance | ||
| Private | Referent | Referent |
| Medicaid | 2.12 (1.25–3.61)* | 1.99 (0.91–4.36) |
| Medicare | 1.36 (0.98–1.89) | 1.77 (1.15–2.72)* |
| Uninsured | 1.42 (0.85–2.37) | 1.66 (0.69–4.00) |
| Other government/unknown | 1.31 (0.82–2.10) | 2.03 (1.00–4.12) |
| Facility Location | ||
| Eastern | Referent | Referent |
| Midwest | 1.52 (1.12–2.07)* | 1.13 (0.71–1.79) |
| South | 1.41 (1.04–1.93)* | 1.14 (0.73–1.77) |
| West | 1.03 (0.70–1.51) | 0.67 (0.37–1.21) |
| Facility Type | ||
| Community cancer | Referent | Referent |
| Academic/research | 0.97 (0.62–1.51) | 0.96 (0.56–1.64) |
| Comprehensive community cancer | 0.84 (0.54–1.31) | 1.16 (0.70–1.93) |
| Other | 2.60 (1.54–4.39)* | - ╪ |
| Urban/Rural | ||
| Metropolitan | Referent | Referent |
| Urban | 0.90 (0.67–1.21) | 1.30 (0.85–1.97) |
| Rural | 0.52 (0.20–1.32) | 0.36 (0.04–3.02) |
| Unknown | 2.05 (1.39–3.02)* | 1.60 (0.86–2.96) |
| Histology | ||
| Serous | Referent | Referent |
| Mucinous | 1.24 (0.91–1.68) | 0.99 (0.66–1.49) |
| Endometrioid | 0.85 (0.64–1.13) | 0.76 (0.51–1.12) |
| Clear cell | - | - |
| Transitional cell | -╪ | - |
| Epithelial tumor NOS | 0.93 (0.60–1.44) | 0.66 (0.36–1.24) |
| Stage | ||
| 1A | Referent | Referent |
| 1B | 1.64 (1.16–2.31)* | 1.49 (0.93–2.40) |
| 1C | - | - |
| Year of Diagnosis | ||
| 1998 | Referent | Referent |
| 1999 | 1.29 (0.78–2.14) | 1.00 (0.53–1.87) |
| 2000 | 1.64 (1.04–2.58)* | 1.04 (0.56–1.90) |
| 2001 | 1.27 (0.76–2.12) | 0.77 (0.40–1.45) |
| 2002 | 1.30 (0.79–2.13) | 0.86 (0.44–1.67) |
| 2003 | 1.02 (0.58–1.78) | 0.86 (0.43–1.71) |
| 2004 | 1.42 (0.81–2.49) | 1.11 (0.51–2.44) |
| 2005 | 1.84 (1.12–3.01)* | 0.68 (0.34–1.34) |
| 2006 | 1.01 (0.53–1.93) | 0.79 (0.34–1.81) |
| 2007 | 1.13 (0.63–2.03) | 1.04 (0.48–2.28) |
| 2008 | 1.12 (0.56–2.21) | 0.90 (0.38–2.10) |
| 2009 | 0.90 (0.44–1.83) | 1.56 (0.65–3.75) |
| 2010 | 0.84 (0.30–2.34) | 1.61 (0.61–4.20) |
| 2011 | 1.35 (0.58–3.12) | 0.69 (0.15–3.26) |
Adjusted hazard ratio (95% confidence interval).
Non-estimable.
306 patients with missing follow-up time, vital status, or facility identifier were excluded; 2,735 and 736 patients were modeled in lymph nodes dissection performed and node dissection not performed.
Age, race, insurance status, facility region, facility type, facility location, histology, stage, lymph node examination, and year of diagnosis were included in the marginal Cox proportional hazard models accounting for facility-level clustering.
Figure 2.
Kaplan-Meier analysis of survival with use of chemotherapy. A. Low risk (P=.17); B. intermediate risk (P<.001); C. high risk (P<.001).
Among low-risk women, 5-year survival was 93.3% (95% CI, 90.7–95.2%) among those who received chemotherapy versus 90.9% (95% CI, 89.7–91.9%) in those who did not receive chemotherapy (P=0.17). For those women with intermediate risk cancers, 5-year survival was 90.4% (95% CI, 88.6–92.0%) for those who received chemotherapy compared to 85.9% (95% CI, 84.2–87.5%) in those not treated with chemotherapy. Finally, survival at 5-years was 85.1% (95% CI, 84.3–85.9%) in those treated and 80.4% (95% CI, 79.0–81.7%) among those high-risk patients not treated with chemotherapy (P<0.001).
Discussion
We noted widespread variation in the use of chemotherapy for stage I ovarian cancer. Nearly 16% of women with low-risk tumors in which chemotherapy is not beneficial received treatment, while over 30% of women with high-risk tumors who benefit from chemotherapy were not treated. Importantly, we found that chemotherapy was associated with improved survival for women with stage IA/IB grade 2 tumors.
The optimal treatment of stage IA and IB grade 2 tumors remains controversial. Data from the GOG in the 1980’s found no benefit from chemotherapy for women with stage IA and IB, grade 1 and 2 tumors.8 However, more recent data from randomized trials in Europe have suggested a greater role for chemotherapy in women with early-stage ovarian cancer and the treatment of patients with stage IA/IB grade 2 has been debated.9,24,25 Among women with stage IA or IB, grade 2 neoplasms, the National Comprehensive Cancer Network (NCCN) guidelines advocate either observation or treatment with 3–6 cycles of platinum and taxane based chemotherapy.7 We noted that mortality was reduced by 26% in women with stage IA/IB grade 2 tumors. These findings clearly warrant further investigation.
In a subset analysis, we found that the survival benefit associated with chemotherapy for the intermediate risk stage IA/IB, grade 2 patients was limited to women who underwent lymphadenectomy. This has important implications and suggests that the survival benefit associated with chemotherapy in intermediate risk women is not due to the treatment of occult, advanced stage disease, but rather, is an effect in women with ovarian-confined disease. Like prior studies, we found that staging procedures such as lymphadenectomy were often omitted in apparent early-stage ovarian cancer.11–17,26
Our study is in line with prior work that has demonstrated no benefit for chemotherapy in women with low-risk, stage IA and IB, grade 1 neoplasms.8,17 Despite the lack of benefit for chemotherapy in this subset of patients, use remains common. We found that approximately 16% of low-risk women received chemotherapy and this remained relatively stable from 1998 to 2012. A prior study examining Medicare beneficiaries found that use of chemotherapy in stage IA or IB, grade 1–2 tumors was 33% and actually increased between 1992 and 2009.17 While differences in use based on stage and histology explain some of the difference, it remains unclear why chemotherapy continues to be used in low-risk women.
In contrast, for women with high-risk (stage IA–IB grade 3 or IC any grade), early-stage ovarian cancer, there is general consensus that chemotherapy is associated with improved outcomes.2,7,10,17,27–29 Despite the survival benefit associated with treatment, chemotherapy is frequently omitted in these patients.17 Within our cohort, 30% of women with high-risk, early stage ovarian cancer did not receive chemotherapy. A prior study of elderly women in the U.S. noted similar findings; 28% of patients with high-risk tumors did not receive chemotherapy.17 Encouragingly, we noted that the use of chemotherapy in this group increased over the years of study.
We recognize a number of important limitations. Although we can examine the use of chemotherapy, data on the specific drugs used, doses, number of cycles is lacking. Further work to examine the specific characteristics of chemotherapy would be of great value. Likewise, NCDB does not capture the specialty of the treating physician; this would be of great interest in future studies. While our models were extensively adjusted for measured confounders, we cannot exclude the possibility that other unmeasured confounding factors influenced use of chemotherapy and outcomes. Additionally, although we recorded use of chemotherapy at any facility, we cannot exclude the possibility that use of chemotherapy was miscoded in a small number of women. Lastly, as with any large study of administrative data, a statistically significant finding does not necessarily imply that it is of clinical importance.
These data have a number of practical implications for the management of women with early-stage ovarian cancer. First, the widespread variation in the adherence to recommended care clearly indicates that the quality of care for women with early-stage ovarian cancer can be improved. Performance of surgical staging, avoidance of chemotherapy in women unlikely to derive benefit, and use of chemotherapy in patients when indicated can improve outcomes as well as reduce toxicity. Second, prior studies grouping stage IA/IB, grade 2 tumors with grade 1 tumors as a low-risk may be insufficient.2,27 Our findings of improved survival among women with stage IA/IB, grade 2 tumors who received chemotherapy suggest that this subset of patients constitutes a separate intermediate risk group where chemotherapy should be strongly considered.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute. Dr. Tergas is the recipient of a fellowship from the NCI (NCI R25 CA094061-11).
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented at the 2016 Society of Gynecologic Oncology Annual Meeting.
References
- 1.Wright JD, Chen L, Tergas AI, et al. Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol. 2015;125:1345–52. doi: 10.1097/AOG.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JK, Tian C, Teoh D, et al. Survival after recurrence in early-stage high-risk epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecologic oncology. 2010;116:307–11. doi: 10.1016/j.ygyno.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Ovarian Cancer Treatment Guidelines. at http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- 4.Trimbos B, Timmers P, Pecorelli S, et al. Surgical staging and treatment of early ovarian cancer: long-term analysis from a randomized trial. Journal of the National Cancer Institute. 2010;102:982–7. doi: 10.1093/jnci/djq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harlan LC, Clegg LX, Trimble EL. Trends in surgery and chemotherapy for women diagnosed with ovarian cancer in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3488–94. doi: 10.1200/JCO.2003.01.061. [DOI] [PubMed] [Google Scholar]
- 6.Winter-Roach BA, Kitchener HC, Lawrie TA. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst Rev. 2012;3:CD004706. doi: 10.1002/14651858.CD004706.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN. Clinical Practice Guidelines in Oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. 2015. Version 2.2015. [Google Scholar]
- 8.Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990;322:1021–7. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
- 9.Colombo N, Guthrie D, Chiari S, et al. International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J Natl Cancer Inst. 2003;95:125–32. doi: 10.1093/jnci/95.2.125. [DOI] [PubMed] [Google Scholar]
- 10.Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. Journal of the National Cancer Institute. 2003;95:105–12. [PubMed] [Google Scholar]
- 11.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109:2031–42. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 12.Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecologic oncology. 2006;103:383–90. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Trope C, Kaern J. Adjuvant chemotherapy for early-stage ovarian cancer: review of the literature. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2909–20. doi: 10.1200/JCO.2007.11.1013. [DOI] [PubMed] [Google Scholar]
- 14.Young RC, Decker DG, Wharton JT, et al. Staging laparotomy in early ovarian cancer. Jama. 1983;250:3072–6. [PubMed] [Google Scholar]
- 15.Le T, Adolph A, Krepart GV, Lotocki R, Heywood MS. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecologic oncology. 2002;85:351–5. doi: 10.1006/gyno.2002.6636. [DOI] [PubMed] [Google Scholar]
- 16.Timmers PJ, Zwinderman K, Coens C, Vergote I, Trimbos JB. Lymph node sampling and taking of blind biopsies are important elements of the surgical staging of early ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20:1142–7. doi: 10.1111/igc.0b013e3181ef8e03. [DOI] [PubMed] [Google Scholar]
- 17.Dinkelspiel HE, Tergas AI, Zimmerman LA, et al. Use and duration of chemotherapy and its impact on survival in early-stage ovarian cancer. Gynecol Oncol. 2015;137:203–9. doi: 10.1016/j.ygyno.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–34. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 19.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb.
- 20.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patankar SS, Tergas AI, Deutsch I, et al. High versus low-dose rate brachytherapy for cervical cancer. Gynecol Oncol. 2015;136:534–41. doi: 10.1016/j.ygyno.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Raigani S, Hardacre JM, Kim J, Ammori JB. Trends in the surgical treatment of gastric adenocarcinoma. Ann Surg Oncol. 2014;21:569–74. doi: 10.1245/s10434-013-3314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95:105–12. [PubMed] [Google Scholar]
- 25.Trimbos JB, Vergote I, Bolis G, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113–25. [PubMed] [Google Scholar]
- 26.Temkin SM, Terplan M. Trends in Relative Survival for Ovarian Cancer From 1975–2011. Obstet Gynecol. 2015;126:898. doi: 10.1097/AOG.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 27.Bell J, Brady MF, Young RC, et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecologic oncology. 2006;102:432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Chan JK, Tian C, Monk BJ, et al. Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer. 2008;112:2202–10. doi: 10.1002/cncr.23390. [DOI] [PubMed] [Google Scholar]
- 29.Kolomainen DF, A’Hern R, Coxon FY, et al. Can patients with relapsed, previously untreated, stage I epithelial ovarian cancer be successfully treated with salvage therapy? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3113–8. doi: 10.1200/JCO.2003.06.119. [DOI] [PubMed] [Google Scholar]