Abstract
Dopamine (DA) is a neuromodulator that regulates different brain circuits involved in cognitive functions, motor coordination, and emotions. Dysregulation of DA is associated with many neurological and psychiatric disorders such as Parkinson’s disease and substance abuse. Several lines of research have shown that the midbrain DA system is regulated by the central adrenergic system. This review focuses on adrenergic interactions with midbrain DA neurons. It discusses the current neuroanatomy including source of adrenergic innervation, type of synapses, and adrenoceptors expression. It also discusses adrenergic regulation of DA cell activity and neurotransmitter release. Finally, it reviews several neurological and psychiatric disorders where changes in adrenergic system are associated with dysregulation of the midbrain DA system.
Norepinephrine (NE) and epinephrine (E) enhance or dampen neural activity by acting through G-coupled receptors. Adrenoceptors α1 and β1, β2, and β3 are classified as activators, as binding of agonist to these receptors leads to Gαq- and Gαs-mediated signaling. Adrenoceptor α2, and in some cases β2, can be classified as inhibitors, as binding of agonist to these receptors leads to leads to Gαi-mediated signaling. The adrenergic system is incredibly complex with adrenoceptors ubiquitously present at postsynaptic and presynaptic elements of neurons, glia, and blood vessels. This complexity makes it difficult to determine how NE and E modulate the activity of brain circuits. The goal of this review is to summarize experimental data on how NE and E modulate the activity of midbrain dopamine (DA) neurons, what the substrates of their actions are and how these may relate to neurological diseases and neuropsychiatric disorders.
Anatomy of midbrain DA regions
Midbrain DA neurons are clustered into three groups originally described by Dahlstrom and Fuxe (1964). In the original description, DA neurons were identified by their green fluorescence induced by a formaldehyde treatment and were named under an A nomenclature. Three clusters were segregated by their location in association to anatomical landmarks. Cells located within the midbrain reticular formation were named A8, those at the substantia nigra (SN) were named A9, and those dorsal and lateral to the interpeduncular nucleus were named A10. Boundaries among these clusters are not well defined; especially in the anteriorlateral part of A10 and the ventromedial part of group A8, and the lateral part of SN and the lateral A8, where DA neurons formed a continuum (Dahlstroem and Fuxe, 1964).
Current anatomical atlases locate A8 in the retrorubral field (RRF). A9 is divided into SN pars compacta and pars lateralis, but with some DA neurons are scattered in the SN pars reticulata. The A10 encompasses many subregions in the midbrain. It is usually associated with the ventral tegmental area (VTA), but it extends to other regions including dorsal raphe, caudal linear nucleus of the raphe (CLi), rostral linear nucleus of the raphe (RLi), interfascicular nucleus (IF), and medial supramammillary nucleus (Phillipson, 1979; Swanson, 1982). In rats, the distribution of DA neurons in the midbrain is 6% in A8, and 47% in A9 and 47% in A10 (German and Manaye, 1993).
DA neurons have a differentiated output with few axon collaterals. With the exception of cell in the medial part of the SN and lateral parts of the VTA, most midbrain DA neurons tend to send axons to discrete brain regions (Fallon, 1981; Fallon and Loughlin, 1982; Swanson, 1982). The axons of DA neurons follow two topographical arrangements. On one hand, DA neurons located medially project to the medial and somewhat anterior sectors of the forebrain, while DA neurons located laterally project to the lateral and somewhat posterior sectors of the forebrain (Fallon, 1988). On the other hand, ventral SN and VTA neurons project preferentially to dorsal structures such as caudate-putamen and septum whereas dorsal SN and VTA neurons project preferentially to ventral structures such as the amygdala and olfactory tubercle (Fallon, 1988). It is important to note that DA neuron topographical segregation also correlates to distinct physiological features and have become important feature in our understanding on how DA might be altered under different psychopathologies (Lammel et al., 2011; Margolis et al., 2008; Mejias-Aponte et al., 2015).
It is important to recognize that in addition to DA neurons, GABA and glutamate neurons are also major cell types within the midbrain DA regions (Steffensen et al., 1998; Yamaguchi et al., 2011; Yamaguchi et al., 2013). These neurons synapse onto DA neurons providing local inhibitory and excitatory inputs to DA neurons (Dobi et al., 2010; van Zessen et al., 2012; Yamaguchi et al., 2013). Recently two pathways with preferential inputs to non-DA neurons have been described; a GABAergic input by nucleus accumbens (NAcc) medium spiny neurons (Xia et al., 2011), and GABAergic and glutamatergic inputs from the bed nucleus of the stria terminalis (Jennings et al., 2013). This organization provides a path to selectively affect DA neuron function through feed forward excitation or inhibition.
Another feature of GABA and glutamate neurons is that they also send afferents to many of the same brain regions that DA neurons innervate (Brown et al., 2012; Carr and Sesack, 2000; Taylor et al., 2014; Yamaguchi et al., 2011). Some of these neurons co-transmit DA (Li et al., 2013; Stuber et al., 2010; Tecuapetla et al., 2010; Tritsch et al., 2014; Zhang et al., 2015). In the case of glutamate neurons, DA release occurs in different subcellular domains to those of glutamate (Zhang et al., 2015). These studies highlight that, in addition to DA, GABA and glutamate are also major neurotransmitters of the system.
Anatomy of adrenergic innervation to midbrain DA regions
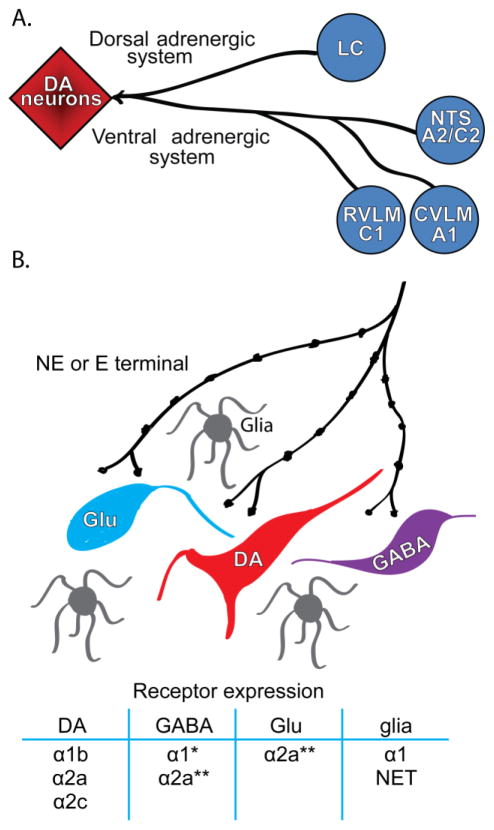
Adrenergic innervation to midbrain DA regions arises from locus coeruleus (LC) and brainstem adrenergic nuclei (Mejias-Aponte et al., 2009; Robertson et al., 2013)(Figure 1). The major NE afferents originate from areas A1, A2, and the LC, whereas the major E afferents originate from area C1. The A1 NE group is located in the caudal ventrolateral medulla, A2 is located in the nucleus of the solitary tract, and C1 is located in the rostral ventrolateral medulla (Dahlstroem and Fuxe, 1964; Hokfelt et al., 1974; Ungerstedt, 1971). The NE and E innervation is topographically distributed. Retrorubral field (RRF, area A8) receives the highest innervation followed by the VTA and midline DA nuclei of A10 (RLi, CLi, IF), and SN (area A9). There is also a small group of DA neurons that belong to A10 within the periaqueductal gray in the dorsal raphe; their source of adrenergic innervation includes the LC and area C1 (Card et al., 2006; Kim et al., 2004).
Figure 1.
Major adrenergic inputs to midbrain DA regions. A. Both ventral and dorsal adrenergic systems innervate midbrain DA regions. The source of ventral adrenergic systems inputs include the noradrenergic and epinephrine neurons in area A2/C2 located in the nucleus of the solitary tract (NTS), noradrenergic neurons in area A1 located in the cadual vetrolateral medulla (CVLM), and epinephrine neurons in area C1 located in the rostral ventrolateral medulla (RVLM). Dorsal noradrenergic innervation arises from the locus coeruleus (LC). B. Midbrain DA regions are heterogeneous in their cellular components. GABA and glutamate neurons are abundant in the VTA and RRF, and to a lesser extent in the SN. These neurons make synapses on DA neurons. Local effects of NE and E can be mediated directly at DA neurons or through their interaction with GABA and glutamate neurons and glia. Known receptors expressed on neurons and glia are listed in the table. *, expression was suggested by electrophysiological data, but lack histological confirmation; **, α2a adrenoceptors are present in non-DA neurons, but cell type is still undetermined.
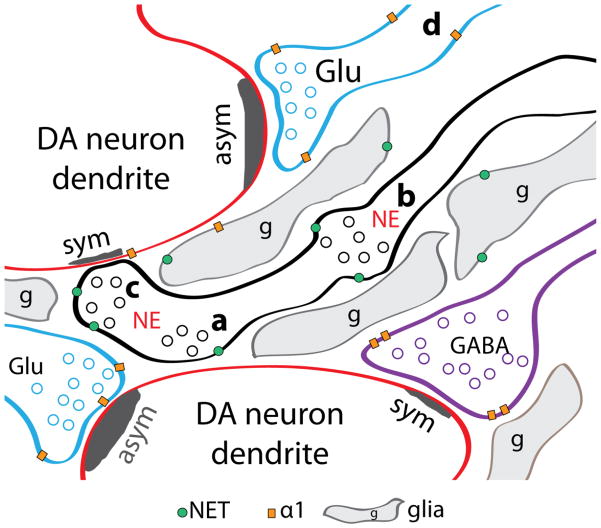
At the ultrastructure level, adrenergic axons within the VTA terminals are primarily associated with synaptic appositions without making synaptic contacts (Liprando et al., 2004). These appositions represent 77% and 49% of samples analyzed from rat and monkey, respectively. The second most frequent synaptic arrangement is synaptic appositions separated by glia processes; 27% on samples analyzed from rat and 40% on samples analyzed from monkey. Lastly, adrenergic terminals with junctional specializations, constituting 7% and 11% in samples analyzed from rat and monkey, respectively. These junctional specializations are often symmetric (associated with inhibitory synapses), but some are asymmetric (associated with excitatory synapses). Furthermore, it was also observed by Liprando and colleagues that some adrenergic terminals were associated with non-DA dendrites; indicating that non-DA neurons within the VTA also receive adrenergic innervation. Taken together, the higher prevalence of synaptic appositions over junctional specializations observed in the ultrastructure indicates a paracrine release of neurotransmitter, where neurotransmitter release occurs extrasynaptically and produces changes in nearby cells (Figure 2). This system of neurotransmitter release is also known as volume transmission (Fuxe et al., 2010).
Figure 2.
Ultrastructure organization of adrenergic innervation to midbrain DA neurons. NE axons have three different synaptic specializations. The most prevalent adrenergic terminals are synaptic appositions in close proximity to synapses (a). The second most prevalent are synaptic apposition surrounded by glia (b). The less prevalent is adrenergic terminal making synaptic junctions (c). Because of the low instances of synaptic junctions, adrenergic innervation is considered a paracrine or volume transmission system. Other known detail of the adrenergic innervation is the location of NET and the α1 adrenoceptors. In addition to the expected location of NET at NE terminals, some glia also expresses NET. NE is also capable of regulating glutamate and GABA release though adrenoceptors at presynaptic terminals. The α1 adrenoceptors are present in both glutamatergic and GABAergic terminals. It is most often found perysynaptically rather than at the synapse. Most often α1 adrenoceptors are found extrasynaptically in unmyelinated axons (d). The α1 adrenoceptors are also present in some glia. The ultrastructural detail of the α2 adrenoceptors is unknown, although pharmacological and electrophysiological evidence indicate their presence in adrenergic, glutamatergic and GABAergic terminals. Abbreviations: symmetric synapse, sym; asymmetric synapse, asym; glutamate terminal, Glu; GABA terminal, GABA; NE terminal, NE.
Adrenergic receptors expression within midbrain DA regions
Actions of NE and E on midbrain DA regions are dependent on the location of adrenoceptors and the type of neurons expressing these receptors. Whereas in the SN DA and non-DA neurons are mostly segregated, this is not the case in the VTA and RRF (Figure 1). In addition to DA neurons, GABA and glutamate neurons are also present in midbrain DA regions (Yamaguchi et al., 2007; Yamaguchi et al., 2013). The heterogeneity of cell types is a challenge to the integration of the available anatomical data mostly established through in-situ hybridization, autoradiography, and immunohistochemistry, and, recently complemented with few ultrastructure studies.
In-situ hybridization studies have shown low levels or no expression of α and β adrenoceptors. RRF, VTA and SN express α1A adrenoceptors, SN also expresses α1B adrenoceptors (Day et al., 1997). Recently, quantitative real-time polymerase chain reaction (qPCR) was used for gene expression profiling of VTA and SN DA neurons and the α1B receptor was detected with a 2-fold higher expression in the VTA than SN (Greene et al., 2005). Conversely, α2 and β1 mRNA expression were undetected in midbrain DA regions (Nicholas et al., 1993a; Nicholas et al., 1993b). It is important to acknowledge that most in-situ hybridization comes from whole-brain sampling studies, where the assays might not been optimized for midbrain DA region samples. This is an area that should be revisited and expanded to cover both DA and non-DA midbrain neurons.
Receptor binding autoradiography has detected mild to moderate levels of α and β adrenoceptors in SN and VTA. Using the selective α1 antagonist, HEAT, low expression has been reported in SN and VTA (Jones et al., 1985). Figure 8 in this report also showed α1 adrenoceptors labeling in the midline A10 nuclei, CLi and IF (Jones et al., 1985). Using the selective α2 antagonist, idaxozan, moderately high levels were detected in the RLi and VTA, and moderate levels in SN pars compacta (Boyajian et al., 1987). Similarly, moderate to low expression was detected with the α2 antagonist, rauwolscine primarily in SN pars lateralis and to a lesser extent in SN pars compacta and VTA. Beta adrenoceptors were studied with the β adrenoceptor antagonist, dihydroalprenolol, revealing moderate expression in both SNc and VTA (Palacios and Kuhar, 1980).
Expression of α2A and α2C adrenoceptorss in midbrain DA regions has been investigated using immunohistochemistry. Moderate levels of α2A were observed in both SNc and VTA. However, few DA neurons expressed the receptor, only 4% and 14% in the SNc and VTA, respectively (Rosin et al., 1993). This indicates that α2A is expressed mainly on non-DA neurons, but whether is in glutamate of GABA neurons is still undetermined. In contrast to the α2A adrenoceptors, α2C is expressed by many DA neurons; co-expression is as high as 63% in RRF, 77–83% in SN, 67% in VTA (Lee et al., 1998).
There is abundant evidence of the presence of adrenoceptors in midbrain DA regions from in-situ hybridization, receptor autoradiography, and immunohistochemistry studies. Nevertheless, there is a lack of cellular detail of their location and their relative expression on DA versus non-DA neurons. The in-situ hybridization studies have established that there are neurons within midbrain DA regions expressing adrenoceptors. Both autoradiography and immunohistochemistry have established associations of adrenoreceptors and neurons in the midbrain DA regions, but ultrastructure studies are needed to conclusively determine whether the adrenoceptors are present at axon terminals or on postsynaptic elements. Conversely, evidence of postsynaptic expression has been supported by electrophysiological studies; these are described in the next section.
Recent ultrastructural studies have started to shed light on the cellular detail of α1 adrenoceptors cellular location. The α1 adrenoceptors were detected almost exclusively extrasynaptically, with <10% of these receptors found peri-synaptically or directly associated with the synaptic active zones of either asymmetric or symmetric specializations. When associated with synaptic elements, the α1 adrenoceptors have been observed primarily in presynaptic elements, with scarce postsynaptic labeling in VTA neurons (Mitrano et al., 2012; Rommelfanger et al., 2009). The majority of presynaptic labeling is associated with symmetric perisynaptic elements and to a lesser extent with asymmetric synapses. Importantly, α1 adrenoceptors are present in VGluT1 and VGluT2 expressing glutamatergic terminals, and in GABAergic terminals (Mitrano et al., 2012), indicating presynaptic regulation of glutamate and GABA release.
In addition to the presence of adrenoceptors in neurons, there is also evidence of α1 adrenoceptors in glia (Mitrano et al., 2012; Rommelfanger et al., 2009). Moreover, the noradrenergic transporter (NET) has also been detected in glia (Liprando et al., 2004). Adrenoceptors α2 and β are also expressed by astrocytes and microglia (Fuxe et al., 2015); however, their presence within midbrain DA regions have not been studied. The separation of synaptic appositions of adrenergic axon terminals by glia suggests an important role of glia in NE neurotransmission.
In summary, the current anatomical data support preferential paracrine release of NE within midbrain DA regions indicated by the greater proportion of synaptic appositions over synaptic junctions. Many of these of synaptic appositions are separated by glia. This synaptic arrangement offers NE access to different cellular elements in the circuit, both at postsynaptic and presynaptic sites, and regulation though interaction with glia (Figure 1 and 2). Evidence of direct postsynaptic actions is supported by mRNA expression of the α1B and protein detection of α2A and α2C adrenoceptors on VTA and SN DA neurons. The α2A adrenoceptors were also found on non-DA neurons; thus, highlighting the possibility of adrenergic regulation of DA neurons indirectly by local connection of non-DA neurons with DA neurons. At the presynaptic site, α1 adrenoceptors are present in glutamatergic and GABAergic terminals supporting presynaptic regulation of neurotransmitter release. Moreover, the presence of NET in glia indicates an active participation of glia in the regulation of NE levels.
Many aspects of the neuroanatomy of the adrenergic innervation require further research. Among these, a revision of the receptor expression is highly needed. Particularly, determining the distribution among DA neurons, non-DA neurons and glia would provide insight into the local circuitry. Moreover, ultrastructural studies focusing on the α2 adrenoceptors are also needed. Conversely, tracing studies are needed to identify the origin of neurons that are expressing the α1 adrenoceptors presynaptically.
Adrenergic regulation of midbrain DA neuron activity
DA neuron activity is characterized as regular with intermittent bursts (Grace and Bunney, 1984). Regular firing is characterized by constant inter-spike intervals between action potentials while bursts are identified by an increase in firing pace of action potentials clustering together for a brief time. Burst firing produces a massive DA release at terminals (Floresco et al., 2003). DA neurons fire in bursts in response to events of behavioral relevance such a conditioned stimulus or the consumption of a reward (Schultz, 2007).
Changes in firing regularity were among the first effects described for adrenergic drugs on DA neurons (Table 1). When given intravenously, clonidine, an α2 agonist, increased firing regularity of midbrain DA neurons without changes in firing rate. Conversely, idaxozan, an α2 antagonist, increased burst firing and prevented clonidine effects (Grenhoff and Svensson, 1988; Grenhoff and Svensson, 1989). Moreover, prazosin, an α1 antagonist, decreased burst firing and prevented idazoxan-increased burst firing (Grenhoff and Svensson, 1993). Given that adrenergic drugs were administered systemically, these studies did not discern whether the effects are caused by direct regulation of DA neurons or regulation of inputs to DA neurons. Nevertheless, these studies established that adrenergic drugs were capable of altering burst firing of DA neurons.
Table 1.
Adrenergic modulation of DA neuron physiology studied in in-vivo anesthetized animals
| Receptor | Midbrain DA Region | Drug | Administration Route | Effect | Reference |
|---|---|---|---|---|---|
| α1 | VTA | Prazosin | i.v. | Decreased burst and regulate cell firing | (Grenhoff and Svensson, 1993) |
| VTA | Prazosin | i.v. | Blocked LC activation of DA neurons | (Grenhoff et al., 1993) | |
| VTA | Phenylephrine | Pressure | Increased firing rate and burst activity | (Goertz et al., 2015) | |
| α2 | VTA/SN | Clonidine | i.v. | Regularized firing without affecting the firing rate | (Grenhoff and Svensson, 1988; Grenhoff and Svensson, 1989) |
| VTA | Clonidine | iontophoresis | Decreased firing rate | (White and Wang, 1984) | |
| VTA/SN | Idazoxan, Yohimbine | i.v. | Increased firing rate and burst firing | (Grenhoff and Svensson, 1988; Grenhoff and Svensson, 1993) | |
| VTA | Piperoxine | iontophoresis | Blocked NE inhibition on firing rate | (White and Wang, 1984) | |
| VTA | Idazoxan | Iontophoresis | Blocked NE inhibition on firing rate | (Guiard et al., 2008) | |
| β | VTA/SN | Isoprotenerol | iontophoresis | Decreased firing rate | (Aghajanian and Bunney, 1977; White and Wang, 1984) |
| D2 | VTA/SN | NE | Iontophoresis | Decreased firing rate | (Aghajanian and Bunney, 1977; White and Wang, 1984) |
| NET | VTA | Nisoxetine | i.v | Increased slow oscillations and burst firing | (Shi et al., 2004) |
Few in-vivo studies have demonstrated changes in firing activity of DA neurons by local administration of NE or adrenoceptor agonists and antagonists (Table 1). Applied iontophoretically, NE decreased DA neuron discharge activity. This effect is blocked by sulpiride, a D2 antagonist, or piperxane and idaxozan, α2 antagonists (Aghajanian and Bunney, 1977; Guiard et al., 2008; White and Wang, 1984); thus indicating that NE inhibits DA neurons through activation of D2 and α2 receptors. Moreover, iontophoresis of α2 agonist, clonidine, weakly decreased firing rate (White and Wang, 1984). Inhibition of firing rate is also evoked by the β adrenoceptor agonist, isoproterenol (Aghajanian and Bunney, 1977; White and Wang, 1984). Contrary to the inhibitory effects of α2 and β adrenoceptors, activation of α1 adrenoceptors by phenylephrine increased both firing rate and burst firing of DA neurons (Goertz et al., 2015).
NE modulatory effects on glutamate-evoked excitation have also been studied in-vivo (Almodovar-Fabregas et al., 2002). In this experiment glutamate-mediated excitation was evoked using short iontophoresis pulses of glutamate while local NE levels were increased using long iontophoresis pulses of NE. This produces an on and off pattern of glutamate-evoked excitation in the presence or absence of local increases in NE. Three different forms of neuromodulation were observed: potentiation (increase in glutamate-evoked excitation independent of changes in spontaneous activity), enhancement (increase in glutamate-evoked excitation relative to spontaneous activity), and suppression (decrease in glutamate-evoked excitation relative to spontaneous activity). Modulation of glutamate-evoked excitation by NE was observed in 92 % of putative VTA DA neurons. Of these 19% of neurons showed potentiation, 27% of neurons showed enhancement, and 46% of neurons showed suppression.
Electrical stimulation of brain regions containing NE and E cell bodies has also shown changes in the activity of DA neurons. Single-pulse stimulation of the LC evoked excitation followed by inhibition. This excitation was sensitive to the α1 adrenoceptor antagonist prazosin. The inhibition was insensitive to α1, α2 and β adrenoceptor antagonists (Grenhoff et al., 1993). Similarly, electrical stimulation of the nucleus of the solitary tract, the brain regions where A2 NE and C2 E neurons reside, also evoked responses of VTA DA neurons; some neurons are excited while others are inhibited (Kirouac and Ciriello, 1997; Mejias-Aponte and Aston-Jones, 2005). Although, A1 NE and C1 E neurons also project to midbrain DA regions, the effects of their stimulation is on the activity of DA neurons have not yet been investigated.
Postsynaptic actions of α1 adrenoceptors have been determined in ex-vivo recordings on midbrain slices (Table 2). NE depolarized 36% of putative DA neurons, which leads to an increase in firing rate for some neurons. NE-induced depolarization is blocked by the α1 antagonist prazosin and mimicked by phelyephrine, an α1 agonist. Phenylephrine-induced membrane depolarization persisted in the presence of tetradotoxin, a sodium channel blocker, confirming its independence from incoming inputs (Grenhoff et al., 1995). Two conductances are affected by α1 adrenoceptors activation, calcium-activated potassium channel currents are decreased and hyperpolarization-activated cation currents are increased. Acting on theses conductances, NE increased intra-burst frequency of aspartate-evoked burst firing (Goertz et al., 2015).
Table 2.
Adrenergic modulation of DA neuron physiology studied in ex-vivo brain slices
| Receptor | Site of action | Midbrain DA Region | Drug | Effect | Reference |
|---|---|---|---|---|---|
| α1 | Postsynaptic | VTA | NE | Activation of calcium-activated K+ channels | (Paladini and Williams, 2004) |
| VTA | Phenylephrine | Heterologous desensitization of mGluR inhibitory postsynaptic currents | (Paladini and Williams, 2004) | ||
| VTA | Phenylephrine | Activation of inward K+ current | (Grenhoff et al., 1995; Paladini and Williams, 2004 | ||
| Presynaptic | VTA | Phenylephrine | Decrease in frequency of spontaneous IPSCs | (Velasquez-Martinez et al., 2015) | |
| VTA\RLi | Phenylephrine, Methoxamine | Increase in frequency of spontaneous and miniature EPSCs | (Velasquez-Martinez et al., 2012; Williams et al., 2014) | ||
| α2 | Postsynaptic | VTA | Clonidine UK-14304 | Inhibition of Ih current | (Inyushin et al., 2010) |
| SNc | UK-14304 | Activation of non-specific cationic current | (Cathala et al., 2002) | ||
| Presynaptic | VTA | Clonidine UK14304 | Decrease in frequency of spontaneous IPSCs | (Velasquez-Martinez et al., 2015) | |
| VTA\RLi | NE UK-14304 | Increase in frequency of spontaneous and miniature EPSCs | (Velasquez-Martinez et al., 2012; Williams et al., 2014) | ||
| D2 | Postsynaptic | VTA | NE | Activation of the Ih current | (Arencibia-Albite et al., 2007) |
Presynaptic modulation of inputs to DA neurons by NE has also been established. Activation of α1 adrenoceptors decreased the frequency of spontaneous GABAA inhibitory postsynaptic currents. This activation is input dependent, as miniature inhibitory postsynaptic currents are unaffected. The underlying mechanism relies on phosphokinase C signaling and the activation of the voltage and calcium-activated potassium channels (Velasquez-Martinez et al., 2015). Contrary to the effect of inhibitory postsynaptic currents, activation of α1 adrenoceptors increased presynaptic glutamate release. Phenylephrine increased the amplitude of evoked and frequency of spontaneous AMPA excitatory postsynaptic currents (Velasquez-Martinez et al., 2012; Williams et al., 2014). Taken together, the presynaptic α1 actions are in accordance to an activation of DA neurons favoring glutamate over GABA inputs.
Postsynaptic modulation by α2 adrenoceptors modulates excitation of DA neurons. Clonidine and UK14304, α2 agonists, both inhibited the hyperpolarization-activated cation current by activating the protein kinase C signaling pathway (Inyushin et al., 2010). Similarly, NE and UK14304 activate a non-specific cationic conductance that produced a small inward current (Cathala et al., 2002). In conjunction, these two postsynaptic actions render DA neurons more excitable and might play a role in the transition to burst firing observed with systemic administration of α2 antagonists (Grenhoff and Svensson, 1988; Grenhoff and Svensson, 1989) These action of α2 adrenoceptors may also synergize with the postsynaptic activation of α1 adrenoceptors (Goertz et al., 2015).
Presynaptic modulation by α2 adrenoceptors affects both inhibitory and excitatory inputs to DA neurons. Clonidine and UK14304, both α2 agonist, decreased the frequency of spontaneous and miniature EPSCs (Jimenez-Rivera et al., 2012; Williams et al., 2014). Conversely, both clonidine and UK14304 increased spontaneous IPSCs (Cathala et al., 2002). This suggests a net increase in inhibition by inputs into DA neurons through activation of α2 receptors.
Another mechanism of adrenergic actions is heterosynaptic interaction with metabotropic glutamate receptor-mediated inhibitory postsynaptic potential (mGluR IPSPs). Brief exposure of aspartate or a train of electrical pulses produces burst firing on DA neurons. This stimulation also activates the mGluR resulting in a negative feedback control that regulates DA neuron burst activity. In the presence of NE, mGluR IPSPs are attenuated in an α1-dependent manner. NE, by itself, also evoked an IPSPs through activation of the postsynaptic α1 receptor, but this effect was susceptible to receptor desensitization, while NE the effect on mGluR was not (Paladini and Williams, 2004). This relationship suggests that, when the coincidence of NE and glutamate inputs into DA neurons is strong enough, DA neurons will fire longer bursts of action potentials (Goertz et al., 2015).
It is important to emphasize that NE produces a rapid desensitization of α1 adrenoceptor-mediated IPSPs (Paladini and Williams, 2004); thus, this current is likely to be engaged during brief phasic NE release and not when there is a high NE tonic level. Importantly, high levels of NE are common throughout the brain during stress (Aston-Jones and Cohen, 2005; Valentino and Van Bockstaele, 2008), and under NET blockade by psychostimulants such as amphetamine and cocaine (Chen and Reith, 1994a; Pan et al., 1996; Pan et al., 2007; Reith et al., 1997). Under high NE levels produced by amphetamine, the α1 adrenoceptor-mediated IPSPs should be desensitized; therefore, providing circumstances for a preferential α1-dependent inhibition of mGluR IPSPs (Paladini et al., 2001), which could lead to the increased α1-dependent burst firing on DA neurons observed in in-vivo recordings (Shi et al., 2000; Shi et al., 2004).
Although research has been centered on NE actions on DA neurons, one report has shown that NE also acts on local non-DA neurons. Namely, phenylephrine has been shown to depolarize non-DA neurons (Grenhoff et al., 1995). Because of the local connections of glutamate and GABA neuron with midbrain DA neurons, future studies are needed to identify other adrenergic actions on non-DA neurons that might impose a regulation on DA neurons.
In summary, there is ample evidence for adrenergic modulation of DA neural activity. This modulation is complex involving a variety of presynaptic and postsynaptic elements. In-vivo studies indicate control of firing regularity of DA neurons, where activation of α1 adrenoceptors increases burst firing. The regulation of burst firing is associated with regulation of conductances residing in the postsynaptic site. Moreover, there is also support for presynaptic modulation, where α1 adrenoceptors has net excitatory drive by dampening GABA and enhancing glutamate. On the other hand, α2 adrenoceptors can enhance an inhibitory drive dampening glutamate and enhancing GABA release.
The development of optogenetics and designer receptors exclusively activated by designer drugs (DREADDs) (Bernstein and Boyden, 2011; Urban and Roth, 2015) can help decipher the specific role for each of the multiple adrenergic pathways that send afferents to midbrain DA regions. The use of transgenic animals can be very helpful; among these, the mice developed by Jensen and colleagues that allows selective targeting of LC and non-LC NE neurons (Robertson et al., 2013). Another question centers on the source of glutamatergic and GABAergic presynaptic terminals that are sensitive to adrenoceptor pharmacology. Future research should also explore in more detail adrenergic action on local non-DA neurons, as these could be a major player in the regulation of DA neural activity.
Regulation of DA release by the adrenergic system
DA levels within midbrain DA regions and at terminal sites are affected by changes in NE levels. Three factors have been identified: direct regulation of DA neurons, presynaptic regulation of DA terminals, and clearance and release of DA through NE terminals. Most of the mechanisms of direct regulation of DA neurons were reviewed in the previous section. Here, I discuss experiments where DA levels were measured locally within midbrain DA regions or at terminal sites while manipulating NE levels at midbrain DA regions.
There is clear evidence that altering brain NE levels changes DA release. Chemical lesions of the LC or its afferents decreased DA levels in the NAcc, caudate, and prefrontal cortex (PFC) (Carboni et al., 1990; Haidkind et al., 2002; Lategan et al., 1990; Lategan et al., 1992; Masana et al., 2011). Similarly, chronic inhibition of NE synthesis by genetic lesion of DA-beta hydroxylase (DBH), the enzyme responsible for converting DA into NE in adrenergic neurons, also decreased DA levels in NAcc and caudate-putamen, but not in the PFC (Schank et al., 2006). Taken together, these findings suggest that NE exerts an excitatory tone on DA levels.
A limited number of studies have provided insight on how altering local NE levels in midbrain DA regions affect the local DA levels. In these studies NE levels were increased by blocking NET or α2 adrenoceptors. Reverse dialysis of desipramine in the VTA, a selective NET blocker, and cocaine and amphetamine, non-selective monoamine blockers, increased NE (Chen and Reith, 1994a; Pan et al., 1996; Pan et al., 2007; Reith et al., 1997). Similarly, reverse dialysis of yohimbine, an α2 antagonist, increased NE release (Chen and Reith, 1994b). This increase on NE levels by antagonizing the α2 adrenoceptor was associated with the blockade of α2 autoreceptor at adrenergic axon terminals. These findings indicate that NE tone exists at the VTA and it is susceptible to regulation at terminal sites. Concomitant to the increase in NE levels, NET blockers and α2 antagonists, also increased local DA levels (Chen and Reith, 1994a; Pan et al., 1996; Pan et al., 2007; Reith et al., 1997). These results are in accordance with an excitatory effect of NE on DA neurons. Although in the case of cocaine and amphetamine, DAT blockade as the mechanism contributing to the increased DA levels cannot be rule out, the increase in DA levels observed with desipramine and yohimbine indicate a NE-mediated activation of DA neurons.
Despite a thorough search of the literature, few experiments have sought to answer how local changes of NE within midbrain DA regions affect DA output at terminal sites. Intra-VTA infusion of cocaine, which increases NE, DA and 5-HT, decreased DA in the NAc and PFC (Chen et al., 1996; Pan et al., 1996). This decrease in extracellular DA levels is associated with heightened local increases of DA in the VTA that can lead to D2 autoreceptor regulation (Brodie and Dunwiddie, 1990; Chen and Reith, 1994b). However, it is important to note that DA PFC projecting neurons lacks the D2 autoreceptor rendering them non-responsive to the inhibitory actions of DA (Lammel et al., 2008). Therefore, a DA-independent mechanism must underlie the decrease of DA in the PFC.
In contrast to cocaine, intra-VTA infusion of amphetamine, increased DA at the PFC. This increase in PFC DA is associated to a heightened increase in local VTA NE over DA as amphetamine induced a 5-fold increase in NE levels compare to those of cocaine (Pan et al., 2007). Because these findings are drawn on the bases of non-specific monoamine transporter inhibitor, future studies using selective NET blockers or α2 adrenoceptors antagonists may provide a better insight on how increasing NE levels at midbrain DA regions affect DA release at terminal sites. Furthermore, a more direct approach could be selective activation of NE terminals utilizing optogenetics or DREADDs. This later approach could also be used to investigate different adrenergic inputs by selective targeting of areas A1, A2, C1 or LC.
Interestingly, contrary to the decrease in DA levels in the NAc and PFC levels by local infusion of cocaine into the VTA, systemic administration of cocaine increased NAc and PFC DA levels (Tanda et al., 1997). This discrepancy is expected as cocaine, which blocks DAT at terminals, leads to local increases in DA at terminal sites. However, DAT blockade is not the only mechanism regulating DA release after systemic cocaine administration. Cocaine increases in DA levels at terminal sites also requires DA neurons being active (Mejias-Aponte et al., 2015; Sombers et al., 2009). Importantly, two mechanisms linking activation of α1 adrenoceptors has been described. Local intra-VTA administration of prazosin, an α1 antagonist, blocked cocaine-evoked DA release in the NAcc shell by regulating burst activity of DA neurons (Goertz et al., 2015). NE regulation also includes presynaptic activation of α1 adrenoceptors at NAc shell DA terminals; local infusion of α1 antagonist, terazosin, blocked cocaine-induced increase in DA extracellular levels (Mitrano et al., 2012).
There are aspects of the regulation of DA release by NE that require further research. Most intra-VTA reverse dialysis experiments were performed using a single dose of the drugs. Future experiments using several doses are needed. This is of special interest as NE actions often follow an inverted-U shape that reflect different states of activity of NE neurons (Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003).
DA uptake and release by NE terminals
Another mechanism regulating DA levels is reuptake and release of DA by NE terminals. Taking advantage of the differential distribution of DA and NE terminals, and DAT and NET expression in the cortex of rats, a series of studies have provided evidence of NE terminals as a source of DA. In rats, DA innervation is preferential to PFC while there is very small innervation at other cortices such as the occipital cortex (OCC) (Descarries et al., 1987). In contrast to DA innervation, NE innervation is much higher and ubiquitous throughout cortical regions (Morrison et al., 1978). If the source of DA is from DA terminals, few outcomes are expected: including increases of extracellular DA by DAT blockers and D2 antagonist. However, local infusion of the selective DAT antagonist GBR12909 in either PFC or OCC did not increase extracellular DA, while only small increases were observed with the D2 antagonist haloperidol (Devoto et al., 2004). The outcome of these experiments argues against DA terminals as the source of DA.
It is possible to argue that the lack of an effect by DAT blockers and the small effect of D2 antagonist on extracellular DA can be explained by low expression of DAT and D2 DA autoreceptors or lack of a functional D2 receptor in cortical projecting neurons (Chiodo et al., 1984; Lammel et al., 2008; Sesack et al., 1998). However, noradrenergic pharmacology supports that DA reuptake and release is occurring at the NE terminals. Extracellular DA and NE are increased in PFC and OCC after local infusion of the NET blocker desipramine (Devoto et al., 2004). Conversely, inhibition of LC activity by local infusion of the α2 adrenoceptor agonist clonidine decreased extracellular DA and NE in the PFC and OCC (Devoto et al., 2003). Because of the scarce DA innervation in the OCC, these findings indicate that reuptake and release of DA occurs at NE terminals.
Another line of evidence supporting DA reuptake by NE terminals comes from genetic studies. Synaptosomes harvested from PFC of NET knock-out mice showed a 55% reduction in DA uptake. A similar reduction on DA uptake effect was also observed on synaptosomes harvested from PFC of wild-type mice treated with the selective NET inhibitor nisoxitine (Moron et al., 2002).
NE terminals are a source of neurotransmitter acting on D1 and D4 receptors
Support for NE terminals as the source of neurotransmitter for both, the activation of D1 receptor in the hippocampus and D4 receptors in the lateral habenula, has been recently demonstrated (Root et al., 2015; Smith and Greene, 2012). In the hippocampus, D1-dependent amphetamine enhancement of glutamate signaling is blocked by blockade NET or NE transmission from LC terminals, but not by blockade DAT or DA transmission from VTA terminals. Similarly, D4-mediated current in lateral habenula are susceptible to lesion of NE terminals, but not DA terminals.
These two studies highlight NE terminals as the source of neurotransmitter. In the experiment performed in the habenula, both DA and NE evoked the D4-mediated current (Root et al., 2015); thus indicating that NE itself could be the agonist. However, in the hippocampus, NE did not evoke the D1-mediated enhancement of glutamate signaling (Smith and Greene, 2012). The authors proposed that amphetamine increases intracellular DA at NE terminals by inhibiting the vesicular monoamine transporter-2 (VMAT2) and monoamine oxidase (MAO). The inhibition of VMAT2 prevents the transport of DA into presynaptic vesicles where DBH convert DA to NE, whereas inhibition of MAO prevents DA conversion to DOPAC. These two effects lead to the increases in intracellular DA that is reversely transported by NET.
Adrenergic and dopaminergic receptor cross-activation by catecholamines
NE inhibits DA neurons via D2 DA receptor activation(Aghajanian and Bunney, 1977). Both NE and E are agonists at] of D2 DA receptors (Lanau et al., 1997; Onali and Olianas, 1987). It worth noticing a gradient in the expression of the D2 DA receptors, higher in the SN and lateral parts of VTA and lower or absent in DA neurons in the medial parts of the VTA, RLi, CLi and IF (Li et al., 2013); thus, regulation of NE and E of DA neuronal activity through the D2 DA receptor is more likely to affect a subgroup of neurons. Moreover, given the fact that NE and E affinity to the D2 receptor is 20–30 fold lower than DA (Werle et al., 1988), activation of D2 receptor will depend on circumstances were NE levels are high such as in the presence of psychostimulants.
Another possible cross-activation of DA receptors by NE and E is at the D4 DA receptors (Lanau et al., 1997). NE and E have affinities in the nanomolar range similar to that of DA for the D4 receptor. In functional assays, based on GTPγS stimulation and changes cAMP levels, NE and E were 2–5 fold less potent than DA (Czermak et al., 2006; Lanau et al., 1997). Recently, NE was shown to be the neurotransmitter that activates D4 receptors in the lateral habenula (Root et al., 2015), supporting NE cross-talk through D4 receptors. Another brain region in which NE D4 receptor activation may be of importance is the PFC, where D4 DA receptors are highly expressed (de Almeida and Mengod, 2010).
There is biochemical evidence of α2 adrenoceptors present in axon terminals of DA neurons (Lahdesmaki et al., 2003); although anatomical confirmation is still lacking. Given that DA binds to α2 adrenoceptors (Cornil and Ball, 2008), the contribution of DA versus NE or E will be dependent on the presence of NE or E axons terminals in areas innervated by DA neurons. For example, dorsal striatum has scarce adrenergic input and the agonist to the α2 adrenoceptors is most likely to be DA.
Adrenergic adrenoceptors α1B and β1 form heteromers with D4 DA receptors. Functional activation of these heteromers has been characterized in the pineal gland, where D4 DA receptors follow a circadian expression (Gonzalez et al., 2012). At hours of dark, when D4 DA receptor expression is high, synthesis of melatonin was blocked by activation of D4 agonist site on heteromers preventing the adrenergic agonist-mediated effects. Conversely, at hours of light, in the absence D4 expression and formation of hetoromers, synthesis of melatonin was increased by adrenergic agonists. By changing the adrenoceptor signaling, hetoromers provide a functional switch in the pineal gland physiology. It will be important to find what other brain regions express heteromers, as they may provide novel targets for targeted receptor-mediated signaling.
Associations of adrenergic modulation of the midbrain DA system and neurological and psychiatry disorders
The direct association of the adrenergic modulation of the midbrain DA system and diseases is scarce. As reviewed in previous sections, there is ample evidence of adrenergic modulation of midbrain DA systems; however, a causal link between disease-related changes in DA as a consequence of changes in NE function is an ongoing area of research. Nevertheless, several animal models suggest that this association is of importance.
Parkinson’s disease (PD) is mostly associated with degeneration of DA neurons of the SN. However, the disease also affects NE neurons (German et al., 1992). A protective role of NE has been observed in animals showing that lesion of the LC exacerbate PD pathology and symptomology (Rommelfanger and Weinshenker, 2007). Moreover, in rodents, motor deficits associated with PD require NE depletion and can be ameliorated with DA agonist; thus indicating a dysregulation of DA by depleting NE (Rommelfanger et al., 2007).
Another interaction between NE and DA that is beneficial for PD patients is blockade of α2 adrenoceptors. L-DOPA, a precursor of DA, is one effective treatment for delaying PD. However, L-DOPA also produces dyskinesia after long-term use. Notably, L-DOPA-induced dyskinesia can be treated with α2 adrenoceptors antagonists (Rascol et al., 2001), which have been linked to a decrease in extracellular DA (Buck et al., 2010), supporting the notion that NE regulation of DA relates to the improvement of motor dyskinesia.
Similar to Parkinson’s disease, neurodegeneration of DA innervation is observed in animals after prolonged exposure or high doses of methamphetamine (Ferrucci et al., 2013). In amphetamine users, low level of DA, TH and DAT have been observed in postmortem tissue (Wilson et al., 1996). Midbrain D2/D3 DA receptors binding positively correlates with grey-matter striatal volume suggesting a compromised DA function relates to a smaller striatum (Morales et al., 2015). Moreover, methamphetamine use is correlated with an enhanced risk to develop PD (Callaghan et al., 2012). The methamphetamine-induced damage of the DA system is exacerbated in animals with lesions of LC-NE axons. Moreover, DA damage is exacerbated in NE deficient mice and in mice where NE synthesis is pharmacologically blocked (reviewed by (Ferrucci et al., 2013; Weinshenker et al., 2008).
It is unclear how NE exerts a neuroprotective role against methamphetamine toxicity. One possibility is anti-inflammatory actions of NE by suppressing tumor necrosis factor, interleukin-1b, and the inducible nitric oxide synthase (Feinstein et al., 2002). Conversely, a possible link is the activation of the α1B adrenoceptors. In both, α1B knock-out and prazosin pretreated wild-type mice, methamphetamine damage of DA axons is reduced (Battaglia et al., 2003).
The interaction of DA and NE has also been described in the development of antidepressants that selectively target NET and DAT. One of the most studied of these antidepressants is bupropion. Given systemically, bupropion increased NE and DA at terminals in the NAcc and PFC (Cooper et al., 1994; Li et al., 2002). The antidepressant effect of bupropion is associated with NE. In mice lacking DBH, the enzyme that converts DA into norepinephrine in adrenergic neurons, bupropion fails to exhibit any effects in the tail suspension test (Cryan et al., 2004). Conversely, increased motivation is associated with bupropion-induced increase in DA (Randall et al., 2014). Interestingly, a study has linked the activity of DA neurons to depressive phenotypes in rodents (Chaudhury et al., 2013). Phasic activation of DA neurons projecting NAcc leads to a susceptible phenotype whereas inhibition of this projection promotes a resilience phenotype. Conversely, inhibition of DA neurons that projects to the PFC leads to susceptible phenotype. This research highlights a complex role of DA action in different circuits.
Attention deficit and hyperactivity disorder (ADHD), a neurodevelopmental disorder associated with inattention, hyperactivity, and impulsivity symptoms, is also associated with changes in the DA and NE systems. Among the neuronal circuits affected in patients with ADHD are prefrontal cortices and basal ganglia (Nakao et al., 2011; Valera et al., 2007). The treatment of choice for ADHD is psychostimulants, methylphenidate and amphetamine (Elia et al., 1999). By acting at DAT and NET, methylphenidate and amphetamine increase DA and NE at terminal sites.
Doses of methylphenidate that are clinically effective produce blood plasma levels between 8–40 ng/ml (Swanson and Volkow, 2002). Equivalent doses in animal studies produced improvements on working memory and sustain attention, without enhanced locomotion as would be produced by higher doses of psychostimulants (Berridge et al., 2006; Kuczenski and Segal, 2002). Behavioral improvements correlate with preferential changes in NE and DA and increase in neuronal responsiveness in the PFC (Berridge et al., 2006; Devilbiss and Berridge, 2008). Working memory improvement can also be obtained with local dorsal medial PFC injection of methylphenidate (Spencer et al., 2015), indicating that methylphenidate actions at terminal sites is sufficient to improve working memory. Methylphenidate effects on working memory are mediated through activation of PFC α2 adrenoceptors and D1 DA receptors; these are blocked by intra-PFC co-infusion of α2 and D1 receptor antagonists (Spencer et al., 2015).
Association between DA and NE is also shown in the substance abuse literature. Although a DA centered view of addiction is predominant, regulation of NE has been highlighted for certain aspects of addition (Weinshenker and Schroeder, 2007). The reinforcing properties of psychostimulants are greatly attenuated or abolished by interventions of the midbrain DA system, but not the adrenergic system (Roberts et al., 1977; Yokel and Wise, 1975; Yokel and Wise, 1976). Early literature focuses on the maintenance phase of self-administration, but latter studies show a contribution of NE adrenoceptors in extinction as well as in reinstatement of drug taking behavior evoked by drug priming, cues associated with the drug environment, and stress (review by (Schmidt and Weinshenker, 2014; Weinshenker and Schroeder, 2007).
Chronic inhibition of NE synthesis with inhibitors of DBH attenuates cocaine seeking as measured by a reduced break point responding for cocaine in a progressive ratio schedule, and attenuated lever press responding to a cocaine-primed injection, cues associated with cocaine self-administration, and stress (Schroeder et al., 2010; Schroeder et al., 2013). The underlying mechanism for drug-primed reinstatement relates to activation of α1 adrenoceptors, as it is attenuated by prazosin, an α1 adrenoceptor antagonist (Zhang and Kosten, 2005). Similarly, cue-induced relapse is attenuated by a cocktail of α1 and β adrenoceptor antagonists (Smith and Aston-Jones, 2011). Cocaine history relates a differential activation of NE areas. NE A2 and C2 neurons are activated after acute cocaine. These neurons also exhibited an enhanced activation after extinction training, but not during cocaine self-administration. However, LC was not activated in any of these conditions (Buffalari and Rinaman, 2014). These studies highlight adrenergic regulation of cocaine seeking; however, whether the mechanism of action is through regulation of DA neurotransmission has not been directly established.
Recently, a link between NE terminals and DA release in PFC as a mechanism involved in the regulation of drug-primed reinstatement was established. As with chronic inhibition of DBH, acute inhibition of DBH one hour before a primed injection of cocaine blocked reinstatement (Devoto et al., 2014a). The mechanism underlying the effect of the DBH treatment relates with an increase of DA release in the PFC that leads to activation of D1 receptors; PFC blockade of D1 receptor reverted the effect of the DBH inhibitor (Devoto et al., 2014b). The source of the heightened PFC DA comes from NE terminals. It was sensitive to α2 adrenoceptor presynaptic modulation and it was also observed in the OCC that it almost devoid of DA terminals in rodents (Devoto et al., 2012; Devoto et al., 2014b).
A strong relationship between NE and modulation of DA release has been established for the cocaine’ seeking and its psychomotor effects measured using conditioned place preference and locomotor behavior. Local depletion of NE in the PFC prevented the development of place preference and increased DA release in the NAcc, indicating that noradrenergic transmission in the PFC is a necessary condition for accumbal DA release (Ventura et al., 2007). Similarly, enhanced locomotion and increased accumbal DA release was attenuated by blockade of α1 adrenoceptors in the NAcc (Mitrano et al., 2012). Blockade of α1 adrenoceptors in the VTA blunted cocaine-evoked increase in DA and the elevation in locomotor activity. The elevation of DA relates to an increase in burst firing through modulation of both calcium-activated potassium channel current and the hyperpolarization-activated cation currents (Goertz et al., 2015).
Summary and future directions
There is clear evidence of adrenergic regulation of midbrain DA neural activity either directly though the activation of postsynaptic adrenoceptors or regulation of inputs at presynaptic glutamaterigic or GABAergic terminals. Adrenergic modulation of DA also occurs at terminal sites, where DA release and uptake can be mediated through adrenergic terminals. This latter NE regulation might be a major player in systems where DA innervation is less dense than NE innervation such as in the cortex or hippocampus (Devoto and Flore, 2006; Smith and Greene, 2012), or in systems where DA neurons lack the machinery to effectively regulate its release, as in the case in the lateral habenula (Root et al., 2015).
Although adrenergic modulation of the midbrain DA system has been a subject of research for four decades, there are many unanswered questions. In terms of basic neurobiology, a better characterization of the receptors expressed postsynaptically, presynaptically and glia within midbrain DA regions is greatly needed. There is also a need to understand how tonic and phasic release NE and E change the activity of midbrain DA regions. This can be achieved utilizing transgenic animals to selectively express opsins and receptors activated by designer drugs in combination with electrophysiology and neurochemical measurements. Similarly with respect to translational science, it is mostly unknown when NE and E levels are altered within midbrain DA regions in different animal models or under different behavioral paradigms.
Most of the interest in adrenergic regulation of midbrain DA regions is focused on the VTA, and to a lesser extent the SN. However, the strongest adrenergic innervation is to the RRF(Mejias-Aponte et al., 2009). DA neurons of the RRF are less studied than those of VTA and SN, in part because there are fewer DA neurons and more non-DA neurons in this region (Yamaguchi et al., 2013). Nevertheless, RRF might be a better research target for understanding the fundamentals of adrenergic modulation of DA neuron activity and output.
It is important to emphasize that the E system also targets the midbrain DA regions. Of the three E groups, C1 is the most prominent (Mejias-Aponte et al., 2009). These neurons are activated by physical stressors including hypoglycemia, infection or inflammation, hypoxia, nociception and hypotension (review by (Guyenet et al., 2013). They are also activated by an acute injection of cocaine (Buffalari and Rinaman, 2014), and psychological stressors including restrain stress, noise and forced swim (Dayas et al., 2001). How activation of C1 affects the activity of DA neurons is at present an unanswered question.
Highlights.
LC and medullary NE and E neurons sends afferents to midbrain DA neurons.
Adrenergic axons mainly form synaptic appositions indicative of a parachrine release of neurotransmitter.
Both, α1 and α2 adrenoceptor, modulates DA neurons firing.
NE axons are one of the sources of DA in the cortex, hippocampus and lateral habenula.
In the absence of NE, DA neurons are more susceptible to toxicity.
Acknowledgments
Research is supported by the National Institute on Drug Abuse Intramural Research Program. My gratitude goes to Vadim Kashtelyan, Gustavo Mejias-Torres and David Barker for proofreading the manuscript and providing edits to improve its readability.
Abbreviations
- CLi
caudal linear nucleus of the raphe
- DA
dopamine
- DBH
dopamine β-hydroxylase
- E
epinephrine
- IF
interfascicular nucleus
- LC
locus coeruleus
- MAO
monoamine oxidase
- NAcc
nucleus accumbens
- NE
norepinephrine
- Occ
occipital cortex
- PFC
prefrontal cortex
- RLi
rostral linear nucleus of the raphe
- RRF
retrorubral field
- SN
substantia nigra
- VTA
ventral tegmental area
- VMAT2
vesicular monoamine transporter-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Almodovar-Fabregas LJ, Segarra O, Colon N, Dones JG, Mercado M, Mejias-Aponte CA, Vazquez R, Abreu R, Vazquez E, Williams JT, Jimenez-Rivera CA. Effects of cocaine administration on VTA cell activity in response to prefrontal cortex stimulation. Ann N Y Acad Sci. 2002;965:157–71. doi: 10.1111/j.1749-6632.2002.tb04158.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Lembo G, Nicoletti F, De Blasi A. Alpha-1B adrenergic receptor knockout mice are protected against methamphetamine toxicity. Journal of neurochemistry. 2003;86:413–21. doi: 10.1046/j.1471-4159.2003.01867.x. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends in cognitive sciences. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. Brain research reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Boyajian CL, Loughlin SE, Leslie FM. Anatomical evidence for alpha-2 adrenoceptor heterogeneity: differential autoradiographic distributions of [3H]rauwolscine and [3H]idazoxan in rat brain. The Journal of pharmacology and experimental therapeutics. 1987;241:1079–91. [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. Cocaine effects in the ventral tegmental area: evidence for an indirect dopaminergic mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:660–5. doi: 10.1007/BF00175709. [DOI] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–6. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Buck K, Voehringer P, Ferger B. The alpha(2) adrenoceptor antagonist idazoxan alleviates L-DOPA-induced dyskinesia by reduction of striatal dopamine levels: an in vivo microdialysis study in 6-hydroxydopamine-lesioned rats. Journal of neurochemistry. 2010;112:444–52. doi: 10.1111/j.1471-4159.2009.06482.x. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Rinaman L. Cocaine self-administration and extinction alter medullary noradrenergic and limbic forebrain cFos responses to acute, noncontingent cocaine injections in adult rats. Neuroscience. 2014;281C:241–250. doi: 10.1016/j.neuroscience.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug and alcohol dependence. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. Journal of neurochemistry. 1990;55:1067–70. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–59. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–23. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cathala L, Guyon A, Eugene D, Paupardin-Tritsch D. Alpha2-adrenoceptor activation increases a cationic conductance and spontaneous GABAergic synaptic activity in dopaminergic neurones of the rat substantia nigra. Neuroscience. 2002;115:1059–65. doi: 10.1016/s0306-4522(02)00542-0. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NH, Reith ME. Effects of locally applied cocaine, lidocaine, and various uptake blockers on monoamine transmission in the ventral tegmental area of freely moving rats: a microdialysis study on monoamine interrelationships. Journal of neurochemistry. 1994a;63:1701–13. doi: 10.1046/j.1471-4159.1994.63051701.x. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME. Autoregulation and monoamine interactions in the ventral tegmental area in the absence and presence of cocaine: a microdialysis study in freely moving rats. The Journal of pharmacology and experimental therapeutics. 1994b;271:1597–610. [PubMed] [Google Scholar]
- Chen SY, Burger RI, Reith ME. Extracellular dopamine in the rat ventral tegmental area and nucleus accumbens following ventral tegmental infusion of cocaine. Brain research. 1996;729:294–6. [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1994;11:133–41. doi: 10.1038/npp.1994.43. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to alpha2-adrenergic receptors in birds and mammals. The Journal of comparative neurology. 2008;511:610–27. doi: 10.1002/cne.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8186–91. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak C, Lehofer M, Liebmann PM, Traynor J. [35S]GTPgammaS binding at the human dopamine D4 receptor variants hD4.2, hD4.4 and hD4.7 following stimulation by dopamine, epinephrine and norepinephrine. European journal of pharmacology. 2006;531:20–4. doi: 10.1016/j.ejphar.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. Evidence For The Existence Of Monoamine-Containing Neurons In The Central Nervous System. I. Demonstration Of Monoamines. The Cell Bodies Of Brain Stem Neurons Acta Physiol Scand Suppl SUPPL. 1964;232:1–55. [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–39. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European journal of neuroscience. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Mengod G. D2 and D4 dopamine receptor mRNA distribution in pyramidal neurons and GABAergic subpopulations in monkey prefrontal cortex: implications for schizophrenia treatment. Neuroscience. 2010;170:1133–9. doi: 10.1016/j.neuroscience.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–24. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biological psychiatry. 2008;64:626–35. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Longu G, Pira L, Gessa GL. Origin of extracellular dopamine from dopamine and noradrenaline neurons in the medial prefrontal and occipital cortex. Synapse. 2003;50:200–5. doi: 10.1002/syn.10264. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pira L, Longu G, Gessa GL. Alpha2-adrenoceptor mediated co-release of dopamine and noradrenaline from noradrenergic neurons in the cerebral cortex. Journal of neurochemistry. 2004;88:1003–9. doi: 10.1046/j.1471-4159.2003.02239.x. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G. On the origin of cortical dopamine: is it a co-transmitter in noradrenergic neurons? Current neuropharmacology. 2006;4:115–25. doi: 10.2174/157015906776359559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Cadeddu R, Gessa GL. Disulfiram stimulates dopamine release from noradrenergic terminals and potentiates cocaine-induced dopamine release in the prefrontal cortex. Psychopharmacology. 2012;219:1153–64. doi: 10.1007/s00213-011-2447-5. [DOI] [PubMed] [Google Scholar]
- Devoto P, Fattore L, Antinori S, Saba P, Frau R, Fratta W, Gessa GL. Elevated dopamine in the medial prefrontal cortex suppresses cocaine seeking via D1 receptor overstimulation. Addiction biology. 2014a doi: 10.1111/adb.12178. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Bini V, Gessa GL. The dopamine beta-hydroxylase inhibitor nepicastat increases dopamine release and potentiates psychostimulant-induced dopamine release in the prefrontal cortex. Addiction biology. 2014b;19:612–22. doi: 10.1111/adb.12026. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–29. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. The New England journal of medicine. 1999;340:780–8. doi: 10.1056/NEJM199903113401007. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1981;1:1361–8. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. Monoamine innervation of the forebrain: collateralization. Brain Res Bull. 1982;9:295–307. doi: 10.1016/0361-9230(82)90143-5. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Heneka MT, Gavrilyuk V, Dello Russo C, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochemistry international. 2002;41:357–65. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Ferrucci M, Giorgi FS, Bartalucci A, Busceti CL, Fornai F. The effects of locus coeruleus and norepinephrine in methamphetamine toxicity. Current neuropharmacology. 2013;11:80–94. doi: 10.2174/157015913804999522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature neuroscience. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Progress in neurobiology. 2010;90:82–100. doi: 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Marcoli M, Borroto-Escuela DO. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochemical research. 2015 doi: 10.1007/s11064-015-1574-5. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Annals of neurology. 1992;32:667–76. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. The Journal of comparative neurology. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Goertz RB, Wanat MJ, Gomez JA, Brown ZJ, Phillips PE, Paladini CA. Cocaine increases dopaminergic neuron and motor activity via midbrain alpha1 adrenergic signaling. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1151–62. doi: 10.1038/npp.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Moreno-Delgado D, Moreno E, Perez-Capote K, Franco R, Mallol J, Cortes A, Casado V, Lluis C, Ortiz J, Ferre S, Canela E, McCormick PJ. Circadian-related heteromerization of adrenergic and dopamine D(4) receptors modulates melatonin synthesis and release in the pineal gland. PLoS biology. 2012;10:e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiology of disease. 2005;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Clonidine regularizes substantia nigra dopamine cell firing. Life Sci. 1988;42:2003–9. doi: 10.1016/0024-3205(88)90500-0. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Clonidine modulates dopamine cell firing in rat ventral tegmental area. Eur J Pharmacol. 1989;165:11–8. doi: 10.1016/0014-2999(89)90765-6. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, North RA, Johnson SW. Alpha 1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midbrain slice. Eur J Neurosci. 1995;7:1707–13. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Crosstalk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus coeruleus, and dorsal hippocampus. Mol Pharmacol. 2008 doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body’s EMTs. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R187–204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidkind R, Kivastik T, Eller M, Kolts I, Oreland L, Harro J. Denervation of the locus coeruleus projections by treatment with the selective neurotoxin DSP-4 [N (2-chloroethyl)-N-ethyl-2-bromobenzylamine] reduces dopamine release potential in the nucleus accumbens shell in conscious rats. Neuroscience letters. 2002;332:79–82. doi: 10.1016/s0304-3940(02)00817-0. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Fuxe K, Goldstein M, Johansson O. Immunohistochemical evidence for the existence of adrenaline neurons in the rat brain. Brain Res. 1974;66:235–251. [Google Scholar]
- Inyushin MU, Arencibia-Albite F, Vazquez-Torres R, Velez-Hernandez ME, Jimenez-Rivera CA. Alpha-2 noradrenergic receptor activation inhibits the hyperpolarization-activated cation current (Ih) in neurons of the ventral tegmental area. Neuroscience. 2010;167:287–97. doi: 10.1016/j.neuroscience.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Rivera CA, Figueroa J, Vazquez-Torres R, Velez-Hernandez ME, Schwarz D, Velasquez-Martinez MC, Arencibia-Albite F. Presynaptic inhibition of glutamate transmission by alpha2 receptors in the VTA. The European journal of neuroscience. 2012;35:1406–15. doi: 10.1111/j.1460-9568.2012.08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain research. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ciriello J. Cardiovascular afferent inputs to ventral tegmental area. Am J Physiol. 1997;272:R1998–2003. doi: 10.1152/ajpregu.1997.272.6.R1998. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7264–71. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Sirvio J, Scheinin M. Alpha2-adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the alpha2A-adrenoceptor subtype. Neuropharmacology. 2003;44:882–92. doi: 10.1016/s0028-3908(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–62. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanau F, Zenner MT, Civelli O, Hartman DS. Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. Journal of neurochemistry. 1997;68:804–12. doi: 10.1046/j.1471-4159.1997.68020804.x. [DOI] [PubMed] [Google Scholar]
- Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebral microdialysis. Brain research. 1990;523:134–8. doi: 10.1016/0006-8993(90)91646-x. [DOI] [PubMed] [Google Scholar]
- Lategan AJ, Marien MR, Colpaert FC. Suppression of nigrostriatal and mesolimbic dopamine release in vivo following noradrenaline depletion by DSP-4: a microdialysis study. Life sciences. 1992;50:995–9. doi: 10.1016/0024-3205(92)90093-5. [DOI] [PubMed] [Google Scholar]
- Lee A, Wissekerke AE, Rosin DL, Lynch KR. Localization of alpha2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience. 1998;84:1085–96. doi: 10.1016/s0306-4522(97)00578-2. [DOI] [PubMed] [Google Scholar]
- Li SX, Perry KW, Wong DT. Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology. 2002;42:181–90. doi: 10.1016/s0028-3908(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain structure & function. 2013;218:1159–76. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–44. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–13. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masana M, Bortolozzi A, Artigas F. Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: therapeutic opportunities in schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011;14:53–68. doi: 10.1017/S1461145710000908. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Aston-Jones G. Afferents from the A2 area of the nucleus of the solitary tract to A8 dopaminergic neurons of the retrorubral area. Society for Neuroscience Abstract; 35th Annual Meeting; 2005. 114.4. [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–26. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Ye C, Bonci A, Kiyatkin EA, Morales M. A subpopulation of neurochemically-identified ventral tegmental area dopamine neurons is excited by intravenous cocaine. J Neurosci. 2015;35:1965–78. doi: 10.1523/JNEUROSCI.3422-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, Weinshenker D. alpha-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2161–72. doi: 10.1038/npp.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED. Gray-matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Molecular psychiatry. 2015;20:764–71. doi: 10.1038/mp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:389–95. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Grzanna R, Molliver ME, Coyle JT. The distribution and orientation of noradrenergic fibers in neocortex of the rat: an immunofluorescence study. J Comp Neurol. 1978;181:17–39. doi: 10.1002/cne.901810103. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. The American journal of psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993a;328:575–94. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hokfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience. 1993b;56:1023–39. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Onali P, Olianas MC. Pharmacological and biochemical characterization of dopamine receptors mediating stimulation of a high affinity GTPase in rat striatum. Biochemical pharmacology. 1987;36:2839–45. doi: 10.1016/0006-2952(87)90274-7. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Kuhar MJ. Beta-adrenergic-receptor localization by light microscopic autoradiography. Science. 1980;208:1378–80. doi: 10.1126/science.6246585. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–81. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–75. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WH, Chen NH, Tsai FY, Liao HY. Intrategmental infusion of cocaine decreases dopamine release and enhances norepinephrine release in the medial prefrontal cortex. European journal of pharmacology. 1996;317:205–13. doi: 10.1016/s0014-2999(96)00724-8. [DOI] [PubMed] [Google Scholar]
- Pan WH, Hsieh MC, Wu HH, Lin SK. Difference in magnitude of psychostimulant-induced extracellular norepinephrine in the ventral tegmental area contributes to discrepant prefrontal dopamine outflow. Addiction biology. 2007;12:51–8. doi: 10.1111/j.1369-1600.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. The cytoarchitecture of the interfascicular nucleus and ventral tegmental area of Tsai in the rat. The Journal of comparative neurology. 1979;187:85–98. doi: 10.1002/cne.901870106. [DOI] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M, Rowland M, Lopez-Cruz L, Correa M, Salamone JD. Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;18 doi: 10.1093/ijnp/pyu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Arnulf I, Peyro-Saint Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C, Bonnet AM, Descombes S, Bejjani B, Fabre N, Montastruc JL, Agid Y. Idazoxan, an alpha-2 antagonist, and L-DOPA-induced dyskinesias in patients with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2001;16:708–13. doi: 10.1002/mds.1143. [DOI] [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–17. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–20. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nature neuroscience. 2013;16:1016–23. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13804–9. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochemical pharmacology. 2007;74:177–90. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Mitrano DA, Smith Y, Weinshenker D. Light and electron microscopic localization of alpha-1 adrenergic receptor immunoreactivity in the rat striatum and ventral midbrain. Neuroscience. 2009;158:1530–40. doi: 10.1016/j.neuroscience.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, Lupica CR, Morales M. Norepinephrine activates dopamine D4 receptors in the rat lateral habenula. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:3460–9. doi: 10.1523/JNEUROSCI.4525-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Zeng D, Stornetta RL, Norton FR, Riley T, Okusa MD, Guyenet PG, Lynch KR. Immunohistochemical localization of alpha 2A-adrenergic receptors in catecholaminergic and other brainstem neurons in the rat. Neuroscience. 1993;56:139–55. doi: 10.1016/0306-4522(93)90569-2. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2221–30. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- Schmidt KT, Weinshenker D. Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Molecular pharmacology. 2014;85:640–50. doi: 10.1124/mol.113.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2440–9. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1032–8. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–11. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:2160–7. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6072–80. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biological psychiatry. 2011;70:712–9. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–42. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RC, Devilbiss DM, Berridge CW. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biological psychiatry. 2015;77:940–50. doi: 10.1016/j.biopsych.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–15. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8229–33. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behavioural brain research. 2002;130:73–8. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain research bulletin. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. The European journal of neuroscience. 1997;9:2077–85. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. The Journal of comparative neurology. 2014;522:3308–34. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7105–10. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu C, Sabatini BL. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. eLife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annual review of pharmacology and toxicology. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European journal of pharmacology. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological psychiatry. 2007;61:1361–9. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–94. doi: 10.1016/j.neuron.2012.02.016. Epub 2012 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez-Martinez MC, Vazquez-Torres R, Jimenez-Rivera CA. Activation of alpha1-adrenoceptors enhances glutamate release onto ventral tegmental area dopamine cells. Neuroscience. 2012;216:18–30. doi: 10.1016/j.neuroscience.2012.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez-Martinez MC, Vazquez-Torres R, Rojas LV, Sanabria P, Jimenez-Rivera CA. Alpha-1 adrenoreceptors modulate GABA release onto ventral tegmental area dopamine neurons. Neuropharmacology. 2015;88:110–21. doi: 10.1016/j.neuropharm.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5181–6. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1433–51. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Ferrucci M, Busceti CL, Biagioni F, Lazzeri G, Liles LC, Lenzi P, Pasquali L, Murri L, Paparelli A, Fornai F. Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. Journal of neurochemistry. 2008;105:471–83. doi: 10.1111/j.1471-4159.2007.05145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle E, Lenz T, Strobel G, Weicker H. 3- and 4-O-sulfoconjugated and methylated dopamine: highly reduced binding affinity to dopamine D2 receptors in rat striatal membranes. Naunyn-Schmiedeberg’s archives of pharmacology. 1988;338:28–34. doi: 10.1007/BF00168808. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984;231:275–80. [PubMed] [Google Scholar]
- Williams MA, Li C, Kash TL, Matthews RT, Winder DG. Excitatory drive onto dopaminergic neurons in the rostral linear nucleus is enhanced by norepinephrine in an alpha1 adrenergic receptor-dependent manner. Neuropharmacology. 2014;86:116–24. doi: 10.1016/j.neuropharm.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–6. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–18. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–90. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Morales M. Glutamate neurons in the substantia nigra compacta and retrorubral field. The European journal of neuroscience. 2013;38:3602–10. doi: 10.1111/ejn.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–9. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology. 1976;48:311–8. doi: 10.1007/BF00496868. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nature neuroscience. 2015;18:386–92. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–4. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]