Abstract
Background
Analysis of the functional consequences and treatment response of rare CFTR variants is challenging due to the limited availability of primary airways cells.
Methods
A Flp recombination target (FRT) site for stable expression of CFTR was incorporated into an immortalized CF bronchial epithelial cell line (CFBE41o−). CFTR cDNA was integrated into the FRT site. Expression was evaluated by western blotting and confocal microscopy and function measured by short circuit current. RNA sequencing was used to compare the transcriptional profile of the resulting CF8Flp cell line to primary cells and tissues.
Results
Functional CFTR was expressed from integrated cDNA at the FRT site of the CF8Flp cell line at levels comparable to that seen in native airway cells. CF8Flp cells expressing WT-CFTR have a stable transcriptome comparable to that of primary cultured airway epithelial cells, including genes that play key roles in CFTR pathways.
Conclusion
CF8Flp cells provide a viable substitute for primary CF airway cells for the analysis of CFTR variants in a native context.
Keywords: CFBE, CFTR, Cystic fibrosis model, Ivacaftor, RNA sequencing
1. Introduction
Over 2000 different variants have been identified in CFTR [1]. A large majority of these variants are considered rare (~1850) and have yet to be evaluated for their effect on CFTR. An appropriate in vitro model is needed to study these rare variants. Primary cells and tissues provide the most relevant context to determine the consequences of disease-associated variants upon epithelial ion transport since mutant CFTR is expressed at endogenous levels in a native context [2]. Both primary airway epithelium and intestinal epithelium [3] have been used for functional studies of mutant CFTR. However, for most CFTR variants, primary tissues are not available due to limited access to the small number of patients carrying these variants.
In lieu of primary tissues, cell culture based systems can serve as reasonable proxies for primary cells. Fischer rat thyroid cells have been used extensively to evaluate mutant CFTR function and response to small molecule therapy [4–7]. However, the rat thyroid cells are not of human origin, so interactions with orthologous proteins such as chaperones, kinases, and ion channels may differ from what occurs in human airway epithelial cells. In addition, it has been shown that folding of CFTR is dependent on the cell type in which it is expressed [8]. Therefore, an epithelial cell line of human origin should more closely model the processing and function of CFTR in vivo.
CFBE41o− (CFBE) is an immortalized cell line created from the bronchial epithelium of a CF patient homozygous for F508del [9]. CFBE cells have been used to study CFTR function and response to small molecules due to their clinical relevance to CF and their ability to polarize and form tight junctions [10–12]. CFBE cell lines have been transduced to stably express CFTR but this process generates lines with variable numbers of integrated sequences expressing exogenous CFTR at high levels [13,14]. We report the creation of a CF8Flp, a CFBE cell line that contains a single recombination target site for the stable integration and expression of a single cDNA, mini-gene, or complete gene. RNA sequencing was performed on the CF8Flp cells and revealed both the transcriptional background and CFTR expression level to be comparable to native bronchial epithelial cells. Thus, the introduction of a single coding sequence into the CF8Flp line allows for regulated expression of CFTR mutants in a cellular context that approximates native airway cells.1
2. Methodology
2.1. Cell culture
Cells were grown in MEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Corning, Corning, NY, USA) and 1% penicillin/streptomycin (Quality Biological, Gaithersburg, MD, USA) in a humidified incubator at 37° in the presence of 5% CO2 on fibronectin/collagen coated plastic ware. For details, see Supplemental materials and methods.
2.2. Transfection and selection of resistant clones
Parental cells were transfected with pFRT/lacZeo plasmid (Thermo Fisher) using Lipofectamine 2000 (Thermo Fisher). Two days after transfection, media was changed to contain 100 μg/mL Zeocin. Individual clones or pools of clones were isolated ~2 weeks post transfection. For details on selection of hygromycin resistant cells, see Supplemental materials and methods.
2.3. Southern blot
10 μg of genomic DNA were blotted and probed with P32 labeled PCR products of approximately 500 bp in length specific to the lacZeo region of pFRT/lacZeo plasmid. For details, see Supplemental materials and methods.
2.4. Inverse PCR
Genomic DNA was digested overnight, electrophoresed, and fragments ranging in size from ~3 kb–6 kb were gel extracted. Varying low amounts of DNA, ranging from 10 ng–200 ng, were ligated overnight at 4° using a rapid ligation kit (Roche). Ligated DNA served as a template for PCR using primers Span1R and Span4F. For details, see Supplemental materials and methods.
2.5. FISH
Probes were labeled using the Nick Translation Kit (Abbot Molecular/Vysis, Des Plaines, IL, USA). Cells were fixed, washed, and dried on slides. 10 μL of chromosome 8 painting probe (Cytocell, Oxford Gene Technology, Tarrytown, NY, USA) were mixed with 1–2 μL of labeled probe and hybridized for 2 min at 72 °C in LSI/WCP hybridization buffer (Abbott Molecular/Vysis) followed by overnight at 37 °C. The following day, slides were washed and mounted with 10 μL of DAPI with antifade (Cytocell). For details, see Supplemental materials and methods.
2.6. Western blot
Whole cell lysates were blotted using mouse monoclonal antibody “596” (UNC antibody distribution program sponsored by Cystic Fibrosis Foundation Therapeutics) to detect CFTR, anti-GFP antibody (#A11122, Thermo Fisher) to detect GFP, and anti-GAPDH antibody (#G9545, Sigma) to detect GAPDH for the loading control. Membrane was incubated with HRP-conjugated secondary antibodies for 1 h at room temp followed by washing with PBST. Membranes were developed using ECL Prime (GE Healthcare, Pittsburgh, PA, USA). For details, see Supplemental materials and methods.
2.7. Laser scanning confocal microscopy
Fluorescence was imaged using a Zeiss LSM510 confocal microscope at the Johns Hopkins Microscope Facility. For details, see Supplemental materials and methods.
2.8. Short circuit current
Short circuit current (Isc) measurements were taken in an Easy-Mount chamber system (Physiologic Instruments, San Diego, CA). Isc was measured with a VCCMC6 multichannel voltage–current clamp amplifier (Physiologic Instruments) in the voltage-clamp mode. A Cl− gradient was used to increase measured response. After stabilization, forskolin (10 μM) was added to the basolateral chamber followed by Ivacaftor (10 μM) and CFTR Inhibitor 172 (10 μM) to the apical chamber. For details, see Supplemental materials and methods.
2.9. RNA sequencing
See Fig. 3A for plating strategy. RNA-seq libraries were constructed using the TruSEQ RNA Sample Prep Kit v2 (Illumina, San Diego, CA, USA) and pair-end sequenced using Illumina HiSeq 2000/2500 (Johns Hopkins Medical Institutions Deep Sequencing and Microarray Core Facility). Raw reads were mapped to the reference genome (hg19) using the Bowtie2 algorithm [15] and Tophat2 (v2.0.13) [16] from the Tuxedo software suite. CuffQuant and Cuffdiff (Cufflinks v2.2.1) [17] were then used to determine relative abundance of gene transcripts and differential expression values among samples. For details, see Supplemental materials and methods. RNA-seq analysis example commands and arguments can be found in the Supplemental worksheet.
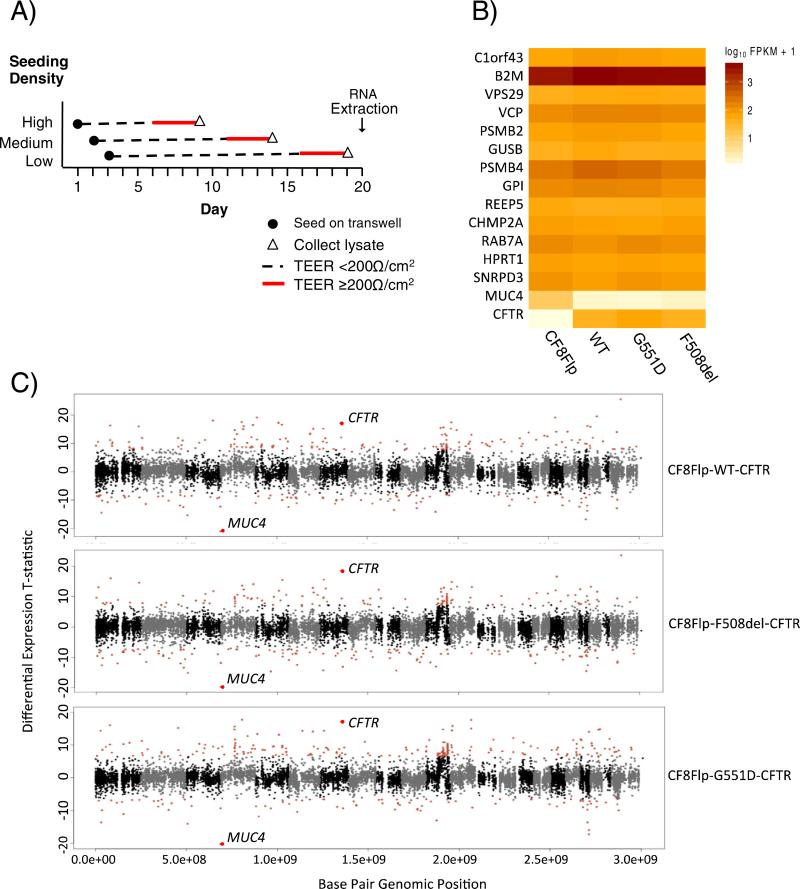
Fig. 3. The transcriptome of CF8Flp cells is stable between independently derived pools.
(A) Diagram of timing for cell plating and harvesting for RNA-seq. Transepithelial electrical resistance (TEER) to monitor tight junction formation was measured every day until a resistance of ≥200 Ω/cm2 was reached for three consecutive days, at which point cell lysate was collected and placed at −80 °C. RNA was harvested from all frozen lysates simultaneously. B) Heat map of 13 housekeeping genes based on FPKM levels. Each row represents a gene while each column represents a cellular pool. C) Manhattan plots of CF8Flp parental cells compared to CF8Flp cells expressing either WT, F508del, or G551D CFTR. Red points indicate ≥3 SD from the mean of the statistic. CFTR and MUC4 are labeled for each plot.
Generated by CuffDuff software [17].
3. Results
3.1. Integration of a single Flp-In target site into chromosome 8 of CFBE cells
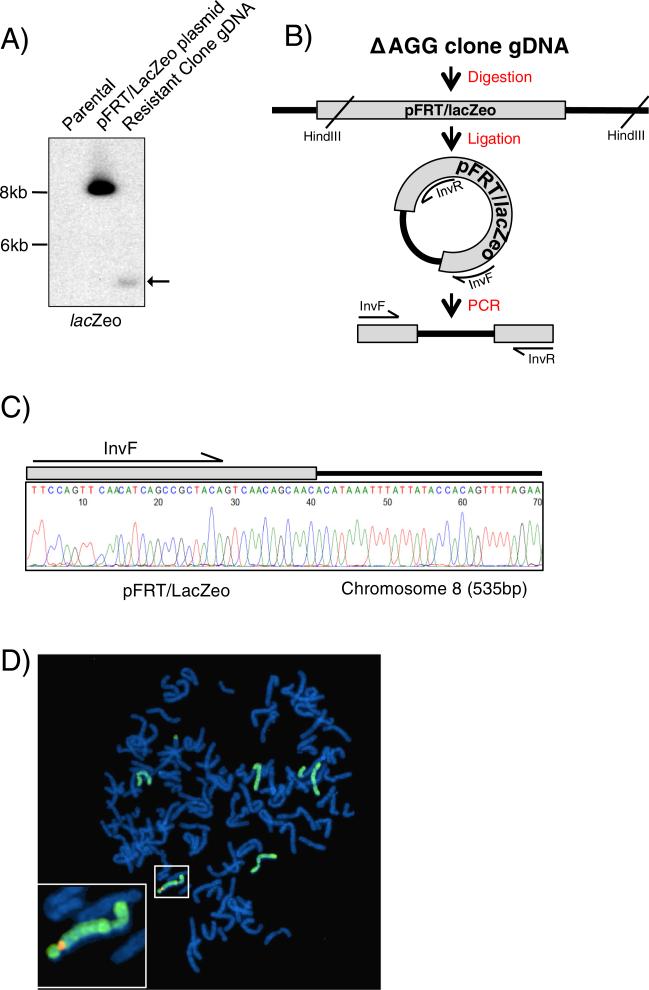
CFBE41o− (CFBE) is an immortalized cell line created from the bronchial epithelium of a CF patient homozygous for F508del that does not express CFTR (Supplemental Fig. 1) [10]. To allow for the targeted integration of heterologous sequences, we elected to incorporate the Flp recombination target (FRT) site into the genomic DNA of CFBE cells using the pFRT/lacZeo plasmid (Thermo Fisher Scientific). To disrupt episomal replication within the Adenovirus 12/SV40 immortalized CFBE cells, the SV40 promoter of the pFRT/lacZeo plasmid was mutated (Supplemental Fig. 2). Following selection for Zeocin resistant cells, Southern blot analysis of genomic DNA revealed one clone with a novel DNA fragment of approximately 4 kb hybridizing to a probe for the lacZ–Zeocin fusion gene (Fig. 1A; highlighted by the arrow). To determine the genomic location of the pFRT/lacZeo plasmid integration, an inverse PCR was performed (Fig. 1B). A DNA fragment of approximately 700 bp, consistent with the size difference between the novel DNA fragment seen on the Southern blot (estimated ~4 kb) and the distance between InvR and InvF (~3.3 kb) was amplified from circularized fragments (Supplemental Fig. 3A). Sequencing of the inverse PCR product revealed 37 bp corresponding to the pFRT/lacZeo plasmid immediately adjacent to 535 bp that mapped to a region on the long arm of chromosome 8 (Ch8:128,569,810) (Fig. 1C). The location of the integration was verified using additional primers designed to encompass the plasmid/genomic DNA junction (Supplemental Fig. 3B/C). The CFBE ‘ΔAGG clone’ with the FRT insertion on chromosome 8 was re-named CF8Flp.
Fig. 1. pFRT/lacZeo/ΔAGG is stably integrated onto chromosome 8.
(A) Southern blot of hygromycin resistant clone using lacZeo specific probe. Arrow indicates band of genomic integration for clone. (B) Diagram of inverse PCR strategy to determine the genomic location of the integrated pFRT/lacZeo. Gray rectangle represents the pFRT/lacZeo plasmid integrated into the genomic DNA depicted by black lines. (C) Chromatogram of the inverse PCR product Sanger sequenced using the InvR primer. The resulting sequence contained a portion of pFRT/lacZeo along with 535 bp of genomic DNA from chromosome 8. (D) Fluorescence in situ hybridization of CF8Flp cells in metaphase. Chromosome 8 was painted and appears green. A probe specific for pFRT/lacZeo is shown in orange. The inset shows a 3× magnification of the chromosome containing the positive signal for the pFRT/lacZeo probe.
Sequencing of the integration region revealed that 4556 bp of the original 8401 bp of pFRT/lacZeo plasmid DNA was integrated and included the FRT site, modified SV40 promoter, and a full length lacZeo gene for selection. Fourteen bp of the genomic DNA were absent due to the insertion of the plasmid between positions 128,569,810 (hg19) and 128,569,825 (data not shown). Inspection of the site occupied by the Flp-In plasmid in the UCSC genome browser revealed the nearest annotated gene, CASC8, is located 114 kb upstream. The region has features of open chromatin as it is enriched for the H3K27Ac histone mark, is sensitive to DNAse digestion, and is <500 bp from a predicted binding site for multiple transcription factors (Supplemental Fig. 3D) as reported by the ENCODE project. This region is transcriptionally active in the CF8Flp cells resulting in stable expression of the Sh ble gene from the integrated plasmid conferring Zeocin resistance to the cells.
Florescent in situ hybridization (FISH) using a probe specific for the Flp-In sequence (pFRT/lacZeo) and probes specific for chromosome 8 revealed a single signal for the pFRT/lacZeo probe on chromosome 8 in 14 out of 20 cells examined (Fig. 1D). Interestingly, the remaining six cells also had a single pFRT/lacZeo locus integrated within a fragment of chromosome 8 that was translocated to another chromosome (Supplemental Fig. 4). G-banded karyotyping revealed that the CF8Flp cells have chromosome amplification characteristic of immortalized cell lines [18]. Chromosomal amplification and structural abnormalities were also observed in parental CFBE cells, CFBE cells that have been transduced with WT-CFTR, and HBE cells (Supplemental Fig. 6). The CFBE derived lines shared specific abnormalities across all three cell lines, such as a derivative chromosomes (5;14) (p10;q10) and (9;10) (q10;q10), while also harboring changes unique to each cell line (Supplemental Table 1).
3.2. Expression of wild type and mutant forms of CFTR from integrated cDNA at Flp-In site of CF8Flp cells
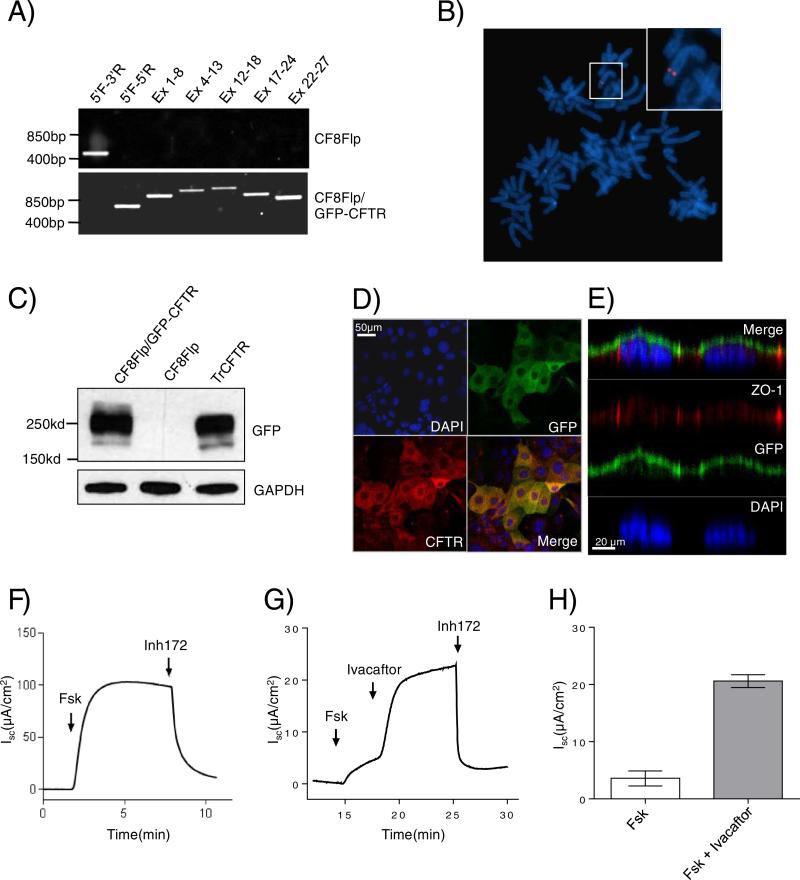
CF8Flp cells were transfected with pcDNA5/FRT plasmid (Thermo Fisher Scientific) containing the cDNA for N-terminal GFP-tagged WT-CFTR and the recombinase expressing plasmid, pOG44, to facilitate integration of the plasmid. Integration of the expression vector at the FRT site confers hygromycin resistance. Integrations at the Flp-In site for ~10 hygromycin resistant clones were confirmed using PCR primers that differentiate between the targeted and untargeted integrated Flp-In site (Fig. 2A). To detect any deletions or rearrangements in the cDNA, PCR primers were used to amplify the entire CFTR cDNA in overlapping segments. The pooled hygromycin resistant cells produced a product of the expected size for targeted Flp-In specific primers and for the five CFTR spanning primer pairs.
Fig. 2. The FRT site of CF8Flp cells is targetable and expresses functional CFTR.
(A) PCR of genomic DNA from CF8Flp cells and CF8Flp cells containing GFP-CFTR to confirm integration. Primers to confirm integration were used for lanes one and two while lanes three through seven used primers spanning the length of CFTR cDNA. (B) FISH of CF8Flp cells containing GFP-CFTR using a probe specific to pFRT/lacZeo (C) Western blot of 40 μg of whole cell lysate probed with antibody raised against GFP. CFBE cells transiently transfected with GFP-CFTR construct served as a positive control. (D) XY image of scanning disc laser confocal microscopy. CFTR is in red, GFP in green, and blue indicates DAPI stained nuclei. (E) XZ images of the cells show apical localization of the CFTR. Antibody specific to ZO-1 (red) hybridizes to the tight junctions of the polarized cells. (F) Representative tracing of electrophysiologic assessment of CF8Flp cells expressing WT-CFTR after sequential addition of 10 μM forskolin and 10 μM CFTR inhibitor 172 (G) Representative tracing from cells expressing G551D-CFTR after sequential addition of 10 μM forskolin, 10 μM Ivacaftor and 10 μM CFTR inhibitor 172 (H) Average change in current after addition of forskolin or Ivacaftor plus forskolin (Mean ± SEM, n = 6 independent tracings).
To assess whether hygromycin selection amplified the number of chromosome segments containing the FRT site, FISH was performed on the targeted cells using a pcDNA5/FRT/GFP-CFTR probe. A single signal was seen in 7 of 7 cells scored indicating that amplification did not occur (Fig. 2B). Western blotting of CF8Flp-GFP/WT-CFTR cells using a GFP specific antibody revealed fully processed GFP-tagged CFTR (Fig. 2C). Confocal laser scanning microscopy of polarized CF8Flp-GFP/WT-CFTR cells demonstrated that GFP-tagged CFTR was located apically in polarized CF8Flp cells (Fig. 2E). Images captured of the XY plane show co-localization of the GFP and CFTR antibody signals in the merged image (Fig. 2D).
The pcDNA5/FRT expression vector utilizes the cytomegalovirus promoter which was silenced over time in our cell line, similar to what has been shown previously in other cell types [19,20]. To prevent loss of expression, we elected to use the pEF5/FRT/V5-D-TOPO vector containing the elongation factor 1α promoter (Thermo Fisher Scientific). Using this vector, an untagged version of WT-CFTR was integrated into CF8Flp and expression was confirmed via western blotting (Supplemental Fig. 5A). A single clone of CF8Flp-WT-CFTR cells were grown to confluence on transwells and short circuit current was measured using an Ussing chamber system. Voltage was clamped and current was measured with the sequential addition of forskolin and CFTR inhibitor 172. Forskolin increased current to around 100 μA/cm2 with WT-CFTR. A representative tracing is shown (Fig. 2F). The level of WT-CFTR forskolin stimulated current was compared between CF8Flp cells and stably transduced CFBEs. The transduced cells showed ~3 fold higher current than the CF8Flp cells (Supplemental Fig. 5B, C). Of note, the transduced cells utilize the CMV promoter and were passaged over 30 times, therefore, a lower passage of cells have the potential for an even higher current recording.
The utility of CF8Flp cells for testing the response of CFTR mutants to therapeutic compounds was evaluated by applying Ivacaftor to cells expressing G551D-CFTR. Ivacaftor increased forskolin stimulated current by ~4 fold in the CF8Flp cells expressing G551D-CFTR (3.58 ± 0.54 to 20.58 ± 0.46 μA/cm2, n = 6) (Fig. 2H). The level of response of G551D-CFTR is comparable to that observed in Fischer rat thyroid cells stably expressing G551D-CFTR as well as primary CF bronchial epithelial cells bearing one copy of the G551D mutation [4].
3.3. Global gene expression patterns of parental CF8Flp cells and derived clones appear unaffected by the selection process
Karyotype analysis of CF8Flp cells with a stable integration of WT-CFTR revealed numerous chromosomal differences from the parental CF8Flp cells (Supplemental Fig. 5 and Supplemental Table 1). To determine if these chromosomal differences were associated with alterations in gene expression, we compared the RNA transcriptome of CF8Flp parental cells to the transcriptomes of three separately derived CF8Flp cell lines: CF8Flp-WT-CFTR, CF8Flp-G551D-CFTR, and CF8Flp-F508del-CFTR. Each CFTR expressing line contained an untagged form of CFTR under control of the elongation factor 1α promoter created in the same fashion as described above. Cells were grown in triplicate on transwell supports in a liquid–liquid interface at three seeding densities and harvested when the transepithelial resistance reached ≥200 Ω/cm2 for three consecutive days (Fig. 3A). Total RNA was extracted and levels of transcription were examined for ~14,000 genes with testable levels of expression. Thirteen housekeeping genes examined showed no significant difference in expression (denoted as fragments per kilobase of exon per million fragments mapped [FPKM]) between the cell lines (Fig. 3B.) The expression patterns of three of these genes, B2M, HPRT1, and GUSB were confirmed via qRT-PCR across 10 different pools of CF8Flp cells containing variants of CFTR (Supplemental Fig. 7A). Additionally, all genes expressed at testable levels (191 of 208) of the CFTR interactome [21] were compared between the four cells lines and showed no significant differences in expression between replicates (Supplemental Fig. 7B). A heat map displaying all ~14,000 expressed genes can be found in the Supplemental material (Supplemental Fig. 8).
Expression of the entire transcriptome was examined by plotting a t-statistic for each gene indicative of the magnitude and directionality of differential RNA expression based on its chromosomal location (Fig. 3C). Each CFTR variant cell line was compared to the parental CF8Flp cells. For WT, F508del, and G551D only 253, 263, and 297 genes out of 14,311, 14,193, and 14,286 testable genes, respectively were differentially expressed at levels ≥3 SD from the mean of the statistic. Notably, the expression level of MUC4 demonstrated the greatest difference in parental cells versus WT, F508del, and G551D with FPKM values of 10.43, 0.58, 0.38, and 0.67 respectively (p = 3 × 10−4) (Fig. 3B). Reduction of MUC4 expression in the presence of CFTR has been reported previously in pancreatic cells [22]. Thus, despite varying chromosomal content among cell lines, widespread imbalance was not observed at the transcriptional level among cell lines.
3.4. Transcription profiles of CFTR related genes are similar between CF8Flp cells and primary airway epithelial cells
To compare the expression profile of genes within the CF8Flp cell line to other cell types, CF8Flp cells expressing WT-CFTR were analyzed with 17 different samples consisting of primary cells, cultured cells, and immortalized cell lines from RNA sequencing experiments (study accession numbers in Supplemental workbook). To assay genes that play key roles in CFTR pathways, expression data for the top 50 genes related to CFTR by shared gene ontologies (GO) were used. Briefly, gene-lists from all biological process ontologies containing CFTR were selected from the GO database accessed via http://geneontology.org/ on 10 Aug 2015. Genes were ranked by frequency with which they co-occurred with CFTR in these ontologies. The top 50 genes most often occurring in these GO were selected for expression analysis (Supplemental workbook).
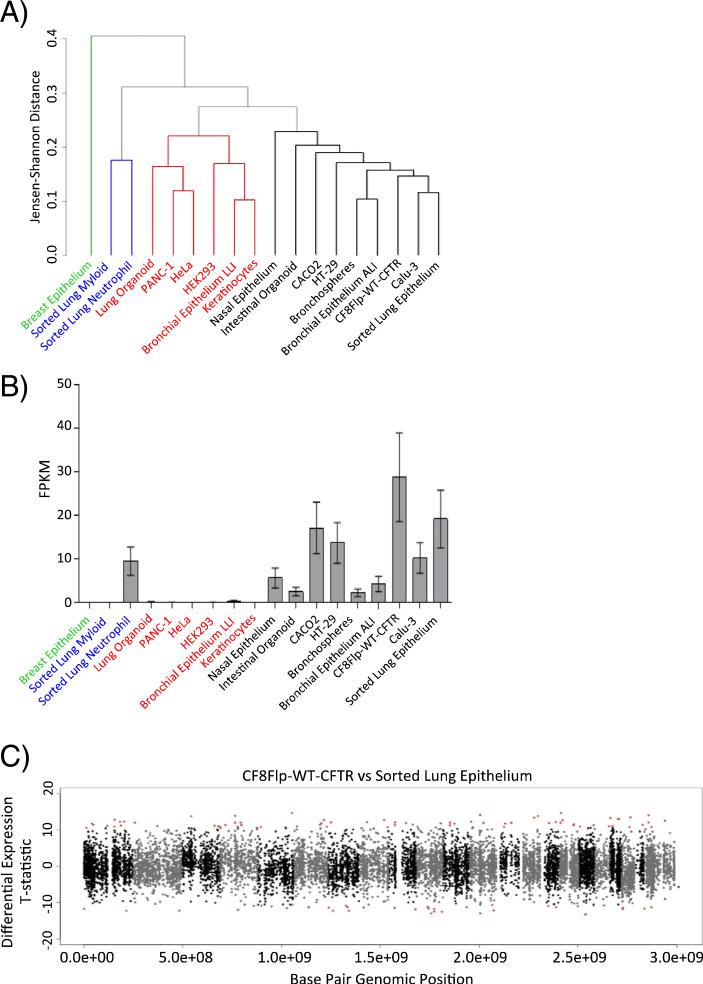
The relative degree of similarity between samples was estimated using Jensen–Shannon distances derived from expression data of 50 selected genes and then visualized as a dendrogram (Fig. 4A). The resulting dendrogram consisted of four key clades. The CF8Flp-WT-CFTR cells share a clade with several other CF relevant cell types including primary airway cells (FACS sorted lung epithelial cells), primary cultured airway cells (bronchial epithelial cells grown in air–liquid interface), and other immortalized cell lines used in CF research (Calu-3, CACO2, HT-29) (Fig. 4A, black font). Two other clades include cell types less related to airway epithelial cells including FACS sorted myeloid cells, neutrophils, HeLa, and HEK293 (Fig. 4A, red and blue font). Finally, breast epithelial cells are shown here as a single sample, distantly to all other samples assayed (green font).
Fig. 4. The CF8Flp transcriptome resembles that of primary bronchial epithelial cells.
(A) Dendrogram derived from Jensen–Shannon distances calculated by using the top 50 genes with shared gene ontologies to CFTR. Data were obtained from the Sequence Read Archive and processed via the same RNA-seq pipeline as CF8Flp samples prior to normalization. (B) CFTR FPKM values from RNA-seq data (mean with 95% CI) (C) Manhattan plot of CF8Flp-WT-CFTR compared to FACS sorted lung epithelial cells. Red points indicate ≥3 SD from the mean of the statistic.
A principle component analysis was performed to determine whether our candidate list of 50 CFTR-related genes significantly contributed to the observed variance across these cell lines. Briefly, genes with FPKM ≥ 1 were used for the analysis. As a result of our gene expression analysis, the first PC represents global changes in FPKM (59.4% explained variance). Components 2 and 4 (8.4% and 4.9% explained variance, respectively) were significantly enriched for our CFTR associated gene set, as well as additional gene sets with biological significance to CF (e.g. fluid shear stress GO pathways) (Supplemental Table 2). These results support our previous findings that the transcriptional profiles of genes relevant to CFTR and epithelial cells are shared between the CF8Flp cell line and other epithelial cells within the clade (black font) formed in our dendrogram.
The expression of CFTR relative to other transcripts was compared between CF8Flp-WT-CFTR cells and the 17 other cell types used in the RNA sequencing analysis (Fig. 4B). Although the CF8Flp cells had the highest expression of CFTR, the difference in level was not statistically significant when compared to the FACS sorted lung epithelial cells expressing endogenous CFTR (p = 0.0812). The sorted lung epithelium had the second highest expression level of CFTR out of the 18 cell types. This may be attributed to the sample being a uniform population of airway epithelial cells. Meanwhile, the lower CFTR levels measured in other primary cell samples could be a consequence a heterogeneous cellular composition. Finally, the transcriptome of the aneuploid CF8Flp-WT-CFTR cells was compared to that of diploid FACS sorted lung epithelial cells (Fig. 4C). The method used to compare the transcriptome of the CF8Flp cells to those containing CFTR variants shown in Fig. 3C was employed. Out of a total of 13,951 testable genes, only 514 (3.7%) were differentially expressed at levels ≥3 SD from the mean of the statistic. The relatively low number of genes differentially expressed between the cell types indicates that the aneuploidy seen in the CF8Flp cell line does not dramatically alter the transcriptome compared to that of a diploid cell line.
4. Discussion
The development and implementation of targeted therapy for CFTR has ushered in new era for CF. The recent approval of the combination drug Orkambi for F508del homozygotes will substantially increase the fraction of CF patients eligible for CFTR-targeted treatment [23]. However, attaining the goal of molecular treatment for every CF patient will be challenging due to the ~1700 variants reported in CF patients that have not been tested for drug response. To date, primary airway epithelial cells expressing select CFTR mutants have been used to demonstrate efficacy of drugs and to gain FDA approval for clinical trials [4,24,25]. Each CFTR variant will likely have to be tested against new and available compounds in vitro to evaluate efficacy before being approved for clinical trials. This is a daunting task given the number of variants to be tested as well as the limited availability of primary tissue expressing rare mutations. In the place of primary cells, Fischer rat thyroid cells have been used extensively to evaluate the function of CFTR bearing a wide range of CFTR variants. However, the Fischer cell line is of non-human origin and also displays functional differences from airway epithelium. As an example, human CFTR is located on both apical and basolateral membranes in Fischer rat thyroid cells [26]. Therefore, a clinically relevant cell type of human origin is needed to more closely model what is observed in vivo in patients.
Assessment of the effects of disease-associated mutations upon CFTR function requires expression levels to approximate those observed in vivo. Comparison of the functional consequences of mutant CFTR to wild-type CFTR requires equivalent levels of expression, otherwise partially functional mutants might be misinterpreted. The CF8Flp line addresses these issues by providing a model that is both biologically relevant to CF and can express various CFTR mutants from a single integration site at levels within the biological range of expressed genes observed in vivo.
Karyotyping revealed the CFBE cells to be aneuploid with multiple markers, translocations, breaks, and fragments. Chromosomal instability as noted in the CFBE cells is a feature of both transformed as well as spontaneously immortalized cell lines [18,27–29]. Despite the karyotypic abnormalities, CF8Flp cells retain the physiologic properties of airway epithelial cells and maintain a relatively stable transcriptome between individually derived pools of cells [10]. In addition, RNA sequencing revealed the CF8Flp cells to be a more similar cell type to primary airway epithelial cells compared to several other commonly used cell types in CF research. As research moves towards examining expression at the whole transcriptome level, it is becoming apparent that there is more variation from tissue to tissue than previously believed [30,31]. This is a critical point to consider when studying a single gene in vitro. The biological context in which a variant is expressed may play a critical role in both its processing and function. It has previously been shown that folding of CFTR is dependent on the cell type in which it is expressed [8]. Therefore, using a CFBE derived cell line that shows a similar transcription profile to primary bronchial epithelial cells provides a useful model to determine the processing and function of CFTR variants in context that is reasonably close to primary airway cells. Additionally, the cell line could serve as a model for studying other airway diseases where CFTR has been implicated to play a role, such as COPD [32].
There are several technical issues that have to be addressed when using cells employing the Flp-In system. Expression levels can vary among clones with full-length integration of the same CFTR mutant. The reasons for this variation is unclear but it necessitates generation of multiple clones so that clones with similar RNA expression levels based on quantitative real time reverse transcriptase PCR can be chosen. Selection based on mRNA levels has been previously shown to alleviate expression variability between cells when using a Flp-In modified Fischer rat thyroid cell line [5,6].
Finally, we have observed duplication of the lacZeo target site on chromosome 8 when CF8FlpIn cells are maintained on Zeocin selection. The presence of multiple integration targets can be detected by staining hygromycin resistant clones for β galactosidase expression from functional lacZeo sequences in cells that were not disrupted by integration. We recommend using low passage cells to decrease the likelihood of a duplication event occurring as a result of the cells accumulating additional genomic abnormalities over time.
In summary, we describe the creation and characterization of CF8Flp, an immortalized epithelial cell line for the study of gene function in the absence of WT-CFTR. The CF8Flp line will serve as a model to efficiently analyze CFTR mutations as well as CF modifiers in a cell type that is physiologically relevant to CF.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Dieter Gruenert for the kind gift of the parental CFBE41o− cells, Dr. William Guggino and Dr. Jie Cheng for their guidance and input regarding the short circuit current experiments, Dr. Peter Mogayzel and Dr. Svetlana Lutsenko for their valuable ideas and discussions on this project, and Janet Biscoe, Susan Morsey, and Barbara Jackson for all their hard work on karyotyping and nomenclature for the cell types. The authors would also like to acknowledge the generous financial contributions from the Cystic Fibrosis Foundation (Grant numbers CUTTIN13A1 and CUTTIN15XX0) and the National Institutes of Health (grant number R01DK044003) for funding this project.
Footnotes
Flp recombination target (FRT), fragments per kilobase of exon per million fragments mapped (FPKM).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jcf.2015.11.010.
References
- 1.Tsui LC. Cystic Fibrosis Mutation Database. 2011 Website: http://www.genet.sickkids.on.ca.
- 2.Randell SH, Fulcher ML, O'Neal W, Olsen JC. Primary epithelial cell models for cystic fibrosis research. Methods Mol Biol. 2011;742:285–310. doi: 10.1007/978-1-61779-120-8_18. [DOI] [PubMed] [Google Scholar]
- 3.Dekkers JF, Wiegerinck CL, De Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013 Jul;19(7):939–45. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 4.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009 Nov 3;106(44):18825–30. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012 May;11(3):237–45. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Van Goor F, Yu H, Burton B, Hoffman BJ. Effect of Ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014 Jan;13(1):29–36. doi: 10.1016/j.jcf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, et al. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005 Sep;115(9):2564–71. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collawn JF, Fu L, Bebok Z. Targets for cystic fibrosis therapy: proteomic analysis and correction of mutant cystic fibrosis transmembrane conductance regulator. Expert Rev Proteomics. 2010 Aug;7(4):495–506. doi: 10.1586/epr.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncz KK, Kunzelmann K, Xu Z, Gruenert DC. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum Mol Genet. 1998 Nov;7(12):1913–9. doi: 10.1093/hmg/7.12.1913. [DOI] [PubMed] [Google Scholar]
- 10.Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, et al. Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o–. Cell Tissue Res. 2006 Mar;323(3):405–15. doi: 10.1007/s00441-005-0062-7. [DOI] [PubMed] [Google Scholar]
- 11.Phuan PW, Veit G, Tan J, Roldan A, Finkbeiner WE, Lukacs GL, et al. Synergy-based small-molecule screen using a human lung epithelial cell line yields DeltaF508-CFTR correctors that augment VX-809 maximal efficacy. Mol Pharmacol. 2014 Apr 18;86(1):42–51. doi: 10.1124/mol.114.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, et al. Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci Transl Med. 2014 Jul 23;6(246):246ra97. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, et al. Failure of cAMP agonists to activate rescued DeltaF508 CFTR in CFBE41o–airway epithelial monolayers. J Physiol. 2005 Dec 1;569(Pt 2):601–15. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, et al. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell. 2012 Nov;23(21):4188–202. doi: 10.1091/mbc.E12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012 Apr;9(4):357–9. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-seq. Bioinformatics. 2009 May 1;25(9):1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013 Jan;31(1):46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sack GH., Jr Human cell transformation by simian virus 40—a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- 19.Meilinger D, Fellinger K, Bultmann S, Rothbauer U, Bonapace IM, Klinkert WE, et al. Np95 interacts with de novo DNA methyltransferases, Dnmt3a and Dnmt3b, and mediates epigenetic silencing of the viral CMV promoter in embryonic stem cells. EMBO Rep. 2009 Nov;10(11):1259–64. doi: 10.1038/embor.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004 Apr;6(4):395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Venable J, Lapointe P, Hutt DM, Koulov AV, Coppinger J, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006 Nov 17;127(4):803–15. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Singh AP, Chauhan SC, Andrianifahanana M, Moniaux N, Meza JL, Copin MC, et al. MUC4 expression is regulated by cystic fibrosis transmembrane conductance regulator in pancreatic adenocarcinoma cells via transcriptional and post-translational mechanisms. Oncogene. 2007 Jan 4;26(1):30–41. doi: 10.1038/sj.onc.1209764. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor–Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015 May 17;373(3):220–31. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18843–8. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awatade NT, Uliyakina I, Farinha CM, Clarke LA, Mendes K, Sole A, et al. Measurements of functional responses in human primary lung cells as a basis for personalized therapy for cystic fibrosis. EBioMedicine. 2015 Feb;2(2):147–53. doi: 10.1016/j.ebiom.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard DN, Carson MR, Ostedgaard LS, Denning GM, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am J Physiol. 1994 Apr;266(4 Pt 1):L405–13. doi: 10.1152/ajplung.1994.266.4.L405. [DOI] [PubMed] [Google Scholar]
- 27.Kaighn ME, Narayan KS, Ohnuki Y, Jones LW, Lechner JF. Differential properties among clones of simian virus 40-transformed human epithelial cells. Carcinogen. 1980 Aug;1(8):635–45. doi: 10.1093/carcin/1.8.635. [DOI] [PubMed] [Google Scholar]
- 28.Stanbridge EJ, Der CJ, Doersen CJ, Nishimi RY, Peehl DM, Weissman BE, et al. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–9. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- 29.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–9. [PubMed] [Google Scholar]
- 30.Human genomics The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015 May 8;348(6235):648–60. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013 Jun;45(6):580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013 Aug;144(2):498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.