Abstract
Background and Purpose
Bone marrow derived mononuclear cells (BMMNCs) offer the promise of augmenting post-stroke recovery. There is mounting evidence of safety and efficacy of BMMNCs from pre-clinical studies of ischemic stroke (IS), however their pooled effects have not been described.
Methods
Using PRIMSA guidelines, we conducted a systematic review of pre-clinical literature for intravenous use of BMMNCs followed by meta-analyses of histological and behavioral outcomes. Studies were selected based on pre-defined criteria. Data were abstracted by two independent investigators. Following quality assessment, the pooled effects were generated using mixed effect models. Impact of possible biases on estimated effect size was evaluated.
Results
Standardized mean difference (SMD), 95% confidence interval (CI) for reduction in lesion volume was significantly beneficial for BMMNC treatment (SMD −3.3, 95% CI: −4.3, −2.3), n = 113 each for BMMNC and controls. BMMNC treated animals (n = 161) also had improved function measured by cylinder test (SMD −2.4, 95% CI: −3.1, −1.6), as compared to controls (n = 205). A trend for benefit was observed for adhesive removal test and neurological deficit score. Study quality score (median: 6, Q1-Q3: 5-7) was correlated with year of publication. There was funnel plot asymmetry, however the pooled effects were robust to the correction of this bias and remained significant in favor of BMMNC treatment.
Conclusions
BMMNCs demonstrate beneficial effects across histological and behavioral outcomes in animal IS models. Though study quality has improved over time, considerable degree of heterogeneity calls for standardization in the conduct and reporting of experimentation.
Keywords: Stroke, Bone Marrow Cells, Animal Experimentation
Introduction
Stroke imposes tremendous mortality and morbidity burden.1 Despite the established benefit of intravenous tissue plasminogen activator (IV rtPA), it is estimated that only about 7% of ischemic stroke (IS) patients receive IV rtPA in the US,2 and intra-arterial therapy is beneficial in only a selected subset of IS patients.3 Cellular therapy is another investigative modality that offers considerable hope and promise to promote post stroke recovery.4
A number of cell types have been investigated in pre-clinical studies and in clinical trials. Bone marrow derived mono-nuclear cells (BMMNCs) are a heterogeneous group of cells consisting of varying proportions of differentially matured B-cells, T-cells, monocytes, as well as a smaller proportion of progenitor cells such as hematopoietic stem cells, mesenchymal stem cells (MSCs), endothelial progenitor cells, and very small embryonic-like cells. The relative ease of processing, potential for intravenous (IV) or intra-arterial (IA) administration, and opportunity of an autologous harvest make them an attractive option for pre-clinical testing and clinical applications.
The evidence of beneficial effect of BMMNCs in animal models of IS has been mounting over the past decade. It has been demonstrated that they lead to a reduction in ischemic lesion volume and improvement in behavioral outcomes.5-9 There is evidence that BMMNCs cross the blood brain barrier,10 exert neuro-protective effects,11, 12 and lead to post-ischemic angiogenesis and neurogenesis.13-15 It has also been demonstrated that IS may lead to activation of BMMNCs resulting in paracrine mediated modulation of post-stroke inflammatory responses.16
The growing evidence of safety and benefit of BMMNCs in pre-clinical models of IS has led to initial clinical testing of these cells by different investigators.17-28 Despite testing in pre-clinical models and application in the clinical milieu, there are a number of unanswered questions regarding the use of BMMNCs in IS patients pertaining to dose, timing, route of administration and autologous vs. allogeneic approach. It is therefore important to study the pooled treatment effects of BMMNCs in relevant pre-clinical models of IS and explore sources of heterogeneity. We therefore aimed to conduct a systematic review and meta-analysis of BMMNCs in animal models of IS.
Methods
The protocol was developed based on Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.29 It was approved by all authors and an external member. For detailed protocol, methods, and PRISMA checklist please see http://stroke.ahajournals.org.
Study selection
Studies were included if they described experiments exclusively on IV administration of autologous, allogeneic, or xenogeneic BMMNCs for pre-clinical models of focal cerebral ischemia in mice and rats.
Search Strategy
We conducted search for literature in MEDLINE, PUBMED, EMBASE, SCOPUS, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Animal Welfare Information Center (AWIC) databases. Elements of the research question were divided into search components (SC), and searched separately followed by combination of SCs. The search results were documented at each step to ensure repeatability. The abstracts were reviewed by hand for relevance, and studies were excluded based on pre-defined criteria.
Data Extraction and Quality Assessment
Data were extracted by two independent abstractors and entered electronically. One abstractor was blinded to the journal, title, and the authors. For articles reporting data only as figures, quantitative methods were used as described in the protocol (https://stroke.ahajournal.org). Each selected study was assessed for quality based on published standards.30
Statistical Analysis
Study characteristics are provided using descriptive analyses. Effects sizes i.e. improvement in outcome for BM MNC treated animals relative to the control group, and were calculated using Hedges’ G.31 Heterogeneity was quantified using the I2 statistic, and weights were assigned using mixed effect models. Sources of heterogeneity were explored by meta-regression.32 Publication and / or selection bias was evaluated using funnel plots,33 and symmetry was formally tested using the Egger test.34 Trim and fill approach was used to correct for funnel plot asymmetry.35 Robustness of estimates to the effect of potentially missed or negative studies was evaluated using Fail-Safe N approach.36, 37 Alpha of 0.05 was used for statistical testing, and analyses were performed using STATA 13 and Comprehensive Meta-Analysis.
Results
Study Characteristics
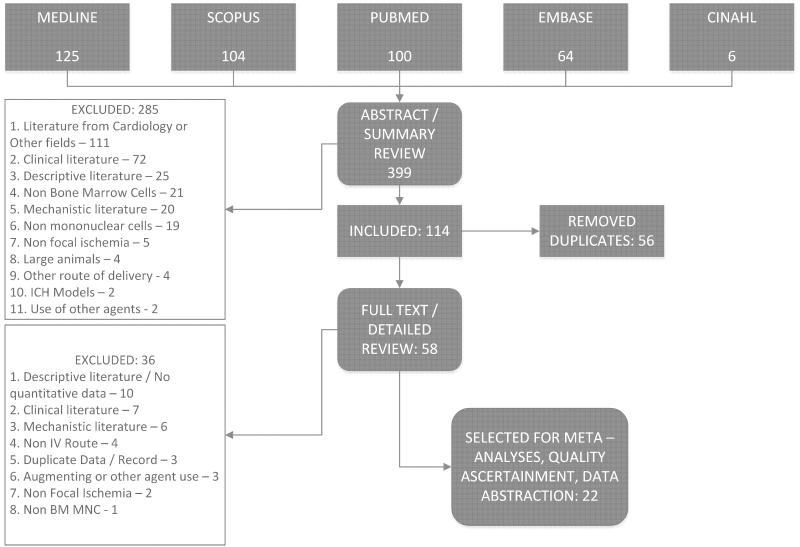
Initial search generated a total of 399 records. Figure 1 illustrates the review process leading to finally selected 22 manuscripts; all published in peer-reviewed journals.5-7, 9, 13, 38-54 An experiment within a study was considered independent if data for a separate control group were available. More than 90% of experiments were done on various species of rats, with 66.3% using allogeneic BMMNCs. The most commonly employed doses were 10 and 30 million cells/ kg in about 63% of the studies. In approximately 75% of experiments BMMNCs were injected within 24 hours of stroke onset. Table 1 summarizes characteristics of the included studies.
Figure 1.
PRISMA flow diagram for review and selection process of studies included in systematic review and meta-analyses of BM MNCs in animal models of cerebral ischemia. The number of search results at each stage of selection along with reasons for exclusion are documented.
Table 1.
Summary characteristics of the 22 studies included in meta-analysis
| Characteristics | Summary Data |
|---|---|
|
| |
| Study Characteristics | |
| Year of publication – n (%) | |
| 2004 – 2008 | 2 (9.1) |
| 2009 – 2010 | 3 (13.6) |
| 2011 – 2012 | 8 (36.4) |
| 2013 – 2014 | 9 (40.9) |
| Journal Impact Factor – median (Q1 – Q3) | 2.96 (2.54 – 4.13) |
| Quality Score – median (Q1 – Q3) | 6 (5 – 7) |
| Presence of additional non IV BM MNC arms | 13 (59.1) |
|
| |
| Animal Characteristics | |
|
| |
| Animal Type – n (%) | |
| Rats | 20 (90.9) |
| Mice | 2 (9.1) |
| Animal Species / Type – n (%) | |
| Rats (n = 20) | |
| Wistar | 8 (40.0) |
| Sprague-Dawley | 6 (30.0) |
| Long Evans | 3 (15.0) |
| SHR* / SHR – SP† | 3 (15.0) |
| Mice (n = 2) | |
| SCID‡ | 1 (50.0) |
| BALB / c§ | 1 (50.0) |
| Animal Gender – n (%) | |
| Male | 19 (86.4) |
| Female | 1 (4.6) |
| Both | 1 (4.6) |
| Not specified | 1 (4.6) |
| Animal Weight Categories – n (%) | |
| 18 – 20 gr | 1 (4.6) |
| 220 – 450 gr | 15 (68.2) |
| 600 – 800 gr | 1 (4.6) |
| Not specified | 5 (22.7) |
|
| |
| Cell Characteristics | |
|
| |
| Cell source – n (%) | |
| Allogeneic | 14 (63.3) |
| Autologous | 7 (31.8) |
| Human | 1 (4.6) |
| Bone Marrow Harvest (autologous cell source) – n (%) | |
| After experimental stroke | 5 (71.4) |
| Before experimental stroke | 2 (28.5) |
| Cell Dose (n = 24, more than 1 experiment / study included) | |
| 30 million cells | 7 (29.2) |
| 20 million cells | 1 (4.2) |
| 10 million cells | 8 (33.3) |
| 8 million cells | 1 (4.2) |
| 5 million cells | 3 (12.5) |
| 3 million cells | 1 (4.2) |
| 1 million cells | 3 (12.5) |
| Timing (n = 37, more than 1 experiment / study included) | |
| ≤ 12 hours | 11 (29.7) |
| 24 hours | 17 (45.9) |
| 48 hours | 2 (5.4) |
| 72 hours | 3 (8.1) |
| > 72 hours | 4 (10.8) |
| Site of delivery - n (%) | |
| Femoral Vein | 7 (31.8) |
| Tail Vein | 7 (31.8) |
| Jugular | 5 (22.7) |
| Not specified | 3 (13.6) |
|
| |
| Stroke type Characteristics | |
|
| |
| Mechanism of ischemia - n (%) | |
| MCAO∥ - Intraluminal Occlusion | 8 (36.4) |
| MCAO - Coagulation / Ligation | 6 (27.3) |
| Thermocoagulation | 5 (22.7) |
| Vasoconstrictor Peptide | 2 (9.1) |
| Cortical Ablation | 1 (4.6) |
| Type of ischemia - n (%) | |
| Permanent | 14 (63.6) |
| Transient | 8 (36.4) |
| Duration of transient ischemia (n = 8) | |
| 180 minutes | 2 (25.0) |
| 90 minutes | 4 (50.0) |
| 60 minutes | 1 (12.5) |
| 45 minutes | 1 (12.5) |
SHR: Spontaneously Hypertensive Rats.
SHR - SP: Spontaneously Hypertensive Rats -Stroke Prone.
SCID: Severe combined immunodeficiency.
BALB/c: Bagg Albino (inbred research mouse strain).
MCAO: Middle Cerebral Artery Occlusion
Outcome Measures
A total of 15 outcomes were identified from included studies and relevant data were abstracted. Five outcomes were measured in 77% of experiments. These ‘Major Outcomes’, and number of animals in control / experimental groups for pooled analyses are: stroke lesion size absolute reduction (n = 113/113) and relative reduction (n = 83/66), cylinder test (n = 161/205), adhesive removal by use of paralyzed limb (n = 69/62) and by time to removal (n = 67/49), neurological deficit score (NDS) (n = 74/74), and modified neurological deficit score (mNDS) (n = 48/48). For details on major and other outcomes please see https://stroke.ahajournals.org.
Pooled estimates
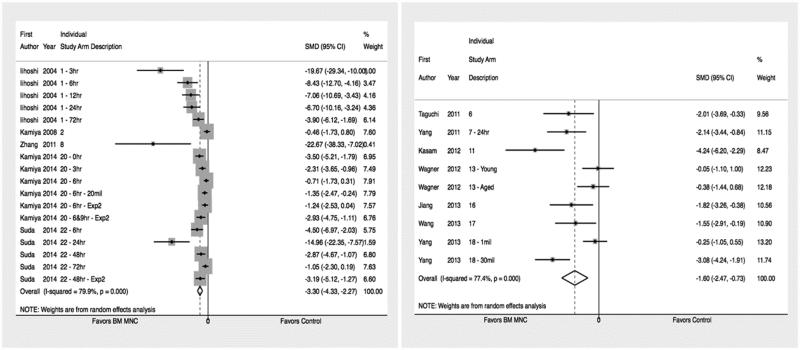
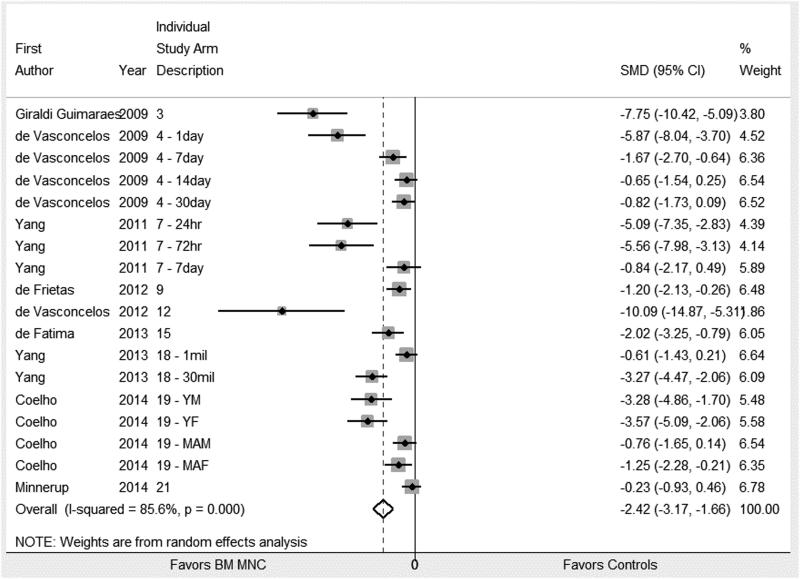
The BMMNC treated animals had significantly reduced stroke lesion volume and enhanced recovery of sensorimotor modalities as measured by cylinder test, adhesive removal test, and NDS. Standardized mean difference (SMD) and 95% confidence interval along with number of animals in the control and intervention group for each of the five major outcomes are summarized in table 2. The corresponding forest plots for lesion size and cylinder test are show in Figures 2a/2b, and 3. Forest plots for other major outcomes are included in the online supplement, please see https://stroke.ahajournals.org.
Table 2.
Pooled estimates from meta-analysis of major outcomes
| Outcome | Number of animals Control / Intervention |
Pooled SMD (95% CI) |
P value |
|---|---|---|---|
| Lesion Size | |||
| Absolute Reduction | 113 / 113 | −3.3 (−4.33, −2.27)* | < 0.001 |
| Percent Reduction | 83 / 66 | −1.6 (−2.47, −0.73)* | < 0.001 |
| Cylinder Test | 161 / 205 | −2.42 (−3.17, −1.66)* | < 0.001 |
| Adhesive Removal Test | |||
| Use of paralyzed limb | 69 / 62 | 1.17 (0.51, 1.84)* | 0.001 |
| Time to adhesive removal | 67 / 49 | −1.96 (−4.48, 0.56) | 0.13 |
| Neurological Deficit Score | 74 / 74 | −1.04 (−1.8, −0.27)* | 0.008 |
| Modified Neurological Deficit Score | 48 / 48 | −1.6 (−3.38, 0.18) | 0.078 |
SMD: Standardized Mean Difference
SMD shows significantly favorable effect of BMMNC treatment
Figure 2a and 2b.
Forrest plot for effect size for IV BM MNCs on absolute reduction (figure 2a) and relative change to the non-infarct side (figure 2b). Weights have been calculated using random effects model. Degree of heterogeneity in the pooled estimates is represented at I2 statistic. The studies included in meta-analysis of absolute5,6,41,54 and relative39,40,44,46,49,50 decrease in infarct size are cited.
Figure 3.
Forrest plot for effect size for IV BM MNCs on cylinder test. Weights have been calculated using random effects model. Degree of heterogeneity in the pooled estimates is represented at I2 statistic. The studies included in the meta-analysis for effect of IV BMMNC on cylinder test are cited.7,38,40,42,45,48,51-53
Exploration of heterogeneity and meta-regression
The pooled estimated for included experiments in all meta-analyses exhibited considerable degree of heterogeneity (I2 values > 70% for all analyses). Univariate meta-regression was conducted to study the effect of dose, timing, and study quality on observed heterogeneity for lesion volume and cylinder test. No significant effects were observed.
Study Quality
The median (Q1,Q3) quality score for was 6 (5-7) and the range was 4 – 10. The experimental quality criteria that were least adhered to were reporting of power and sample size calculations, use of animal models with relevant comorbidities, and reporting of allocation concealment procedures. The coefficient of meta-regression for study quality with effect size for lesion volume was 1.44 (p = 0.06), and there was a statistically significant correlation between study quality and year of publication (p = 0.03). Only six (27.2%) published articles directly or in-directly reported details on immunophenotyping of BMMNCs.
Assessment of bias and sensitivity analysis
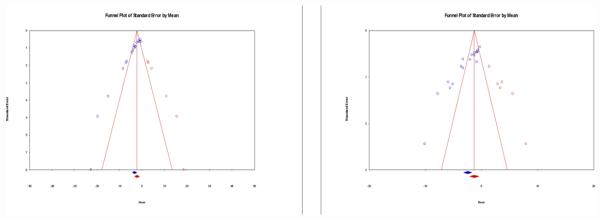
Funnel plots for effect size of BMMNCs as measured by lesion size and cylinder test were asymmetric (p < 0.001 for both). However, the pooled effect size under the random effects model remained statistically significant in favor of BMMNCs for lesion volume (SMD: −2.03, 95% CI: −3.48, −1.06) and cylinder test (SMD: −1.24, 95% CI: −2.09, −0.39) after the trim and fill procedure (Figure 4a/4b). The classic Fail-Safe N analysis yielded the lesion volume and cylinder test effect size of BMMNCs to be robust against 748 and 846 potentially missed null studies, respectively. Furthermore, the Orwin Fail-Safe N analysis indicated combined effect sizes to rise above −0.5 if 19 and 12 studies are added respectively to lesion volume and cylinder test analyses with SMD of 1.
Figure 4a / 4b.
Funnel plot with standardized mean (X Axis) and standard error (Y Axis) for studies included in meta-analysis for absolute reduction in lesion size (4a) and cylinder test (4b). The bubbles in blue are estimates from actual studies, whereas the bubbles in red are hypothetical studies included during the trim and fill approach to correct for asymmetry of the funnel plot. The diamonds below the X Axis represent actual estimates of effect (blue) and correct estimates (red) after trim and fill. Null value is represented by Zero on the X Axis.
Discussion
In the rapidly evolving field of cellular therapy for IS, there are a number of un-answered questions with respect to the choice of cell type, timing, route of administration, safe and effective dose, and the purported mechanism of action. As the evidence generated from pre-clinical studies forms the basis for designing clinical trials, it is important to explore the pooled effects of animal studies, and investigate the various sources of heterogeneity. Prior reviews have either pooled results for a number of neurological disorders,55 or have included multiple different cell types for IS.56 Other reviews have focused solely on MSCs manufactured from various tissues.57 Some of these studies did not generate an effect size or analyze study quality,58 whereas others pooled results by including various routes of delivery.57 To our knowledge, this is the first systematic review and meta-analysis of BMMNCs in experimental stroke models. The aim was to focus on bone marrow MNCs, administered solely via IV delivery, in a clearly defined disease model of small animal focal cerebral ischemia – while examining study quality, and pooling estimates of most commonly and homogenously measured outcomes.
We employed a comprehensive search and robust data assimilation procedure. For the 22 studies that were finally selected, 15 different outcomes were analyzed. A number of behavioral tests in pre-clinical models of stroke have been reviewed in the literature.59 Meta-analyses were only performed for outcomes that were consistent in measurement and reporting. The pooling procedures employed and outcome reported were similar to other meta-analyses.57
Based on arbitrarily defined quantification of effect size,60 our observed effect seizes for beneficial effect of BMMNCs on histological and behavioral outcomes were very large (between −3.3 and −1.04). All estimates other than modified neurological severity score and time to adhesive removal were statistically significant. The number of animals included for these two outcomes in the pooled analyses were small; it is therefore possible that lack of statistical significance for these end-points is a function of small sample size. Though methodological differences do not permit a direct comparison with previously conducted meta-analyses, a prior meta-analysis has reported similar favorable effect sizes for MSCs in IS models for modified neurological severity scale and adhesive removal test respectively.57 Also, another meta-analysis that included multiple cell types, reported a comparable SMD for reduction in infarct lesion size in stem cell treated animals.56 We therefore believe that observing large beneficial effect sizes in pre-clinical pooled data is not unique to our analysis.
Study quality was assessed using Stroke Therapy Academic Industry Roundtable (STAIR) recommended objective scoring ciretira.30 The importance of assessing study quality has been repeatedly emphasized, and a prior review of MSCs reported a positive correlation between effect size and study quality.57 Meta-regression yielded a similar trend in our analysis, showing a 44% increase in effect size for one point score increase in study quality (p = 0.06). All included studies were published within the last 10 years, (95% and 77% during the last 7 and 4 years, respectively). We also noted a statistically significant correlation between study quality and year of publication. This result may be indicative of better implementation of and adherence to quality standards over time. The quality criteria that were not addressed in most studies were sample size / power calculations, concealment of allocation, and testing of animals with relevant comorbidities. Lack of sample size justification in pre-clinical experimentation in neuroscience is prevalent, and attention has been drawn to its detrimental influence on overestimation of effect size.61 Standardization in experimentation and measurement, along with development of data repositories for pre-clinical disease models may provide these estimates for investigators. Allocation concealment is necessary to minimize selection bias, and lack thereof is another factor potentially leading to exaggeration of treatment effects.62 The importance of using disease specific animal models was emphasized in various STAIR publications, and is regarded by some as necessary for any successful translation of a purported new therapy for IS.63
We recognize that our results are not immune to publication and small study effect biases. We used funnel plots to examine the possibility of these biases, and observed considerable asymmetry resulting from lack of null or negative studies. This asymmetry was also quantified using Egger’s test which was found to be statistically significant. We made corrections for apparent asymmetry of the funnel plots, using trim and fill approach, and found that our corrected estimates, though reduced in magnitude of effect, remained statistically significant in favor of BMMNC therapy. We further explored the sensitivity of our estimates to the effect of addition of non-significant studies, and found that a considerably large number of null or negative studies would need to be added to make our estimates statistically not significant. We are also limited by a relatively small number of studies compared with other meta-analyses that fit the specific inclusion criteria. We chose to be specific in our search criteria in order to describe the effects of a specific type of cell therapy in a relevant pre-clinical model using an intravenous delivery. Despite these restrictive selection criteria, a considerable degree of heterogeneity in estimates was observed. We performed univariate meta-regression to study the possible effects of measured variables on effect sizes but did not find any significance. A possible reason could have been a small number of experiments per each outcome. Having fewer studies has also resulted in a relatively small number of animals in experimental and control groups for our pooled analyses. We acknowledge the impact of small sample size on pooled estimates, as has been discussed in literature.64
Our results indicate the IV BMMNCs have significantly beneficial pooled effects on IS lesion size, the cylinder test, the adhesive removal test (as measured by proportional use of the paralytic limb), and neurological deficit score in experimental models of IS. These behavioral tests indicate that BMMNCs carry the potential to improve both modality-specific limb function and overall neurological outcome on a composite score. Estimated effects seem large but are overall robust to potential biases. Compared to other cell therapies, BMMNCs have similar effect sizes and carry the advantage that they can be prepared from patients and re-administered intravenously in more acute time windows after stroke. However, there is a considerable degree of unexplained heterogeneity within experiments despite using restrictive inclusion criteria for study selection. Although the overall study quality has significantly improved over time, standardization of conduct and measurement of pre-clinical experimentation for various structural and behavioral outcomes of cerebral ischemia may be an important focus area for experts in the field.
Supplementary Material
Acknowledgments
Sources of Funding: SIS is supported by NIH R01 NS071127.
Footnotes
Disclosures: SIS is a site investigator for industry clinical trials by SanBio, Let’s Cure CP, Mission Connect, Athersys, and Aldagen. SIS is consultant for Neural Stem, Mesoblast, ReNeuron, and San Bio (all funding goes to UT Health).
References
- 1.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of us health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 3.Grotta JC, Hacke W. Stroke neurologist's perspective on the new endovascular trials. Stroke. 2015;46:1447–1452. doi: 10.1161/STROKEAHA.115.008384. [DOI] [PubMed] [Google Scholar]
- 4.Savitz SI. Developing cellular therapies for stroke. Stroke. 2015;46:2026–2031. doi: 10.1161/STROKEAHA.115.007149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83:433–437. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Giraldi-Guimardes A, Rezende-Lima M, Bruno FP, Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009;1266:108–120. doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 8.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr., Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamiya F, Ueda M, Nito C, Kamiya N, Inaba T, Suda S, et al. Effect of repeated allogeneic bone marrow mononuclear cell transplantation on brain injury following transient focal cerebral ischemia in rats. Life sciences. 2014;95:22–28. doi: 10.1016/j.lfs.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakar S, Muthaian R, Chabbra R, Anand A. Analysis of homing potential of marrow-derived mononuclear cells in an experimentally-induced brain stroke mouse model. Brain Inj. 2010;24:1485–1490. doi: 10.3109/02699052.2010.520298. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Ihara M, Ushiki T, Hirai H, Kizaka-Kondoh S, Hiraoka M, et al. Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke. 2010;41:2938–2943. doi: 10.1161/STROKEAHA.110.596379. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, et al. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88:2869–2876. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, et al. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem cells. 2010;28:1292–1302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Yu L, Jiang C, Chen M, Ou C, Wang J. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and angiogenesis. Brain Behav Immun. 2013;34:56–66. doi: 10.1016/j.bbi.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, et al. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the vegf-vegfr2 signaling pathway in a rat model of vascular dementia. Behavioural brain research. 2014;265:171–180. doi: 10.1016/j.bbr.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Xi X, Aronowski J, Savitz SI. Ischemic stroke may activate bone marrow mononuclear cells to enhance recovery after stroke. Stem cells and development. 2012;21:3332–3340. doi: 10.1089/scd.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr., Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 18.Mendonca ML, Freitas GR, Silva SA, Manfrim A, Falcao CH, Gonzales C, et al. [safety of intra-arterial autologous bone marrow mononuclear cell transplantation for acute ischemic stroke] Arq Bras Cardiol. 2006;86:52–55. doi: 10.1590/s0066-782x2006000100008. [DOI] [PubMed] [Google Scholar]
- 19.Correa PL, Mesquita CT, Felix RM, Azevedo JC, Barbirato GB, Falcao CH, et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin Nucl Med. 2007;32:839–841. doi: 10.1097/RLU.0b013e318156b980. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa da Fonseca LM, Battistella V, de Freitas GR, Gutfilen B, Dos Santos Goldenberg RC, Maiolino A, et al. Early tissue distribution of bone marrow mononuclear cells after intra-arterial delivery in a patient with chronic stroke. Circulation. 2009;120:539–541. doi: 10.1161/CIRCULATIONAHA.109.863084. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa da Fonseca LM, Gutfilen B, Rosado de Castro PH, Battistella V, Goldenberg RC, Kasai-Brunswick T, et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp Neurol. 2010;221:122–128. doi: 10.1016/j.expneurol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regenerative medicine. 2011;6:45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(Suppl 1):S13–21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- 24.Prasad K, Mohanty S, Bhatia R, Srivastava MV, Garg A, Srivastava A, et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J Med Res. 2012;136:221–228. [PMC free article] [PubMed] [Google Scholar]
- 25.Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- 26.Bhasin A, Srivastava MV, Mohanty S, Bhatia R, Kumaran SS, Bose S. Stem cell therapy: A clinical trial of stroke. Clin Neurol Neurosurg. 2013;115:1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Rosado-de-Castro PH, Schmidt Fda R, Battistella V, Lopes de Souza SA, Gutfilen B, Goldenberg RC, et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med. 2013;8:145–155. doi: 10.2217/rme.13.2. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A, Sane H, Nagrajan A, Gokulchandran N, Badhe P, Paranjape A, et al. Autologous bone marrow mononuclear cells in ischemic cerebrovascular accident paves way for neurorestoration: A case report. Case reports in medicine. 2014;2014:530239. doi: 10.1155/2014/530239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Annals of internal medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 30.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durlak JA. How to select, calculate, and interpret effect sizes. Journal of Pediatric Psychology. 2009;34:917–928. doi: 10.1093/jpepsy/jsp004. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. Bmj. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- 37.Orwin RG. A fail-safe n for effect size in meta-analysis. Journal of Educational Statistics. 1983;8:157–159. [Google Scholar]
- 38.de Vasconcelos Dos Santos A, da Costa Reis J, Diaz Paredes B, Moraes L, Jasmin Giraldi-Guimaraes A, et al. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010;1306:149–158. doi: 10.1016/j.brainres.2009.09.094. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi A, Zhu P, Cao F, Kikuchi-Taura A, Kasahara Y, Stern DM, et al. Reduced ischemic brain injury by partial rejuvenation of bone marrow cells in aged rats. J Cereb Blood Flow Metab. 2011;31:855–867. doi: 10.1038/jcbfm.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang XM, Du F, Yang D, Wang R, Yu CJ, Huang XN, et al. Granulocyte colony-stimulating factor increases the therapeutic efficacy of bone marrow mononuclear cell transplantation in cerebral ischemia in mice. BMC neuroscience. 2011;12:61. doi: 10.1186/1471-2202-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Freitas HT, da Silva VG, Giraldi-Guimaraes A. Comparative study between bone marrow mononuclear fraction and mesenchymal stem cells treatment in sensorimotor recovery after focal cortical ablation in rats. Behavioral and brain functions : BBF. 2012;8:58. doi: 10.1186/1744-9081-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco EC, Cardoso MM, Gouveia A, Pereira A, Gomes-Leal W. Modulation of microglial activation enhances neuroprotection and functional recovery derived from bone marrow mononuclear cell transplantation after cortical ischemia. Neurosci Res. 2012;73:122–132. doi: 10.1016/j.neures.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Kasam M, Yang B, Strong R, Schaar K, Misra V, Xi X, et al. Nitric oxide facilitates delivery and mediates improved outcome of autologous bone marrow mononuclear cells in a rodent stroke model. PLoS One. 2012;7:e32793. doi: 10.1371/journal.pone.0032793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasconcelos-dos-Santos A, Rosado-de-Castro PH, de Souza SAL, da Costa Silva J, Ramos AB, de Freitas GR, et al. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem cell research. 2012;9:1–8. doi: 10.1016/j.scr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Wagner D-C, Bojko M, Peters M, Lorenz M, Voigt C, Kaminski A, et al. Impact of age on the efficacy of bone marrow mononuclear cell transplantation in experimental stroke. Exp Transl Stroke Med. 2012;4:17. doi: 10.1186/2040-7378-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardoso MM, Franco EC, de Souza CC, da Silva MC, Gouveia A, Gomes-Leal W. Minocycline treatment and bone marrow mononuclear cell transplantation after endothelin-1 induced striatal ischemia. Inflammation. 2013;36:197–205. doi: 10.1007/s10753-012-9535-5. [DOI] [PubMed] [Google Scholar]
- 48.dos Santos Sampaio MdF, dos Santos Marcilio F, Giraldi-Guimarães A. Does treatment with bone marrow mononuclear cells recover skilled motor function after focal cortical ischemia? Analysis with a forelimb skilled motor task in rats. Brain research. 2013;1492:130–139. doi: 10.1016/j.brainres.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Jiang C, Wang J, Yu L, Ou C, Liu X, Zhao X, et al. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behavioural brain research. 2013;250:222–229. doi: 10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Yu L, Jiang C, Chen M, Ou C, Wang J. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and angiogenesis. Brain, behavior, and immunity. 2013;34:56–66. doi: 10.1016/j.bbi.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang B, Migliati E, Parsha K, Schaar K, Xi X, Aronowski J, et al. Intra-arterial delivery is not superior to intravenous delivery of autologous bone marrow mononuclear cells in acute ischemic stroke. Stroke. 2013;44:3463–3472. doi: 10.1161/STROKEAHA.111.000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coelho BP, Giraldi-Guimarães A. Effect of age and gender on recovery after stroke in rats treated with bone marrow mononuclear cells. Neuroscience research. 2014;88:67–73. doi: 10.1016/j.neures.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Minnerup J, Wagner DC, Strecker JK, Posel C, Sevimli-Abdis S, Schmidt A, et al. Bone marrow-derived mononuclear cells do not exert acute neuroprotection after stroke in spontaneously hypertensive rats. Frontiers in cellular neuroscience. 2014;7:288. doi: 10.3389/fncel.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suda S, Katsura KI, Saito M, Kamiya N, Katayama Y. Valproic acid enhances the effect of bone marrow-derived mononuclear cells in a rat ischemic stroke model. Brain research. 2014;1565:74–81. doi: 10.1016/j.brainres.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: A meta-analysis of preclinical results. Stem cells and development. 2010;19:5–16. doi: 10.1089/scd.2009.0271. [DOI] [PubMed] [Google Scholar]
- 56.Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, et al. Stem cell-based therapy for experimental stroke: A systematic review and meta-analysis. International journal of stroke : official journal of the International Stroke Society. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 57.Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Experimental & translational stroke medicine. 2010;2:13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 61.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 62.Schulz KF, Grimes DA. Allocation concealment in randomised trials: Defending against deciphering. Lancet. 2002;359:614–618. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- 63.Mergenthaler P, Meisel A. Do stroke models model stroke? Disease models & mechanisms. 2012;5:718–725. doi: 10.1242/dmm.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sena ES, Briscoe CL, Howells DW, Donnan GA, Sandercock PA, Macleod MR. Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: Systematic review and meta-analysis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1905–1913. doi: 10.1038/jcbfm.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.