Abstract
Objective
Compared to conventional intravenous platinum and taxane-based chemotherapy for ovarian cancer, both intraperitoneal chemotherapy and more frequent dose-dense intravenous chemotherapy have been associated with improved survival in some studies. We examined the utilization and toxicity of these three methods of chemotherapy delivery in women with ovarian cancer.
Methods
We performed a population-based study and analyzed data on women with ovarian cancer who underwent primary surgery followed by platinum and taxane-based chemotherapy from 2009–2013 who were recorded in the MarketScan database. Adjuvant chemotherapy was classified as: intraperitoneal chemotherapy, dose-dense chemotherapy (weekly administration of chemotherapy), or standard chemotherapy (every 3 weeks). Hospitalizations and emergency department visits for chemotherapy-associated complications and costs were recorded and compared using χ2 tests.
Results
A total of 5,892 patients, including 4,135 (70.2%) who received standard chemotherapy, 859 (14.6%) who received intraperitoneal chemotherapy, and 898 (15.2%) treated with dose-dense chemotherapy were identified. From 2009 to 2013, use of intraperitoneal chemotherapy remained constant (16.3% to 16.3%) while use of dose-dense therapy increased (8.7% to 18.1%) (P<0.001). Hospitalizations for chemotherapy-associated complications occurred in 21.3% of women receiving standard chemotherapy, 34.7% of patients treated with intraperitoneal therapy, and in 25.2% of those receiving dose-dense treatment (P<0.001), while emergency department visits occurred in 18.3%, 26.3%, and 20.3%, respectively (P<0.001). The largest differences in hospitalizations and emergency visits were seen for gastrointestinal toxicities and electrolyte disorders. The per-patient costs of hospitalization were higher for intraperitoneal chemotherapy than other treatment modalities.
Conclusion
Intraperitoneal chemotherapy was used in less than 15% of women with ovarian cancer, while use of dose-dense chemotherapy is increasing. While we did not examine survival, intraperitoneal chemotherapy is significantly more toxic than the other methods of treatment.
Introduction
Advances in chemotherapy have contributed to the improved survival of ovarian cancer seen over the last 3 decades.1 In the 1980s, the activity of platinum analogs was recognized.2 In the 1990’s, paclitaxel in combination with cisplatin was demonstrated to be superior to cisplatin and cyclophosphamide and since that time, combination platinum and taxane-based chemotherapy has remained the standard of care for advanced stage ovarian cancer.3,4 Platinum and taxane-based chemotherapy is most commonly administered every 3 weeks.
More recently, alternative methods of delivery of these drugs have shown improved efficacy compared to standard therapy with carboplatin and paclitaxel.5–10 Intraperitoneal chemotherapy allows for the delivery of drugs directly into the abdominal cavity, the common site of metastatic disease for ovarian cancer.5,6,10 In contrast, dose-dense chemotherapy regimens deliver drugs intravenously but at a more frequent schedule with at least one drug delivered weekly.7,8 Dose-dense chemotherapy is now used for a variety of solid tumors, including breast cancer. Both intraperitoneal chemotherapy and dose-dense chemotherapy have demonstrated superior survival compared to standard chemotherapy.5–8,10 However, a drawback of both regimens is that they are associated with substantially greater toxicity than standard therapy.5–10
In the United States, studies have consistently shown that patients often do not receive treatments that demonstrate efficacy in randomized controlled trials.11 A major concern is that while new treatments may be efficacious in highly selected trial subjects, these findings may not be generalizable and the toxicities may be greater in the broader population.12 We performed a population-based study, first to analyze the trends in use of adjuvant therapy for ovarian cancer and second, to explore the toxicity associated with various regimens.
Materials and Methods
We performed a retrospective cohort study of women with ovarian cancer receiving adjuvant chemotherapy using the Truven Health MarketScan database.13 The dataset includes a sample of patients enrolled in commercial health plans sponsored by approximately 100 employers from across the U.S. The database captures claims on over 50 million covered lives, includes all inpatient, outpatient and office claims as well as data on prescription drug use.13 The database collects detailed information on monthly enrollment and allows longitudinal data capture patient follow-up. The data source has been used in a large number of studies of healthcare utilization and outcomes. All data was de-identified and deemed exempt by the Columbia University Institutional Review Board.
We selected patients with a primary diagnosis of ovarian cancer (ICD-9 183.x) who underwent primary surgery with ovarian resection and/or hysterectomy (Appendix 1, available online at http://links.lww.com/xxx). The cohort was limited to only women who had complete coverage from 2 months prior until 6 months after surgery. Patients who received any chemotherapy within the 2-month period prior to surgery were excluded from the analysis. The cohort was limited to patients who received at least one infusion of chemotherapy with carboplatin and a taxane in the 6-month period after surgery.
The cohort was stratified into three groups based on the dosing and method of delivery of chemotherapy. Intraperitoneal chemotherapy was defined as at least one billing code for the intraperitoneal delivery of a chemotherapeutic agent. We recorded the number of infusions of intraperitoneally administered chemotherapy for women in the intraperitoneal chemotherapy cohort. We report the number of infusions and not number of cycles; in the Gynecologic Oncology Group’s protocol 172, 1 cycle of chemotherapy consisted of 2 infusions of intraperitoneal therapy (1 each of cisplatin and paclitaxel). In accord with clinical trials, patients who discontinued intrapertioneal chemotherapy often received intravenous chemotherapy. These patients were included in the intraperitoneal therapy group. Patients without a code for intraperitoneal chemotherapy were classified as either standard chemotherapy or dose-dense chemotherapy based on the schedule of administration.
Dose-dense chemotherapy for ovarian cancer may be administered as carboplatin every 21 days in combination with weekly paclitaxel or as administration of both drugs on a weekly basis.7–9 As patients receiving dose-dense chemotherapy may not receive chemotherapy every week due to toxicity or disruption of treatment cycles, we defined dose-dense chemotherapy as a ratio of a taxane to carboplatin of ≥1.5 (patients receiving standard chemotherapy would have a ratio of 1:1) or as the cumulative receipt of >9 infusions of carboplatin and ≥9 infusions of taxane within the 6 month period (more infusions than would be received with standard chemotherapy every 21 days). Patients who did not meet the criteria for either intraperitoneal chemotherapy or dose-dense chemotherapy were classified as standard chemotherapy.
The primary outcome of the analysis was acute care requiring hospitalization or use of emergency department services for the management of a chemotherapy-associated complication. Based on prior work, we classified chemotherapy-associated complications into 9 categories: electrolyte disorders, constitutional symptoms, gastrointestinal disorders, malnutrition, anemia/red cell transfusion, neutropenia, thrombocytopenia, venous thromboembolism, and infection (Appendix 1, http://links.lww.com/xxx).14 Hospitalization was defined as admission to an acute care facility, while emergency department services were defined as a billing code for care in an emergency department. For each group, we measured the number of patients who were hospitalized or cared for in the emergency department as well as the total number of hospitalizations or emergency department visits.
Clinical and demographic characteristics of the cohort analyzed included age at the time of surgery (<35, 35–44, 45–54, 55–64 and ≥65 years), year of surgery (2009–2013), and region (northeast, north central, south, west, unknown). Comorbid medical conditions were measured using the Charlson comorbidity score and classified as 0, 1, or ≥2.15
Utilization of each method of chemotherapy delivery is reported descriptively by year of diagnosis. Frequency distributions between categorical variables were compared across the groups using χ2 tests. Continuous variables were compared using ANOVA or Wilcoxon rank sums tests. Point estimates are presented with 95% confidence intervals.
Cost data is reported as per patient costs with 95% confidence intervals. All costs are adjusted for inflation and reported in 2013 dollars. Given that cost data is highly skewed, costs were winsorized with values <5th percentile reported at the 5th percentile and costs >95th percentile reported at the 95th percentile as previously described.16,17 All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
A total of 5,892 patients were identified. The cohort included 4,135 (70.2%) women who received standard chemotherapy, 859 (14.6%) who received intraperitoneal chemotherapy, and 898 (15.2%) who were treated with dose-dense chemotherapy (Table 1). The use of intraperitoneal chemotherapy was 16.3% (95% CI, 13.9–18.8%) in 2009, decreased to 13.2% (95% CI, 10.9–15.7%) in 2010, and then increased back to 16.3% (95% CI, 12.5–20.5%) in 2013 (Figure 1). In contrast, use of dose-dense chemotherapy rose year after year from 8.7% (95% CI, 6.2–11.2%) in 2009 to 18.1% (95% CI, 14.3–22.3%) in 2013, while the use of standard chemotherapy declined from 75.0% (95% CI, 72.5–77.5%) to 65.6% (95% CI, 61.8–69.7%) over the same time period. Bevacizumab was used in 4.0% of women receiving standard chemotherapy, 5.2% of women treated with intraperitoneal chemotherapy, and 8.2% of those receiving dose-dense therapy.
Table 1.
Clinical and demographic characteristics of the cohort.
| Standard | Intraperitoneal | Dose Dense | P-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N | % | N | % | N | % | ||
| 4,135 | (70.2) | 859 | (14.6) | 898 | (15.2) | ||
| Year | <0.001 | ||||||
| 2009 | 849 | (75.0) | 185 | (16.3) | 98 | (8.7) | |
| 2010 | 991 | (71.1) | 184 | (13.2) | 218 | (15.7) | |
| 2011 | 1,016 | (68.0) | 217 | (14.5) | 261 | (17.5) | |
| 2012 | 917 | (69.4) | 183 | (13.9) | 221 | (16.7) | |
| 2013 | 362 | (65.6) | 90 | (16.3) | 100 | (18.1) | |
| Age (years) | <0.001 | ||||||
| <35 | 101 | (76.5) | 15 | (11.4) | 16 | (12.1) | |
| 35–44 | 329 | (68.3) | 83 | (17.2) | 70 | (14.5) | |
| 45–54 | 1,143 | (69.2) | 276 | (16.7) | 234 | (14.2) | |
| 55–64 | 1,647 | (68.9) | 376 | (15.7) | 366 | (15.3) | |
| ≥65 | 915 | (74.0) | 109 | (8.8) | 212 | (17.2) | |
| Region | <0.001 | ||||||
| Northeast | 920 | (68.9) | 232 | (17.4) | 184 | (13.8) | |
| North Central | 990 | (70.9) | 198 | (14.2) | 209 | (15.0) | |
| South | 1,333 | (72.8) | 254 | (13.9) | 245 | (13.4) | |
| West | 810 | (66.8) | 154 | (12.7) | 248 | (20.5) | |
| Unknown | 82 | (71.3) | 21 | (18.3) | 12 | (10.4) | |
| Comorbidity | <0.001 | ||||||
| 0 | 3,249 | (69.1) | 725 | (15.4) | 727 | (15.5) | |
| 1 | 685 | (74.1) | 112 | (12.1) | 128 | (13.8) | |
| ≥2 | 201 | (75.6) | 22 | (8.3) | 43 | (16.2) | |
| Concurrent bevacizumab | 165 | (4.0) | 45 | (5.2) | 74 | (8.2) | <0.001 |
Outcomes were compared using χ2 tests.
Figure 1.
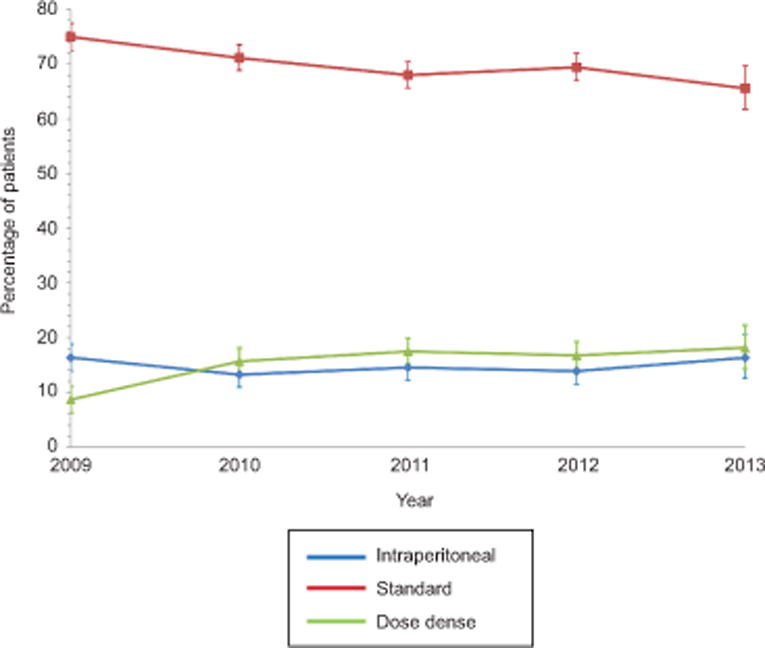
Trends in use of intraperitoneal, dose dense intravenous, and standard intravenous chemotherapy among women with ovarian cancer (P<.001). Error bars represent 95% confidence intervals. Outcomes were compared using chi-square tests.
The median number of infusions of chemotherapy delivered intraperitoneally among the women in the intraperitoneal cohort was 6 (IQR, 3–10). Within this group, 12.7% received ≥12 infusions of intraperitoneal treatment (corresponding to 6 cycles of treatment). In contrast, 21.8% received ≤2 infusions of intraperitoneal treatment, the equivalent of only 1 cycle of therapy (Appendix 2, available online at http://links.lww.com/xxx).
Within the cohort, 21.3% who had standard chemotherapy, 34.7% of women receiving intraperitoneal chemotherapy, and 25.2% of those receiving a dose-dense regimen were hospitalized with a claim for a chemotherapy-related complication (P<0.001) (Table 2). Two or more hospitalizations were recorded in 6.3%, 12.6%, and 6.8% for each chemotherapy regimen, respectively (P<0.001). Emergency department visits for a chemotherapy-related complication were required in 18.3% of women administered standard chemotherapy, 26.3% of patients treated with intraperitoneal chemotherapy, and 20.3% for those receiving dose-dense treatment (P<0.001). Two or more ED visits were required in 5.4%, 8.5%, and 7.6% of the groups, respectively (P<0.001).
Table 2.
Complications stratified by type of chemotherapy received.
| Standard | Intraperitoneal | Dose Dense | P-value | ||||
|---|---|---|---|---|---|---|---|
| Patients hospitalized | 880 | (21.3) | 298 | (34.7) | 226 | (25.2) | <0.001 |
| Number of hospitalizations | 1,352 | – | 479 | – | 313 | – | – |
| Hospitalization per patient (median, IQR) | 1 | (1–2) | 1 | (1–2) | 1 | (1–2) | 0.046 |
| Hospitalization per Patient | <0.001 | ||||||
| 0 | 3,255 | (78.7) | 561 | (65.3) | 672 | (74.8) | |
| 1 | 621 | (15.0) | 190 | (22.1) | 165 | (18.4) | |
| ≥2 | 259 | (6.3) | 108 | (12.6) | 61 | (6.8) | |
| Patients with emergency department visits | 756 | (18.3) | 226 | (26.3) | 182 | (20.3) | <0.001 |
| Number of emergency department visits | 1,173 | – | 391 | – | 374 | – | – |
| Emergency department visits per patient (median, IQR) | 1 | (1–2) | 1 | (1–2) | 1 | (1–2) | 0.06 |
| Emergency department visits per patient | <0.001 | ||||||
| 0 | 3,379 | (81.7) | 633 | (73.7) | 716 | (79.7) | |
| 1 | 533 | (12.9) | 153 | (17.8) | 114 | (12.7) | |
| ≥2 | 223 | (5.4) | 73 | (8.5) | 68 | (7.6) | |
| Cost per Patients for Hospitalizations1 | $6,353 | ($5,790–$6,917) | $7,974 | ($6,804–$9,144) | $7,516 | ($6,202–$8,831) | 0.03 |
Costs were winsorized with values <5th percentile reported at the 5th percentile and costs >95th percentile reported at the 95th percentile and reported in 2013 dollars.
Outcomes were compared using χ2 tests.
Women who received intraperitoneal chemotherapy had a higher rate of complications overall, and in each of the subcategories, compared to the other groups (Table 3). The most frequent chemotherapy-associated complication was gastrointestinal disorders, which were noted in 13.3% after standard therapy, 24.7% of women who received intraperitoneal chemotherapy, and 13.7% of those treated with dose-dense therapy (P<0.001). Electrolyte disorders were seen in 11.6%, 22.7%, and 12.9% (P<0.001) of women respectively, while infectious complications were documented in 15.4%, 18.9%, and 15.4% of the three groups, respectively (P=0.04). The individual complications are displayed in Appendix 3, available online at http://links.lww.com/xxx.
Table 3.
Distribution of complications stratified by type of chemotherapy received.
| Standard | Intraperitoneal | Dose Dense | P-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N | % | N | % | N | % | ||
| Hospitalization or emergency department visits | |||||||
| Electrolyte disorders | 478 | (11.6) | 195 | (22.7) | 116 | (12.9) | <0.001 |
| Constitutional symptoms | 349 | (8.4) | 100 | (11.6) | 79 | (8.8) | 0.01 |
| Gastrointestinal disorders | 548 | (13.3) | 212 | (24.7) | 123 | (13.7) | <0.001 |
| Malnutrition | 180 | (4.4) | 56 | (6.5) | 53 | (5.9) | 0.01 |
| Anemia & red cell transfusion | 448 | (10.8) | 133 | (15.5) | 126 | (14.0) | <0.001 |
| Neutropenia | 205 | (5.0) | 67 | (7.8) | 43 | (4.8) | 0.002 |
| Thrombocytopenia | 82 | (2.0) | 18 | (2.1) | 16 | (1.8) | 0.89 |
| Venous thromboembolism | 208 | (5.0) | 72 | (8.4) | 51 | (5.7) | <0.001 |
| Infection | 636 | (15.4) | 162 | (18.9) | 138 | (15.4) | 0.04 |
Outcomes were compared using χ2 tests.
Among those who were hospitalized, the per-patient winsorized mean cost of hospitalizations was $6353 (95% CI, $5790–6917) after standard chemotherapy, $7974 (95% CI, $6804–9144) after intraperitoneal chemotherapy, and $7516 (95% CI, $6202–8831) for dose-dense chemotherapy (P=0.03) (Table 1).
Discussion
Despite the efficacy of intraperitoneal chemotherapy for ovarian cancer, we noted only modest use of the treatment. In contrast, the use of dose-dense chemotherapy appears to be increasing rapidly. Complications and side effects are substantially more common after intraperitoneal chemotherapy than other treatment modalities.
The efficacy of intraperitoneal chemotherapy has been demonstrated in multiple randomized controlled trials.5,6,10 In the Gynecologic Oncology Group’s protocol 172, intraperitoneal chemotherapy was associated with a 16-month improvement in survival compared to standard intravenous chemotherapy (66 vs. 50 months), however, intraperitoneal therapy was also substantially more toxic.5 We also noted a higher rate of hospitalizations and ED visits with intraperitoneal chemotherapy compared to both standard and dose-dense treatment regimens.
Despite the survival advantage of intraperitoneal chemotherapy, uptake has been poor.18–20 In an analysis of six National Comprehensive Cancer Network (NCCN) Institutions, only 41% of eligible patients received intraperitonealchemotherapy.20 A report of Medicare beneficiaries found that just 3.5% of women received intraperitoneal chemotherapy.19 Our findings were similar; only 15% of ovarian cancer patients receiving chemotherapy in the community were treated with intraperitoneal therapy. Similar to the data from the NCCN, in our cohort the use of intraperitoneal treatment plateaued from 2009 to 2013.
Toxicity and logistical challenges are major barriers to the utilization and completion of intraperitoneal chemotherapy.21,22 In the GOG’s study, only 42% of patients completed all six cycles of intraperitoneal treatment, while in a study of NCCN institutions, patients received a median of 5 cycles of intraperitoneal therapy.5,20 We found that women frequently received a limited amount of therapy intraperitoneally. In our cohort, 22% of women only received 1 or 2 infusions of intraperitoneal therapy. While suboptimal, receipt of even a limited number of intraperitoneal infusions appears to confer a survival benefit over standard therapy.5,23 To improve tolerability and maximize drug delivery, a number of modified intraperitoneal regimens have been described.20,24,25
Although use of intraperitoneal therapy plateaued, administration of dose-dense chemotherapy increased substantially over time. The first large, randomized trial of dose-dense chemotherapy was reported in 2009.7,8 Long-term follow-up of this cohort demonstrated a median survival of 100.5 months for women with advanced stage ovarian cancer treated with dose-dense chemotherapy compared to 62 months for those who received conventional therapy.7 However, a recent cooperative group in the United States failed to show a benefit for dose-dense chemotherapy compared to conventional 3-week dosing.26 In our cohort, use of dose-dense chemotherapy more than doubled from 8.7% in 2009 to 18.1% by 2013. Hospitalizations and chemotherapy-associated complications were slightly greater than conventional chemotherapy.
We acknowledge a number of important limitations. First, claims data may undercapture side effects and toxicity, especially symptoms not captured well on billing claims. To mitigate this bias, we selected only major complications that are likely to generate a claim. We recognize that these complications may not necessarily be attributable to chemotherapy itself, but may be due to surgical complications or other underlying medical conditions. Second, given missed infusions and schedule alterations, classification of dose-dense chemotherapy has to be based on a ratio or number of infusions of each drug. We performed a series of sensitivity analyses of the data and chose a conservative definition of dose-dense chemotherapy. While we cannot exclude the possibility of misclassification of a small number of women, any misclassification would bias our findings toward the null hypothesis. Further, because of this classification schema, it is difficult to ascertain the true number of cycles obtained for comparisons. Third, we are unable to capture dose reductions and alterations in treatment. Fourth, MarketScan lacks data on a number of clinical and demographic factors as well as tumor characteristics. Importantly, the goal of our study was not to examine survival, but rather toxicity based on the type of chemotherapy used.
Our data has a number of important implications. First, the toxicity profiles and complications we noted for all three regimens were greater than what has been reported in clinical trials and selected studies from referral centers. Hospitalization rates in our series were 2.5 times higher for both intraperitoneal and conventional chemotherapy than reported for patients treated at comprehensive cancer centers.20 As such, caution should be used when generalizing the results of patients treated on protocol and at selected referral centers to the general population.12 Second, there was substantial variability in not only the choice of chemotherapy regimens, but also the quality of treatment. In our cohort a large majority of women receiving intraperitoneal chemotherapy received a small number of infusions of drug intraperitoneally. Prior work has shown that the quality of chemotherapy for ovarian cancer is highly variable; chemotherapy is frequently omitted when indicated or delivered in a suboptimal manner.27,28 Going forward, strategies to optimize adjuvant chemotherapy for women with ovarian cancer are clearly needed.
Supplementary Material
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Wright JD, Chen L, Tergas AI, et al. Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol. 2015;125:1345–52. doi: 10.1097/AOG.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omura GA, Bundy BN, Berek JS, Curry S, Delgado G, Mortel R. Randomized trial of cyclophosphamide plus cisplatin with or without doxorubicin in ovarian carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 1989;7:457–65. doi: 10.1200/JCO.1989.7.4.457. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 7.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–6. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 8.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 9.Pignata S, Scambia G, Katsaros D, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 10.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 11.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 12.Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju002. dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truven Health Analytics. MarketScan. Accessed June 21, 2015, at http://truvenhealth.com/your-healthcare-focus/life-sciences/marketscan-databases-and-online-tools.
- 14.Hassett MJ, O’Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–17. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Joynt KE, Orav EJ, Jha AK. Association between hospital conversions to for-profit status and clinical and economic outcomes. Jama. 2014;312:1644–52. doi: 10.1001/jama.2014.13336. [DOI] [PubMed] [Google Scholar]
- 17.Ly DP, Jha AK, Epstein AM. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26:1291–6. doi: 10.1007/s11606-011-1815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowles EJ, Wernli KJ, Gray HJ, et al. Diffusion of Intraperitoneal Chemotherapy in Women with Advanced Ovarian Cancer in Community Settings 2003–2008: The Effect of the NCI Clinical Recommendation. Front Oncol. 2014;4:43. doi: 10.3389/fonc.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairfield KM, Murray K, LaChance JA, et al. Intraperitoneal chemotherapy among women in the Medicare population with epithelial ovarian cancer. Gynecol Oncol. 2014;134:473–7. doi: 10.1016/j.ygyno.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Wright AA, Cronin A, Milne DE, et al. Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.61.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naumann RW, Sukumvanich P, Edwards RP. Practice patterns of intraperitoneal chemotherapy in women with ovarian cancer. Gynecol Oncol. 2009;114:37–41. doi: 10.1016/j.ygyno.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100:27–32. doi: 10.1016/j.ygyno.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460–6. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry E, Matthews KS, Singh DK, et al. An outpatient intraperitoneal chemotherapy regimen for advanced ovarian cancer. Gynecol Oncol. 2009;113:63–7. doi: 10.1016/j.ygyno.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Gray HJ, Shah CA, Swensen RE, Tamimi HK, Goff BA. Alternative intraperitoneal chemotherapy regimens for optimally debulked ovarian cancer. Gynecol Oncol. 2010;116:340–4. doi: 10.1016/j.ygyno.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 26.Chan JK, Brady MF, Penson RT, et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N Engl J Med. 2016;374:738–48. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairfield KM, Murray K, Lucas FL, et al. Completion of adjuvant chemotherapy and use of health services for older women with epithelial ovarian cancer. J Clin Oncol. 2011;29:3921–6. doi: 10.1200/JCO.2010.34.1552. [DOI] [PubMed] [Google Scholar]
- 28.Wright JD, Ananth CV, Tsui J, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer. 2014;120:1246–54. doi: 10.1002/cncr.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


