Abstract
Bioprinting is a 3D fabrication technology used to precisely dispense cell-laden biomaterials for the construction of complex 3D functional living tissues or artificial organs. While still in its early stages, bioprinting strategies have demonstrated their potential use in regenerative medicine to generate a variety of transplantable tissues, including skin, cartilage, and bone. However, current bioprinting approaches still have technical challenges in terms of high-resolution cell deposition, controlled cell distributions, vascularization, and innervation within complex 3D tissues. While no one-size-fits-all approach to bioprinting has emerged, it remains an on-demand, versatile fabrication technique that may address the growing organ shortage as well as provide a high-throughput method for cell patterning at the micrometer scale for broad biomedical engineering applications. In this review, we introduce the basic principles, materials, integration strategies and applications of bioprinting. We also discuss the recent developments, current challenges and future prospects of 3D bioprinting for engineering complex tissues. Combined with recent advances in human pluripotent stem cell technologies, 3D-bioprinted tissue models could serve as an enabling platform for high-throughput predictive drug screening and more effective regenerative therapies.
Keywords: Bioprinting, bioink, tissue engineering, 3D printing, hydrogel, drug screening, regenerative medicine
1. Introduction
In the United States, one name is added to the organ transplant waiting list every 15 minutes (Abouna 2008). While this list grows rapidly, less than one-third of waiting patients can receive matched organs from donors (Ozbolat and Yu 2013). This growing deficit, however, is unlikely to be met by a supply of transplantable organs that has stagnated over the last decade (Bajaj et al. 2014). One of the most promising techniques to alleviate this organ shortage crisis is tissue engineering, the use of a combination of cell, engineering, and material methods to generate artificial tissues and organs (Langer and Vacanti 1993). In tissue engineering, three strategies are used to replace or induce targeted tissues: (1) the use of cells alone, (2) the use of biocompatible biomaterials, (3) the use of a combination of both cells and biomaterials (Khademhosseini et al. 2006). These cells and biomaterials are combined into scaffolds through a variety of processes, which can generally be classified as either top-down, or bottom-up. In top-down approaches cells are often seeded sparsely and homogenously in biomaterials shaped to resemble biological geometries. On the other hand, in bottom-up approaches modular units of cells and biomaterials are combined to form macro tissues. Top-down methods have been in wide use for years, however, these methods often cannot accurately control the distribution of cells, and fail to generate the appropriate extracellular matrix (ECM) (Khademhosseini et al. 2006). Without a proper ECM microenvironment, cells cannot function as tissues properly. This limitation is addressed in bottom-up approaches that build up tissues brick by brick via micro- and nano-technologies. As a result, cell distribution can be defined at the micrometer scale, which significantly improves the controllability of scaffold fabrication (Jiao et al. 2014). Motivated by developments in nanotechnology, techniques like self-assembly and soft-lithography have been applied to bottom-up tissue engineering (Kim et al. 2013, 2014a; Shapira et al. 2014). Among the micro-scale bottom-up techniques recently applied to tissue engineering, bioprinting, a form of additive manufacturing, has become one of the most promising and advanced fabrication methods (Table 1).
Table 1.
Comparison of Tissue Engineering Methods
| Assembly Method | ||||
|---|---|---|---|---|
| Bioprinting | Molding | Porous Scaffolds | References | |
| Materials | Natural and synthetic polymers High concentration cell solutions |
Natural and synthetic polymers High concentration cell solutions Cell sheets |
Natural and synthetic polymers Ceramics Metals |
(Agarwal et al. 2013; Skardal and Atala 2014) |
| Resolution | 10 – 1000 μm | >500 nm | 100 nm – 1000 μm | (Kim et al. 2010; Lu et al. 2013; Bajaj et al. 2014) |
| Advantages | Control of tissue geometry across a wide range of scales; Rapid production of scaffolds; Precise cell and material patterning | Accurate control of small (< 100 μm) features; Scaffold fabrication is rapid and molds are often reusable; Gentle on encapsulated cells | Controllable material properties (e.g. porosity, modulus); Wide range of materials available for use | (Lu et al. 2013; Bajaj et al. 2014; Jiao et al. 2014; Murphy and Atala 2014) |
| Disadvantages | Printing techniques may reduce cell viability or have unknown consequences; Limited material selection due to crosslinking speed | Scaffolds are generally homogenous or require combination of multiple scaffolds to create patterns | Scaffold geometry is less controllable; Technique may damage encapsulated cells or require seeding after assembly; Less control of cell patterning | |
| Techniques | Extrusion Laser-assisted Inkjet Stereolithography |
Cell sheet stacking Lithography Injection molding |
Electrospinning Phase Separation Freeze drying Self-Assembly |
(Ballyns et al. 2008; Zheng et al. 2012; Lu et al. 2013; Jiao et al. 2014) |
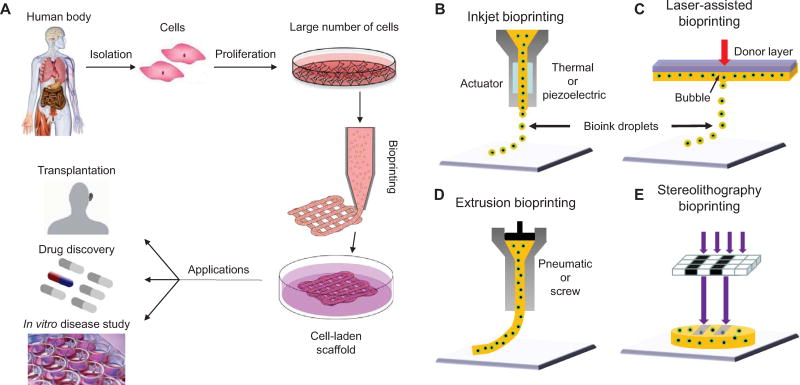
In bioprinting, small units of cells and biomaterials are dispensed with micrometer precision to form tissue-like structures (Figure 1). Unlike conventional 3D printing techniques that have been used to print temporary cell-free scaffolds for use in surgery (Bracci et al. 2013), bioprinting requires a different technical approach that is compatible with depositing living cells. The advantages of bioprinting include accurate control of cell distribution, high-resolution cell deposition, scalability, and cost-effectiveness. For those reasons, the development and subsequent applications of bioprinting have greatly increased during the last five years. In this review, we discuss the basic principles of bioprinting, including bioprinter device design, workflow, biomaterial options, and current and potential applications.
Figure 1. Bioprinting process, techniques, and applications.
(A) For human therapeutic applications, the typical workflow of bioprinting would involve the isolation and expansion of human cells prior to printing the desired cell-laden scaffold. These scaffolds could then ultimately be used as therapeutic devices themselves, as a testing platform for drug screening and discovery, or as an in vitro model system for disease. (B) Inkjet printers eject small droplets of cells and hydrogel sequentially to build up tissues. (C) Laser bioprinters use a laser to vaporize a region in the donor layer (top) forming a bubble that propels a suspended bioink to fall onto the substrate. (D) Extrusion bioprinters use pneumatics or manual force to continuously extrude a liquid cell-hydrogel solution. (E) Stereolithographic printers use a digital light projector to selectively crosslink bioinks plane-by-plane. In (C) and (E), colored arrows represent a laser pulse or projected light, respectively.
2. Bioprinting Techniques
To date, no single bioprinting technique enables the production of all scales and complexities of synthetic tissues. The three major bioprinting techniques of inkjet, laser-assisted, and extrusion bioprinting each have specific strengths, weaknesses, and limitations. A concise comparison of these approaches is also provided in Table 2.
Table 2.
Comparison of four types of bioprinting techniques
| Parameters | Inkjet | Laser-assisted | Extrusion | Stereolithography | Reference |
|---|---|---|---|---|---|
| Cost | Low | High | Moderate | Low | (Billiet et al. 2012; Ozbolat and Yu 2013; Orloff et al. 2014; Ozbolat et al. 2014a) |
| Cell viability | > 85% | > 95% | 40% – 80% | > 85% | (Xu et al. 2005; Catros et al. 2011b) |
| Print speed | Fast | Medium | Slow | Fast | (Xu et al. 2009b; Guillotin et al. 2010; Murphy and Atala 2014) |
| Supported viscosities | 3.5 to 12 mPa/s | 1 to 300 mPa/s | 30 mPa/s to above 6×107 mPa/s | No limitation | (Guillemot et al. 2010; Chang et al. 2011; Murphy and Atala 2014) |
| Resolution | High | High | Moderate | High | (Ozbolat and Yu 2013) |
| Quality of vertical structure | Poor | Medium | Good | Good | (Wang et al. 2016) |
| Cell density | Low < 106 cells/mL | Medium < 108 cells/mL | High (Cell spheroids) | Medium < 108 Cells/mL | (Murphy and Atala 2014) |
| Representative materials for bioinks | Alginate, PEGDMA, Collagen | Collagen, Matrigel | Alginate, GelMA, Collagen | GelMA, GelMA-PEGDA hybrid hydrogel | (Nahmias et al. 2005; Cui et al. 2012a; Xu et al. 2012; Kolesky et al. 2014; Ozbolat et al. 2014b; Wang et al. 2016) |
| Reported applications | Tissue Engineering (Blood Vessel, Bone, Cartilage, and Neuron) | Tissue Engineering (Blood Vessel, Bone, Skin, and Adipose) | Tissue Engineering (Blood Vessel, Bone, Cartilage, Neuron, Muscle, Tumor) Controlled release of biomacromolecules, Organ-on-a-chip |
Tissue Engineering (Blood Vessel and Cartilage) Organ-on-a-chip |
(Chang et al. 2010; Ker et al. 2011; Gou et al. 2014; Huang et al. 2014) |
2.1 Inkjet printing
Inkjet bioprinting was the first bioprinting technology (Tuan et al. 2003) and is very similar to conventional 2D inkjet printing (Singh et al. 2010). A hydrogel pre-polymer solution with encapsulated cells (called a bioink) is stored in the ink cartridge. The cartridge is then connected to a printer head and acts as the bioink source during the electronically controlled printing process. During printing, the printer heads are deformed by a thermal or piezoelectric actuator and squeezed to generate droplets of a controllable size, as shown in Figure 1B. The advantages of inkjet printing include: (1) low cost due to similar structure with commercial printers, (2) high printing speed conferred by the ability of the printer heads to support parallel work mode, and (3) relatively high cell viability (usually from 80% to 90%), as determined by many experimental results (Cui et al. 2012a, 2012b, 2013).
However, because current printer heads are based on microelectromechanical systems (MEMS) devices, there is a relatively small deformation generated by either thermal or piezoelectric actuation at the nozzle opening. As a result, MEMS-based printer heads cannot squeeze out high viscosity materials (>15 mPa/s) and do not work well with bioinks with high cell density (>1×106 cells/mL). High cell density increases the average viscosity of bioinks, resulting in clogging of the head (Xu et al. 2005; Guillotin et al. 2010; Pepper et al. 2011, 2012). Recent research has highlighted another disadvantage of inkjet printing, named the settling effect (Pepper et al. 2011, 2012). When bioinks are initially loaded into the ink cartridge, they are well mixed. Over the entire printing process, however, cells begin to settle in the cartridge, increasing the viscosity of the bioink and often clogging the printer head.
The simplest way to build inkjet bioprinter is to modify a commercial printer. HP 26 printer heads (Hewlett-Packard, Palo Alto, USA) were combined with a controller to print bioinks (Mattimore et al. 2010). Similar print heads were further integrated with a modified HP G3110 scanner (Hewlett-Packard, Palo Alto, USA) to build a low-cost bioprinter (~$700) (Orloff et al. 2014). Such a low-cost system was achieved by using commercial print heads as the dispenser, a scanner as a 2-axis servo stage, and free control software. The resolution of the servo stage was approximately 500 μm, however, which is too coarse for micro-positioning. Additionally these print heads and cartridges are not capable of storing enough bioink to print large tissues, limiting the applications of this simple bioprinter.
Many efforts have been made to improve the stage resolution and enlarge the reservoir capacity. Screw-based servo stages with less than the 100 μm resolution in each direction were used to provide sub-micro meter positioning (Nishiyama et al. 2009; Arai et al. 2011). External jugs and bottles were modified and connected to multiple print heads to increase the maximum bioink capacity. After adopting high accuracy stages and larger reservoirs, this inkjet bioprinter was able to achieve 10 μm positioning accuracy and 20 picoliter droplet volume.
2.2 Laser-assisted printing
Laser-assisted printing originated from laser direct-write (Bohandy et al. 1986) and laser-induced transfer technologies (Duocastella et al. 2007; Kattamis et al. 2007). Figure 1C shows a schematic of laser-assisted printing. The critical part of the laser-assisted printing system is a donor layer that responds to laser stimulation. The donor layer comprises a ‘ribbon’ structure containing an energy-absorbing layer (e.g., titanium or gold) on the top and a layer of bioink solution suspended on the bottom. During printing, a focused laser pulse is applied to stimulate a small area of the absorbing layer. This laser pulse vaporizes a portion of the donor layer, creating a high-pressure bubble at the interface of the bioink layer and propelling the suspended bioink. The falling bioink droplet is collected on the receiving substrate and subsequently crosslinked. Compared to inkjet printing, laser-assisted printing can avoid direct contact between the dispenser and the bioinks. This non-contact printing method does not cause mechanical stress to the cells, which results in high cell viability (usually higher than 95%). In addition, laser-assisted printing can also print highly viscous materials, and more types of bioinks can be used than in inkjet printing. These features of laser bioprinting are promising, but the side effects of laser exposure on the cell are not yet fully understood. Moreover, laser diodes with high-resolution and intensity are expensive compared to other nozzle-based printing methods, and control of the laser printing system is complex, limiting the technique’s adoption.
Due to the high cost, there are few laser-assisted bioprinters, which are usually cumbersome and complex compared to other types of printers. A laser printing prototype was developed by combining optical laser sources with a lens (Nahmias et al. 2005). A more compact, high-throughput laser printing system was also built (Guillemot et al. 2010) and this system was further developed into a highly accurate version with 10 μm resolution (Guillotin et al. 2010). In addition to the high equipment cost, laser-assisted printing is still immature because of unexplored parameters affecting the droplet size and quality. Instead of building prototypes of laser-assisted bioprinters, more researchers have focused on investigating the relationships between laser parameters, such as wavelength, intensity, and pulse time, with the quality of printed patterns (Duan et al. 2013; Duarte Campos et al. 2013).
2.3 Extrusion printing
Extrusion printing is a modification of inkjet printing. In order to print to print the viscous materials inkjet printers cannot deposit, extrusion printing uses either an air-force pump or a mechanical screw plunger to dispense bioinks, as shown in Figure 1D. By applying a continuous force, extrusion printing can print uninterrupted cylindrical lines rather than a single bioink droplet. Almost all types of hydrogel pre-polymer solutions of varying viscosity as well as aggregates with high cell density can be printed with extrusion bioprinters. While extrusion bioprinters can print a wider range of materials, they also expose the encapsulated cells to larger mechanical stresses that are thought to reduced cell viability (Khalil and Sun 2007; Murphy and Atala 2014).
Most existing commercial bioprinters, including the Bioplotter (EnvisionTec, Gladbeck, Germany) and NovoGen 3D Bioprinting platform (Organovo, San Diego, USA), are based on extrusion technology. Extrusion bioprinting provides good compatibility with photo, chemical and thermal crosslinkable hydrogels of very different viscosities at a reasonable cost (Khalil and Sun 2007; Murphy and Atala 2014). A typical extrusion printer, the Multi-head tissue/organ building system from the Cho group, includes three-axis motion control with six dispensing heads, supporting up to six different bioinks (Lee et al. 2014). The substrate plate contains heating and cooling functions to control thermally sensitive hydrogels. Similar designs have been reported by two other groups (Chang et al. 2010; Bertassoni et al. 2014a). The latest versions of extrusion printers include tissue-vessel parallel printing (Ozbolat et al. 2014a) and parallel multi-bioink printing (Kolesky et al. 2014).
Dispensers in current extrusion systems have a few differences (Khalil and Sun 2007). Pneumatic micro nozzles powered by compressed gases support a wider range of viscosity, but have difficulty precisely controlling the deposited mass. Screw-based nozzles can print without inlet air and are much cheaper, but they experience problems in high viscosity dispensing.
2.4 Other technical approaches
While these three printing methods are most commonly used by bioprinting researchers, the bioprinting paradigm itself has been challenged and novel printing methodologies remain under investigation. Rather than directly printing tissues, Miller et al (Miller et al. 2012) used a pneumatically controlled syringe to print molten sugar glass in the shape of a desired vascular network. Once printed, this artificial vascular network was embedded within a variety of hydrogels and could then be dissolved to form open channels within cross-linked tissues. While this approach sacrificed the ability to carefully control the deposition of cells within the bulk matrix, it enabled previously unachieved engineered vascular complexity in a synthetic tissue.
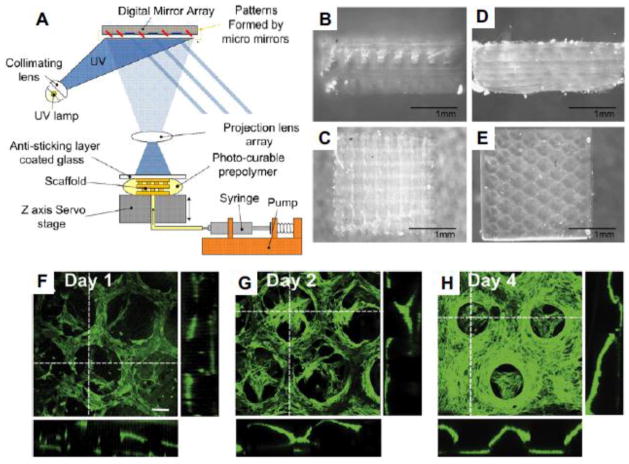
Stereolithography has also been modified for bioprinting purposes (Figure 1E) (Gauvin et al. 2012; Gou et al. 2014). Like laser-assisted printing, stereolithography bioprinters use light to selectively solidify a bioink in a layer-by-layer process that additively builds up objects (Figure 2A). These printers use a digital light projector to cure bioinks plane-by-plane and have several advantages over traditional bioprinting methods. No matter how complex pattern in one layer is, the printing time is same because the entire pattern is projected over the printing plane. As a result, the printer only needs a moveable stage in vertical direction, which significantly simplifies the control of the printer. This reported stereolithography bioprinting system can achieve 100 μm resolution and printing times less than one hour (Gauvin et al. 2012; Gou et al. 2014) while maintaining very high cell viability (>90%). Figure 2B–E show the woodpile and hexagonal structures printed by the stereolithography system. The fluorescent images of the hexagonal structures encapsulated with human umbilical vein endothelial cells (HUVEC) are given in Figure 2F–H. A recent advance in stereolithographic 3D printing technology by the DeSimone group (Tumbleston et al. 2015) referred to as “continuous liquid interface production (CLIP)” dramatically improved both resolution and printing time for some materials. While this has not yet been applied to bioprinting, it may be an approach that enables the formation of more complex tissue architectures.
Figure 2. Stereolithographic bioprinting.
(A) Schematic illustration of stereolithography system (Gauvin et al. 2012). (B)–(E) The side view of woodpile (B) and hexagonal (D), as well as the top view of woodpile (C) and hexagonal (E) structures generated by their stereolithography system. (F)–(H) 3D confocal images showing the proliferation of encapsulated HUVEC cells in day 1 (F), day 2 (G) and day 4 (H). (Scale bar: 100 μm)
2.5 Bioprinting CAD, Modeling, and the printing process
Bioprinters cannot print without instructions. To successfully create bioprinted tissues, it is necessary to generate the printing paths, select appropriate bioinks, control the bioprinter and perform quality control after printing (Murphy and Atala 2014). The typical bioprinting process is as follows: (1) designers draw the printing geometry and manually verify its feasibility; (2) designers select appropriate cell types and hydrogels, and load the bioinks into the bioprinting system (3) through control language and protocols, such as RS 274 (G-Code, Massachusetts Institute of Technology, Cambridge, USA) and LabView (National Instruments, Austin, USA), the designed paths are sent to the bioprinting system; (4) the bioprinter builds structures by depositing bioinks under the control of a computer; (5) bioprinted tissues are checked manually via microscopy after bioprinting. After the bioprinting process, successfully printed constructs are transferred to an incubator for culturing. The bioprinting process is not currently highly automated and many manual operations at a variety of steps can result in slow processing speeds and increase the chance for mistakes and errors. To ensure printing quality and to improve the printing process, many researchers have investigated computer-aided design (CAD) and modeling technology for bioprinting. These CAD techniques can utilize computer automation systems to assist and accelerate the design process.
Bioprinting models, like models used in conventional rapid prototyping, are often converted to the STereoLithography (STL) file format as an intermediate between model and print path generation (Mironov et al. 2009; Mondy et al. 2009). These files contain accurate surface information of complex 3D geometries, and can be designed via graphic user interfaces, or created from clinical images, including those from Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) (Keriquel et al. 2010; Arai et al. 2011). In a process analogous to histologic sectioning, printing paths are created by “slicing” these STL model into layers and creating bioprinter toolpaths that trace out the perimeter and interior features of each slice. The thickness of these layers is often referred to as the resolution of a particular printer and is usually in the range of 100–500 μm depending on the machine and material used. These toolpaths are the instructions read and executed by the bioprinter for each layer and can include material selections. Layers are formed sequentially and stacked as the model is built up in an additive process forming a 3D object from a collection of 2D layers. All other things being equal, smaller resolutions are associated with higher quality and longer print times.
Clinical images can provide information regarding the in vivo tissue distribution of patients, and anatomically realistic tissue geometries can be determined via image processing. Clinical image-based STLs therefore have the potential to become the starting point for on-demand tissue production in the future. In addition, a smart program was coded for planning and optimizing bioprinting experimental design (Weiss et al. 2005). In summary, the introduction of Bio-CAD techniques has significantly improved the automation of bioprinting path generation.
Computer aided techniques, known as bio-computer-aided-manufacturing (Bio-CAM), also play an important role during and after bioprinting. Bio-CAM aims to predict the feasibility of the fabrication process by simulating relevant physical models on computers. To simulate bioprinting, both classical formula calculations and the finite element method (FEM) are applied. Currently, the most widely used physical model for bioprinting is laminar multiphase flow. Although this model is oversimplified, ignoring complex issues generated by the inclusion of cells, simulations are still helpful for checking and optimizing the feasibility of specific designs. Many researchers are already attempting to model bioprinting results with the corresponding printing parameters. For extrusion printing, relationships between dispensing pressure, printing time, and nozzle diameter have been tested and modeled (Yu et al. 2013). Cell settling effects in inkjet printers, which are highly related to clogging and viscosity, change during printing and were modeled by both analytical and finite element methods (FEM) (Pepper et al. 2011, 2012). For laser printing, the effects of laser energy, substrate film thickness, and hydrogel viscosity on the viability of cells (Catros et al. 2011b), as well as droplet size (Duocastella et al. 2007; Mézel et al. 2010; Gruene et al. 2011b), cell differentiation (Gruene et al. 2011a), and cell proliferation (Gruene et al. 2011a) have been investigated. Some researchers also focused on post-printing modeling of cellular dynamics (McCune et al. 2014), fusion (Yang et al. 2012, 2013; Sun and Wang 2013; Thomas et al. 2014), deformation (Sim et al. 2007) and stiffness (Tirella et al. 2011; Mobed-Miremadi et al. 2012), as well as modeling of the typical types of printed tissues, including tumors (Zhao et al. 2014) and soft tissues (Zhang et al. 2013). Bio-CAM research not only provides a fast way to check design feasibility, but also gives designers a chance to better understand the physical and chemical principles governing printing. With the integration of Bio-CAD and Bio-CAM, an advanced design flow for bioprinting begins to take shape. Bio-CAD can accelerate the speed of the whole bioprinting process, and Bio-CAM can guarantee the quality of what is printed.
3. Materials for bioprinting
Bioinks typically consist of a hydrogel pre-polymer solution and cells. The desired properties of hydrogels are presented at the beginning of this section, and the characteristics of various types of crosslinkable hydrogels are summarized. Resources for the cells and materials used in bioprinting applications are briefly reviewed at the end.
3.1 Hydrogel bioink characteristics
Hydrogels play an essential role in bioprinting. They not only have direct contact with cells to provide structural support, but they also dominate the chemical and physical properties of bioinks (Williams 2008). Ideally, hydrogels used for bioprinting should be characterized by the properties described below.
3.1.1 Printability and crosslinkability
Printability refers to the relationships between bioinks and substrates that results in printing an accurate, high-quality pattern (Murphy and Atala 2014). In bioprinting, printability is usually associated with surface tension, which is measured by the contact angles between two media. Research has shown that the surface tension of supporting structures has significant and profound implications on cell attachment and development (Discher et al. 2005). To form 3D scaffolds, the printed hydrogel pre-polymer solution should not be too flat on the substrate. This means that the hydrogel pre-polymers are expected to maintain tension in the vertical direction and have a large contact angle with the substrate. Since glass slides and petri dishes are the most commonly employed substrates, ideal hydrogel pre-polymer solutions should be able to build highly vertical structures after printing on glass and plastic substrates. Unfortunately, most of the glass slide substrates have poor contact angles. This problem can be solved by coating the substrates with a thin layer of material, such as 3-(Trimethoxysilyl)propyl methacrylate (TMSPMA) (Zhang et al. 2008), to enhance their hydrophobicity before printing (Bauer et al. 2012; Nikkhah et al. 2012).
Printability is also influenced by how easily materials can be crosslinked. The three types of bioprinting technologies currently available are only capable of dispensing liquid materials and consequently hydrogels must be in liquid or paste-like form during printing. To accommodate different cell densities and printing technologies, the viscosity of the hydrogel pre-polymer solutions should be controllable over a wide range. At odds with this condition, bioinks must form a quasi-scaffold structure to support cell proliferation after printing. These conditions have effectively limited hydrogel pre-polymer solutions to either photo (Weiner et al. 2007; Nichol et al. 2010), chemically (Li et al. 2005; Glowacki and Mizuno 2008; Liu et al. 2009; Balakrishnan et al. 2012; Araujo et al. 2014), or thermally (Gao et al. 2012; Wu et al. 2012) crosslinkable polymers (Murphy et al. 2013; Bajaj et al. 2014).
3.1.2 Mechanical properties
Hydrogels should maintain sufficient mechanical properties after polymerization to provide the cells with a stable environment for attachment, proliferation and differentiation (Limpanuphap and Derby 2002; Murphy et al. 2013). These mechanical properties include strain, shear stress, compressive modulus and mass swelling ratio. It is well understood that cell adhesion is significantly affected by the dynamic interactions between cells and hydrogels (Dou et al. 2012; Benson et al. 2014). In fact, mechanical properties are considered to be highly essential for soft tissues, such as cartilage and skin, because the functions of such tissues mainly rely on their mechanical properties (Hutmacher 2000; Kim et al. 2012).
3.1.3 Biocompatibility and controllability of by-products and degradation
Biocompatibility refers to the ability of a material to perform with an appropriate host response in a specific situation (Hobkirk 1988). In general, for in vitro applications, biocompatibility requires that the material itself is not harmful to cell proliferation and has the ability to provide proper binding with cells (Williams 2008). For in vivo applications, biocompatibility adds the requirement that the material can be degraded by or integrated with the ECMs of cells without generating harmful by-products or having negative interactions with cells (Williams 2008). It is desirable for implanted tissue to eventually fuse with other in vivo tissues. Therefore, hydrogel scaffolds need to be degraded or integrated with the in vivo ECM environment and hydrogels with a natural and controllable degradation rate which is similar to the ECM growth rate is highly desired (Murphy and Atala 2014).
3.2 Bioinks
From the perspective of hydrogel design, there are basically two types of hydrogels: those based on natural polymers and those based on synthetic polymers (Zorlutuna et al. 2013). Natural hydrogels include polymers existing in ECM components, such as gelatin, collagen, laminin and fibronectin, as well as other natural polymers such as alginate, chitosan and silk fibroin. Interactions between natural hydrogels and cells have been well investigated (Zorlutuna et al. 2013). Synthetic polymers, unlike natural polymers, are made through chemical synthesis and are typically more controllable in terms of their chemical and mechanical properties (Zhu and Marchant 2011). Their interactions with and effects on cells, however, have not yet been studied systematically (Zorlutuna et al. 2013). Natural polymers are widely used in bioprinting research, but some researchers have used a combination of natural and synthetic polymers (Schuurman et al. 2011; Xu et al. 2013).
Decellularized extracellular matrices have been an increasingly promising material in tissue engineering as decellularization protocols have steadily improved. Recently, Pati et al (Pati et al. 2014) showed a that dECMs from three tissues could be solubilized into bioinks and bioprinted. While many bioinks are compositionally simple, dECM bioinks contain the diverse array of ECM components characteristic of different tissues and as a result, more closely resemble the native tissue. Although the mechanical properties of dECM bioinks do not mirror the original tissue, they represent a promising addition to the bioinks available in bioprinting.
On the synthetic side, there is significant interest in developing conductive biomaterials (Balint et al. 2014). Recently Jakus et al (Jakus et al. 2015) developed a printable high-content graphene:polyactide-co-glycolide bioink with high conductivity (800 S/m). Scaffolds printed with this material were able to support the growth of hMSC and have interesting possible applications in both biomedical devices and biologic scaffolds where enhanced conductivity is desirable. For example, conductive tracks through scaffolds could be pre-patterned in printed tissues simply by changing the bioink. This would complement a previously demonstrated method for installing these tracks by using α-hemolysin containing droplets (Villar et al. 2013). For a more complete discussion of existing biomaterials for bioprinting as well as the interaction and trade-off between desired hydrogel properties, we refer the reader to recent reviews (Bajaj et al. 2014; Skardal and Atala 2014).
3.3 Cells
To form a highly mimetic tissue or organ on a macro scale, bioprinted cells must proliferate. Two main factors are considered when selecting cells for bioprinting: how closely the bioprinted cells can mimic the physiological state of cells in vivo, and to what degree the bioprinted cells can maintain or develop their in vivo functions under optimized microenvironments (Murphy and Atala 2014). Artificial tissues are seeded by either printing functional primary cells with supporting cells (Keriquel et al. 2010; Cui et al. 2012a; Duan et al. 2013; Michael et al. 2013; Xu et al. 2013; Zhang et al. 2013; Dolati et al. 2014) or printing progenitors or stem cells for further differentiation (Gruene et al. 2011a; Xu et al. 2011; Duarte Campos et al. 2013; Hong et al. 2013; Owens et al. 2013; Visser et al. 2013). Direct printing of primary cells can rapidly increase the complexity of bioprinting. Since multiple types of cells embedded within the same or different hydrogels need to be printed in parallel, many bioinks need to be prepared for each print. Real-time alignment and printing step control are complicated by using many bioinks as each switch between bioinks has the possibility to introduce error into the bioprinting process. Printing with stem cells will usually reduce the total number of bioinks used for a given print, but also adds its own set of complications. Additional bioink formulations with different growth factor and small molecule signals may be desirable to attempt to guide site-specific differentiation. Even without this kind of approach, there is added difficulty in post-printing culture as growth factors and other differentiation stimulators must be deposited precisely to ensure the control of differentiation, especially when vascularization is desired.
Reliable cell sourcing poses a perennial problem to bioprinting. For clinical applications, cells for bioprinting would ideally be isolated from the patients themselves to avoid negative immune responses (Ozbolat and Yu 2013). Because not all types of cells can regenerate after damage (e.g. cardiac muscle cells), stem cells (e.g. adipose derived stem cells) with the ability to proliferate and differentiate into the desired cell types are the most promising cell source. Examples of some of the cell types and organ systems targeted by recent bioprinting publications are presented in Table 3.
Table 3.
Examples of bioprinted tissues and organs
| Tissue | Cell sources | Materials | Printing method | Reference |
|---|---|---|---|---|
| Vessel | Smooth muscle cells | Carbon nanotube encapsulated alginate | Extrusion | (Dolati et al. 2014) |
| Smooth muscle cells and aortic valve leaflet interstitial cells | Gelatin and alginate | Extrusion | (Duan et al. 2013) | |
| Human umbilical vein endothelial cells (HUVEC) | PEG-DA, Matrigel, fibrin gel, alginate, agarose, and GelMA | Extrusion | (Miller et al. 2012; Kolesky et al. 2014) | |
| Rat heart endothelial cells | Alginate | Extrusion | (Khalil and Sun 2009) | |
| Ea.hy926 endothelial cells | Nano-hydroxyapatite (n-HA) | Laser-assisted | (Catros et al. 2011b) | |
| Fibroblasts (L929), mouse endothelial cells and human mesenchymal stem cells | Acrylated hyaluronic acid-PEG (HA-PEG), and Matrigel | Inkjet | (Hong et al. 2013) | |
| HUVEC | GelMA | Stereolithography | (Gauvin et al. 2012) | |
| Bone | Mouse osteoblastic cells | n-HA | Inkjet | (Keriquel et al. 2010) |
| MG-63 cells | Alginate | Extrusion | (Loozen et al. 2013) | |
| Human osteoprogenitor cells | n-HA | Laser-assisted | (Catros et al. 2011a) | |
| Cartilage | Patient’s cartilage | poly(ethylene glycol) dimethacrylates (PEGDMA) | Inkjet | (Cui et al. 2012a) |
| Minced cartilage cells | Poly (ε-caprolactone) (PCL), and fibrin-collagen hydrogels | Inkjet | (Xu et al. 2013) | |
| Equine chondrocytes and mesenchymal stromal cells (MSCs) | PCL, GelMA, and GelMA-gellan hydrogels | Extrusion | (Visser et al. 2013) | |
| Human meniscus cells | GelMA | Stereolithography | (Grogan et al. 2013) | |
| Skin | NIH3T3 fibroblast, HaCaT keratinocytes | Collagen | Laser-assisted | (Michael et al. 2013) |
| Neuronal tissue | Mouse bone marrow stem cells | Collagen, and agarose | Extrusion | (Owens et al. 2013) |
| Embryonic stem cells | N/A | Inkjet | (Xu et al. 2011) | |
| Skeletal muscle | C2C12 mouse myoblasts | Polyurethane (PU), and PCL | Extrusion | (Merceron et al. 2015) |
| C2C12 mouse myoblasts | Alginate, and gelatin | Extrusion | (Zhang et al. 2013) | |
| Tumor | Hela cells | Gelatin-alginate- fibrinogen hydrogel | Extrusion | (Zhao et al. 2014) |
| Adipose tissue | Adipose derived stem cells | Alginate | Laser-assisted | (Gruene et al. 2011a) |
4. Applications of bioprinting
In this section, the current applications of bioprinting are reviewed in terms of several popular tissue types and its role in drug screening.
4.1 Vessels
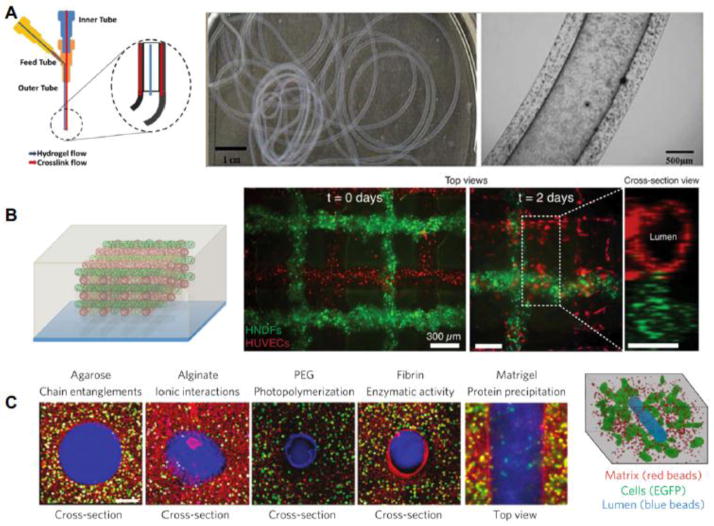
While the ability to create vascular features in bioprinted tissues is often limited, novel bioprinting techniques may resolve this problem. Dolati et al (Dolati et al. 2014), for example, utilized a coaxial nozzle system to print vascular conduits more than a meter long (Figure 3A). These carbon nanotube reinforced alginate conduits were perfusable and supported the growth of human coronary artery smooth muscle cells within the matrix. Using this technique, the authors were able to fabricate conduits with diameters in the sub-millimeter range, but did not show an ability to print closer to capillary diameters. Another possible solution is to add magnetically controlled nanoparticles to bioinks and use these to print vessels. With this technique the position of the vessels within tissues could then be controlled by applying a magnetic field (Mironov et al. 2008; Talelli et al. 2009). However, further research is needed to determine the efficiency and the potential effects of magnetic particles on cells and ECM. To reduce the size of vascular channels and to incorporate them directly into printed tissues, others have employed sacrificial inks to some success. Kolesky et al (Kolesky et al. 2014) used a Pluronic F127 fugitive bioink to print channels as small as 45 μm and were able to subsequently endothelialize them with HUVECs. This approach, combined with printing fibroblasts encapsulated in a gelatin methacrylate bioink, yielded multicellular bioprinted constructs (Figure 3B). Once the constructs were printed and crosslinked, the temperature was lowered to 4°C to liquefy and remove the Pluronic F127, leaving behind open vascular channels ready to be seeded. Previously, Miller et al (Miller et al. 2012) encapsulated and dissolved printed carbohydrate glass in various bulk extracellular matrices to form seedable channels as small as 150 μm (Figure 3C). Rather than dissolving away the sacrificial material, Bertassoni et al (Bertassoni et al. 2014b) cast hydrogels around printed agarose fibers and then aspirated or manually removed the fiber. The resulting lumen were perfusable and HUVEC could form an endothelial monolayer. These sacrificial techniques are exciting advances that may simplify not only the prepatterning of vascular features in bioprinted tissues, but also the speed at which large tissues can be printed.
Figure 3. Bioprinting strategies for vascularization.
(A) Fabrication of long (> 1 meter) vascular conduits using a coaxial nozzle system yielding internal lumen diameters below 1 mm (Dolati et al. 2014). (B) Pluronic F127 as a sacrificial bioink to form open lumens (red) while concurrently printing encapsulated cells around the vessels (green) (Kolesky et al. 2014). (C) Carbohydrate glass to cast vascular features into a variety of hydrogels, forming perfusable vessels that support cell growth (Miller et al. 2012). Figure adapted from (Miller et al. 2012; Dolati et al. 2014; Kolesky et al. 2014).
4.2 Bone and cartilage
The bone engineering space is interesting in that both conventional and bioprinting are poised to influence the field. Made to order metal 3D printed devices (Hsu and Ellington 2015), 3D printed models for surgical planning (Pietrabissa et al. 2015; Scawn et al. 2015), and 3D printed tools (Burleson et al. 2015) highlight some of the current and future biomedical applications of conventional 3D printing technologies. Bioprinting techniques have also been applied to bone tissue engineering. Yao et al (Yao et al. 2015) used anatomic data from CT scans of rabbits to print and test polycaprolactone-hydroxyapatite scaffolds which supported physiologically relevant loads. Wang et al (Wang et al. 2015a) printed poly(propylene fumarate) porous scaffolds, characterized the degradation process over a 224 day period, and showed the printed scaffolds were suitable for bone tissue engineering applications. Pati et al (Pati et al. 2015) enhanced the osteogenic potential of 3D-printed PCL/PLGA/β-TCP scaffolds by using human nasal inferior turbinate tissue-derived mesenchymal stromal cells to deposit bone-like ECM. After a brief culture period, the scaffolds were decellularized and then investigated both in vitro and in vivo where they showed improved osteoinductive and osteoconductive properties.
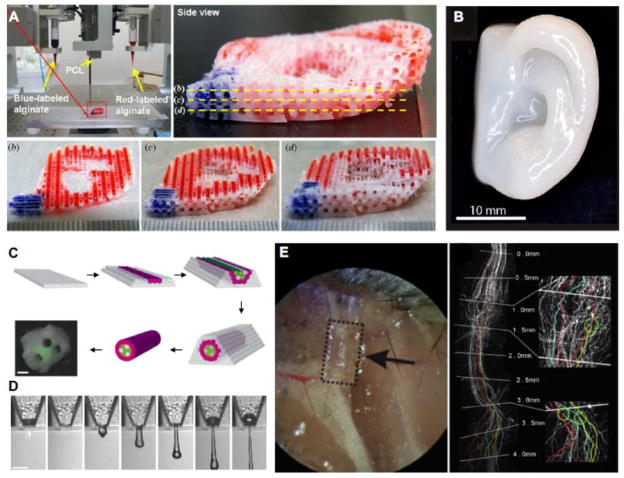
Cartilaginous tissues have also been an area of interest in tissue engineering (Tatman et al. 2015). Kundu et al (Kundu et al. 2013) printed alginate encapsulated chondrocytes with a supportive PCL structure and in vivo experiments suggested cartilage production. Lee et al (Lee et al. 2014) printed a PEG and PCL construct containing chondrocytes and showed this material mixture could be used to print ear-shaped constructs (Figure 4A). Similarly, Markstedt et al (Markstedt et al. 2015) developed a novel nanocellulose-alginate bioink with desirable printing properties. This ink supported the culture of human nasoseptal chondrocytes in printed tissues and could also be printed into complex shapes (Figure 4B). Collectively, studies like these highlight the promise of bioprinting to produce unique 3D structures suitable for bone and cartilage tissue engineering.
Figure 4. Examples of bioprinted tissues and organs.
(A) Printed ear-shaped PCL and alginate scaffolds with bioinks localized to certain tissue regions (Lee et al. 2014). (B) Cartilaginous ear scaffolds printed using a novel nanocellulose-alginate bioink supported human chondrocytes (Markstedt et al. 2015). (C) Fabrication of a synthetic nerve graft by printing cell-dense tubes of Schwann cells and BSMC (Owens et al. 2013). (D) Demonstration of the feasibility of printing mouse ganglion and glial cells (Lorber et al. 2014). (E) Printed PEG-based guidance conduits for nerve repair studies, showing their biocompatablity and efficacy (Pateman et al. 2015). Figure adapted from (Owens et al. 2013; Lee et al. 2014; Lorber et al. 2014; Markstedt et al. 2015; Pateman et al. 2015).
4.3 Neuronal tissues
Bioprinting nervous tissue is another application that has been explored by researchers. Large synthetic tissues will need to integrate with the host nervous system, and bioprinting may be a means to generate new nervous tissue or to enhance the innervation of tissue engineered constructs. Owens et al (Owens et al. 2013) printed a synthetic nerve graft using cells alone. Isolated mouse bone marrow stem cells and Schwann cells were cast into 500 μm diameter tubes and then loaded into a bioprinter which extruded discrete tubes to form a dense nerve conduit of Schwann cell tubes surrounded by mouse bone marrow stem cell tubes for use in animal studies (Figure 4C). These early stage proof-of-principle printed grafts performed similarly to control tissues and remain promising as the methodology is refined and improved. Lorber et al (Lorber et al. 2014) also provided important validation on the feasibility of printing cells of the nervous system, showing rat retinal ganglion cells and glia can be used in inkjet printing systems (Figure 4D). Pateman, Harding et al (Pateman et al. 2015) used a microsterolithographic technique to print PEG-based nerve guidance conduits for nerve repair studies (Figure 4E). Printed conduits had a finer resolution than those made through previously reported methods and performed comparably to autograft controls.
4.4 Construction of drug screening systems
Bioprinting is also promising in the design of drug screening systems. Compared to manual methods, bioprinting can deposit cells uniformly on the surface of micro devices. Such uniformity is highly desirable for testing and screening the interactions between cells and drugs (Huh and Kim 2015; Nam et al. 2015). Existing examples of bioprinted drug testing platforms include those for the liver (Snyder et al. 2011). Chang et al (Chang et al. 2010) developed an air-pressure based extrusion bioprinter to prototype a drug testing platform for the liver with alginate encapsulated immortalized hepatocytes. In this system, the authors were able to show differential drug metabolism. Snyder et al (Snyder et al. 2011) expanded on this system by printing microfluidic channels in a co-culture system of liver and mammary cells to investigate tissue damage from radiation. Bioprinting has also been used to seed cell layers uniformly on each side of the interface of micro devices for the formation of organ-on-a-chip devices (Chang et al. 2010). Organ-on-a-chip systems mimic parts of typical organ functions to investigate the interactions between drugs and their potential effects on tissues (Wang et al. 2015b). Bioprinting may play an important role in organ-on-a-chip technology, given it is a practical solution for the formation of uniform and highly controllable tissue layers at low cost.
5. Present limitations and future prospects
5.1 Current limitations for bioprinting
5.1.1 Limitations of the current bioprinting approach
Although these three common bioprinting techniques have different printing principles and features, there are a few limitations to the typical bioprinting process as it stands today. All three techniques are based on a layer-by-layer printing method, which generally have difficulty printing complex hollow structures. In the simplest case of printing with a single material, each layer must be connected and mechanically supported as it is printed. When voids are introduced in one layer, subsequent layers that deposit material over the void may collapse causing a cascade of offset features and inaccurate geometries. One possible solution to this problem is to incorporate a sacrificial material, which is a method widely employed in the fabrication of suspended structures in MEMS (Taylor et al. 2013; Bertassoni et al. 2014b). This sacrificial material provides the mechanical support each layer needs during fabrication and is then removed from the completed object in a post-processing step. This approach has been taken by several groups using several fugitive materials, including carbohydrate glass, (Miller et al. 2012) Pluronic F-127, (Kolesky et al. 2014) and gelatin microparticles (Hinton et al. 2015). The introduction of extra materials, however, can increase the complexity of the printing process as the bioprinting platform must support rapid material exchanges or multiple nozzles loaded with different inks. Sacrificial materials must be printable under conditions compatible with non-sacrificial biomaterials and cells, and their method of removal and breakdown products must be cytocompatible. These difficulties have likely limited the development and adoption of new sacrificial materials.
The lack of reliable methods to print pre-vascularized tissues is a hurdle that cannot be overlooked. This problem is not unique to bioprinting, but bioprinting is unique in its ability to create large tissues with high metabolic demands relatively quickly. Many of the small-scale tissues researchers currently print can survive through diffusion alone, but full-scale organs and large tissue constructs will require an embedded vasculature as well as mechanically robust conduits to connect to host arteries and veins. Small bioprinted tissues may take only minutes or hours to print, but the question of cell viability both within a pre-polymer bioink and within the polymerized early regions of large multi-day prints must be addressed. Self-assembly of vascular features is too slow a process to rely on when there is the threat of necrosis in partially assembled tissues still sitting on the printer. These sacrificial techniques represent the most promising approach in the current bioprinter’s toolbox, but innovation could lead to better printed tissues.
In addition to the difficulty in fabricating hollow vascular features, bioink preparation can take several days to weeks due to cell culturing and biomaterial synthesis (Murphy and Atala 2014). Once fully prepared, the working time of bioinks may also become an issue. This issue of time may be overcome by incorporating additional features into the bioprinter that support the maintenance of partially printed structures, the development of increasingly parallel bioprinters (e.g. multiple print heads working simultaneously) or other refinements to the printing process (e.g. CLIP (Tumbleston et al. 2015)). Faster bioprinters with higher resolution would be poised to solve some of the problems faced by modern technology.
5.1.2 Cell and material limitations
Material selection remains a major concern and limitation for bioprinting. More biomimetic materials like dECM bioinks often lack the mechanical strength to be the sole material in printed tissues, requiring support from stronger but less bioactive inks, like PCL (Ousterout et al. 2013). Tunable bioinks with a wide range of material properties could be a solution to this problem and may be achievable through the creation of new composite mixtures to enhance crosslinking or other desirable features while maintaining the properties of the base bioink. Poly(ethylene glycol) (PEG) has received attention because of its tunable mechanics (Zustiak and Leach 2010; Kim et al. 2014b) and represents a suitable component for composite bioinks. The Khademhosseini group developed PEG:gelatin methacrylate (PEG:GelMA) and carbon nanotube-incorporated photocrosslinkable gelatin methacrylate (CNT:GelMA) composites with tunable mechanical and degradation properties that could have such applications (Shin et al. 2013). Similarly, the West group (Zhang et al. 2015) developed a low molecular weight-high molecular weight PEG composite which could mimic the anisotropy of heart valve leaflet moduli. These kind of composites further expand the options available to researchers in bioprinting, and may lead to more complex and biomimetic structures.
Incorporating multiple materials also remains a challenge. For most bioprinters, materials to be printed are prepared in bulk before printing begins and switching materials involves changing to secondary pre-loaded reservoirs (e.g. a separate syringe or bioink cartridge). For example, the commercially available 3-D Bioplotter® (EnvisionTEC) is limited to three material cartridges for a single print job. While this approach enables multi-material printing, it makes creating smooth gradients of cells or growth factors impossible or arduous due to the need to prepare many independent solutions. To address this, the Lewis group recently developed an impeller based active mixing system for use in extrusion style printers (Ober et al. 2015). The inclusion of active mixing would reduce the number of solutions that need to be prepared and can enable more precise control over the concentration of deposited components. Although this does introduce some non-trivial complexity to the printing system, the benefits of on the fly mixing are significant. Such a system may also alleviate other concerns for long prints where cell suspensions, pre-polymer solutions, growth factors and other components can be stored in independently controlled reservoirs optimized for their contents.
5.2 Future prospects
In the future, bioprinting may be considered as much a nano-biofabrication technique as a tool for artificial organ generation. Due to its advantages on the micrometer scale, and highly controllable dispensing of live cells, bioprinting may fill a vital role in biofabrication. Bioprinting can be applied wherever the deposition or integration of live cells is desired. Bio-sensors (Xu et al. 2009a) and protein and DNA arrays of stem cells (Tasoglu and Demirci 2013) have already been fabricated by bioprinting. These diverse applications illustrate the versatility and potential of bioprinting as a technology still in its infancy. Moreover, bioprinting remains a promising solution for addressing the growing international organ shortage. The ability to generate tissues for transplant on-demand with reduced immune response risk holds significant promise in the fabrication of artificial organs. Recent progress in hydrogel science, including the development of dynamic switchable hydrogels (Gillette et al. 2010) and oxygen producing hydrogels (Harrison et al. 2007), provide researchers with more and more methods to control cell microenvironments. In order to realize the potential of bioprinting and rapid prototyping, the printing speed, characteristics of hydrogels, preparation time for cells and hydrogels, vascularization of tissues, innervation of tissues, and the controllability of on-demand scaffold and cell maturation must be improved further. As the technology matures, bioprinting is poised to become a key technique in the fabrication of human-on-a-chip systems as well as on-demand anatomically realistic artificial organs.
6. Conclusions
Bioprinting is an advanced fabrication technique for the dispensing of cell-laden hydrogels, with a bright future accompanying numerous challenges and problems. Bioprinting has shown great potential in tissue engineering applications at its early research stage where many in vitro and even in vivo experiments have already hinted at the feasibility of bioprinted artificial organs. Due to advantages in micro scale, high-throughput, cell deposition, the applications of bioprinting are expanding rapidly. Bioprinting has become a strong fabrication tool to create complex micro- and macro-scale biomedical systems. Even with the progress that has been made, bioprinting remains an emerging and growing technology with incredible potential.
Acknowledgments
This work was supported by a National Institutes of Health R21 Grant (R21AR064395) and a Muscular Dystrophy Association Research Grant (MDA255907). This work was also supported by the Natural Sciences and Engineering Research Council of Canada Discovery Grant (Application No. RGPIN-2014-04010)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouna GM. Organ Shortage Crisis: Problems and Possible Solutions. Transplant Proc. 2008;40(1):34–8. doi: 10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Greiner A, Wendorff JH. Functional materials by electrospinning of polymers. Prog Polym Sci. 2013;38(6):963–91. [Google Scholar]
- Arai K, Iwanaga S, Toda H, Genci C, Nishiyama Y, Nakamura M. Three-dimensional inkjet biofabrication based on designed images. Biofabrication. 2011:034113. doi: 10.1088/1758-5082/3/3/034113. [DOI] [PubMed] [Google Scholar]
- Araujo JV, Davidenko N, Danner M, Cameron RE, Best SM. Novel porous scaffolds of pH responsive chitosan/carrageenan-based polyelectrolyte complexes for tissue engineering. J Biomed Mater Res - Part A. 2014:4415–26. doi: 10.1002/jbm.a.35128. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D Biofabrication Strategies for Tissue Engineering and Regenerative Medicine. Annu Rev Biomed Eng. 2014;16:247–76. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Jayakrishnan A, Kumar SSP, Nandkumar AM. Anti-bacterial properties of an in situ forming hydrogel based on oxidized alginate and gelatin loaded with gentamycin. Trends Biomater Artif Organs. 2012;26(3):139–45. [Google Scholar]
- Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10(6):2341–53. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Ballyns JJ, Gleghorn JP, Niebrzydowski V, Rawlinson JJ, Potter HG, Maher SA, et al. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng Part A. 2008;14(7):1195–202. doi: 10.1089/ten.tea.2007.0186. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kim K, Qiu Y, Calpe B, Khademhosseini A, Liao R, et al. Spot identification and quality control in cell-based microarrays. ACS Comb Sci. 2012;14(8):471–7. doi: 10.1021/co300039w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson K, Galla HJ, Kehr NS. Cell adhesion behavior in 3D hydrogel scaffolds functionalized with D - Or L -aminoacids. Macromol Biosci. 2014;14(6):793–8. doi: 10.1002/mabi.201300485. [DOI] [PubMed] [Google Scholar]
- Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo Wa, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014a;6(2):024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014b;14(13):2202–11. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012:6020–41. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- Bohandy J, Kim BF, Adrian FJ. Metal deposition from a supported metal film using an excimer laser. J Appl Phys. 1986;60(4):1538–9. [Google Scholar]
- Bracci R, Maccaroni E, Cascinu S. Transient sunitinib resistance in gastrointestinal stromal tumors. N Engl J Med. 2013;368(21):2042–3. doi: 10.1056/NEJMc1301237. [DOI] [PubMed] [Google Scholar]
- Burleson S, Baker J, Hsia AT, Xu Z. Use of 3D printers to create a patient-specific 3D bolus for external beam therapy. J Appl Clin Med Phys. 2015;16(3):5247. doi: 10.1120/jacmp.v16i3.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catros S, Fricain J-C, Guillotin B, Pippenger B, Bareille R, Remy M, et al. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication. 2011a;3(2):025001. doi: 10.1088/1758-5082/3/2/025001. [DOI] [PubMed] [Google Scholar]
- Catros S, Guillotin B, Bačáaková M, Fricain JC, Guillemot F. Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by laser-assisted bioprinting. Appl Surf Sci. 2011b:5142–7. [Google Scholar]
- Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res - Part B Appl Biomater. 2011:160–70. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R, Emami K, Wu H, Sun W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication. 2010;2(4):045004. doi: 10.1088/1758-5082/2/4/045004. [DOI] [PubMed] [Google Scholar]
- Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng Part A. 2012a:1304–12. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Breitenkamp K, Lotz M, D’Lima D. Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol Bioeng. 2012b;109(9):2357–68. doi: 10.1002/bit.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Gao G, Qiu Y. Accelerated myotube formation using bioprinting technology for biosensor applications. Biotechnol Lett. 2013;35(3):315–21. doi: 10.1007/s10529-012-1087-0. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Dolati F, Yu Y, Zhang Y, De Jesus AM, Sander EA, Ozbolat IT. In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology. 2014;25(14):145101. doi: 10.1088/0957-4484/25/14/145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou X-Q, Yang X-M, Li P, Zhang Z-G, Schönherr H, Zhang D, et al. Novel pH responsive hydrogels for controlled cell adhesion and triggered surface detachment. Soft Matter. 2012:9539. [Google Scholar]
- Duan B, Hockaday La, Kang KH, Butcher JT. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res - Part A. 2013;101 A(5):1255–64. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte Campos DF, Blaeser A, Weber M, Jäkel J, Neuss S, Jahnen-Dechent W, et al. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication. 2013;5(1):015003. doi: 10.1088/1758-5082/5/1/015003. [DOI] [PubMed] [Google Scholar]
- Duocastella M, Colina M, Fernández-Pradas JM, Serra P, Morenza JL. Study of the laser-induced forward transfer of liquids for laser bioprinting. Appl Surf Sci. 2007;253(19):7855–9. [Google Scholar]
- Gao H, Sun LN, Wu YP. Preparation and Dimming Performance Study of PNIPAm Thermal Hydrogel. Appl Mech Mater. 2012;236–237:99–104. [Google Scholar]
- Gauvin R, Chen Y-C, Lee JW, Soman P, Zorlutuna P, Nichol JW, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33(15):3824–34. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette BM, Jensen Ja, Wang M, Tchao J, Sia SK. Dynamic hydrogels: Switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv Mater. 2010;22(6):686–91. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–44. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, et al. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat Commun. 2014;5:3774. doi: 10.1038/ncomms4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan SP, Chung PH, Soman P, Chen P, Lotz MK, Chen S, et al. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013;9(7):7218–26. doi: 10.1016/j.actbio.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene M, Pflaum M, Deiwick A, Koch L, Schlie S, Unger C, et al. Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication. 2011a;3(1):015005. doi: 10.1088/1758-5082/3/1/015005. [DOI] [PubMed] [Google Scholar]
- Gruene M, Unger C, Koch L, Deiwick A, Chichkov B. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed Eng Online. 2011b;10(1):19. doi: 10.1186/1475-925X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010:2494–500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–6. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28(31):4628–34. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9):e1500758–e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobkirk JA. Definitions in biomaterials. Clin Mater. 1988:261. [Google Scholar]
- Hong S, Song SJ, Lee JY, Jang H, Choi J, Sun K, et al. Cellular behavior in micropatterned hydrogels by bioprinting system depended on the cell types and cellular interaction. J Biosci Bioeng. 2013;116(2):224–30. doi: 10.1016/j.jbiosc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Hsu AR, Ellington JK. Patient-Specific 3-Dimensional Printed Titanium Truss Cage With Tibiotalocalcaneal Arthrodesis for Salvage of Persistent Distal Tibia Nonunion. Foot Ankle Spec. 2015 doi: 10.1177/1938640015593079. [DOI] [PubMed] [Google Scholar]
- Huang TQ, Qu X, Liu J, Chen S. 3D printing of biomimetic microstructures for cancer cell migration. Biomed Microdevices. 2014;16(1):127–32. doi: 10.1007/s10544-013-9812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh DD, Kim D-H. JALA special issue: microengineered cell- and tissue-based assays for drug screening and toxicology applications. J Lab Autom. 2015;20(2):79–81. doi: 10.1177/2211068215574458. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- Jakus AE, Secor EB, Rutz AL, Jordan SW, Hersam MC, Shah RN. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano. 2015;9(4):4636–48. doi: 10.1021/acsnano.5b01179. [DOI] [PubMed] [Google Scholar]
- Jiao A, Trosper NE, Yang HS, Kim J, Tsui JH, Frankel SD, et al. Thermoresponsive nanofabricated substratum for the engineering of three-dimensional tissues with layer-by-layer architectural control. ACS Nano. 2014;8:4430–9. doi: 10.1021/nn4063962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattamis NT, Purnick PE, Weiss R, Arnold CB. Thick film laser induced forward transfer for deposition of thermally and mechanically sensitive materials. Appl Phys Lett. 2007;91(17):171120. [Google Scholar]
- Ker EDF, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, et al. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32(32):8097–107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amédée J, et al. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication. 2010;2(1):014101. doi: 10.1088/1758-5082/2/1/014101. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Tissue Engineering Special Feature: Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci. 2006;103(8):2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil S, Sun W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater Sci Eng C. 2007;27(3):469–78. [Google Scholar]
- Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J Biomech Eng. 2009;131(11):111002. doi: 10.1115/1.3128729. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107:565–70. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E-S, Ahn EH, Dvir T, Kim D-H. Emerging nanotechnology approaches in tissue engineering and regenerative medicine. Int J Nanomedicine. 2014a;9(Suppl 1):1–5. doi: 10.2147/IJN.S61212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HN, Jiao A, Hwang NS, Kim MS, Kang DH, Kim D-H, et al. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev. 2013;65(4):536–58. doi: 10.1016/j.addr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HN, Kang D-H, Kim MS, Jiao A, Kim D-H, Suh K-Y. Patterning methods for polymers in cell and tissue engineering. Ann Biomed Eng. 2012;40(6):1339–55. doi: 10.1007/s10439-012-0510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Yuan A, Nam K-H, Jiao A, Kim D-H. Fabrication of poly(ethylene glycol): gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication. 2014b;6:024112. doi: 10.1088/1758-5082/6/2/024112. [DOI] [PubMed] [Google Scholar]
- Kolesky DB, Truby RL, Gladman aS, Busbee Ta, Homan Ka, Lewis Ja. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–30. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- Kundu J, Shim J-H, Jang J, Kim S-W, Cho D-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lee J-S, Hong JM, Jung JW, Shim J-H, Oh J-H, Cho D-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2):024103. doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–28. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Limpanuphap S, Derby B. Manufacture of biomaterials by a novel printing process. J Mater Sci Mater Med. 2002:1163–6. doi: 10.1023/a:1021146106442. [DOI] [PubMed] [Google Scholar]
- Liu X, Smith La, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30(12):2252–8. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loozen LD, Wegman F, Öner FC, Dhert WJa, Alblas J. Porous bioprinted constructs in BMP-2 non-viral gene therapy for bone tissue engineering. J Mater Chem B. 2013;1(48):6619. doi: 10.1039/c3tb21093f. [DOI] [PubMed] [Google Scholar]
- Lorber B, Hsiao W-K, Hutchings IM, Martin KR. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2014;6(1):015001. doi: 10.1088/1758-5082/6/1/015001. [DOI] [PubMed] [Google Scholar]
- Lu T, Li Y, Chen T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int J Nanomedicine. 2013;8:337–50. doi: 10.2147/IJN.S38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015;16(5):1489–96. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- Mattimore JP, Groff RE, Burg T, Pepper ME. A general purpose driver board for the HP26 ink-jet cartridge with applications to bioprinting. Conf Proc - IEEE SOUTHEASTCON. 2010:510–3. [Google Scholar]
- McCune M, Shafiee A, Forgacs G, Kosztin I. Predictive modeling of post bioprinting structure formation. Soft Matter. 2014;10(11):1790. doi: 10.1039/c3sm52806e. [DOI] [PubMed] [Google Scholar]
- Merceron TK, Burt M, Seol Y-J, Kang H-W, Lee SJ, Yoo JJ, et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication. 2015;7(3):035003. doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- Mézel C, Souquet A, Hallo L, Guillemot F. Bioprinting by laser-induced forward transfer for tissue engineering applications: jet formation modeling. Biofabrication. 2010;2(1):014103. doi: 10.1088/1758-5082/2/1/014103. [DOI] [PubMed] [Google Scholar]
- Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, et al. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS One. 2013;8(3):e57741. doi: 10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11(9):768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov V, Kasyanov V, Markwald RR. Nanotechnology in vascular tissue engineering: from nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008:338–44. doi: 10.1016/j.tibtech.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Mironov V, Zhang J, Gentile C, Brakke K, Trusk T, Jakab K, et al. Designer “blueprint” for vascular trees: morphology evolution of vascular tissue constructs. Virtual Phys Prototyp. 2009:63–74. [Google Scholar]
- Mobed-Miremadi M, Asi B, Parasseril J, Wong E, Tat M, Shan Y. Comparative diffusivity measurements for alginate-based atomized and inkjet-bioprinted artificial cells using fluorescence microscopy. Artif Cells, Blood Substitutes Biotechnol. 2012;41(3):1–6. doi: 10.3109/10731199.2012.716064. [DOI] [PubMed] [Google Scholar]
- Mondy WL, Cameron D, Timmermans J-P, De Clerck N, Sasov A, Casteleyn C, et al. Computer-aided design of microvasculature systems for use in vascular scaffold production. Biofabrication. 2009;1(3):035002. doi: 10.1088/1758-5082/1/3/035002. [DOI] [PubMed] [Google Scholar]
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res - Part A. 2013;101 A(1):272–84. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005;92(2):129–36. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- Nam K-H, Smith AST, Lone S, Kwon S, Kim D-H. Biomimetic 3D Tissue Models for Advanced High-Throughput Drug Screening. J Lab Autom. 2015;20(3):201–15. doi: 10.1177/2211068214557813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials. 2012;33(35):9009–18. doi: 10.1016/j.biomaterials.2012.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Nakamura M, Henmi C, Yamaguchi K, Mochizuki S, Nakagawa H, et al. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009;131(3):035001. doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- Ober TJ, Foresti D, Lewis JA. Active mixing of complex fluids at the microscale. Proc Natl Acad Sci U S A. 2015;112(40):12293–8. doi: 10.1073/pnas.1509224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff ND, Truong C, Cira NJ, Koo S, Hamilton AL, Choi S, et al. Integrated bioprinting and imaging for scalable, networkable desktop experimentation. RSC Adv. 2014;4(65):34721. [Google Scholar]
- Ousterout DG, Perez-Pinera P, Thakore PI, Kabadi AM, Brown MT, Qin X, et al. Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchenne muscular dystrophy patients. Mol Ther. 2013;21(9):1718–26. doi: 10.1038/mt.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens CM, Marga F, Forgacs G, Heesch CM. Biofabrication and testing of a fully cellular nerve graft. Biofabrication. 2013;5(4):045007. doi: 10.1088/1758-5082/5/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbolat IT, Chen H, Yu Y. Development of “Multi-arm Bioprinter” for hybrid biofabrication of tissue engineering constructs. Robot Comput Integr Manuf. 2014a;30(3):295–304. [Google Scholar]
- Ozbolat IT, Chen H, Yu Y. Development of “Multi-arm Bioprinter” for hybrid biofabrication of tissue engineering constructs. Robot Comput Integr Manuf. 2014b;30(3):295–304. [Google Scholar]
- Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–9. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- Pateman CJ, Harding AJ, Glen A, Taylor CS, Christmas CR, Robinson PP, et al. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials. 2015;49:77–89. doi: 10.1016/j.biomaterials.2015.01.055. [DOI] [PubMed] [Google Scholar]
- Pati F, Jang J, Ha D-H, Won Kim S, Rhie J-W, Shim J-H, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati F, Song T-H, Rijal G, Jang J, Kim SW, Cho D-W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230–41. doi: 10.1016/j.biomaterials.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Pepper ME, Seshadri V, Burg T, Booth BW, Burg KJL, Groff RE. Cell settling effects on a thermal inkjet bioprinter. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2011:3609–12. doi: 10.1109/IEMBS.2011.6090605. [DOI] [PubMed] [Google Scholar]
- Pepper ME, Seshadri V, Burg TC, Burg KJL, Groff RE. Characterizing the effects of cell settling on bioprinter output. Biofabrication. 2012:011001. doi: 10.1088/1758-5082/4/1/011001. [DOI] [PubMed] [Google Scholar]
- Pietrabissa A, Marconi S, Peri A, Pugliese L, Cavazzi E, Vinci A, et al. From CT scanning to 3-D printing technology for the preoperative planning in laparoscopic splenectomy. Surg Endosc. 2015 doi: 10.1007/s00464-015-4185-y. [DOI] [PubMed] [Google Scholar]
- Scawn RL, Foster A, Lee BW, Kikkawa DO, Korn BS. Customised 3D Printing: An Innovative Training Tool for the Next Generation of Orbital Surgeons. Orbit. 2015:1–4. doi: 10.3109/01676830.2015.1049367. [DOI] [PubMed] [Google Scholar]
- Schuurman W, Khristov V, Pot MW, van Weeren PR, Dhert WJa, Malda J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3(2):021001. doi: 10.1088/1758-5082/3/2/021001. [DOI] [PubMed] [Google Scholar]
- Shapira A, Kim D-H, Dvir T. Advanced micro- and nanofabrication technologies for tissue engineering. Biofabrication. 2014;6(2):020301. doi: 10.1088/1758-5082/6/2/020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7(3):2369–80. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JH, Puria S, Steele CR. Mechanics of Materials and Structures. 2007;2(October) [Google Scholar]
- Singh M, Haverinen HM, Dhagat P, Jabbour GE. Inkjet printing-process and its applications. Adv Mater. 2010;22(6):673–85. doi: 10.1002/adma.200901141. [DOI] [PubMed] [Google Scholar]
- Skardal A, Atala A. Biomaterials for Integration with 3-D Bioprinting. Ann Biomed Eng. 2014;43(3):730–46. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, et al. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication. 2011;3(3):034112. doi: 10.1088/1758-5082/3/3/034112. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Q. Modeling and simulations of multicellular aggregate self-assembly in biofabrication using kinetic Monte Carlo methods. Soft Matter. 2013;9(7):2172. [Google Scholar]
- Talelli M, Rijcken CJF, Lammers T, Seevinck PR, Storm G, Van Nostrum CF, et al. Superparamagnetic iron oxide nanoparticles encapsulated in biodegradable thermosensitive polymeric micelles: Toward a targeted nanomedicine suitable for image-guided drug delivery. Langmuir. 2009;25(4):2060–7. doi: 10.1021/la8036499. [DOI] [PubMed] [Google Scholar]
- Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol. 2013:10–9. doi: 10.1016/j.tibtech.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatman PD, Gerull W, Sweeney-Easter S, Davis JI, Kim D-H, Gee A. Multi-scale Biofabrication of Articular Cartilage: Bioinspired and Biomimetic Approaches. Tissue Eng Part B Rev. 2015 doi: 10.1089/ten.TEB.2015.0142. [DOI] [PubMed] [Google Scholar]
- Taylor RE, Kim K, Sun N, Park SJ, Sim JY, Fajardo G, et al. Sacrificial layer technique for axial force post assay of immature cardiomyocytes. Biomed Microdevices. 2013;15(1):171–81. doi: 10.1007/s10544-012-9710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GL, Mironov V, Nagy-Mehez A, Mombach JCM. Dynamics of cell aggregates fusion: Experiments and simulations. Phys A Stat Mech its Appl. 2014;395:247–54. [Google Scholar]
- Tirella A, Vozzi F, De Maria C, Vozzi G, Sandri T, Sassano D, et al. Substrate stiffness influences high resolution printing of living cells with an ink-jet system. J Biosci Bioeng. 2011;112(1):79–85. doi: 10.1016/j.jbiosc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, et al. Continuous liquid interface production of 3D objects. Science (80-) 2015;347(6228):1349–52. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- Villar G, Graham AD, Bayley H. A tissue-like printed material. Science. 2013;340(6128):48–52. doi: 10.1126/science.1229495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJa, Melchels FPW, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5(3):035007. doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- Wang MO, Piard CM, Melchiorri A, Dreher ML, Fisher JP. Evaluating changes in structure and cytotoxicity during in vitro degradation of three-dimensional printed scaffolds. Tissue Eng Part A. 2015a;21(9–10):1642–53. doi: 10.1089/ten.tea.2014.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication. 2016 doi: 10.1088/1758-5090/7/4/045009. In press. [DOI] [PubMed] [Google Scholar]
- Wang Z, Roya S, Kyo-in K, Kim K. Organ-on-a-Chip Platforms for Drug Delivery and Cell Characterization: A Review. Sensors Mater. 2015b;27(6):487–506. [Google Scholar]
- Weiner Aa, Shuck DM, Bush JR, Prasad Shastri V. In vitro degradation characteristics of photocrosslinked anhydride systems for bone augmentation applications. Biomaterials. 2007;28(35):5259–70. doi: 10.1016/j.biomaterials.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Weiss LE, Amon CH, Finger S, Miller ED, Romero D, Verdinelli I, et al. Bayesian computer-aided experimental design of heterogeneous scaffolds for tissue engineering. CAD Comput Aided Des. 2005;37(11):1127–39. [Google Scholar]
- Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wei W, Zhou M, Wang Y, Wu J, Ma G, et al. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33(7):2351–60. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- Xu C, Chai W, Huang Y, Markwald RR. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioeng. 2012;109(12):3152–60. doi: 10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- Xu F, Moon S, Emre AE, Lien C, Turali ES, Demirci U. Cell bioprinting as a potential high-throughput method for fabricating Cell-Based Biosensors (CBBs) Proc IEEE Sensors. 2009a:387–91. [Google Scholar]
- Xu F, Sridharan B, Wang S, Gurkan UA, Syverud B, Demirci U. Embryonic stem cell bioprinting for uniform and controlled size embryoid body formation. Biomicrofluidics. 2011;5(2):22207. doi: 10.1063/1.3580752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Baicu C, Aho M, Zile M, Boland T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication. 2009b;1(3):035001. doi: 10.1088/1758-5082/1/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1):015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26(1):93–9. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Yang X, Mironov V, Wang Q. Modeling fusion of cellular aggregates in biofabrication using phase field theories. J Theor Biol. 2012;303:110–8. doi: 10.1016/j.jtbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Yang X, Sun Y, Wang Q. A phase field approach for multicellular aggregate fusion in biofabrication. J Biomech Eng. 2013;135(7):71005. doi: 10.1115/1.4024139. [DOI] [PubMed] [Google Scholar]
- Yao Q, Wei B, Guo Y, Jin C, Du X, Yan C, et al. Design, construction and mechanical testing of digital 3D anatomical data-based PCL-HA bone tissue engineering scaffold. J Mater Sci Mater Med. 2015;26(1):5360. doi: 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang Y, Martin Ja, Ozbolat IT. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J Biomech Eng. 2013;135(9):91011. doi: 10.1115/1.4024575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu K, Ai H, Chen D, Xv L, Chen M. Synthesis, characterization and solution properties of hydrophobically modified polyelectrolyte poly(AA-co-TMSPMA) J Solution Chem. 2008;37(8):1137–48. [Google Scholar]
- Zhang T, Yan KC, Ouyang L, Sun W. Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication. 2013;5(4):045010. doi: 10.1088/1758-5082/5/4/045010. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu B, Puperi DS, Yonezawa AL, Wu Y, Tseng H, et al. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015;14:11–21. doi: 10.1016/j.actbio.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 2014;6(3):035001. doi: 10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109(24):9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8(5):607–26. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorlutuna P, Vrana NE, Khademhosseini A. The expanding world of tissue engineering: The building blocks and new applications of tissue engineered constructs. IEEE Rev Biomed Eng. 2013;6:47–62. doi: 10.1109/RBME.2012.2233468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zustiak SP, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11(5):1348–57. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]