Abstract
The dentate gyrus (DG) is a hippocampal region that has long been characterized as a critical mediator of enduring memory formation and retrieval. As such, there is a wealth of studies investigating this area. Most of these studies have either treated the DG as a homogeneous structure, or examined differences in neurons along the septal-temporal axis. Recent data, however, have indicated that a functional distinction exists between the suprapyramidal and infrapyramidal blades of the DG, with the former showing more robust responses during spatial tasks. To date, few anatomical studies have addressed this functional gradient in rats, and no study has done so in the mouse. To address this, we investigated dendritic morphology and spine density in hippocampal granule cells of rats and mice using the Golgi-Cox technique. We find that granule cells from the suprapyramidal blade of the DG contains greater dendritic material in the region receiving spatial information from the medial perforant path. This provides a potential anatomical substrate for the asymmetric response of the DG to spatial input.
Keywords: dendrites, spines, Sholl, Golgi, hippocampus
Graphical Abstract
Introduction
A wealth of evidence links the hippocampus with episodic memory (reviewed in Davachi, 2006; Manns and Eichenbaum, 2006; Squire and Wixted, 2011). Consistent with the complexity of memory phenomena, individual regions of the hippocampal formation are thought to make unique contributions to memory encoding and retrieval. Most theories on the contribution of the dentate gyrus (DG) emphasize pattern separation. That is, the DG is thought to extract unique information from events to differentiate their internal representation from similar memories (Aimone et al., 2011; Schmidt et al., 2012).
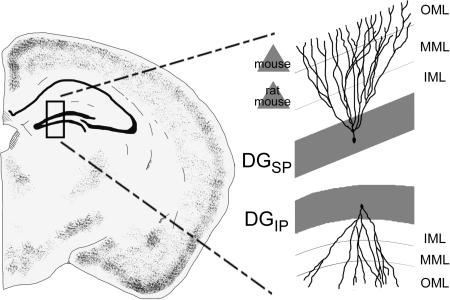
Consistent with the critical role the DG plays in memory, a great deal of research has investigated the structure and function of this region. The majority of this work, however, has either treated the DG as a homogeneous structure (e.g., Treves et al., 2008), or has examined the functional gradient from the septal to temporal poles of the hippocampus, emphasizing spatial information in the former and non-spatial associations like those that drive fear conditioning in the latter (e.g., Kesner, 2013). Although this is an important functional gradient within the hippocampus, recent data show that an additional gradient may exist along the transverse axis of the DG. Along this axis, the DG may be divided into the suprapyramidal (DGsp) and infrapyramidal (DGip) blades (Fig. 1a). Although unit recordings have been performed almost exclusively in the DGsp, immediate-early gene markers suggest that granule cells in the DGsp are an order of magnitude more active during spatial tasks than the neighboring DGip (Chawla et al., 2005; Ramirez-Amaya et al., 2006; Marrone et al., 2012a, 2012b; Satvat et al., 2012). Moreover, the blunted response of the DGip occurs much later, typically several hours following behavior (Ramirez-Amaya et al., 2005; 2013). These observations have renewed interest in the factors mediating this functional gradient, including anatomical differences between the DGsp and DGip.
Figure 1.
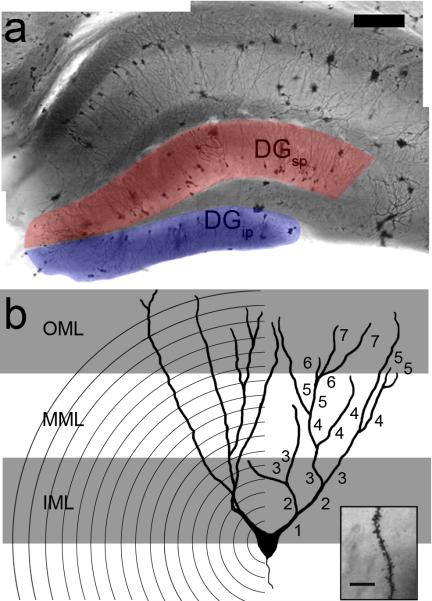
Overview of the methods used in the current experiment. (a) Following Golgi-Cox staining, granule cells were selected from the suprapyramidal (DGsp, red overlay) and infrapyramidal (DGip, blue overlay) blades of the dentate gyrus (shown in a, scale bar = 200 μm). (b) Schematic of a granule cell demonstrating each of the parameters measured. The size of the dendritic arbor was measured using the Sholl method, which overlays concentric circles spaced 20 μm apart and counts the number of intersections (left). In addition, the complexity of the dendritic tree was measured by counting the number of intersections and assigning each branch a consecutively higher branch order number (right). Finally, dendritic spines were counted (inset, scale bar = 10μm) under 100x magnification under oil in each of the inner (IML, grey area nearest the soma), middle (MML), and outer (OML, grey area furthest from soma) third of the molecular layer.
Unfortunately, only a handful of studies have compared the DGsp to the DGip (Seress and Pokorny, 1981; Desmond and Levy, 1982, 1985; Green and Juraska, 1985; Claiborne et al., 1990). These studies indicate that granule cells in the DGsp have greater dendritic length and spine density. However, few of these studies have systematically examined the extent of both dendritic branches and spines across the depth of the dendritic tree. This is an important issue since the arbor of granule cells can be split into 3 parts, each with unique inputs (Amaral et al., 2007). The inner third, known as the inner molecular layer (IML, closest to the soma) contains predominantly commissural and associational fibers as well as recurrent fibers from mossy cells (e.g., Buckmaster et al., 1992), while the middle third, termed the middle molecular layer (MML) receives input from the medial perforant path, and the outer third, termed the outer molecular layer (OML) receives lateral perforant path input (e.g., Steward, 1976). Moreover, these pathways carry different kinds of information. The MML input, for instance, carries much more spatial information relative to the OML (e.g., Knierim et al., 2013). Thus, knowing the molecular layer in which the arbor of granule cells of the DGsp and DGip differ may provide insight into the mechanism for the unique physiological responses of these cells during behavior. To the authors’ knowledge, no study has examined this anatomical gradient in mice, despite their widespread use in studies of DG function and the fact that the mouse DG differs from the rat in several subtle but potentially impactful ways. For instance, adult-born granule cells are greater in number and have a greater impact on the behavioral response of the rat DG relative to the mouse (Snyder et al., 2009). Moreover, the pattern of projections from the entorhinal cortex shows topographical distinctions in the two species (van Groen et al., 2002). These subtle differences may lead to considerable divergence in DG morphology or function. To address this issue, the dendritic arborization and spine density across this gradient is examined in both rats and mice using the Golgi-Cox technique.
Materials and Methods
Subjects
The current study included eight F344 rats (males aged 3-4 months) and six C57/Bl6 mice (males aged 2-6 months). All animals were housed in 12:12 light cycles with ad lib food and water, and all procedures were approved by either the Animal Care Committee of Wilfrid Laurier University (rat procedures) or the Institutional Animal Care and Use Committee of the University of Arizona (mouse). All animal work followed the guidelines of either the National Institutes of Health or Canadian Council on Animal Care.
Histology
The tissue from all animals was prepared using the Golgi-Cox technique, as previously described (Gibb and Kolb, 1998). Briefly, animals were decapitated under isofluorane and the brains were rapidly extracted, rinsed in 0.9% saline and immersed in Golgi-Cox solution for at least 14 days followed by 30% sucrose for at least 3 days. Brains were then sectioned on the coronal plane using a Vibratome at 200 μm and mounted on gelatin-coated slides. Ten fully-impregnated cells were selected from the DGsp and DGip of each animal, and were imaged in a z-stack throughout the thickness of the section using a bright-field microscope (Leica Mcrosystems, Concord, ON) equipped with a digital camera (Pixellink, Ottawa, ON). Granule cells were included in the analysis if (a) the cell could be identified as a granule cell on the basis of morphology, (b) the dendritic tree appeared well impregnated and largely intact in the plane of section, and (c) the cell was not obscured with precipitate, blood vessels, or astrocytes.
Cells were selected from the septal third (rat: −2.28mm to −3.24mm from Bregma, Paxinos & Watson, 2006; mouse: −1.35mm to −2.35mm from Bregma, Allen Mouse Brain Atlas, 2015) of the hippocampus. Granule cells were randomly selected along the longitudinal axis as well as throughout the depth of the granule cell layer. However, depth within the granule cell layer affects morphology (Claiborne et al., 1990, Green and Juraska, 1985). More specifically, significant differences in granule cell morphology can be found between cells that are superficial (i.e., with cell bodies closer to the molecular layer) relative to cells that are deep (i.e., with cell bodies closer to the hilus). Because of this, the sampled granule cells were classified post-hoc (see Table 1) and analyzed to determine if there were any significant differences in the number of superficial vs deep granule cells across regions or species. The classification of cells involved bisecting the thickness granule cell layer in each perpendicular to its long axis. Those cells that contained the majority of their soma with the half closest to the hilus were classified as deep, while those predominantly in the furthest half were classified as superficial.
Table 1.
Characteristics of granule cells sampled
| Rat | Mouse | |||
|---|---|---|---|---|
| DGsp | DGip | DGsp | DGip | |
| Total granule cells | 64 | 64 | 80 | 80 |
| Superficial | 34 | 32 | 46 | 45 |
| Deep | 30 | 32 | 34 | 35 |
Analysis
Using ImageJ (NIH, Bethesda, MD) and Metamorph (Molecular Devices, Sunnyvale, CA) software, several measures of dendritic arborization were obtained (Fig. 1). The amount of dendritic material was estimated using the Sholl technique (Sholl, 1953), which involves overlaying the cell with a series of concentric circles (20 μm apart) centered on the soma and recording the number of dendritic processes intersecting each circle to a maximal distance of 380 μm. Note that although this analysis was conducted on a 3-dimensional image stack, the measure is inherently 2-dimensional.
In addition, branching order was calculated for all cells. The primary dendrites originating from the soma are assigned a branch order of one, while dendritic processes originating from that dendrite are second order branches, and each subsequent bifurcation is assigned a progressively higher branch order (Uylings et al., 1986). Dendritic spine density was analyzed at 100x under oil immersion. In each section, the IML, MML, and OML were defined by dividing the length of the molecular layer into 3 equal parts. Dendritic spines were counted in a sample of 2 random 20-μm-long segments within each zone (i.e., the IML, MML, and OML) in each included cell. Sholl data were analyzed by repeated-measures ANOVA, while branching order and spine density measures used general factorial ANOVA, with region (i.e., DGsp vs DGip) and species (i.e., rat vs mouse) as between-subject factors.
The statistical models described above were also assessed with the addition of depth (i.e., deep vs superficial granule cells) as an additional factor in order to assess possible differences in morphology based on cell depth.
Results
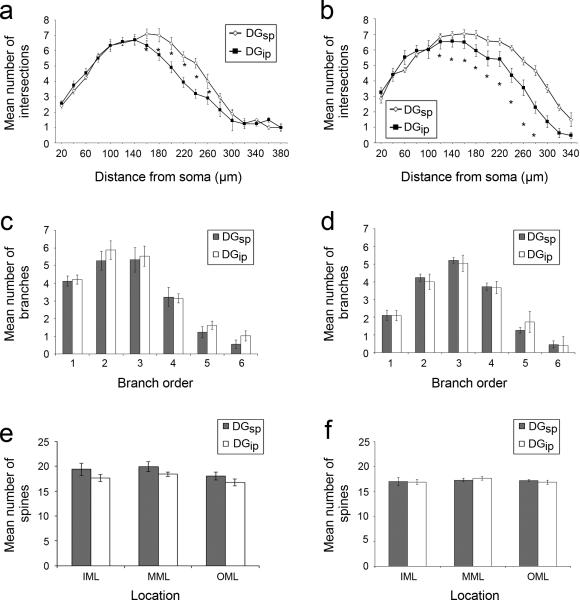
Consistent with previous reports in rats (Desmond and Levy, 1982; Green and Juraska, 1985), DGsp granule cells contain more dendritic material than DGip granule cells (main effect of region: F1,24 = 6.03; p = 0.02). Moreover, this increase was consistently observed in both rats and mice (main effect of species: F1,24 = 2.37; p = 0.14). Post-hoc testing with Tukey's HSD shows that the dendritic arbor is selectively increased in the middle third of the dendritic tree (corresponding to the MML) in both rats and mice, as well as the outer third (corresponding to the OML) in mice (see Fig. 2a,b). This increase occurred without an increase in branching complexity (F1,24 = 0.31; p = 0.58) or a change in spine density (F3,22 = 0.37; p = 0.14), as neither parameter shows significant differences on the basis of anatomical location. This pattern of results (i.e., an increase in the number of intersections in Sholl analysis without a change in branch order) suggests an increase in the length of dendritic segments rather than an increase in the number of segments in (and thus the complexity of) the dendritic arbour.
Figure 2.
Quantification of granule cell measurements in rats (a,c,e) and mice (b,d,f). Sholl analysis (a,b) shows an increase in dendritic material in DGsp (open diamonds) relative to the DGip (filled squares) in the MML (i.e., middle third) of both rats and mice, and well as the OML (i.e., outer third) of mice. Analysis of the branch orders (c,d) and the density of spines (e, f) reveals no significant difference between the DGsp (grey) and DGip (white) in either species (*p < 0.05, Tukey's HSD).
The number of deep vs. superficial cells was analyzed, since previous studies have shown that granule cell morphology can vary according to their depth in the granule cell layer (e.g., Claiborne et al., 1990; Green and Juraska, 1985). No significant difference were observed on the basis of granule cell layer depth (p > 0.26 in all cases). These data suggest that differences observed are representative of differences between DGsp and DGip and are not the result of sampling error.
To further determine any potential morphological differences, statistical comparison was conducted between deep and superficial cells in rats and mice in each region of the DG (see Table 2). The addition of cell depth as a factor did not result in any significant differences based on depth, or any significant interactions between depth and region (i.e., DGsp vs DGip) or species (p > 0.18 in all cases). Thus, the current study has failed to replicate previous observations showing significant differences in granule cell morphology based on cell depth. It should be noted, however, that because the study was not originally designed for this comparison, the study may be under-powered for this comparison. As a result, it remains difficult to draw conclusions from this analysis.
Table 2.
Morphology of Deep vs Superficial Cells
| Rat | Mouse | |||||||
|---|---|---|---|---|---|---|---|---|
| DGsp | DGip | DGsp | DGip | |||||
| super2 | deep3 | super | deep | super | deep | super | deep | |
| Sholl1 | ||||||||
| 0-60 μm | 6.23 ± 0.61 | 7.38 ± 0.71 | 6.93 ± 0.92 | 6.67 ± 1.07 | 12.84 ± 0.89 | 11.43 ± 0.85 | 14.43 ± 0.95 | 11.68 ± 0.99 |
| 61-120 μm | 11.13 ± 1.12 | 11.46 ± 1.71 | 11.55 ± 1.60 | 12.17 ± 4.94 | 20.46 ± 0.91 | 17.93 ± 1.24 | 19.38 ± 1.18 | 18.06 ± 0.53 |
| 121-180 μm | 16.85 ± 1.30 | 15.92 ± 0.69 | 14.70 ± 1.85 | 14.74 ± 1.90 | 22.20 ± 1.03 | 21.69 ± 1.41 | 19.23 ± 1.03 | 18.52 ± 1.02 |
| 181-240 μm | 18.86 ± 1.26 | 19.06 ± 1.06 | 18.83 ± 1.68 | 18.01 ± 1.72 | 19.36 ± 0.69 | 20.61 ± 1.44 | 13.74 ± 1.37 | 14.55 ± 1.67 |
| 241-300 μm | 19.42 ± 1.20 | 20.04 ± 1.29 | 21.32 ± 1.21 | 21.11± 0.95 | 13.72 ± 1.04 | 14.26 ± 1.50 | 5.46 ± 0.96 | 7.40 ± 1.29 |
| 300+ μm | 54.88 ± 5.62 | 67.34 ± 17.89 | 49.86 ± 9.77 | 54.76 ± 15.17 | 4.54 ± 0.99 | 4.48 ± 0.78 | 1.14 ± 0.36 | 1.42 ± 0.64 |
| Branch Order | ||||||||
| 1 | 2.60 ± 0.32 | 2.40 ± 0.39 | 2.85 ± 0.20 | 2.75 ± 0.31 | 2.15 ± 0.17 | 1.90 ± 0.19 | 2.36 ± 0.13 | 1.78 ± 0.11 |
| 2 | 5.52 ± 0.70 | 5.10 ± 0.78 | 5.94 ± 0.63 | 6.14± 0.68 | 4.20 ± 0.28 | 3.98 ± 1.34 | 4.24 ± 0.32 | 3.67 ± 0.21 |
| 3 | 7.67 ± 0.61 | 7.31 ± 0.64 | 7.31 ± 0.75 | 7.08 ± 0.73 | 5.61 ± 0.36 | 4.81 ± 0.34 | 4.85 ± 0.26 | 5.13 ± 0.39 |
| 4 | 6.00 ± 0.73 | 6.40 ± 0.63 | 5.78 ± 0.66 | 5.54 ± 0.80 | 4.15 ± 0.37 | 4.13 ± 0.59 | 3.86 ± 0.49 | 3.60 ± 0.57 |
| 5 | 1.73 ± 0.27 | 2.25 ± 0.41 | 1.95 ± 0.36 | 1.31 ± 0.34 | 1.43 ± 0.32 | 1.45 ± 0.30 | 1.71 ± 0.38 | 1.39 ± 0.29 |
| 6+ | 1.02 ± 0.77 | 1.84± 0.78 | 0.86 ± 0.24 | 0.70 ± 0.30 | 0.55 ± 0.19 | 0.67 ± 0.28 | 0.31 ± 0.16 | 0.39 ± 0.17 |
| Spines | ||||||||
| IML | 17.28 ± 1.40 | 17.65 ± 1.12 | 19.13 ± 1.09 | 19.09 ± 0.66 | 16.76 ± 0.70 | 17.13 ± 0.64 | 16.30 ± 0.57 | 17.21 ± 0.52 |
| MML | 18.35 ± 1.06 | 18.58 ± 1.14 | 20.26 ± 0.79 | 19.57 ± 0.69 | 17.29 ± 0.50 | 17.39 ± 0.53 | 17.08 ± 0.34 | 17.34 ± 0.54 |
| OML | 16.40 ± 0.70 | 16.86 ± 0.87 | 18.71 ± 0.72 | 16.95 ± 0.89 | 16.77 ± 0.47 | 18.05 ± 0.46 | 16.55 ± 0.50 | 17.15 ± 0.44 |
Mean number of line crossing in Sholl analysis within each third of the molecular layer.
Superficial: the majority of the cell body was contained within the half of the granule cell layer closest to the hilus
The majority of the cell body was contained within the half of the granule cell layer closest to the hilus
Discussion
The selective increase in perforant path input to DGsp may provide an anatomical substrate for the asymmetric response of the DG to spatial information. The medial perforant path contains the output of highly spatially-tuned cell populations such as grid cells (reviewed in Moser et al., 2014) as well as head-direction and border cells (Zhang et al., 2013). In contrast, the lateral perforant path contains little spatial tuning (Knierim et al., 2013). Consistent with these differences in information content, the medial perforant path also disproportionately supports performance of spatial tasks while the lateral perforant path supports non-spatial tasks such as object recognition (Myhrer, 1988; Hunsaker et al., 2007). It is intriguing to note that the expansion of dendritic material in the OML of the mouse DGsp suggests differences in the DG response to non-spatial information between these species. That is, the rat DGsp receives more synapses selectively from the MML, which contains highly spatially-tuned input from the medial perforant path, and thus it is intuitive that the rat DGsp should show a much stronger response than the DGip during spatial tasks. In contrast, the mouse DGsp receives more input from both the spatially-tuned medial perforant path and the non-spatially tuned lateral perforant path. This result is consistent with earlier observations that the input from the medial entorhinal cortex (to the MML) contains robust bilateral projections in rats but the projections are largely unilateral in the mouse (van Groen et al., 2001). This discrepancy suggests that the mouse DGsp may show greater activity not only during spatial tasks, but also during hippocampus-dependent non-spatial tasks as well. This hypothesis however, needs to be tested experimentally, as the relationship between synaptic density and granule cell activity is necessarily complex.
A prime example of the complex relationship between synapse number and synaptic drive can be observed in the changes that occur in the DG as a result of aging. With progressive age, the number of synapses is reduced (Geinisman et al., 1986; 1992), but the remaining synapses become much more powerful in terms of their ability to drive granule cell responses (Barnes and McNaughton, 1980). As a result, the output of the DG of aged animals remains comparable to that of younger animals under many circumstances, and the changes that do occur are complex (reviewed in Burke and Barnes, 2006; Gray and Barnes, 2015). Thus, it may be too simplistic to assume that a significant increase in spine number will result in a comparable increase in synaptic drive.
Several other observations have been proposed to account for the DG's asymmetric activity. For example, the proliferation and survival of newly-generated granule cells follows a qualitatively different time course in the two blades. In the DGsp, fewer neurons are generated, but those that are produced are more likely to survive (Snyder et al., 2012). Moreover, entorhinal projections activate the DGsp more rapidly in response to a stimulus, which is thought to act along with recurrent inhibition to allow the DGsp to out-compete DGip in response to stimulation (Canning and Leung, 1997). In addition, DGsp receives twice as much supramammillary input (Wyss et al., 1979), although these projections terminate in the IML and thus cannot account for the differences observed here. In addition to the expansion of the MML-recipient zone observed here, these anatomical differences may account for the unique physiological responses of these two parts of the DG, a structure often considered to be homogeneous.
The current observations add to a growing body of literature describing novel functional gradients within the hippocampal formation. As more functional gradients are uncovered, it is likely that many anatomical observations will need to be revisited to build a more accurate model of the hippocampal formation and its contribution to learning and memory.
Supplementary Material
Acknowledgments
Support was provided by the Natural Sciences and Engineering Research Council of Canada, the Ontario Mental Health Foundation (DFM), and the US National Institute of Mental Health award MH097803 (ALG).
Footnotes
The authors declare they have no conflicts of interest.
References
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Mouse Brain Atlas [Internet] Available from: http://mouse.brain-map.org.
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992;2:349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Canning KJ, Leung LS. Lateral entorhinal, perirhinal, and amygdalaentorhinal transition projections to hippocampal CA1 and dentate gyrus in the rat: a current source density study. Hippocampus. 1997;7:643–655. doi: 10.1002/(SICI)1098-1063(1997)7:6<643::AID-HIPO6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. Quantitative, three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J Comp Neurol. 1990;302:206–219. doi: 10.1002/cne.903020203. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. A quantitative anatomical study of the granule cell dendritic fields of the rat dentate gyrus using a novel probabilistic method. J Comp Neurol. 1982;212:131–145. doi: 10.1002/cne.902120204. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Granule cell dendritic spine density in the rat hippocampus varies with spine shape and location. Neurosci Lett. 1985;54:219–224. doi: 10.1016/s0304-3940(85)80082-3. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res. 1986;398:266–275. doi: 10.1016/0006-8993(86)91486-1. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Green EJ, Juraska JM. The dendritic morphology of hippocampal dentate granule cells varies with their position in the granule cell layer: a quantitative Golgi study. Exp Brain Res. 1985;59:582–586. doi: 10.1007/BF00261350. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Kesner RP. An analysis of the dentate gyrus function. Behav Brain Res. 2013;254:1–7. doi: 10.1016/j.bbr.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Ramirez-Amaya V, Barnes CA. Neurons generated in senescence maintain capacity for functional integration. Hippocampus. 2012a;22:1134–1142. doi: 10.1002/hipo.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone DF, Satvat E, Shaner MJ, Worley PF, Barnes CA. Attenuated long-term Arc expression in the aged fascia dentata. Neurobiol Aging. 2012b;33:979–90. doi: 10.1016/j.neurobiolaging.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Roudi Y, Witter MP, Kentros C, Bonhoeffer T, Moser MB. Grid cells and cortical representation. Nat Rev Neurosci. 2014;15:466–481. doi: 10.1038/nrn3766. [DOI] [PubMed] [Google Scholar]
- Myhrer T. The role of medial and lateral hippocampal perforant path lesions and object distinctiveness in rats' reaction to novelty. Physiol Behav. 1988;42:371–377. doi: 10.1016/0031-9384(88)90279-x. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Angulo-Perkins A, Chawla MK, Barnes CA, Rosi S. Sustained transcription of the immediate early gene Arc in the dentate gyrus after spatial exploration. J Neurosci. 2013;33:1631–1639. doi: 10.1523/JNEUROSCI.2916-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satvat E, Gheidi A, Voll S, Odintsova IV, Marrone DF. Location is everything: neurons born during fluoxetine treatment accumulate in regions that do not support spatial learning. Neuropharmacol. 2012;62:1627–1633. doi: 10.1016/j.neuropharm.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res. 2012;226:56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Seress L, Pokorny J. Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. J Anat. 1981;133:181–195. [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices on the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Ferrante SC, Cameron HA. Late maturation of adult-born neurons in the temporal dentate gyrus. PLoS One. 2012;7:e48757. doi: 10.1371/journal.pone.0048757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Topographic organisation of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter MP, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Ruiz-Marcos A, van Pelt J. The metric analysis of three-dimensional dendritic tree patterns: a methodological review. J Neurosci Methods. 1986;18:127–151. doi: 10.1016/0165-0270(86)90116-0. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Species differences in the projections from the entorhinal cortex to the hippocampus. Brain Res Bull. 2002;57:553–556. doi: 10.1016/s0361-9230(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammillary region of the hypothalamus. Anat Embryol (Berl) 1979;156:165–176. doi: 10.1007/BF00300012. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Ye J, Couey JJ, Witter M, Moser EI, Moser MB. Functional connectivity of the entorhinal-hippocampal space circuit. Philos Trans R Soc Lond B Biol Sci. 2013;369:20120516. doi: 10.1098/rstb.2012.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.