Abstract
Background
Acute myocardial infarction (AMI) is frequently associated with transient hyperglycemia even in patients without pre-existing diabetes. Acute stress can lead to increased blood glucose through the effect of catecholamines on alpha2A-adrenergic receptors (α2A-ARs) present in pancreatic islet β-cells. Variation in the gene (ADRA2A) that encodes the α2A-AR affects insulin release and glucose control and may play a particularly important role during times of stress.
Methods
We performed a retrospective cohort study using de-identified electronic medical records linked to a DNA repository in 521 Caucasians and 55 African-American non-diabetic patients with AMI. We examined the association between admission blood glucose concentrations and ten selected ADRA2A SNPs in Caucasians.
Results
Three ADRA2A SNPS were associated with stress-induced hyperglycemia in Caucasians. Individuals homozygous for the rs10885122 variant (n=9) had a 23% lower admission glucose (geometric mean [95% CI], 99 [83 – 118] mg/dl) compared with non-carriers (121 [118–125] mg/dl; n=401; P = 0.001). Admission glucose was 14% higher in rs1800544 variant homozygotes (134 [119–150] mg/dl; n=36) compared to non-carriers (118 [115–121] mg/dl; n=290, P=0.046). Furthermore, homozygotes of the rs553668 variant (n = 13) had a 13% higher glucose (133 [110–160] mg/dl) compared to non-carriers (118 [115–122] mg/dl; n=366; P = 0.056). Haplotypes including these ADRA2A SNPs were associated with higher admission glucose levels.
Conclusions
Three ADRA2A genetic variants are associated with blood glucose and stress-induced hyperglycemia after AMI in Caucasians.
Keywords: Stress, Glucose, Alpha adrenergic receptors
Introduction
Acute serious illnesses, including myocardial infarction, are frequently associated with transient hyperglycemia even in patients without pre-existing diabetes (1). Such stress-induced hyperglycemia is an independent predictor of mortality in the setting of myocardial infarction, stroke, and other serious illnesses (2–5). For example, mortality after myocardial infarction is increased 4-fold in patients with stress-induced hyperglycemia, even after adjusting for other prognostic factors (6). Interestingly, the mortality risk associated with stress-induced hyperglycemia is consistently greater in patients without pre-existing diabetes than in diabetics (6–8).
Stress-induced hyperglycemia is associated with the severity of the underlying acute illness, but no other risk factor has been identified consistently, and it is unclear why some patients develop hyperglycemia and others do not (6, 9). The mechanisms underlying hyperglycemia are thought to include insulin resistance mediated in part through stress-induced sympathetic activation and catecholamine release (10). Catecholamines, among their other actions, act on postsynaptic alpha2A-adrenergic receptors (α2A-ARs) of pancreatic islet β-cells to inhibit insulin secretion and thus increase blood glucose during stress (10). There is substantial variation in the gene (ADRA2A) that encodes the α2A-AR, and recent studies indicate that this genetic variability affects insulin release and glucose control (11–13). A relatively common ADRA2A variant (rs553668), present in approximately 15% of Caucasians and 25% of African-Americans, was associated with increased expression of ADRA2A messenger RNA and thus with greater α2A-AR density on pancreatic islet β-cells, and also with decreased insulin secretion and higher glucose levels in response to a glucose load (11). Furthermore, in vitro studies using pancreatic islet cells from rs553668 carriers showed that the reduced secretion of insulin in response to glucose was normalized by pharmacological α2A-AR antagonists (11, 14). In keeping with these findings, the rs553668 variant was associated with increased risk of diabetes in several large population studies (11, 15, 16). However, there is no information regarding the effect of rs553668 or other ADRA2A variants on glucose control under conditions of stress, a time when catecholamine concentrations are increased and the effects of α2A-ARs on insulin regulation are likely to be most important.
Therefore, we examined the hypothesis that rs553668 and other ADRA2A variants are associated with the risk of stress-induced hyperglycemia after acute myocardial infarction in non-diabetic patients.
METHODS
Setting
We performed a retrospective cohort study using the Vanderbilt de-identified electronic medical record (EMR) database that is linked to the Vanderbilt DNA repository (BioVU).(17) BioVU contains approximately 180,000 DNA samples linked to de-identified EMRs; details about the recruitment, sample acquisition and storage, and data handling have been described previously.(17) Approval for the present study was obtained from the Vanderbilt Institutional Review Board.
Study Cohort
We identified Caucasian and African-American patients admitted to the Vanderbilt University Medical Center with a diagnosis of acute myocardial infarction (AMI) who had at least one blood glucose level measured at admission and who had a DNA sample available in BioVU. We excluded patients who had an AMI after a medical procedure and those admitted for other medical reasons and subsequently had an AMI during the hospital stay. We excluded patients with pre-existing diabetes, defined as having a hemoglobin A1C level of > 6.5% at admission or during hospitalization or a previous history of diabetes mellitus, as determined from medical history and the use of antidiabetic medications.
Phenotyping
Patients fulfilled the third universal definition of myocardial infarction with a rise or fall of a cardiac biomarker (troponin I or troponin T >0.05 ng/ml, CKMB > 6 ng/ml or CKMB ratio >3%) and with at least one of the following: symptoms of ischemia, new or presumed new significant ST-segment-T wave (ST-T) changes or new left bundle branch block; development of pathological Q waves in the ECG; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality; or identification of an intracoronary thrombus by angiography.(18)
We identified patients with AMI in the de-identified EMR using strategies validated in large epidemiological studies.(19, 20) This involved the presence of ICD-9 code 410, excluding 410.x2 (readmission after AMI), on at least two consecutive days to identify potential cases, followed by manual review of the medical records to identify true cases meeting the criteria for AMI. Glucose measurements recorded in the EMR after the onset of signs and symptoms of AMI were extracted. We converted whole blood glucose measurements obtained from capillary samples to plasma glucose values by multiplying by a factor of 1.15, as previously described.(19) Covariates were extracted by both manual chart review and bioinformatic approaches.
Outcomes
The primary outcome was the first glucose concentration recorded after onset of signs and symptoms of AMI, termed ‘admission glucose’.
Genotyping
We genotyped nine ADRA2A tagSNPs previously described and one additional ADRA2A variant (rs10885122) that has been associated with diabetes mellitus or related traits in at least two genome wide association studies.(13, 21, 22) Genotyping was performed by the Center for Human Genetics Research at Vanderbilt University Medical Center according to standard protocols using the Sequenom platform. Two SNPs (rs2484516 and rs1800035) did not pool well in the Sequenom platform and were genotyped using TaqMan assays. For quality control, we examined genotyping call rates and calculated Hardy-Weinberg equilibrium (HWE) of genotype distributions.
Statistical Analysis
Baseline demographics were described using mean and standard deviation for continuous variables and frequencies and percentages for categorical variables and were compared between the two ethnic groups using independent sample t-tests and chi square tests, or non-parametric tests when the statistical assumptions were not met. Blood glucose levels were not normally distributed and were therefore log-transformed for analyses and expressed as geometric means with 95% confidence intervals (CIs). For all genetic analyses, we assumed an additive genetic model, coding the genotypes according to the number of variant alleles (0–2). In single-variant analyses, we used linear regression analyses to examine the association of admission glucose as dependent continuous variable with genotypes of each SNP, with and without adjustment for the following covariates: age, sex, BMI, peak CKMB, ST elevation, in-hospital use of diuretics, in-hospital defibrillation, and, for analyses of the combined cohort, ethnicity. In sensitivity analyses, we categorized the outcome variable (admission glucose) into a dichotomous variable (admission hyperglycemia yes/no) using cut-off values (associated with therapeutic and prognostic thresholds) of 140 mg/dl(19, 23) and assessed the association between admission hyperglycemia with each ADRA2A variant (before and after adjustment for covariates) using logistic regression analyses.
We defined haplotype families in each ethnic group using the 10 ADRA2A SNPs by expectation-minimization algorithms implemented using EM algorithm with the haplo.stats program. Furthermore, we assessed the association of each haplotype with admission glucose, with and without adjustment for covariates.
The primary analyses were performed in Caucasians; only exploratory analyses were performed in the African American group because of the small sample size. Statistical analyses were performed using R software (www.R-project.org) and SPSS (v. 21, IBM® SPSS® Inc., Chicago, IL). All analyses were two-tailed, and a P-value < 0.05 was considered significant.
RESULTS
Baseline Demographics
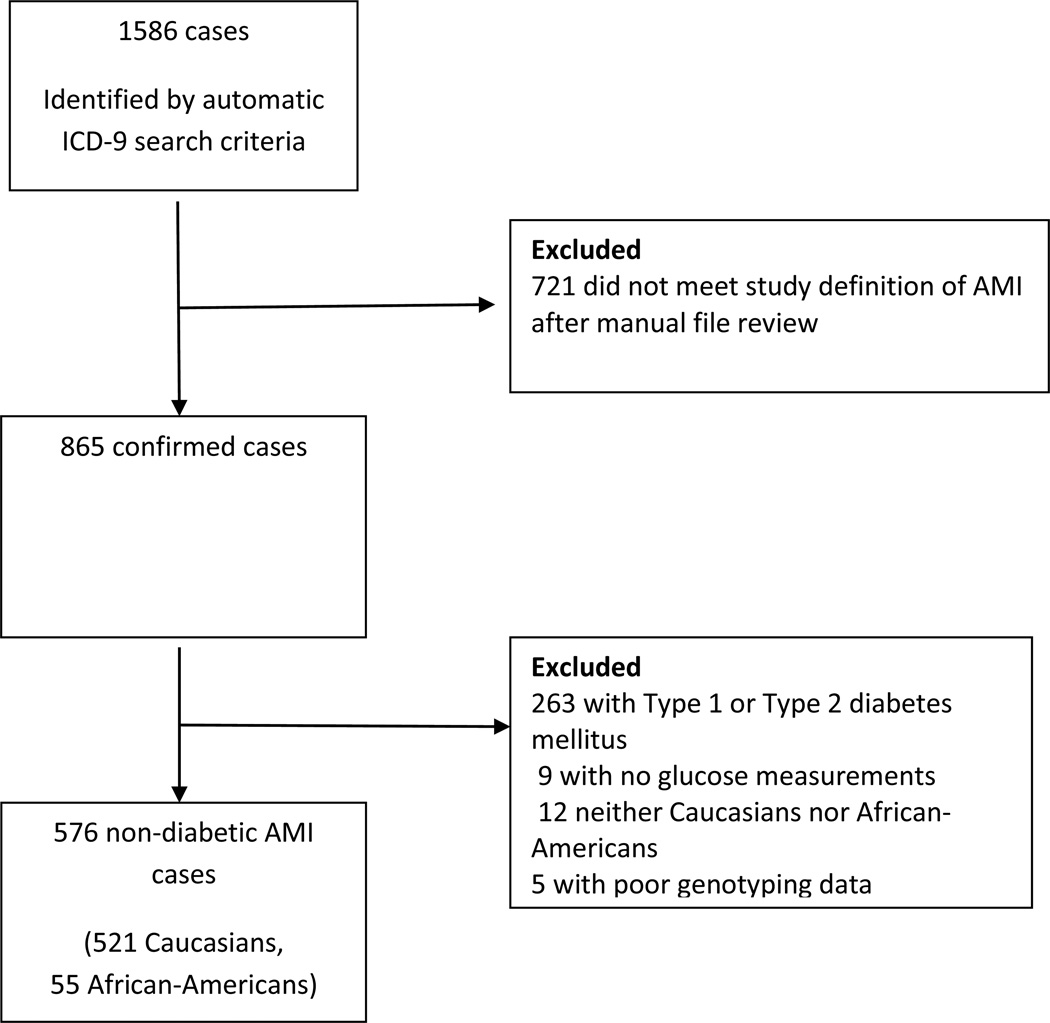
We identified 1586 potential cases of AMI based on ICD-9 code criteria, of whom 576 cases (521 Caucasian, 55 African-American) met the study criteria after manual EMR review (Figure 1). Cases were predominantly Caucasian males (Table 1 and Supplementary Table 1). Among the 521 Caucasian cases, the admission glucose ranged from 60 – 568 mg/dl (median (IQR); 113 mg/dl (100 – 138 mg/dl). 122 Caucasians (23%) had admission glucose > 140 mg/dL. In 32 patients (6.1%), stress-induced hyperglycemia was treated with insulin at some stage of the hospitalization.
Figure 1.
Schematic diagram of selection of 576 AMI cases for the study
Table 1.
Baseline demographics and characteristics of 521 Caucasian patients
| Covariates | Caucasians n = 521 |
|---|---|
| Age, years | 63±14 |
| Female sex, n (%) | 178 (34.2) |
| BMI, kg/m2* | 27±5 |
| Previous history of AMI, n (%) | 117 (22.5) |
| Previous history of PCI/CABG, n (%) | 144 (27.6) |
| ST elevation, n (%) | 209 (40.1) |
| In-hospital events | |
| In hospital ventricular tachycardia/fibrillation, n (%) | 70 (13.4) |
| In hospital PCI, n (%) | 372 (71.4) |
| In hospital CABG, n (%) | 46 (8.8) |
| In hospital thrombolysis, n (%) | 26 (5.0) |
| In hospital defibrillation, n (%) | 38 (7.3) |
| In-hospital Chemistry/Hematology | |
| Creatinine, mg/dl | 1.2±0.9 |
| Peak troponin I, µg/l* | 42.2±155.6 |
| Peak troponin T, µg/l† | 2.0±2.6 |
| Peak CK-MB, U/L‡ | 102.8±142.7 |
| Medication Use In-hospital | |
| Diuretics, n (%) | 222 (42.6) |
| Beta blockers, n (%) | 464 (89.1) |
| ACE Inhibitors, n (%) | 363 (69.7) |
| Statins, n (%) | 443 (85.0) |
| Aspirin, n (%) | 496 (95.2) |
328 of 521 patients had troponin I values;
130 of 521 patients had troponin T values
510 of 521 patients had CKMB values.
Genotyping
Minor allele frequencies for the 10 ADRA2A variants (Supplementary Table S2) were in the expected range, and all genotypes conformed to Hardy-Weinberg equilibrium in each ethnic group. No Caucasians carried the rs34303217 variant.
ADRA2A Genotypes and Admission Glucose
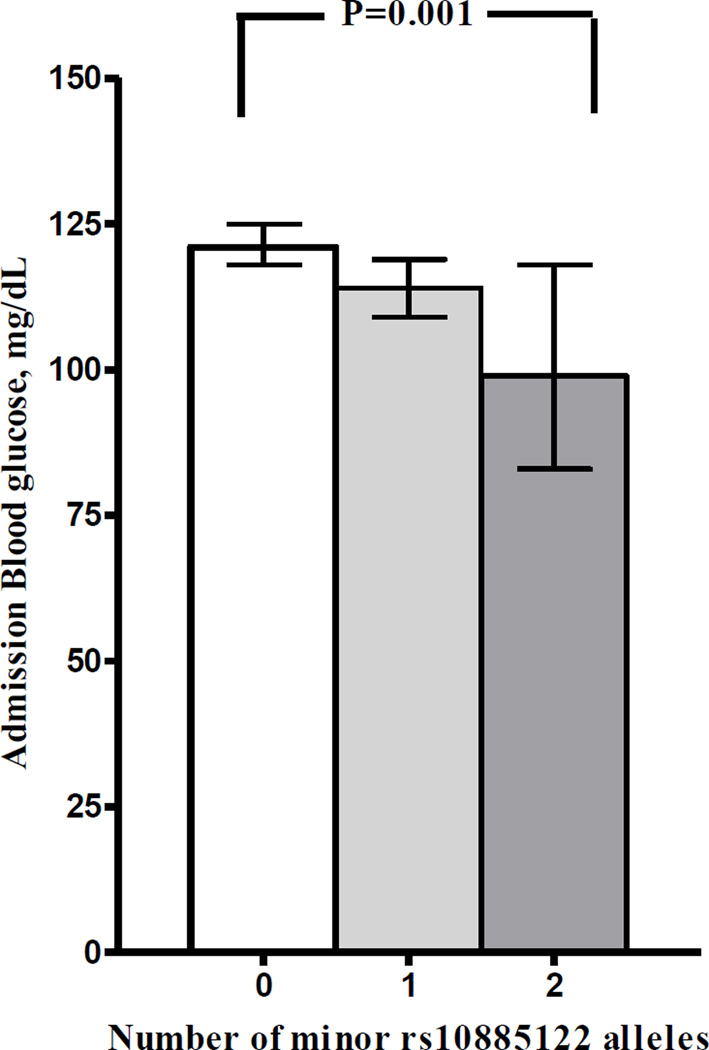
Of the ten ADRA2A variants, three were associated with glucose levels at the time of hospital admission in Caucasians in single-variant analyses (Table 2). The strongest association was found with rs10885122: carriers of the T allele had lower admission blood glucose (β= −0.035; 95% CI, −0.056 to −0.14; adjusted P=0.001, Table 2, Supplementary Table 3) compared to non-carriers. The 9 subjects homozygous for the T allele had a 23% lower geometric mean admission glucose compared with the 401 subjects homozygous for the G allele (P = 0.001), and heterozygotes had intermediate values (Figure 2).
Table 2.
ADRA2A variants associated with admission blood glucose levels in Caucasian patients with acute myocardial infarction.
| Caucasians | ||||
|---|---|---|---|---|
| SNP | Glucose, mg/dl Geometric mean (95% CI) |
P-value*, adjusted |
||
| Number of variant alleles |
0 | 1 | 2 | |
| rs10885122 | 121 (118–125) N = 401 |
114 (109–119) N = 111 |
99 (83 –118) N = 9 |
0.001 |
| rs553668 | 118 (115–122) N = 366 |
121 (116–127) N = 142 |
133 (110–160) N = 13 |
0.056 |
| rs1800544 | 118 (115–121) N = 290 |
119 (114–123) N = 195 |
134 (119–150) N = 36 |
0.046 |
P value adjusted for age, sex, BMI, peak CKMB, ST elevation, in-hospital use of diuretics, in-hospital defibrillation.
Figure 2.
rs10885122 genotype in Caucasians and its association with admission glucose following acute myocardial infarction.
Two additional variants had a weaker association with admission glucose in Caucasians. rs553668 was associated with higher admission glucose (β=0.019; 95% CI, 0.001 to 0.038; adjusted P=0.056). Homozygotes (n = 13) had a 13% higher n admission glucose (133 mg/dl) compared with the 366 non-carriers (118 mg/dl; Table 2). Additionally, rs1800544 was associated with a higher admission glucose (β=0.016; 95% CI, 0.001 to 0.033; adjusted P=0.046). Admission glucose was 14% higher in variant homozygotes (134 mg/dl; n=36) compared to non-carriers (118 mg/dl; n=290; Table 2).
Multiple linear regression models that included covariates either with or without genotype information for all three significant ADRA2A SNPs in the Caucasian cohort revealed that adding genetic information improved the percentage of variability explained (adjusted R2) from 7.0% to 9.5%. Other covariates associated with higher admission glucose included peak CK-MB (P=0.002), ST elevation (P=0.038), and need for defibrillation (P=0.009). In this model, rs10885122 was the only ADRA2A SNP significantly associated with admission glucose (P = 0.001).
In sensitivity analyses with admission glucose categorized as a dichotomous variable (≥ 140 mg/dl (n=122) compared to <140 mg/dl (n=399), only rs10885122 was significantly associated with admission glucose ≥ 140 mg/dl. Of patients homozygous for the G allele, 26% had an admission glucose greater than 140 mg/dl, compared with 11% of homozygous carriers of the T allele (P = 0.015). After adjusting for covariates, the G allele remained significantly associated with admission glucose > 140 mg/dL (OR=2.30; 95% CI, 1.71 to 3.11; P = 0.006). The two other ADRA2A variants were not associated with admission glucose ≥ 140 mg/dl before or after adjusting for covariates (all P > 0.31).
Among the African American subgroup (n = 55), none of the ten ADRA2A variants was significantly associated with admission glucose levels except for rs1800545, which showed a trend to association with glucose levels but with no gene-dose-effect (P = 0.041, Supplementary Table S3).
ADRA2A Haplotypes and Admission Glucose
Thirteen haplotypes were derived in the Caucasian group and 8 in African-Americans, accounting for 99.6% and 80% of the populations, respectively (Supplementary Table 4). Haplotype 4, prevalent in 10% of Caucasians, included the variant alleles for rs1800544, rs553668 and the major G allele for rs10885122 (associated with higher admission glucose) was the only haplotype significantly associated with higher admission blood glucose (P = 0.040). Among African-Americans, haplotype 6 (prevalent in 7% of African Americans and including rs1800544, rs1800545 and rs10885122 variants) was significantly associated with higher admission blood glucose (P = 0.011; Supplementary Table 4B).
DISCUSSION
This is the first study of the genetic contribution of adrenergic receptor variants to stress-induced hyperglycemia. Examining the association of ten selected ADRA2A variants with initial blood glucose levels following an AMI in non-diabetic patients, we found that rs10885122, rs553668, and rs1800544 were associated with stress-induced hyperglycemia in Caucasians.
The increased sympathetic drive following an acute event such as an AMI results in release of stress hormones, including catecholamines. Catecholamines activate post-synaptic α2A-ARs on pancreatic islet β-cells and inhibit insulin release; these actions facilitate an increase in blood glucose levels as part of the fight-or-flight response. Recently, several genetic variants in ADRA2A have been associated with impaired glucose regulation,(15, 21, 22) confirming the relevance of ADRA2A as a candidate gene for the regulation of glucose homeostasis. We have extended these findings and identified three ADRA2A variants associated with blood glucose levels in patients after AMI.
The strongest association with blood glucose concentrations was that of rs10885122. GG homozygotes had glucose levels on admission to hospital that was 23 mg/dL (geometric mean) higher than that of TT homozygotes, and heterozygotes had intermediate values. The G-allele of rs10885122 (approximate allele frequency in Caucasians, 88%) has been associated with impaired fasting glucose in two large GWAS studies.(13, 21, 22) This SNP is located in the intergenic region between the genes TCF7L2 and ADRA2A. TCF7L2, positioned 1.6 Mbp upstream of rs10885122, encodes transcription factor 7-like 2, which has been consistently linked to diabetes mellitus and related traits.(24, 25) Thus, it is unclear whether rs10885122 has a causal association with blood glucose regulation or is only in linkage disequilibrium with a causal variant in either TCF7L2 or ADRA2A.
Two additional variants, rs553668 and rs1800544, were also associated with admission glucose. The rs553668 variant, formerly identified as the DraI restriction fragment length polymorphism, was identified as a marker for type 2 DM, increased fasting glucose, and decreased insulin levels in a number of previous studies.(11, 15, 26) The gain-of-function T-allele of this SNP, located in the 3-′UTR of ADRA2A, was associated with higher mRNA and receptor expression and enhanced α2A-AR activity in different physiological pathways. (27–29) In human pancreatic islet β-cells from T-allele carriers, membrane docking of the insulin vesicles was reduced, and carriers thus had reduced insulin secretion and increased glucose levels.(11) Thus, our finding that patients homozygous for the T-allele had higher admission glucose levels compared to non-carriers is in keeping with findings from previous studies identifying the rs553668 T-allele as a marker for impaired glucose regulation.
The rs1800544 variant, also known as the C1291G polymorphism, was associated with diabetes-related traits such as fasting glucose in one previous study.(30) Moreover, it has been associated with other α2A-AR-related phenotypes suggesting a gain-of-function action such as schizophrenia, weight gain during antipsychotic medication use, body fat accumulation, and vascular reactivity to stress.(31–34) The variant is in the promoter region of the gene, and it may exert its effects by influencing gene expression.(35) In keeping with previous findings related to glucose regulation phenotypes,(31–34) we found that the G allele was associated with higher admission glucose in Caucasians (P = 0.046) with a similar trend among African-American subjects.
Our findings may have implications for clinical practice. Stress-induced hyperglycemia is associated with increased mortality following cardiovascular events.(6) However, achieving stricter hyperglycemic control using insulin has not consistently achieved mortality benefits, suggesting that in such patients stress-induced hyperglycemia may be a marker of a detrimental enhanced adrenergic stimulation rather than representing the causal pathophysiological mechanism of increased mortality.(6, 8) Conceptually, inhibition of genetically defined enhanced α2A-AR pathways has been shown to reduce hyperglycemia: a previous study demonstrated improved insulin secretion using the non-selective α2-AR antagonist, yohimbine, in diabetic patients treated according to their rs553668 genotype.(36) It would be interesting to consider inhibition of the α2A-AR pathway for the prevention or treatment of stress-induced hyperglycemia and its associated morbidity and mortality, particularly for individuals with genetic risk markers. However, there is no selective α2A-AR antagonist available for clinical use; moreover, considering that α2-AR antagonists such as yohimbine can increase blood pressure and sympathetic activity it would be challenging to conduct this type of study in acutely ill patients.(37)
Our study had several limitations. First, this was a retrospective study using a de-identified electronic medical records system. Thus, we could not measure the plasma insulin levels at the time of AMI to assess their relationship with the genetic variants studied. The direct effect of α2A-ARs is to inhibit insulin secretion and thus increase glucose levels. However, blood glucose regulation also involves other pathways including other stress hormones such as cortisol, as well as glucose transporters and other factors that could affect stress-induced hyperglycemia. Second, given the complex phenotype and the hypothesis-driven approach based on known biology, we did not replicate our findings in a separate cohort. However, our findings reproduce the established biological effect of rs553668 and other ADRA2A variants previously associated with impaired glucose homeostasis. Third, due to the small size of the African-American sample, we were unable to adequately examine the genetic determinants of stress-induced hyperglycemia in this group. This likely accounts for the lack of statistically significant associations with most of the ADRA2A variants in the African-American group. Fourth, in this retrospective study, we did not have data on glucose levels before AMI admission, and we therefore could not assess whether ADRA2A variants were associated with blood glucose specifically just under stress conditions or also at baseline. Last, we only studied stress-induced hyperglycemia following AMI. Stress-induced hyperglycemia also occurs following other acute conditions such as stroke, sepsis, and other serious illness.(2–5) It is unclear if our results can be generalized to hyperglycemia following other disease conditions. However, adrenergic activation is a feature common to all these conditions, and given our understanding of how the α2A-AR pathway is relevant to the pathophysiology of stress-induced hyperglycemia, similar associations with ADRA2A variants may be expected.
In conclusion, our findings suggest that three ADRA2A genetic variants, known to be associated with impaired glucose regulation in population studies, are associated with blood glucose concentrations and stress-induced hyperglycemia after AMI in Caucasians. Future studies assessing the prognostic value of these variants on morbidity and mortality and their association with other adrenergic phenotypes in the setting of AMI, as well as other clinical conditions resulting in stress-induced hyperglycemia, will be of interest.
Supplementary Material
Acknowledgments
None
Funding Source: This study was supported by a grant from the National Institutes of Health (HL56693). Drs. Adefurin and Okafor were supported by the National Institute of General Medical Science of the National Institutes of Health under award number T32 GM007569. Dr. Kawai was supported by a grant from the National Institutes of Health supplement 3P01HL056693-17S. Dr. Stein is the recipient of the Dan May Chair in Medicine. The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU, which is supported by institutional funding, the 1S10RR025141-01 instrumentation award, and by the Vanderbilt CTSA grant UL1TR000445 from NCATS/NIH.
Footnotes
Disclosures: None
References
- 1.Bronisz A, Kozinski M, Magielski P, et al. Stress hyperglycaemia in patients with first myocardial infarction. Int J Clin Pract. 2012;66(6):592–601. doi: 10.1111/j.1742-1241.2012.02917.x. [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 3.Kerby JD, Griffin RL, MacLennan P, Rue LW., 3rd Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann Surg. 2012;256(3):446–452. doi: 10.1097/SLA.0b013e3182654549. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JW, Zhou YJ, Cao SJ, Yang Q, Yang SW, Nie B. Impact of stress hyperglycemia on in-hospital stent thrombosis and prognosis in nondiabetic patients with ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24(5):352–356. doi: 10.1097/MCA.0b013e328361a942. [DOI] [PubMed] [Google Scholar]
- 5.Schuetz P, Kennedy M, Lucas JM, et al. Initial management of septic patients with hyperglycemia in the noncritical care inpatient setting. Am J Med. 2012;125(7):670–678. doi: 10.1016/j.amjmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117(12):1610–1619. doi: 10.1161/CIRCULATIONAHA.107.188629. [DOI] [PubMed] [Google Scholar]
- 7.Zarich SW, Nesto RW. Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction. Circulation. 2007;115(18):e436–e439. doi: 10.1161/CIRCULATIONAHA.105.535732. [DOI] [PubMed] [Google Scholar]
- 8.Kosiborod M, McGuire DK. Glucose-lowering targets for patients with cardiovascular disease: focus on inpatient management of patients with acute coronary syndromes. Circulation. 2010;122(25):2736–2744. doi: 10.1161/CIRCULATIONAHA.109.913368. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JW, He LJ, Cao SJ, Yang Q, Yang SW, Zhou YJ. Effect of glycemic variability on short term prognosis in acute myocardial infarction subjects undergoing primary percutaneous coronary interventions. Diabetol Metab Syndr. 2014;6:76. doi: 10.1186/1758-5996-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosengren AH, Jokubka R, Tojjar D, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327(5962):217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 12.Ghimire LV, Muszkat M, Sofowora GG, et al. Variation in the alpha(2A) adrenoceptor gene and the effect of dexmedetomidine on plasma insulin and glucose. Pharmacogenet Genomics. 2013;23(9):479–486. doi: 10.1097/FPC.0b013e3283642f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurnik D, Muszkat M, Li C, et al. Variations in the alpha2A-adrenergic receptor gene and their functional effects. Clin Pharmacol Ther. 2006;79(3):173–185. doi: 10.1016/j.clpt.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Rosengren AH, Braun M, Mahdi T, et al. Reduced insulin exocytosis in human pancreatic beta-cells with gene variants linked to type 2 diabetes. Diabetes. 2012;61(7):1726–1733. doi: 10.2337/db11-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talmud PJ, Cooper JA, Gaunt T, et al. Variants of ADRA2A are associated with fasting glucose, blood pressure, body mass index and type 2 diabetes risk: meta-analysis of four prospective studies. Diabetologia. 2011;54(7):1710–1719. doi: 10.1007/s00125-011-2108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, Zhu X, Wu X, et al. Evaluation of the association between the ADRA2A genetic polymorphisms and type 2 diabetes in a Chinese Han population. Genet Test Mol Biomarkers. 2012;16(12):1424–1427. doi: 10.1089/gtmb.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Glob Heart. 2012;7(4):275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 20.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med. 2009;169(5):438–446. doi: 10.1001/archinternmed.2008.593. [DOI] [PubMed] [Google Scholar]
- 21.Manning AK, Hivert MF, Scott RA, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koracevic GP. Various admission glucose cut-offs for prognostication and for therapeutic threshold in acute myocardial infarction. Am J Emerg Med. 2015;33(1):108–109. doi: 10.1016/j.ajem.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 24.Perry JR, Voight BF, Yengo L, et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012;8(5):e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voight BF, Scott LJ, Steinthorsdottir V, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42(7):579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bo S, Cassader M, Cavallo-Perin P, Durazzo M, Rosato R, Gambino R. The rs553668 polymorphism of the ADRA2A gene predicts the worsening of fasting glucose values in a cohort of subjects without diabetes. A population-based study. Diabet Med. 2012;29(4):549–552. doi: 10.1111/j.1464-5491.2011.03522.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurnik D, Muszkat M, Li C, et al. Genetic variations in the alpha(2A)-adrenoreceptor are associated with blood pressure response to the agonist dexmedetomidine. Circ Cardiovasc Genet. 2011;4(2):179–187. doi: 10.1161/CIRCGENETICS.110.957662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman K, Farrow S, Schmaier A, Freedman R, Schork T, Lockette W. Genetic polymorphism of the alpha 2-adrenergic receptor is associated with increased platelet aggregation, baroreceptor sensitivity, and salt excretion in normotensive humans. Am J Hypertens. 1995;8(9):863–869. doi: 10.1016/0895-7061(95)00155-I. [DOI] [PubMed] [Google Scholar]
- 29.Yabe M, Matsubara Y, Takahashi S, et al. Identification of ADRA2A polymorphisms related to shear-mediated platelet function. Biochem Biophys Res Commun. 2006;347(4):1001–1005. doi: 10.1016/j.bbrc.2006.06.180. [DOI] [PubMed] [Google Scholar]
- 30.Rosmond R, Bouchard C, Bjorntorp P. A C-1291G polymorphism in the alpha2A-adrenergic receptor gene (ADRA2A) promoter is associated with cortisol escape from dexamethasone and elevated glucose levels. J Intern Med. 2002;251(3):252–257. doi: 10.1046/j.1365-2796.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelsey RM, Alpert BS, Dahmer MK, Krushkal J, Quasney MW. Alpha-adrenergic receptor gene polymorphisms and cardiovascular reactivity to stress in Black adolescents and young adults. Psychophysiology. 2012;49(3):401–412. doi: 10.1111/j.1469-8986.2011.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YC, Bai YM, Chen JY, Lin CC, Lai IC, Liou YJ. Polymorphism of the adrenergic receptor alpha 2a −1291C>G genetic variation and clozapine-induced weight gain. J Neural Transm. 2005;112(11):1463–1468. doi: 10.1007/s00702-005-0291-7. [DOI] [PubMed] [Google Scholar]
- 33.Roffeei SN, Reynolds GP, Zainal NZ, et al. Association of ADRA2A and MTHFR gene polymorphisms with weight loss following antipsychotic switching to aripiprazole or ziprasidone. Hum Psychopharmacol. 2014;29(1):38–45. doi: 10.1002/hup.2366. [DOI] [PubMed] [Google Scholar]
- 34.Garenc C, Perusse L, Chagnon YC, et al. The alpha 2-adrenergic receptor gene and body fat content and distribution: the HERITAGE Family Study. Mol Med. 2002;8(2):88–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Kirstein SL, Insel PA. Autonomic nervous system pharmacogenomics: a progress report. Pharmacol Rev. 2004;56(1):31–52. doi: 10.1124/pr.56.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Axelsson AS, Spegel P, et al. Genotype-based treatment of type 2 diabetes with an alpha2A-adrenergic receptor antagonist. Sci Transl Med. 2014;6(257):257ra139. doi: 10.1126/scitranslmed.3009934. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg MR, Hollister AS, Robertson D. Influence of yohimbine on blood pressure, autonomic reflexes, and plasma catecholamines in humans. Hypertension. 1983;5(5):772–778. doi: 10.1161/01.hyp.5.5.772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.