Abstract
Although depression and anxiety are common in youth (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003), factors that put children at risk for such symptoms are not well understood. The current study examined associations between early childhood cognitive control deficits and depression and anxiety over the course of development through school age. Participants were 188 children (at baseline M = 5.42 years, SD = .79 years) and their primary caregiver. Caregivers completed ratings of children's executive functioning at preschool age and measures of depression and anxiety severity over seven assessment waves (a period of approximately 7.5 years). Longitudinal multilevel linear models were used to examine the effect of attention shifting and inhibition deficits on depression and anxiety. Inhibition deficits at preschool were associated with significantly greater depression severity scores at each subsequent assessment wave (up until 7.5 years later). Inhibition deficits were associated with greater anxiety severity from 3.5 to 7.5 years later. Greater shifting deficits at preschool age were associated with greater depression severity up to 5.5 years later. Shifting deficits were also associated with significantly greater anxiety severity up to 3.5 years later. Importantly, these effects were significant even after accounting for the influence of other key predictors including assessment wave/time, gender, parental education, IQ, and symptom severity at preschool age, suggesting that effects are robust. Overall, findings indicate that cognitive control deficits are an early vulnerability factor for developing affective symptoms. Timely assessment and intervention may be beneficial as an early prevention strategy.
Keywords: cognitive control, preschool, depression, anxiety, longitudinal
Although depression and anxiety are common forms of psychopathology in youth (Costello et al., 2003), the factors that put children at risk for such symptoms are not well understood. Several theoretical models of depression have highlighted the importance of cognitive processes, especially attentional control, in order to improve understanding of symptom development and maintenance (e.g., De Raedt & Koster, 2010; Disner, Beevers, Haigh, & Beck, 2011). Although there is strong cross-sectional evidence to support these models in adults (see De Raedt & Koster, 2010 for review), they have yet to be adequately tested using a developmental framework, particularly in young children when cognitive processes are rapidly developing. Even fewer studies have tested the basic validity of the models across development using extended longitudinal methodology. In order to address these gaps in the literature, the current study examined the effect of deficits in cognitive control, a cognitive process key to effective emotion regulation (Gross, 2014), at preschool age on subsequent symptoms of depression and anxiety over the next 7.5 years of development.
The critical role of attention deficits in the vulnerability for depression has been highlighted by one recent conceptual model of depression (De Raedt & Koster, 2010). The model builds on Beck's cognitive schema theory of depression (Beck, Rush, Shaw, & Emery, 1979; Clark, Beck, & Alford, 1999) by integrating its components with recent literature on underlying neurobiological cognitive processes. Beck's cognitive model proposes that negative schemas about the self, others, and the world, when activated by stress, strongly influence information processing. De Raedt and Koster (2010) then extend Beck's theory by linking it with brain-based and biological underpinnings of cognitive control. Broadly, the authors present a model of neurobiological and neuroendocrine psychopathological processes in which hypothalamic-pituitary-adrenal (HPA) axis hyperactivity leads to disrupted serotonergic functioning and in turn, atypical functioning in prefrontal brain regions known to support cognitive control. Cognitive control deficits are hypothesized to then result in failure to inhibit negative elaborative processes (e.g., rumination) when negative cognitive schemas are activated. The authors propose that in this way poor attentional control provides a gateway for negative thoughts. Negative thoughts then combine with sustained attention for negative material in the form of rumination, leading in turn to persistent negative affect (De Raedt & Koster, 2010). The model suggests that this relationship between depressed mood and negative thoughts is strengthened over time with each depressive episode, increasing reliance on cognitive control mechanisms to regulate affect. If such mechanisms fail, they propose, future episodes are more likely.
Cognitive control, also referred to as a component of executive functioning, can be differentiated into three operations, including updating, shifting, and inhibition (Miyake et al., 2000). The loose gateway in De Raedt and Koster's model is hypothesized to be the result of deficits in inhibition, which involves deliberately withholding dominant, automatic, or prepotent responses (Fisk & Sharp, 2004). The sustained attention on negative stimuli described by De Raedt and Koster, however, may reflect deficits in shifting, which involves moving between multiple tasks, operations, or mental sets (Monsell, 1996). Thus, the current study will focus on deficits in inhibition and shifting.
There is ample cross-sectional evidence to support the basic tenets of the model in adult samples with depressive symptoms (see De Raedt & Koster, 2010 for review). Longitudinal studies, while fewer, are also generally consistent with this model and indicate that executive functioning deficits are associated with later affective symptoms. For example, interference control (a measure of inhibition deficits on the flanker task) predicted the maintenance of depressive symptoms and rumination six months later in a sample of undergraduates (Zetsche & Joormann, 2011). Cognitive control deficits have also been shown to moderate the association between stress and later rumination, such that greater cognitive control deficits were related to greater rumination (De Lissnyder et al., 2012). Others have found that rumination mediated the association between cognitive control deficits and depression severity both at baseline and one year later in a sample of adults with remitted depression (Demeyer, De Lissnyder, Koster, & De Raedt, 2012). Relatedly, several recent treatment studies have also shown that targeting neurobiological correlates of cognitive control (e.g., dorsal lateral PFC) with transcranial direct current stimulation (Vanderhasselt, Brunoni, Loeys, Boggio, & De Raedt, 2013) and neurobehavioral therapies (Siegle, Ghinassi, & Thase, 2007) reduces rumination. Overall, data from cross sectional, longitudinal, and novel treatment studies provide support for the hypothesis that cognitive control deficits are closely associated with symptoms of depression in adults.
There is some empirical evidence supporting similar associations between cognitive control deficits and symptoms in youth, although most studies have focused on adolescents. A recent meta-analysis of 17 cross-sectional studies found that children and adolescents with major depressive disorder performed worse than healthy controls on several tests of cognitive function including inhibition capacity, shifting ability, sustained attention, and planning (Wagner, Müller, Helmreich, Huss, & Tadić, 2014). Other studies of adolescents with depression found evidence of deficits in sustained attention (Han et al., 2012) and attentional switching, even after controlling for processing speed (Wilkinson & Goodyer, 2006), compared to healthy controls. However, another cross-sectional study of adolescents failed to find associations between symptoms of depression or a depression diagnosis and executive functioning deficits (Wagner, Alloy, & Abramson, 2014). A longitudinal study of adolescents also failed to find an association between baseline executive functioning scores and symptoms of depression 15 months later (Connolly et al., 2014). Some inconsistency in findings may be due to differences in measures of depression, as some studies used diagnostic interviews while others used self-report symptom scales. Overall, there is some evidence to suggest that cognitive control deficits are associated with affective symptoms in youth, but inconsistent findings and lack of research in young children suggest that additional study is warranted.
While cognitive control deficits have been proposed to explain vulnerability to depression, the basic tenets of De Raedt and Koster's (2010) model can also be extended to anxiety. First, both depression and anxiety are characterized by repetitive negative thinking (i.e., rumination and worry), a key process in the proposed model. Evidence suggests that rumination and worry are highly correlated and are similarly related to symptom levels of anxiety and depression (Segerstrom, Tsao, Alden, & Craske, 2000; Siegle, Moore, & Thase, 2004). Further, transdiagnostic measures of repetitive negative thinking have been linked cross-sectionally with both anxiety and depression (e.g., Ehring & Watkins, 2008) and with anxiety and depression symptom improvement over the course of cognitive-behavioral treatment (Kertz, Koran, Stevens, & Björgvinsson, 2015). Second, like depression, anxiety in children and adolescents has been linked with dysregulated activity in prefrontal brain regions associated with cognitive control (e.g., Fitzgerald et al., 2013; Monk et al., 2008; Sylvester et al., 2013; Telzer et al., 2008). For example, children with an anxiety disorder have shown decreased dorsolateral prefrontal cortex (dlPFC) activity in response to errors on a performance task compared to healthy controls, suggesting failure to recruit brain regions associated with cognitive control (Fitzgerald et al., 2013). Third, similar to the findings with depression, anxiety has also been linked with performance-based cognitive control deficits in adults. Several studies have found that trait anxiety is associated with poor inhibition on behavioral tasks (Berggren & Derakshan, 2014; Derakshan, Ansari, Hansard, Shoker, & Eysenck, 2009) and worry and trait anxiety have also been linked with deficits in shifting (Visu-Petra, Miclea, & Visu-Petra, 2013). There is additional cross-sectional evidence linking cognitive control deficits with anxiety in children. For example, a study of youth (ages 8 to 16 years) found that anxiety and depression diagnoses were associated with cognitive control deficits in the context of emotional processing (Ladouceur et al., 2006). Given that depression and anxiety share similar underlying vulnerabilities, including that both are characterized by sustained cognitive processing (i.e., rumination and worry), deficits in prefrontal areas associated with cognitive control, and inhibition and shifting deficits, it may be that the cognitive control deficits hypothesized to underpin vulnerability to depression also play an important role in the development and maintenance of anxiety.
Overall, although advances have been made in understanding the role of cognitive processes in affective psychopathology, studies to date have relied on cross-sectional designs and older, adolescent samples. Few studies have tested such models in younger samples and no studies to our knowledge have examined longitudinal associations between hypothesized vulnerability factors in the preschool period of development and affective symptoms measured at school age. The use of cross-sectional designs and focus on older adolescent samples limits developmental conclusions, as conceptual models have posited that cognitive control deficits may act as a risk factor for the development of symptoms. This model is particularly salient in childhood when cognitive control skills are on a steep developmental trajectory (e.g., Hare et al., 2009). As such, the cognitive control deficits hypothesized to put children at risk for affective symptoms at school age and adolescence may begin to emerge early in development (i.e., during preschool age). For example, executive functioning deficits in preschool have been linked with both short-term outcomes, including academic and social abilities in childhood (Razza & Blair, 2009; Willoughby, Blair, Wirth, & Greenberg, 2012), as well as long-term outcomes including estimates of physical health and socioeconomic status in adulthood (Moffitt et al., 2011). These findings suggest that identification of early markers or risk may be particularly important, making studies of younger children imperative for understanding risk, occurrence, and course of childhood onset depression and anxiety.
In order to address these limitations, the current study was designed to examine longitudinal associations between deficits in inhibition and attention shifting during preschool age and later symptoms of anxiety and depression over approximately 7.5 subsequent years. We tested two specific hypotheses. First, it was predicted that cognitive control deficits in shifting and inhibition would be associated with increased symptoms of depression over time. Second, we hypothesized that the model can be extended to also predict symptoms of anxiety, and we expect that deficits in shifting and inhibition are also associated with increased symptoms of anxiety over time.
Method
Participants and Procedure
All study procedures were approved by the Washington University School of Medicine's Institutional Review Board in advance of data collection. Parents provided written informed consent and children provided assent to participate in an ongoing, longitudinal study of preschool depression conducted at the Early Emotional Development Program at Washington University School of Medicine. The study sample was recruited from primary care sites in the St. Louis community using a screening checklist. Preschoolers with depressive symptoms were oversampled and psychiatric and healthy control children were also included. Detailed descriptions of recruitment and methods can be found elsewhere see (Luby, Belden, Pautsch, Si, & Spitznagel, 2009; Luby, Si, Belden, Tandon, & Spitznagel, 2009).
The current study sample included children initially enrolled in the study (around ages 3 to 5.11 years) who also completed the subsequent assessment approximately 2.5 years later (Baseline; Mage=5.42, SD = .79) and at least one additional assessment. Additional assessments occurred 1 year (Time 1; Mage=6.42, SD = .78), 3.5 years (Time 3.5 Mage=8.95, SD = .80), 4.5 years (Time 4.5; Mage=10.05, SD = .86), 5.5 years (Time 5.5 Mage=11.10, SD = .87), 6.5 years (Time 6.5 Mage=12.43, SD = .93), and 7.5 years (Time 7.5 Mage=13.42, SD = .84) after the Baseline assessment. Children and their primary caregivers participated in up to 8 annual (and up to 5 semi-annual) assessment waves in which developmental and mental health functioning were comprehensively assessed (see Luby et al., 2009 and Luby et al., 2009, for additional details). With 188 subjects and 7 possible assessment waves, there were a total of 1,316 possible assessment waves. Subjects completed 1057 assessment waves for an 80.3% completion rate.
At Baseline, caregivers completed the Behavior Rating Inventory of Executive Function (BRIEF). At Baseline and subsequent visits, caregivers completed the Preschool Age Psychiatric Assessment (PAPA) or the Child and Adolescent Psychiatric Assessment (CAPA), which yielded depression and anxiety scores. Children completed the Weschsler Abbreviated Scale of Intelligence (WASI) at Time 4.5.
Measures
Behavior Rating Inventory of Executive Function-Preschool Version (BRIEF-P; Gioia et al., 2003) or Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000). Caregivers completed either the BRIEF-P (n = 141) for children younger than age 6 years or BRIEF (n = 47) for children 6 years or older. The BRIEF-P and BRIEF are 63-item and 86-item, standardized parent rating scales of behavioral manifestations of executive function in children aged 2.0 to 5.11 years for the BRIEF-P and ages 5-18 years for BRIEF. Items are rated on a 3-point scale including never, sometimes, and often. Scale sums are reported as age normed T scores (M =50; SD =10) with higher scores indicating greater deficits. Scores at or above 65 are considered clinically significant. The measure assesses multiple domains of executive functioning, including inhibit, shift, emotional control, working memory, and plan/organize. The inhibit and shift scales were used in the current study. Inhibit measures the ability to stop behavior at the appropriate time. Shift measures the ability to move from one task to another, including the ability to problem solve flexibly, switch or alternate attention, and alter focus from one topic to another. The BRIEF-P and BRIEF scores have shown good test-retest reliability, internal consistency, and validity with other similar rating scale measures (see Roth, Isquith, & Gioia, 2014, for full review of psychometric properties). Both the BRIEF and BRIEF-P will subsequently be referred to as the “BRIEF.”
Diagnostic Interviews
The presence of DSM symptoms was assessed at each assessment with age-appropriate, semi-structured diagnostic interviews. Caregivers were administered the Preschool Age Psychiatric Assessment (PAPA; Egger, Ascher, & Angold, 1999) when participants were between ages 3.0 and 7.11 years. Caregivers were administered the Child and Adolescent Psychiatric Assessment (CAPA; Angold et al., 1995) when children were aged 8.0 to 8.11 years, and both children and caregivers were administered the CAPA when children were age 9.0 years or older. The PAPA and CAPA assess for DSM criteria and their age-appropriate expressions. The interviews have good test-retest reliability (Angold & Costello, 2000; Egger et al., 2006). Trained raters, blind to previous assessment waves, completed the interviews and 20% of all interviews were reviewed by a master coder to prevent drift, the method recommended by the authors. Depression scores were the number of DSM-IV major depressive disorder core symptoms endorsed (out of nine possible) at a clinically significant level. Anxiety scores were the number of DSM-IV generalized anxiety disorder and separation anxiety disorder core symptoms endorsed at a clinical level (out of 15 possible). Anxiety and depression scores were moderately positively correlated in the current study (Baseline r = .53, Time 1 r = .64, Time 3.5 r = .63, Time 4.5 r = .67, Time 5.5 r = .60, Time 6.5 r = .60, Time 7.5, r = .40, all ps < .001).
Wechsler Abbreviated Scale of Intelligence (WASI; The Psychological Corporation, 1999) is a measure of cognitive functioning for individuals ages 6 to 89 years and includes four subtests, including Vocabulary, Similarities, Block Design, and Matrix Reasoning. The subtests yield Verbal, Performance, and Full Scale IQ scores. IQ scores are scaled in standard units (M = 100, SD = 15). Concurrent validity of the WASI has been demonstrated by significant positive correlations with theoretically comparable estimates from the Kaufman Brief Intelligence Test (all rs > .84; Hays, Reas, & Shaw, 2002) and the Wide Range Intelligence Test (Canivez, Konold, Collins, & Wilson, 2009).
Statistical Analysis
Longitudinal multilevel linear models (MLM) using restricted maximum likelihood estimation were conducted in SPSS version 22 to model the effect of Baseline BRIEF shift or inhibit T-scores on initial values and change in anxiety and depression severity. MLMs were chosen to test study hypotheses over repeated measures ANOVA for several reasons. The primary reason is the ability of MLMs to handle missing observations where repeated measures ANOVAs do not allow for any missing data. Other advantages of MLM over repeated measures ANOVA include estimation of individual subject growth models including intercepts and slopes, the use of maximum likelihood estimation instead of least squares estimation, and the use of different covariance structures (e.g., compound symmetry, autoregressive, and unstructured) while repeated measures ANOVA only uses a compound symmetry covariance structure. Additionally, MLMs can include time-varying covariates in addition to the time invariant covariates repeated measures ANOVA allows.
As noted above, assessments occurred annually, with the exception of an approximate 2.5-year gap between Time 1 and Time 3.5. For analysis, waves were assigned the following numeric values to correspond to the time in years since the Baseline assessment: Time 1 = 1, Time 3.5 = 3.5, Time 4.5 = 4.5, Time 5.5 = 5.5, Time 6.5 = 6.5, and Time 7.5 = 7.5. Covariates in the models were gender, Baseline parental education, IQ score, Baseline anxiety or depression severity, and the interaction of Baseline anxiety or depression severity with time. Wave was initially centered at Time 1 (1 year after Baseline), but to investigate whether there was a significant main effect of Baseline BRIEF shift or inhibit T-scores at each time after Baseline, the MLM's were each run 6 separate times with time centered at a different assessment wave for each model run. In models with a significant interaction of Baseline BRIEF shift or inhibit T-score with wave, wave squared was added to the model to test for quadratic trajectories. To facilitate interpretation of results, assessment “wave” will be referred to as “time.” The repeated measures dependent variables were anxiety and depression severity scores obtained from PAPA/CAPA interviews at annual assessments after Baseline. A first-order autoregressive covariance structure was used.
Results
Descriptive Statistics
There were N=188 participants with Baseline BRIEF data and PAPA/CAPA symptom data from at least one subsequent annual wave available. Characteristics of the sample are shown in Table 1. The sample was 55% (n = 103) male with an average age of 5.42 (SD = .79) years. The racial composition was 60% (n= 113) White, 27% (n = 50) Black, and 13% (n =25) other race/ethnicity.
Table 1.
Characteristics of the Sample (N=188)
| Total N | Mean (SD) | Range | |
|---|---|---|---|
| Child age at baseline (years) | 188 | 5.42 (0.79) | 4.06 – 6.98 |
| Child baseline depression severity | 188 | 1.93 (1.54) | 0 – 8 |
| Child baseline anxiety severity | 187 | 2.40 (2.03) | 0 – 11 |
| Child baseline BRIEF shift T-score | 188 | 52.31 (11.93) | 37 – 90 |
| Child baseline BRIEF inhibit T-score | 188 | 53.38 (12.39) | 36 – 87 |
| Child IQ score | 188 | 105.47 (14.70) | 71 – 137 |
| Total N | % (N) | ||
|---|---|---|---|
| Child male gender | 188 | 54.8 (103) | |
| Child race | 188 | ||
| Caucasian | 60.1 (113) | ||
| African-American | 26.6 (50) | ||
| Other race | 13.3 (25) | ||
| Parental education at baseline | 188 | ||
| High school diploma or less | 7.4 (14) | ||
| Some college | 42.6 (80) | ||
| 4-year college degree | 22.3 (42) | ||
| Graduate education | 27.7 (52) | ||
| Total family income at baseline | 188 | ||
| $0-20,000 | 17.0 (32) | ||
| $20,001-40,000 | 16.0 (30) | ||
| $40,001-60,000 | 18.6 (35) | ||
| $60,000+ | 41.5 (78) |
Depression Severity
Shift deficits
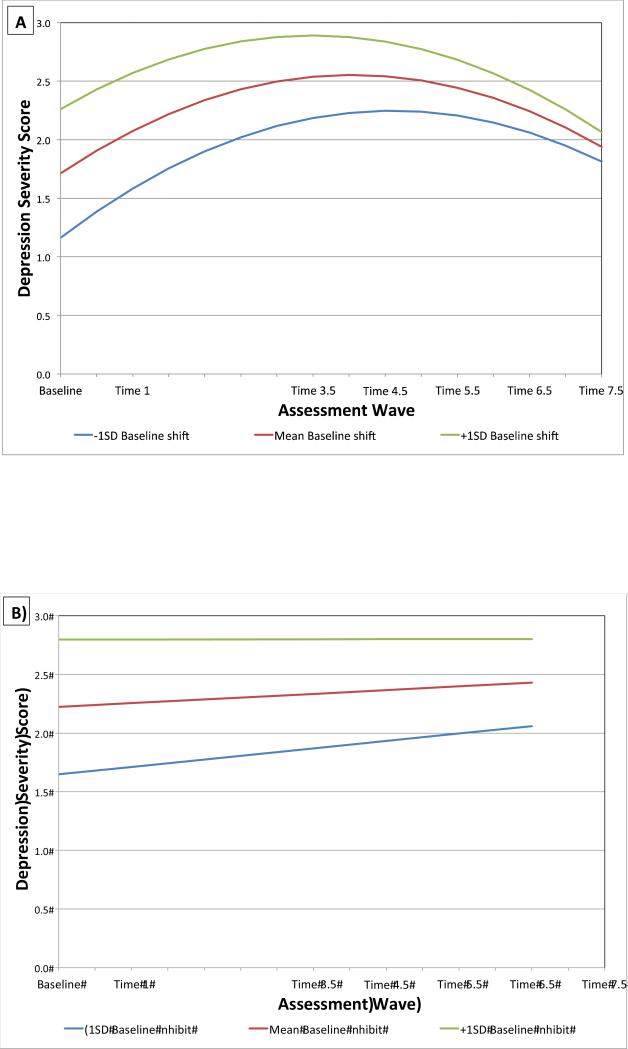
Results of the MLM examining shifting and depression severity are shown in Table 2, model 1 and Figure 1A. Baseline depression severity was a significant covariate; greater depression severity at Baseline was associated with greater depressive symptoms at Time 1. The main effect of Baseline BRIEF shift T-score, time, the interaction between shift T-score and time, and the quadratic effect of time were all significant, ps < .05. Participants with higher Baseline BRIEF shift T-scores had greater depression severity at Time 1, Time 3.5, Time 4.5, and Time 5.5 than those with lower scores. Those with higher Baseline BRIEF shift T-scores also had significantly different depression trajectories than participants with lower shift T-scores, as indicated by the significant shift T-score × time interaction. Specifically, while shift deficits were associated with increased symptoms of depression over time (at 1, 3.5, 4.5, and 5.5 years later), there was a significant difference in the rates of change in symptoms for those with higher (1 SD above the mean) compared to lower (1 SD below the mean) shift deficits (see Figure 1A).. Participants with relatively higher shift deficits showed a faster decrease in depression severity after Time 3.5 5, around age 9 years. In contrast, those with relatively lower shift deficits showed a slower decrease in severity over time after age 9 years.
Table 2.
Multilevel Linear Models of Depression Severity (N=188)
| Model 1. Shift Model for Depression severity | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept | 0.346 | 0.903 | 0.38 | .702 |
| Timea | 0.592 | 0.133 | 4.45 | <.001 |
| Time squared | −0.051 | 0.012 | −4.20 | <.001 |
| Male gender | 0.301 | 0.169 | 1.79 | .076 |
| Baseline parental educationb | −0.068 | 0.043 | −1.59 | .114 |
| IQ score | −0.007 | 0.007 | −0.96 | .338 |
| Baseline depression severity | 0.358 | 0.082 | 4.38 | <.001 |
| Baseline BRIEF shift T-scorec | 0.041 | 0.011 | 3.90 | <.001 |
| Baseline depression severity X Time | −0.016 | 0.018 | −0.88 | .379 |
| Baseline BRIEF shift T-score X Time | −0.005 | 0.002 | −2.03 | .043 |
| Model 2. Inhibition Model for Depression severity | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept | 0.016 | 0.837 | 0.02 | .985 |
| Timea | 0.221 | 0.116 | 1.91 | .056 |
| Male gender | 0.281 | 0.159 | 1.77 | .078 |
| Baseline parental educationb | −0.040 | 0.040 | −1.01 | .316 |
| IQ score | −0.007 | 0.007 | −1.05 | .297 |
| Baseline depression severity | 0.415 | 0.075 | 5.53 | <.001 |
| Baseline BRIEF inhibit T-scored | 0.044 | 0.009 | 4.70 | <.001 |
| Baseline depression severity X Time | −0.028 | 0.018 | −1.59 | .112 |
| Baseline BRIEF inhibit T-score X Time | −0.003 | 0.002 | −1.16 | .248 |
| Model 3. Shift Model for Anxiety severity | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept | 0.106 | 0.922 | 0.12 | .909 |
| Timea | 0.143 | 0.121 | 1.18 | .237 |
| Male gender | 0.131 | 0.171 | 0.77 | .444 |
| Baseline parental educationb | −0.012 | 0.043 | −0.28 | .779 |
| IQ score | −0.004 | 0.007 | −0.55 | .586 |
| Baseline anxiety severity | 0.509 | 0.067 | 7.64 | <.001 |
| Baseline BRIEF shift T-scorec | 0.029 | 0.012 | 2.48 | .014 |
| Baseline anxiety severity X Time | −0.047 | 0.014 | −3.29 | .001 |
| Baseline BRIEF shift T-score X Time | −0.004 | 0.002 | −1.43 | .154 |
| Model 4. Inhibition Model for Anxiety severity | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept | 0.465 | 0.903 | 0.52 | .607 |
| Timea | −0.053 | 0.117 | −0.46 | .649 |
| Male gender | 0.116 | 0.170 | 0.68 | .497 |
| Baseline parental educationb | 0.003 | 0.042 | 0.07 | .945 |
| IQ score | −0.004 | 0.007 | −0.60 | .551 |
| Baseline anxiety severity | 0.549 | 0.063 | 8.72 | <.001 |
| Baseline BRIEF inhibit T-scored | 0.018 | 0.010 | 1.71 | .089 |
| Baseline anxiety severity X Time | −0.057 | 0.014 | −4.24 | <.001 |
| Baseline BRIEF inhibit T-score X Time | 0.001 | 0.002 | 0.29 | .770 |
Time = numeric values corresponding to time in years since the Baseline assessment: Time 1 = 1, Time 3.5 = 3.5, Time 4.5 = 4.5, Time 5.5 = 5.5, Time 6.5 = 6.5, and Time 7.5 = 7.5. Time centered at Time 1 in models shown.
Parental education on 1-11 scale, with 11 being the most education
Higher Baseline BRIEF shift T-score significantly associated with greater depression severity at all time points up to Time 5.5
Higher Baseline BRIEF inhibit T-score significantly associated with greater depression severity at all time points
Time = numeric values corresponding to time in years since the Baseline assessment: Time 1 = 1, Time 3.5 = 3.5, Time 4.5 = 4.5, Time 5.5 = 5.5, Time 6.5 = 6.5, and Time 7.5 = 7.5. Time centered at Time 1 in models shown.
Parental education on 1-11 scale, with 11 being the most education
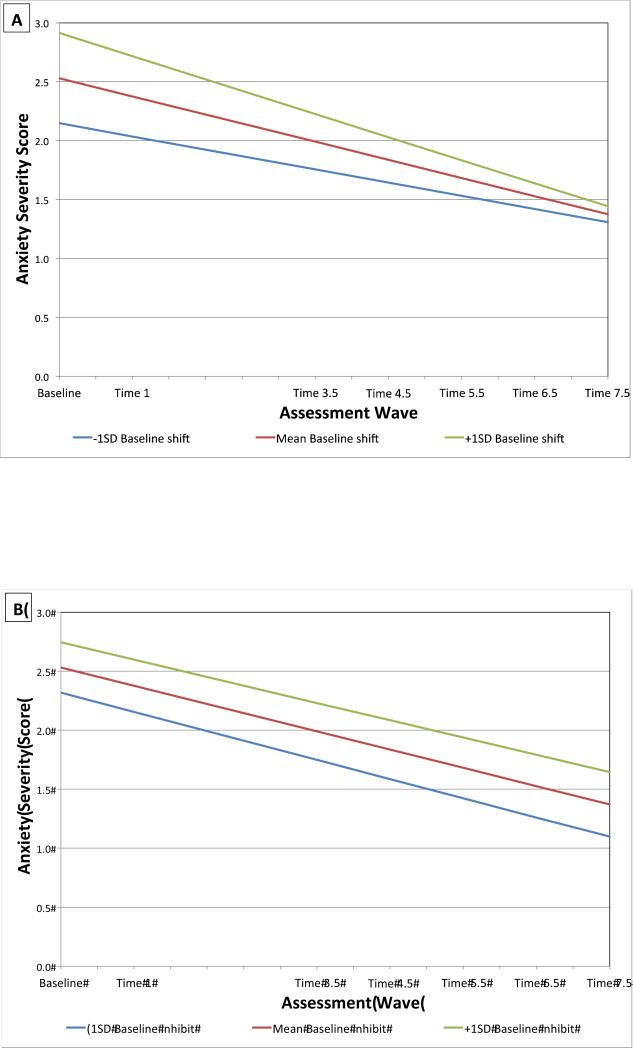
Higher Baseline BRIEF shift T-score significantly associated with greater anxiety severity at Time 1 and Time 3.5
Higher Baseline BRIEF inhibit T-score significantly associated with greater anxiety severity at Time 3.5 and all later waves.
Figure 1.
Estimated Trajectories of Depression Severity by Baseline BRIEF Shift (A) and Inhibit T-Scores (B)
Inhibition deficits
Results of the MLM examining inhibition deficits and depression severity are shown in Table 2, model 1 and Figure 1B. Baseline depression severity was a significant covariate, p < .001, with greater Baseline depression associated with greater depression at Time 1. There was no significant main effect for time, p = .06. The interaction between Baseline BRIEF inhibit T-score and time was non-significant, p = .25. The main effect of Baseline BRIEF inhibit T-score was significant, p < .001, indicating a significant association between higher Baseline BRIEF inhibit T-scores and greater depression severity for all subsequent time points (Time 1, Time 3.5, Time 4.5, Time 5.5, Time 6.5, and Time 7.5), ps < .05.
Anxiety Severity
Shift deficits
Results of the MLM of shift deficits and anxiety severity are shown in Table 3, model 3 and Figure 2A. Baseline anxiety severity was a significant covariate, p < .001, with greater Baseline anxiety severity associated with greater Time 1 anxiety; there was also a significant interaction between Baseline anxiety severity and time, p = .001, with greater Baseline anxiety associated with a sharper decrease in anxiety severity over time. There was no significant interaction between Baseline BRIEF shift T-score and time, p = .15. However, the main effect of Baseline BRIEF shift T-score was significantly associated with greater anxiety severity at Time 1 and Time 3.5, p < .05.
Figure 2.
Estimated Trajectories of Anxiety Severity by Baseline BRIEF Shift (A) and Inhibit T-Scores (B)
Inhibition deficits
Results for the MLM of inhibition deficits and anxiety are presented in Table 3, model 4 and Figure 2B. As before, the Baseline anxiety covariate was positively associated with greater subsequent anxiety at Time 1, p < .001, and the interaction with time was significant, p < .001, suggesting a steeper decrease in anxiety severity over time in participants with greater Baseline anxiety. There was not a main effect of Baseline BRIEF inhibit scores on anxiety at Time 1, p = .09; however greater Baseline BRIEF inhibit T-scores were significantly associated with greater anxiety severity at Time 3.5, Time 4.5, Time 5.5, Time 6.5, and Time 7.5, all ps < .05. There was not a significant interaction between Baseline BRIEF inhibit scores and time on later anxiety severity, p =.77.
Discussion
The current study was designed to examine associations between cognitive control deficits, specifically in inhibition and shifting, early in childhood (ages 3 to 6 years) and symptoms of both depression and anxiety through school age. Overall, results from multilevel models suggest that early deficits in shifting and inhibition were associated with greater depression and anxiety over time, relative to those with lower deficits. Importantly, these effects were significant even after accounting for the influence of other key predictors including assessment wave (time), gender, IQ, and symptoms at Time3, suggesting that effects are relatively robust. Assessments of such deficits, especially in shifting and inhibition, may help to identify children at risk for later symptoms.
The model predicting depression from early shifting deficits resulted in a significant Time × Shift interaction, indicating that shift deficits were associated with distinct independent trajectories of depression symptoms. Specifically, attentional shift deficits were associated with both greater depression severity (1, 3.5, 4.5, and 5.5 years later) and different rates of change in severity, such that relatively higher shift deficits were associated with a more rapid decrease in depression severity after age 9 years, compared to those with relatively lower shift deficits. This significant Shift × Time interaction was unexpected and counter-intuitive at first glance. However, developmental differences in attentional challenges may explain the risk trajectory found. The more rapid decrease in depression severity after age 9 years associated with high shift deficits is hypothesized to be related to the change in psychosocial stresses related to puberty. That is, during the adolescent period, also known as the “storm and stress period of development,” stressors become more socially nuanced. We speculate that for those with early (and presumably ongoing) shift deficits, these psychosocial stressors become less salient or more poorly understood/processed, resulting in more rapid decreases in symptoms. This hypothesis is speculative and should be directly tested in future studies.
Results also indicated that shift deficits were associated with greater anxiety severity 1 year and 3.5 years after Baseline. Inhibition deficits at preschool age significantly predicted both greater depression and anxiety over time, including at each of the six subsequent time points for depression and at each of five subsequent time points, for anxiety. There was no significant interaction between time and shift for the model of anxiety, or for inhibition in the models of depression or anxiety. The nonsignificant interaction suggests that shift deficits were associated with increased depression and inhibition deficits were associated with increased depression and anxiety severity, regardless of developmental stage and/or time. These findings suggests that shift deficits in relation to anxiety and inhibition deficits in relation to both anxiety and depression may be more stable, trait-like risk factors present early in development that persists throughout childhood and do not vary in their association with symptoms across developmental stage.
Relative Prediction of Shift and Inhibition Deficits
Findings from this study suggest that there may be relative differences in how specific cognitive control deficits influence trajectories of depression and anxiety symptom severity. The association between inhibition deficits and both depression and anxiety appeared to persist across development while the association between shift deficits and depression changed across development, such that deficits no longer predicted symptoms at later time points. The longer-term impact of inhibition deficits may be due in part to De Raedt and Koster's (2010) notion of a gateway mechanism for negative thoughts. That is, those with greater inhibition deficits may experience a greater overall number of unwanted negative thoughts, increasing the likelihood of rumination and/or worry and associated negative affect. While shift deficits likely also contribute to the experience of getting “stuck” on negative thoughts, the gateway inhibition deficit may represent a more significant longer-term vulnerability factor.
Conceptual Implications for Models of Depression and Anxiety Symptom Development
The role of cognitive deficits in the development and maintenance of affective problems in youth have yet to be fully integrated into existing models of child psychopathology. Established risk factors for internalizing problems include genetic factors, temperament, attachment, parental mental health and parenting behaviors (harsh discipline, overcontrol [for anxiety], lack of warmth), cognitions, and biased attention (e.g., Bayer et al., 2011; Garber, 2010; Ingram & Price, 2010; Vasey & Dadds, 2001). The influence of cognitive control deficits, such as those assessed in the current study, however, remains understudied. Further, the mechanisms by which recognized biological and environmental risk factors like those above might interact with cognitive deficits to predict symptoms remains largely unknown, and cognitive and developmental psychopathology models remain largely disparate. As an example of how this gap might be addressed, one recent study found that hostile parenting at age three years predicted larger error-related negativity, an event-related potential (ERP) associated with cognitive control (and anxiety) in children, 3 years later (Meyer et al., 2014). Additional work designed to integrate cognitive control deficits into existing models of developmental psychopathology may help to further explain the development of internalizing symptoms over time.
In terms of cognitive models, findings from this longitudinal study are consistent with hypotheses that cognitive control deficits represent an important early vulnerability factor for developing affective symptoms (e.g., Disner, Beevers, Haigh, & Beck, 2011). Cognitive control skills begin developing around one year of age (Rueda, Posner, & Rothbart, 2005) and show substantial growth during preschool age (Zelazo & Müller, 2002). Early measures of cognitive control deficits have been linked with academic and social ability in childhood (Razza & Blair, 2009; Willoughby et al, 2012) and positive health and financial outcomes into the early 30s (Moffitt et al., 2011). Given the rapid rate and perhaps critical timing of the development of cognitive control skills in preschool years (for review see Moriguchi & Hiraki, 2013), its assessment during this developmental period may be important in order to capture influences on later mental health outcomes.
Clinical Implications
The current findings underscore the importance of early cognitive control processes in the longitudinal trajectory of depression and anxiety and have important clinical implications. Incorporating a measure of cognitive control deficits, with predictive utility beyond that provided by assessments of current symptoms (as indicated in the current study), may help to identify youth at risk for worsening symptoms. Importantly, the parent-report measure of deficits in the current study has high clinical utility, as it can be completed by the child's parent in less than 15 minutes and may be scored quickly by hand or with computer software. This relatively simple measure may provide educators, primary care physicians, psychiatrists, and other mental health professionals with additional important information about children's likelihood of developing and maintaining later affective symptoms.
If replicated, findings may also have implications for prevention and intervention. While cognitive-behavioral therapies have shown moderate effect sizes in anxiety and depression symptom reduction and rates of remission (Cartwright-Hatton et al., 2004; James et al. 2013; Weisz et al., 2006), a substantial proportion of children fail to fully recover from affective disorders. Further, given their chronic and recurrent course, many children will likely go on to experience additional symptomatic episodes. Targeting underlying cognitive vulnerabilities that increase the likelihood of symptoms, rather than (or in addition to) the expression of the symptoms themselves, may provide an additional tool for both prevention and intervention. In light of findings from the current study, early intervention on cognitive control in particular may be beneficial in altering children's long-term symptom trajectory.
Limitations and Future Directions
Results of the current study should be interpreted with several limitations in mind. First, parent-reported estimates of cognitive control were used to measure deficits in shifting and inhibition. In general, inconsistent correlations between rating scale and performance based measures of the same construct are of note (Roth, Isquith, & Goia, 2014). However, several studies suggest that the BRIEF has small to moderate correlations with performance based measures of cognitive control (Mahone et al., 2002; McAuley, Chen, Goos, Schachar, & Crosbie, 2010; Shimoni, Engel-Yeger, & Tirosh, 2012; Toplak, Bucciarelli, Jain, & Tannock, 2009), somewhat alleviating this concern. Studies designed to assess cognitive control using brain-based measures of prefrontal activation, such as electroencephalogram (EEG) or other imaging, especially in the context of emotional information, would address this concern. The study is also limited by a single assessment of cognitive control deficits. Additional study of both cognitive control and affective symptoms over time would provide information about bidirectional influences. Further research designed to measure the influence of cognitive control on the development of affective symptoms is needed, especially studies that measure cognitive control across several units of analysis and longitudinally (before, during, and after symptomatic episodes). Future work will also be needed to more fully integrate cognitively and neurobiologically based models of the development of depression and anxiety with known risk factors in order to provide a comprehensive, up-to-date perspective on current knowledge.
References
- Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA). Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA). Psychological Medicine. 1995;25:739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- Bayer JK, Ukoumunne OC, Lucas N, Wake M, Scalzo K, Nicholson JM. Risk factors for childhood mental health symptoms: National longitudinal study of Australian children. Pediatrics. 2011;128:865–879. doi: 10.1542/peds.2011-0491. [DOI] [PubMed] [Google Scholar]
- Beck A, Rush A, Shaw B, Emery G. Cognitive therapy of depression. Guilford Press; Guilford, New York. New York: 1979. [Google Scholar]
- Berggren N, Derakshan N. Inhibitory deficits in trait anxiety: Increased stimulus-based or response-based interference? Psychonomic Bulletin & Review. 2014;21:1339–1345. doi: 10.3758/s13423-014-0611-8. http://doi.org/10.3758/s13423-014-0611-8. [DOI] [PubMed] [Google Scholar]
- Canivez GL, Konold TR, Collins JM, Wilson G. Construct validity of the Wechsler Abbreviated Scale of Intelligence and Wide Range Intelligence Test: Convergent and structural validity. School Psychology Quarterly. 2009;24:252–265. [Google Scholar]
- Cartwright-Hatton S, Roberts C, Chitsabesan P, Fothergill C, Harrington R. Systematic review of the efficacy of cognitive behaviour therapies for childhood and adolescent anxiety disorders. British Journal of Clinical Psychology. 2004;43:421–436. doi: 10.1348/0144665042388928. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory and therapy of depression. Wiley; Hoboken, NJ: 1999. [Google Scholar]
- Connolly SL, Wagner CA, Shapero BG, Pendergast LL, Abramson LY, Alloy LB. Rumination prospectively predicts executive functioning impairments in adolescents. Journal of Behavior Therapy and Experimental Psychiatry. 2014;45:46–56. doi: 10.1016/j.jbtep.2013.07.009. http://doi.org/10.1016/j.jbtep.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. http://doi.org/10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EHW, Goubert L, Onraedt T, Vanderhasselt M-A, De Raedt R. Cognitive control moderates the association between stress and rumination. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43:519–525. doi: 10.1016/j.jbtep.2011.07.004. http://doi.org/10.1016/j.jbtep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. http://doi.org/10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Demeyer I, De Lissnyder E, Koster EHW, De Raedt R. Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: A prospective study in remitted depressed adults. Behaviour Research and Therapy. 2012;50:292–297. doi: 10.1016/j.brat.2012.02.012. http://doi.org/10.1016/j.brat.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness: An investigation using the Antisaccade task. Experimental Psychology. 2009;56:48–55. doi: 10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh E. a P., Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews. Neuroscience. 2011;12:467–477. doi: 10.1038/nrn3027. http://doi.org/10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Egger H, Ascher B, Angold A. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; Durham, NC: 1999. Preschool Age Psychiatric Assessment (PAPA), Version 1.1. [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA). Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Ehring T, Watkins ER. Repetitive Negative Thinking as a Transdiagnostic Process. International Journal of Cognitive Therapy. 2008;1:192–205. [Google Scholar]
- Fitzgerald KD, Liu Y, Stern ER, Welsh RC, Hanna GL, Monk CS, Taylor SF. Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:1183–1191.e1. doi: 10.1016/j.jaac.2013.09.002. http://doi.org/10.1016/j.jaac.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J. Vulnerability to depression in childhood and adolescence. In: Ingram RE, Price JM, editors. Vulnerability to psychopathology: Risk across the lifespan. Guilford Press; New York, NY: 2010. pp. 189–247. [Google Scholar]
- Gioia GA, Espy KA, Isquith PK. Behavior Rating Inventory of Executive Function-Preschool Version: Professional manual. Psychological Assessment Resources; Lutz: 2003. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworhty L. Behavior rating inventory of executive function (BRIEF): Professional manual. Psychological Assessment Resources; Lutz, FL: 2000. [Google Scholar]
- Gross JJ. Emotion regulation: Conceptual and empirical foundations. In: Gross JJ, editor. Handbook of emotion regulation. 2nd ed. Guilford Press; New York, NY: 2014. pp. 3–20. [Google Scholar]
- Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, Cullen K. Selective neurocognitive impairments in adolescents with major depressive disorder. Journal of Adolescence. 2012;35:11–20. doi: 10.1016/j.adolescence.2011.06.009. http://doi.org/10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T. a, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2009;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. http://doi.org/10.1016/j.biopsych.2008.03.015015.Biological. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JR, Reas DL, Shaw JB. Concurrent validity of the Wechsler abbreviated scale of intelligence and the Kaufman brief intelligence test among psychiatric inpatients. Psychological Reports. 2002;90:355–359. doi: 10.2466/pr0.2002.90.2.355. http://doi.org/10.2466/pr0.2002.90.2.355. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Price JM. Vulnerability to psychopathology: Risk across the lifespan. Guilford Press; New York: 2010. [Google Scholar]
- James AC,, James G, Cowdrey FA, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Systematic Review. 2013;6:CD004690. doi: 10.1002/14651858.CD004690.pub3. [DOI] [PubMed] [Google Scholar]
- Kertz SJ, Koran J, Stevens KT, Bjorvinsson T. Repetitive negative thinking predicts depression and anxiety symptom improvement during brief cognitive behavioral therapy. Behaviour Research and Therapy. 2015;68:54–63. doi: 10.1016/j.brat.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. http://doi.org/10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. Journal of Affective Disorders. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. http://doi.org/10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Archives of General Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. http://doi.org/10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Cirino PT, Cutting LE, Cerrone PM, Hagelthorn KM, Hiemenz JR, Denckla MB. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 2002;17:643–662. [PubMed] [Google Scholar]
- McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? Journal of the International Neuropsychological Society : JINS. 2010;16:495–505. doi: 10.1017/S1355617710000093. http://doi.org/10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- Meyer A, Proudift GH, Bufferd SG, Kujawa AJ, Laptoook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error related brain activity in six-year-old children. Journal of Abnormal Child Psychology. 2015;43:821–829. doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. http://doi.org/10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. http://doi.org/10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Control of mental processes. In: Bruce V, editor. Unsolved mysteries of the mind: Tutorial essays in cognition. Lawrence Erlbaum Associates Ltd; Hove, UK: 1996. pp. 93–148. [Google Scholar]
- Moriguchi Y, Hiraki K. Prefrontal cortex and executive function in young children: a review of NIRS studies. Frontiers in Human Neuroscience. 2013;7:867. doi: 10.3389/fnhum.2013.00867. http://doi.org/10.3389/fnhum.2013.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razza RA, Blair C. Associations among false-belief understanding, executive function, and social competence: A longitudinal analysis. Journal of Applied Developmental Psychology. 2009;30:332–343. doi: 10.1016/j.appdev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith P, Gioia GA. Assessment of executive functioning using the Behavior Rating Inventory of Executive Function (BRIEF). In: Goldstein S, Naglieri JA, editors. Handbook of executive functioning. Springer Science + Business Media; New York, New York: 2014. pp. 301–331. [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The development of executive attention: contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28:573–94. doi: 10.1207/s15326942dn2802_2. http://doi.org/10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Tsao JCI, Alden LE, Craske MG. Worry and rumination: Repetitive thought as a concomitant and predictor of negative mood. Cognitive Therapy and Research. 2000;24:671–688. [Google Scholar]
- Shimoni M, Engel-Yeger B, Tirosh E. Executive dysfunctions among boys with Attention Deficit Hyperactivity Disorder (ADHD): performance-based test and parents report. Research in Developmental Disabilities. 2012;33:858–565. doi: 10.1016/j.ridd.2011.12.014. http://doi.org/10.1016/j.ridd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research. 2007;31:235–262. http://doi.org/10.1007/s10608-006-9118-6. [Google Scholar]
- Siegle GJ, Moore PM, Thase ME. Rumination: One construct, many features in healthy individuals, depressed individuals, and individuals with lupus. Cognitive Therapy and Research. 2004;28:645–668. [Google Scholar]
- Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:1326–1336.e5. doi: 10.1016/j.jaac.2013.10.001. http://doi.org/10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, Monk CS. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology. 2008;79:216–222. doi: 10.1016/j.biopsycho.2008.05.004. http://doi.org/10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation . WASI: Wechsler Abbreviated Scale of Intelligence. Author; San Antonio, TX: 1999. [Google Scholar]
- Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology : A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2009;15:53–72. doi: 10.1080/09297040802070929. http://doi.org/10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, Brunoni AR, Loeys T, Boggio PS, De Raedt R. Nosce te ipsum - Socrates revisited? Controlling momentary ruminative self-referent thoughts by neuromodulation of emotional working memory. Neuropsychologia. 2013;51:2581–2589. doi: 10.1016/j.neuropsychologia.2013.08.011. http://doi.org/10.1016/j.neuropsychologia.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Vasey MF, Dadds MR. An introduction to the developmental psychopathology of anxiety. In: Vasey MF, Dadds MR, editors. The developmental psychopathology of anxiety. Oxford University Press; New York, NY: 2001. pp. 3–26. [Google Scholar]
- Visu-Petra L, Miclea M, Visu-Petra G. Individual differences in anxiety and executive functioning: a multidimensional view. International Journal of Psychology : Journal International de Psychologie. 2013;48:649–659. doi: 10.1080/00207594.2012.656132. http://doi.org/10.1080/00207594.2012.656132. [DOI] [PubMed] [Google Scholar]
- Wagner CA, Alloy LB, Abramson LY. Trait rumination, depression, and executive functions in early adolescence. Journal of Youth and Adolescence. 2014;44:18–36. doi: 10.1007/s10964-014-0133-8. http://doi.org/10.1007/s10964-014-0133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Müller C, Helmreich I, Huss M, Tadić A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. European Child & Adolescent Psychiatry. 2014;24:5–19. doi: 10.1007/s00787-014-0559-2. http://doi.org/10.1007/s00787-014-0559-2. [DOI] [PubMed] [Google Scholar]
- Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: A meta-analysis. Psychological Bulletin. 2006;132:132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson PO, Goodyer IM. Attention difficulties and mood-related ruminative response style in adolescents with unipolar depression. Journal of Child Psychology and Psychiatry. 2006;47:1284–1291. doi: 10.1111/j.1469-7610.2006.01660.x. http://doi.org/10.1111/j.1469-7610.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, Greenberg M. The measurement of executive function at age 5: Psychometric properties and relationship to academic achievement. Psychological Assessment. 2012;24:226–239. doi: 10.1037/a0025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development. In: Goaswami U, editor. Blackwell handbook of childhood cognitive development. Blackwell Publishing; Malden: 2002. pp. 445–469. [Google Scholar]
- Zetsche U, Joormann J. Components of interference control predict depressive symptoms and rumination cross-sectionally and at six months follow-up. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:65–73. doi: 10.1016/j.jbtep.2010.06.001. [DOI] [PubMed] [Google Scholar]