Summary
Numerous arthropods harbor maternally transmitted bacteria that induce the preferential death of males [1-7]. This sex-specific lethality benefits the bacteria because males are ‘dead ends’ regarding bacterial transmission and their absence may result in additional resources for their viable female siblings who can thereby more successfully transmit the bacteria [5]. Although these symbionts disrupt a range of developmental processes [8-10], the underlying cellular mechanisms are largely unknown. It was previously shown that mutations in genes of the dosage compensation pathway of Drosophila melanogaster suppressed male killing caused by the bacterium, Spiroplasma [10]. This result suggested that dosage compensation is a target of Spiroplasma. However, it remains unclear how this pathway is affected, and whether the underlying interactions require the male-specific cellular environment. Here we investigated the cellular basis of male embryonic lethality in D. melanogaster induced by Spiroplasma. We found that the dosage compensation complex (DCC), which acetylates X chromatin in males [11], becomes mis-localized to ectopic regions of the nucleus immediately prior to the killing phase. This effect was accompanied by inappropriate histone acetylation and genome-wide mis-regulation of gene expression. Artificially induced formation of the DCC in infected females, through transgenic expression of the DCC-specific gene msl-2, resulted in mis-localization of this complex to non-X regions and early Spiroplasma-induced death, mirroring the killing effects in males. These findings strongly suggest that Spiroplasma initiates male killing by targeting the dosage compensation machinery directly and independently of other cellular features characteristic of the male sex.
Results and Discussion
Spiroplasma are cell wall-less spirochetes belonging to the bacterial Mollicute class that infect the haemolymph and egg cytoplasm of their insect hosts [12,13]. Male killing strains of Spiroplasma are common within the Drosophila genus, having been detected in at least eight different Drosophila species including D. melanogaster [6]. In this latter species, males, but not females, infected with the male-killing MSRO (for melanogaster sex ratio organism) strain of Spiroplasma exhibit disrupted central nervous system (CNS) formation and heightened apoptosis in epithelial cells during the middle stages of embryogenesis, and they fail to reach the larval stages [14,15]. Previous work has suggested that Spiroplasma induces developmental defects in males by altering the male-specific process of dosage compensation [10]. In these studies, genetic crosses were utilized to test if strong loss-of-function mutations in several male specific lethal (msl) genes, including msl-1, msl-2, and msl-3, as well as maleless (mle), and male absent on the first (mof), could modulate male killing [10]. Normally msl-2 is expressed only in males while the other genes are expressed in both sexes. In males the MSL-2 protein associates with the other proteins to form the DCC (also known as the MSL complex), which localizes preferentially to specific regions within the gene-containing euchromatin of the single X [16,17]. The presence of this complex induces acetylation of histone H4 at Lysine residue 16 (H4K16ac) within the body of compensated genes as a result of MOF acetylase activity [11,18,19]. This modification is accompanied by a ~2-fold transcriptional increase of most genes on the X chromosome so that the X-to-autosome (non-sex chromosome) ratio for gene expression in males matches that found in females, which have two active X chromosomes [20]. A substantial portion of Spiroplasma-infected males mutant for any of the DCC components were found to survive past the embryonic male killing phase [10]. This result demonstrated a genetic interaction between the dosage compensation components and the level of male killing. However, it remains to be determined whether this effect reflects a direct bacterial targeting of the dosage compensation machinery or instead an indirect suppression of male killing, perhaps because dosage compensation is downstream of another targeted cellular process.
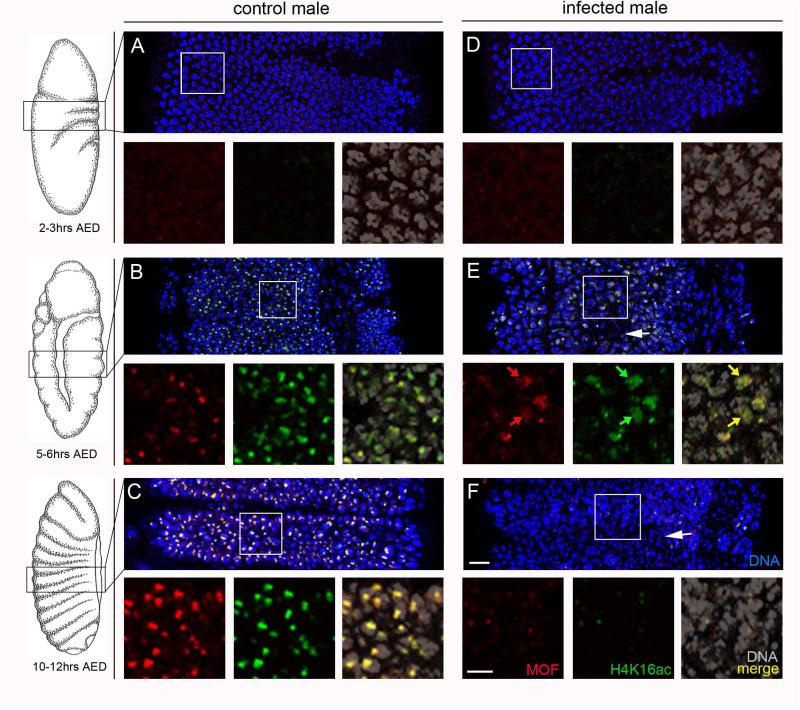
To better understand this phenomenon we cytologically examined the subcellular behavior of the DCC and its histone acetylation mark just before the lethal phase in male D. melanogaster embryos infected with MSRO Spiroplasma (from here onward referred to as Spiroplasma) [14]. This strain was lethal to ≥96% of male progeny in the D. melanogaster lines used in this study (Table 1). In control (uninfected) embryos, very little DCC signal was seen before 3hrs after egg deposition (AED), as indicated by hardly detectable levels of visible MOF or H4K16ac on chromatin (N=7 embryos; Figure 1A), consistent with previous studies showing that DCC formation occurs around this time [21,22]. By 4-6hrs AED, during embryonic germ band extension, MOF appeared as a distinct sub-nuclear focus that was highly enriched with H4K16ac within the epithelial (outer) cells of male embryos (N=13 embryos; Figure 1B). This enrichment of H4K16ac also co-localized with a single bright region of MSL-3 (N=11 embryos; Figure S1) and resided immediately adjacent to or overlapping with X sequences (Figure S2). These signals became strongest and evenly distributed across the embryo's surface by the beginning of segmentation (7+hrs AED) (Figure 1C, Figure S1).
Table 1.
Adult sex ratios for Spiroplasma-infected and uninfected fly lines
| Fly line | Number females | Number males | Total | % males |
|---|---|---|---|---|
| Canton-S | 117 | 99 | 216 | 46 |
| Canton-S (Sp+) | 178 | 0 | 178 | 0 |
| w;msl-3-GFP | 233 | 248 | 481 | 52 |
| w;msl-3-GFP (Sp+) | 265 | 5 | 270 | 2 |
Figure 1. Spiroplasma-infected male embryos exhibit mis-localized MOF and H4K16ac.
(A,B) Both control (uninfected) and infected embryos at 2-3hrs after egg deposition (AED) show very little MOF or H4K16ac signals. (C,D) At 5-6hrs AED, MOF and H4K16ac signals become bright and co-localize to a single focus in each nucleus of control embryos. The H4K16ac signals co-localizes with X sequences and overlap with MSL3 (see Figures S1 and S2 respectively). In contrast, these signals, although still overlapping, become distributed across nuclei in infected embryos (yellow arrows in right panel). Some nuclei in infected embryos appear hyper-condensed (white arrow). (E,F) At 9-11hrs AED, MOF and H4K16ac signals persist as bright foci in the nuclei of control embryos. However, in infected embryos these signals are barely visible in most nuclei, and more nuclei are hyper-condensed (white arrow). Scale bar equals 15 μm in wide panel F and 5 μm in the high magnification panel under F.
In Spiroplasma-infected male embryos, very little MOF and H4K16ac signals were visible before 3hrs AED (N=6 embryos), similar to control male embryos at this time (Figure 1D, compared to 1A). At 4-6hrs, AED MOF and H4K16ac accumulated into sub-nuclear foci corresponding to X sequences within a portion of nuclei (N=15 embryos; Figure 1E). However, these signals only weakly accumulated into foci or not at all, and in many regions of the embryo they were distributed broadly across the nucleus (N=13 of 15 embryos; Figure 1E). In contrast, overall nuclear morphology appeared normal in most cells at this time, although a small portion of nuclei appear hyper-condensed (Figure 1E). By 9+hrs AED, when embryos undergo body segmentation, many nuclei exhibited abnormal hyper-condensation (Figure 1F). Additionally, DCC and H4K16ac signals became very low (N=15 embryos; Figure 1F; Figure S1), which may stem from general chromatin mis-organization as nuclear morphology became more abnormal. No DCC foci were observed in wild type Spiroplasma-infected or uninfected female embryos (N=9 embryos; Figure S3) and, consistent with previous studies [14,15], all were normal in cellular morphology.
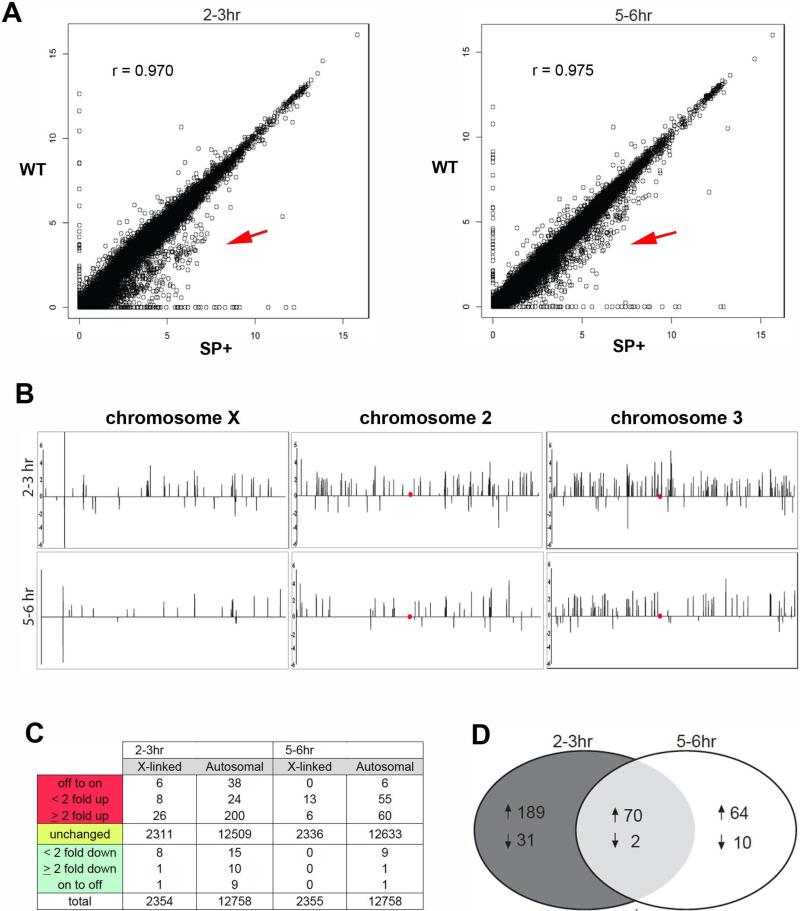
The observation of DCC localization and H4K16 acetylation in ectopic nuclear regions led us to ask if this effect causes abnormal genome-wide mis-regulation of gene expression, which could explain defective male development. In order to address this question, we collected male enriched embryo broods (see Supplemental Experimental Procedures) at both 2-3hrs and 5-6hrs AED, and performed transcriptional profiling of total mRNAs extracted from these samples (Table S1). Although there was a high congruence of gene expression between control and infected embryos, a subset of genes appeared to be mis-expressed with a bias toward over-expression in infected embryos at both developmental times (Figure 2A, Table S1). To further evaluate this trend, we plotted the distribution of expression ratios (infected/uninfected) for autosomal and X-linked genes at both time points and used the Kolmogorov-Smirnov (K-S) test to determine an overall deviation from a 1:1 ratio (i.e., no change in expression between conditions). The distribution of X-linked expression ratios significantly deviated at 2-3hrs AED (P<1×10−4) while the autosomal distribution was found to significantly deviate at both 2-3hrs and 5-6hrs AED (P<1×10−7 and P<1×10−4, respectively; Table S1), confirming the observed bias toward global over-expression (Figure 2A).
Figure 2. Spiroplasma-infected male embryos exhibit genome-wide misregulation of gene expression.
(A) Scatterplots show averaged FPKM values between corresponding replicates for expressed genes in wild type embryos plotted against those of Spiroplasma-infected embryos for both time points. Values are shown in log scale. Red arrow indicates a higher clustering of genes that appear over-expressed in infected embryos. (B) Chromosome maps depicting the positions of significantly mis-expressed genes in Spiroplasma-infected embryos relative to uninfected embryos. Peaks above the horizontal lines represent genes that are significantly over-expressed in Spiroplasma-infected embryos, and peak height reflects expression ratio on the log scale. Red dot corresponds to the chromosome centromeres (C) The number of significantly mis-expressed genes on the X vs. the autosomes (non-sex chromosomes) and their general fold change values between Spiroplasma-infected and uninfected embryos. The patterns in B and C reflect an enrichment of over-expressed genes on all chromosomes and for both developmental times. (D) A Venn diagram depicting the number of over-expressed genes (up arrow) and under-expressed (down arrow) in Spiroplasma-infected embryos that are unique to each developmental time point and those common between these times. See Table S1 for raw data, and Figure S4 for ontogeny groupings of mis-expressed genes common between 2-3hrs and 5-6hrs AED.
Closer inspection of our data sets revealed that a modest number of both X and autosomal (non-sex chromosome) genes were significantly mis-expressed at each developmental time (Figure 2B,C; Table S1), although this number may be underestimated due to large variation in expression between replicates, potentially reflecting the stochastic nature of DCC mis-localization. Mis-expressed genes were located across each chromosome and not clustered at distinct region(s) (Figure 2B), consistent with the observed lack of DCC concentration at distinct non-X regions (Figure 1E,F). The finding of over-expressed genes on the X, however, was surprising given that the DCC normally becomes enriched on X chromatin and overall loss of DCC there would be expected to result in the opposite pattern. These findings are consistent with previous studies showing that dissociation of the DCC from the male X results in MOF localization and H4K16 acetylation across the genome, as well as up-regulation of autosomal genes but not X-linked genes [23,24]. The majority of mis-expressed genes were over-expressed at both developmental times (Figure 2B,C; Table S1), consistent with the H4K16ac mark being associated with increased gene expression [25]. A portion of mis-expressed genes on the X and the autosomes exhibited greater than 2-fold expression level differences in infected embryos (Figure 2C), when, under normal conditions, the DCC transcriptionally compensates at only a ~2-fold level expression change on the X. These patterns may reflect an abnormal chromatin remodeling behavior of the DCC in the presence of Spiroplasma. This idea is consistent with previous studies suggesting that DCC activity is normally constrained by an unknown mechanism so that X gene up-regulation occurs at an ~2-fold level [24,26,27]; it is possible that the male killing effect alleviates this constraint so that the DCC becomes hyperactive. Alternatively, higher-than-2-fold expression changes may also occur from mis-regulation of loci involved in transcriptional repression.
Some genes were consistently mis-expressed at both 2-3hrs and 5-6hrs AED (Figure 2D, Table S1). These genes represent a range of biological functions, with those involved cuticle formation being one of the more highly represented groups (Supplementary Figure 4; Table S1). These genes, and other—such as those involved in muscle assembly—are normally not expressed until late embryogenesis and afterward, suggesting that ectopic DCC mis-localization leads to activation of genes that are not normally expressed at these earlier times. While many of these genes may become mis-expressed due to ectopic DCC activity, others including the immune genes IM1, IM2-3, and IM4 (Table S1) could be induced as a general response to bacterial infection. The majority of immune response genes, however, were not induced at either developmental time analyzed (Table S1), a pattern that is consistent with previous work suggesting that Spiroplasma is capable of largely evading the host's immune system [28]. Of particular interest were two over-expressed regulators of apoptosis, reaper and sickle, at 2-3hrs AED, and just sickle at 5-6hrs AED (Table S1). The nature of our data does not allow us to address whether increased expression of these genes is a direct result of DCC mis-localization or instead a secondary response to broad gene mis-regulation. Nevertheless, elevated expression of these genes is consistent with previous cytological detection of increased cell death due to Spiroplasma infection at later embryonic stages [15].
Based on the above observations, Spiroplasma may initiate male killing by directly altering DCC localization as it forms during early embryogenesis, thereby leading to wide-scale gene mis-expression and developmental abnormalities. Although DCC-directed compensation is a distinguishing characteristic of the male sex during early development, other sex-specific differences exist at this time [29-31]. It is possible that Spiroplasma targets an earlier male-specific factor or process that leads to DCC mis-behavior or, alternatively, that other male factors could be essential for DCC targeting; either of these scenarios is consistent with suppression of male killing by mutational loss of the DCC [10]. To test if the DCC is the sole male-specific factor required for male killing, we assessed the viability of Spiroplasma-infected D. melanogaster females artificially expressing msl-2 via a heat shock-inducible hsp83 promoter [17]. Under heat shock this transgene expresses msl-2 at a high level, an effect that is strongly lethal to females due to a proportionally high level of DCC formation and activity [17]. However, without heat shock this transgene is leaky, expressing a low level of msl-2 that does not affect female viability when in a genetic background that is heterozygous for an msl-1 mutation [17]. DCC formation through leaky expression of this transgene was previously shown in female salivary gland nuclei [17]. Here we observed single foci of MOF in the nuclei of female embryos carrying one transgene copy (Figure 3), demonstrating that this transgene is also active during early development.
Figure 3. Spiroplasma infection causes death of females expressing low levels of MSL-2 and mis-localized DCC during female embryogenesis.
(A) The number of F1 progeny carrying the hsp83-MSL-2 transgene (+Tr) and their siblings without the transgene (no Tr) produced from either uninfected (control) or Spiroplasma-infected mothers. Red asterisk indicates a significant difference between transgenic and non-transgenic females infected by Spiroplasma. (B) Transgenic male and female embryos that are either uninfected or Spiroplasma-infected. Uninfected embryos of both sexes show distinct MOF localization, whereas in infected embryos MOF fails to concentrate and becomes distributed lightly across nuclei. Uninfected females do not exhibit foci of MOF or H4K16ac (see Figure S3). Scale bar equals 5 μm.
To assess the effect of DCC formation on the viability of infected females we crossed uninfected males carrying one copy of the hsp-83 msl-2 transgene to wild type females that were either Spiroplasma-infected or uninfected and scored the viability of the resulting offspring (see Experimental Procedures for details of the crosses and genotypes). The ratio of uninfected, transgenic to uninfected, non-transgenic female progeny was close to 1 (Chi-square goodness of fit test, P=0.55) (Figure 3A), recapitulating that the leaky expression of the msl-2 transgene alone has very little effect on female viability when in an msl-1 heterozygous mutant background. However, the ratio of infected, transgenic to infected, non-transgenic female progeny was 0.411 (Figure 3A), a highly significant deviation from the expected 1:1 ratio of these genotypes that occurred in the absence of infection (Chi-square goodness-of-fit test, P<0.0001). Thus, the presence of the DCC is lethal to female embryos only when they carry the Spiroplasma infection. Consistent with this finding, Spiroplasma-infected female embryos expressing msl-2 were nearly identical in abnormal sub-cellular phenotype to those of wild type infected male embryos: they exhibited mis-localized MOF at 4+hrs AED, and these signals diminished in intensity as nuclear morphology degenerated during later developmental times (Figure 3B). In contrast, transgene-carrying female embryos without infection showed well-formed MOF foci and normal nuclear morphology (Figure 3B).
Although compensation of most X-linked genes involves the DCC, a sub-set of genes involved in early embryogenesis become compensated as much as one hour before DCC formation, thus likely occurring through a DCC-independent mechanism [31]. Although the details of this process are currently unknown, it is clear that gene dosage compensation in Drosophila is more complex than previously thought and extends beyond the DCC pathway. Moreover, this complexity infers that the embryonic cytotype begins to differ substantially between males and females as early as one hour after fertilization. Our ability to reconstitute the killing effect in female embryos by expression of msl-2 alone strongly suggests that DCC mis-localization is a direct effect of Spiroplasma and does not result secondarily from targeting of the earlier, DCC-independent compensation. More generally, DCC mis-localization by Spiroplasma does not require interactions with other cellular processes that are characteristic of male embryos. Thus, DCC mis-localization can be viewed as a sex-independent phenomenon that may arise from a specific interaction of this complex with a bacterium-produced factor in the cells of whichever sex these components are present. This interaction could also involve other host cellular components that must be present in both male and female embryos.
Taken together, our results support a model in which Spiroplasma initiates male killing by altering DCC behavior on both the X and ectopically on non-sex chromosomes, thereby leading to abnormal histone acetylation and gene mis-expression across the genome. The mis-expression of certain genes – such as those involved in apoptosis – may elicit severe cellular responses at even slightly elevated levels [32,33]. Thus, perturbations of several key genes may have profound deleterious effects on early male development. Wild type infected females would be spared this effect because these individuals do not normally express the MSL-2 protein and, therefore, harbor no integral DCC [17,34]. Knowledge that the DCC itself may be a direct target of Spiroplasma in D. melanogaster will help to identify the specific interacting molecules of host and symbiont that underlie this phenomenon through further experimentation.
Experimental Procedures
Fly lines and infection
Embryo collections for cytology were obtained from two different fly lines. One was a Canton-S line carrying the MSRO strain of Spiroplasma that was used previously in [14]. A second was a line homozygous for a transgenic copy of msl-3-GFP (A gift from M. Kuroda). This line was infected for this particular study by haemolymph transfer from the infected Canton-S line (protocol provided by T. Haselkorn). Fly lines were propagated by crossing virgin infected females from each fly line to males from an uninfected stock of the same genetic background. Before crossing, infected females were held to 1 week in order to insure strong penetrance of male killing. All embryo collections, crosses, and propagation lines were maintained at 25°C.
For testing the effects of msl-2 expression in infected females, we crossed w ; msl-1; P{w+ hsp83-msl-2} females (stock obtained from M. Kuroda) to w / Y ; + ; TM3 Ser Sb / TM6 Tub males. F1 Male progeny of the genotype w / Y ; msl-1 / + ; P{w+ hsp83-msl-2} / TM3 Ser Sb were then crossed to either Spiroplasma-infected or uninfected Canton-S females aged 1 week. The resulting progeny from this cross carried either the P{w+ hsp83-msl-2} transgene or the TM3 Ser Sb balancer chromosome and were easily distinguished by the presence or absence of the two co-inherited dominant markers.
Embryo collection and fixation
Mated females of the desired genotype and infection status were allowed to lay on collection plates containing grape agar medium and embryos were aged to the desired developmental stage at 25°C. Embryos were dechorionated in 50% bleach, fixed for 10 minutes in 4% paraformaldehyde/heptane, and devitellinized in methanol. Embryos were either kept in methanol at −20°C for long-term storage or rehydrated for cytolological purposes. Hydration was conducted in a series of methanol:water solutions in order of the following ratios: 9:1, 5:5, and 1:9, followed by three 5min washes in 1xPBT (1xPBS and 0.1%Tween-20).
Immunohistochemical staining and fluorescence in situ hybridization
Hydrated, fixed embryos were incubated in the following primary antibodies overnight at 4°C: mouse anti-GFP (1:50, clone number 4C9, Developmental Studies Hybridoma Bank); goat anti-MOF (1:100, clone number dn-17, Santa Cruz Biotech); rabbit anti-acetyl-Histone H4 Lysine 16 (1:100, catalog number 07-329, Millipore). Following incubation with primary antibodies, embryos were washed 3×15min in 1xPBT before secondary antibody staining. Anti-mouse, anti-rabbit, and anti-goat secondary antibodies were conjugated with either Alexa555 or Alexa633 (Molecular Probes-Invitrogen) and used at a dilution of 1:300. Secondary staining was conducted out of light for 1hr at room temperature and washed three times subsequently.
Fluorescence in situ hybridization was conducted with an oligonucleotide probe specific for a satellite DNA sequence unique to the Y chromosome [35]. The probe consisted of the sequence 5’-AAT ACA ATA CAA TAC AAT ACA ATA CAA TAC-‘3 and was synthesized commercially (IDT, Inc.) with Cy2 conjugated at the 5’ end. Whole mount embryo hybridizations were conducted as described [35], except that embryos used for this purpose were stained first with antibodies, re-fixed in 4% paraformaldehyde for 45 minutes, and washed 3X in 2xSSCT buffer before probe hybridization.
Confocal Imaging and data processing
Fluorescence images were collected on a Leica TCS SPE confocal microscope. Images were collected by using identical laser, gain, and offset settings for both uninfected and infected tissues for direct comparison between these conditions. The specific setting values of the original Leica-formatted images can be obtained at request from the authors. After image capture, image files were exported in TIFF format and processed in Adobe Photoshop CS5 version 12. Images were enhanced with the brightness and contrast settings being manipulated identically between infected and uninfected conditions.
Supplementary Material
Highlights.
The DCC becomes mis-localized in Spiroplasma-infected male fruit fly embryos
Infected males exhibit ectopic H4K16 acetylation and genome-wide gene mis-expression
Infection leads to death of females artificially expressing the DCC
Acknowledgements
We thank M. Kuroda for the w ; msl-1 ; P{w+ hsp83-msl-2} and w ; msl-3-GFP fly lines, C. Staber for the SD fly lines, C-T. Wu and D. Colognori for the X16E FISH probe, and T. Hazelkorn for the haemolymph transfer protocol. We also thank E. Ferree, V. Meller, and J. Birchler for helpful input. This study was supported by an NSF CAREER award (NSF1451839) to P. M. Ferree and an NIH K22 grant (5K22AI113060-02) to O. S. Akbari.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
B.C. performed the cytological experiments and genetic crosses. N.K. performed the genetic crosses. O.S.A. performed the transcriptomic analyses and statistical tests. J.C.A., O.S.A. and P.M.F. analyzed the data. P.M.F. and O.S.A. designed the experiments. P.M.F. and J.C.A. wrote the manuscript. All authors have discussed the results and contributed to the manuscript.
References
- 1.Werren JH, Skinner SW, Huger AM. Male-killing bacteria in a parasitic wasp. Science. 1986;231:990–992. doi: 10.1126/science.3945814. [DOI] [PubMed] [Google Scholar]
- 2.Zeh DW, Zeh JA, Bonilla MM. Wolbachia, sex ratio bias and apparent male killing in the harlequin beetle riding pseudoscorpion. Heredity (Edinb) 2005;95:41–49. doi: 10.1038/sj.hdy.6800666. [DOI] [PubMed] [Google Scholar]
- 3.Jiggins FM, Hurst GD, Schulenburg JH, Majerus ME. Two male-killing Wolbachia strains coexist within a population of the butterfly Acraea encedon. Heredity (Edinb) 2001;86:161–166. doi: 10.1046/j.1365-2540.2001.00804.x. [DOI] [PubMed] [Google Scholar]
- 4.Werren JH, Hurst GDD, Zhang W, Breeuwer JAJ, Stouthamer R, et al. Rickettsial Relative Associated with Male Killing in the Ladybird Beetle (Adalia- Bipunctata). Journal of Bacteriology. 1994;176:388–394. doi: 10.1128/jb.176.2.388-394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst GD, Jiggins FM. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Infect Dis. 2000;6:329–336. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haselkorn TS. The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly (Austin) 2010;4:80–87. doi: 10.4161/fly.4.1.10883. [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb RF. The genus Spiroplasma. Annu Rev Microbiol. 1980;34:677–709. doi: 10.1146/annurev.mi.34.100180.003333. [DOI] [PubMed] [Google Scholar]
- 8.Riparbelli MG, Giordano R, Ueyama M, Callaini G. Wolbachia-mediated male killing is associated with defective chromatin remodeling. PLoS One. 2012;7:e30045. doi: 10.1371/journal.pone.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferree PM, Avery A, Azpurua J, Wilkes T, Werren JH. A bacterium targets maternally inherited centrosomes to kill males in Nasonia. Curr Biol. 2008;18:1409–1414. doi: 10.1016/j.cub.2008.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veneti Z, Bentley JK, Koana T, Braig HR, Hurst GD. A functional dosage compensation complex required for male killing in Drosophila. Science. 2005;307:1461–1463. doi: 10.1126/science.1107182. [DOI] [PubMed] [Google Scholar]
- 11.Gelbart ME, Larschan E, Peng S, Park PJ, Kuroda MI. Drosophila MSL complex globally acetylates H4K16 on the male X chromosome for dosage compensation. Nat Struct Mol Biol. 2009;16:825–832. doi: 10.1038/nsmb.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herren JK, Paredes JC, Schupfer F, Lemaitre B. Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. MBio. 2013;4 doi: 10.1128/mBio.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anbutsu H, Fukatsu T. Tissue-specific infection dynamics of male-killing and nonmale-killing spiroplasmas in Drosophila melanogaster. FEMS Microbiol Ecol. 2006;57:40–46. doi: 10.1111/j.1574-6941.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Chong T, Ferree PM. Male killing Spiroplasma preferentially disrupts neural development in the Drosophila melanogaster embryo. PLoS One. 2013;8:e79368. doi: 10.1371/journal.pone.0079368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harumoto T, Anbutsu H, Fukatsu T. Male-killing Spiroplasma induces sex- specific cell death via host apoptotic pathway. PLoS Pathog. 2014;10:e1003956. doi: 10.1371/journal.ppat.1003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meller VH, Wu KH, Roman G, Kuroda MI, Davis RL. roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell. 1997;88:445–457. doi: 10.1016/s0092-8674(00)81885-1. [DOI] [PubMed] [Google Scholar]
- 17.Kelley RL, Solovyeva I, Lyman LM, Richman R, Solovyev V, et al. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995;81:867–877. doi: 10.1016/0092-8674(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 18.Alekseyenko AA, Larschan E, Lai WR, Park PJ, Kuroda MI. High-resolution ChIP-chip analysis reveals that the Drosophila MSL complex selectively identifies active genes on the male X chromosome. Genes Dev. 2006;20:848–857. doi: 10.1101/gad.1400206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilfillan GD, Straub T, de Wit E, Greil F, Lamm R, et al. Chromosome-wide gene-specific targeting of the Drosophila dosage compensation complex. Genes Dev. 2006;20:858–870. doi: 10.1101/gad.1399406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta V, Parisi M, Sturgill D, Nuttall R, Doctolero M, et al. Global analysis of X-chromosome dosage compensation. J Biol. 2006;5:3. doi: 10.1186/jbiol30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rastelli L, Richman R, Kuroda MI. The dosage compensation regulators MLE, MSL-1 and MSL-2 are interdependent since early embryogenesis in Drosophila. Mech Dev. 1995;53:223–233. doi: 10.1016/0925-4773(95)00438-7. [DOI] [PubMed] [Google Scholar]
- 22.Franke A, Dernburg A, Bashaw GJ, Baker BS. Evidence that MSL-mediated dosage compensation in Drosophila begins at blastoderm. Development. 1996;122:2751–2760. doi: 10.1242/dev.122.9.2751. [DOI] [PubMed] [Google Scholar]
- 23.Bhadra U, Pal-Bhadra M, Birchler JA. Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics. 1999;152:249–268. doi: 10.1093/genetics/152.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhadra MP, Bhadra U, Kundu J, Birchler JA. Gene expression analysis of the function of the male-specific lethal complex in Drosophila. Genetics. 2005;169:2061–2074. doi: 10.1534/genetics.104.036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kind J, Vaquerizas JM, Gebhardt P, Gentzel M, Luscombe NM, et al. Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell. 2008;133:813–828. doi: 10.1016/j.cell.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Prestel M, Feller C, Straub T, Mitlohner H, Becker PB. The activation potential of MOF is constrained for dosage compensation. Mol Cell. 2010;38:815–826. doi: 10.1016/j.molcel.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Fernandez HR, Donohue RC, Li J, Cheng J, et al. Male-specific lethal complex in Drosophila counteracts histone acetylation and does not mediate dosage compensation. Proc Natl Acad Sci U S A. 2013;110:E808–817. doi: 10.1073/pnas.1222542110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herren JK, Lemaitre B. Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol. 2011;13:1385–1396. doi: 10.1111/j.1462-5822.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 29.Cline TW, Meyer BJ. Vive la difference: males vs females in flies vs worms. Annu Rev Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 30.Schutt C, Nothiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development. 2000;127:667–677. doi: 10.1242/dev.127.4.667. [DOI] [PubMed] [Google Scholar]
- 31.Lott SE, Villalta JE, Schroth GP, Luo S, Tonkin LA, et al. Noncanonical compensation of zygotic X transcription in early Drosophila melanogaster development revealed through single-embryo RNA-seq. PLoS Biol. 2011;9:e1000590. doi: 10.1371/journal.pbio.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White K, Tahaoglu E, Steller H. Cell killing by the Drosophila gene reaper. Science. 1996;271:805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- 33.Wing JP, Karres JS, Ogdahl JL, Zhou L, Schwartz LM, et al. Drosophila sickle is a novel grim-reaper cell death activator. Curr Biol. 2002;12:131–135. doi: 10.1016/s0960-9822(01)00664-9. [DOI] [PubMed] [Google Scholar]
- 34.Bashaw GJ, Baker BS. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development. 1995;121:3245–3258. doi: 10.1242/dev.121.10.3245. [DOI] [PubMed] [Google Scholar]
- 35.Ferree PM, Barbash DA. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 2009;7:e1000234. doi: 10.1371/journal.pbio.1000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.