Abstract
Background
Sunitinib and everolimus are standard first-line and second-line therapies, respectively, in clear cell renal cell carcinoma (ccRCC).
Objective
To conduct a randomized phase 2 trial comparing sunitinib and everolimus in non–clear cell RCC (non-ccRCC).
Design, setting, and participants
Patients with metastatic, non-ccRCC, or ccRCC with >20% sarcomatoid features (ccSRCC) were randomized to receive sunitinib or everolimus with crossover at disease progression.
Outcome measurement and statistical analysis
Primary end point was progression-free survival (PFS) in first-line therapy; 108 patients were needed to show improvement in median PFS (mPFS) from 12 wk with sunitinib to 20 wk with everolimus.
Results and limitations
Interim analysis of 68 patients (papillary [27], chromophobe [12], unclassified [10], translocation [7], ccSRCC [12]) prompted early trial closure. The mPFS in first-line therapy was 6.1 mo with sunitinib and 4.1 mo with everolimus (p = 0.6); median overall survival (mOS) was not reached with sunitinib and was 10.5 mo with everolimus, respectively (p = 0.014). At final analysis, mOS was 16.2 and 14.9 mo with sunitinib and everolimus, respectively (p = 0.18). There were four partial responses (PRs) in first-line therapy (sunitinib: 3 of 33 [9%]; everolimus, 1 of 35 [2.8%]) and four PRs in second-line therapy (sunitinib: 2 of 21 [9.5%]; everolimus, 2 of 23 [8.6%]), with mPFS of 1.8 mo and 2.8 mo, respectively. In patients without sarcomatoid features in their tumors (n = 49), mOS was 31.6 mo with sunitinib and 10.5 mo with everolimus (p = 0.075). Genomic profiling of a chromophobe RCC from a patient with a PR to first-line everolimus revealed a somatic TSC2 mutation.
Conclusions
In this trial, everolimus was not superior to sunitinib. Both agents demonstrated modest efficacy, underscoring the need for better therapies in non-ccRCC.
Patient summary
This randomized phase 2 trial provides the first head-to-head comparison of everolimus and sunitinib in patients with metastatic non–clear cell renal cell carcinoma (non-ccRCC). The observed very modest efficacy underscores the need to develop more effective therapies for non-ccRCC.
Keywords: Renal cell carcinoma, Non–clear cell renal cell carcinoma, Sunitinib, Everolimus
1. Introduction
Renal cell carcinoma (RCC) is a heterogeneous malignancy encompassing many distinct histologic subtypes [1]. Clear cell RCC (ccRCC) is the most common subtype among renal epithelial malignancies. The remaining subtypes, referred to as non–clear cell RCC (non-ccRCC), include papillary RCC, chromophobe RCC, unclassified RCC, and rare entities such as collecting duct carcinoma (CDC), renal medullary carcinoma, and translocation RCC [2]. The initiating oncogenic events in non-ccRCC tumors are not driven by the von Hippel-Lindau (VHL) gene. Clinical practice guidelines list participation in clinical trials as the preferred treatment option for patients with metastatic non-ccRCC [3,4].
Most of the landmark trials that led to the approval of targeted agents in advanced RCC excluded patients with non-ccRCC. Available data on the use of targeted therapy in non-ccRCC are based on retrospective cohorts [5], expanded access programs [6,7], and a few single-arm phase 2 trials [8–13]. However, the clinical efficacy of sunitinib and other vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) in non-ccRCC has been questioned. In a phase 2 trial of sunitinib in patients with metastatic non-ccRCC, we reported a 5% objective response rate (ORR) and a 2.7-mo median progression-free survival (PFS) [8]. A phase 3 trial of temsirolimus versus interferon-α in patients with poor-risk RCC included 20% with non-ccRCC [14]. Subsequent analysis of the non-ccRCC subgroup in this study [15] and the results of the everolimus expanded access program [16] provided support for mammalian target of rapamycin (mTOR) inhibitors in non-ccRCC [17,18].
Given the limited prospective data on targeted therapy in non-ccRCC, we designed this randomized phase 2 trial to compare everolimus with sunitinib as first-line therapy in patients with metastatic non-ccRCC.
2. Patients and methods
2.1. Study design
This was a randomized multicenter phase 2 trial of everolimus versus sunitinib in patients with metastatic non-ccRCC. The study was approved by the institutional review board or ethics committee at each participating center, and it was conducted in accordance with the provisions of the Declaration of Helsinki and good clinical practice guidelines. The study was registered with clinicaltrials.gov (NCT01185366).
2.2. Patients
Eligible patients were >18 yr of age and had not received prior systemic therapy for advanced papillary, chromophobe, CDC, Xp11.2 translocation, unclassified RCC, or ccRCC with >20% sarcomatoid features in their primary tumors. We included the latter cohort because of its poor prognosis and lack of response to VEGF-TKI according to a previous report. The histologic diagnosis was confirmed by genitourinary pathologists at the participating institutions. Additional criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0–1, measurable disease, and adequate organ and marrow function. Exclusion criteria included untreated brain metastases, metabolic dysfunction, and uncontrolled medical conditions. All patients signed informed consent.
2.3. Randomization
Patients were stratified by Memorial Sloan Kettering Cancer Center risk group [19] and histologic RCC subtype (papillary vs other), and they were randomized 1:1 to receive either everolimus or sunitinib.
2.4. Procedures
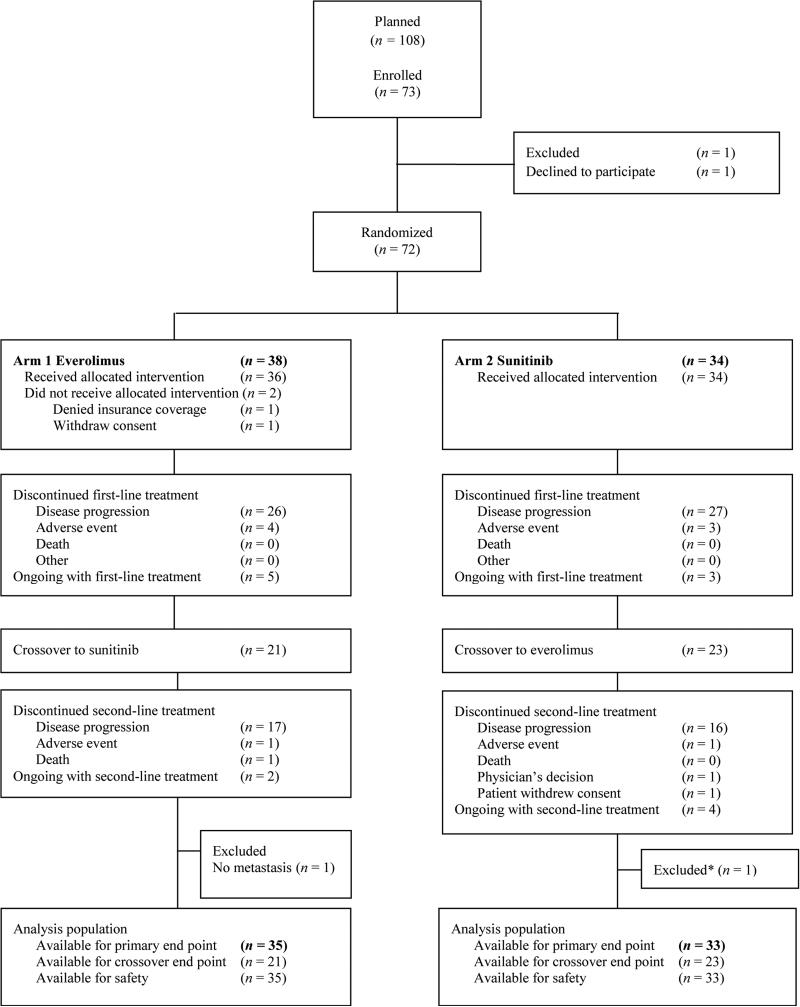
Patients received everolimus 10 mg/d orally or sunitinib 50 mg/d orally for 4 wk on and 2 wk off. Patients were treated until progressive disease (PD), unacceptable adverse events (AEs), or withdrawal of consent. At PD, patients were allowed to receive the agent that they did not receive upfront. Second-line therapy was initiated within 28 d of the last dose of the first agent after a 14-d washout period (Fig. 1).
Fig. 1.
Consolidated Standards of Reporting Trials diagram.
* Patient did not receive treatment in a timely manner due to delay in drug coverage by insurance.
2.5. Outcomes
The primary end point was PFS in first-line therapy, calculated from the date of randomization to the date of first documentation of PD, or death from any cause.
Secondary end points included ORR in first-line therapy, overall survival (OS), PFS, ORR in second-line therapy, and safety. OS was calculated from the date of start of therapy to the date of death. Survival of patients who were lost to follow-up was censored as of the date of last contact. After PD on first-line therapy, patients had the option to receive the alternative agent. PFS in second-line therapy was calculated from initiation of second-line therapy until PD or death from any cause or last follow-up. An independent radiology panel assessed tumor response using Response Evaluation Criteria in Solid Tumors v.1.0, at 6 wk, at 12 wk, and every 12 wk thereafter. Safety was assessed by continuous monitoring of AEs and scheduled monitoring of metabolic abnormalities and physical condition using National Cancer Institute-Common Terminology Criteria (NCI-CTC), v.3.
2.6. Statistical analysis
A one-sided type I error rate was set at 0.05 and power at 0.80. Under the alternative hypothesis, median PFS was 12 wk for sunitinib [8] and 20 wk for everolimus. The maximum sample size to be accrued was 108 patients (54 in each arm).
A stopping rule for futility served as guidance for early termination of patient accrual. An independent data monitoring committee (DMC) provided oversight for treatment efficacy and patient safety. The DMC convened at the beginning of the trial and at two interim time points. The interim stopping rule consisted of a group sequential test based on gamma family error spending functions with the parameter value set to –3. A study stop for futility was planned if the p value was >0.742 or >0.3125 for PFS or OS or either, at first and second interim looks. The distribution of each continuous variable was summarized by its mean, standard deviation, and range. The distribution of each categorical variable was summarized by its frequencies and percentages. Kaplan-Meier methods were used to estimate unadjusted OS and PFS time distributions. The stratified log-rank test was used to compare each time-to-event variable between treatment groups. Exploratory analyses were conducted to compare the time-to-event variable for patients with different histologic subtypes. All computations were performed in SAS v9.3 (SAS Institute, Cary, NC, USA) and TIBCO S-PLUS v8.2 (TIBCO Software Inc., Palo Alto, CA, USA).
2.7. Genomic profiling
Patients who achieved a partial response (PR) had genomic profiling of their tumors at Dana-Farber Cancer Institute (DFCI). High-throughput mutation profiling was performed using massively parallel sequencing as previously described for the OncoPanel assay (Eurofins Panlabs, Inc., Redmond, WA, USA) that surveys exonic DNA sequences of 275 cancer genes and detects copy number variations and structural variants in tumor DNA [20].
3. Results
3.1. Patients
From September 3, 2010, through November 19, 2013, 73 patients were accrued. On February 4, 2014, after 51 PFS events in first-line therapy and 27 deaths, with both OS and first-line PFS results favoring first-line sunitinib (median OS not reached vs 10.5 mo; p = 0.014; median PFS: 6.1 mo vs 4.1 mo), the DMC recommended closure of the trial to new patient enrollment. At final analysis (May 2014), 68 patients were evaluable and 39 (57%) had died, at a median follow-up of 23.6 mo (95% confidence interval [CI], 15.7–30.2). Table 1 summarizes the patient characteristics.
Table 1.
Baseline patient characteristics
| Everolimus | Sunitinib | ||
|---|---|---|---|
| n = 35 | n = 33 | ||
| Age, yr, median (range) | 58 (23–73) | 60 (28–76) | |
| Gender, male:female | 24:11 | 19:14 | |
| Race | White | 28 | 25 |
| Hispanic | 3 | 5 | |
| Black | 2 | 3 | |
| Prior nephrectomy | 27 | 25 | |
| Histology | Papillary | 13 | 14 |
| Clear cell with sarcomatoid features | 6 | 6 | |
| Chromophobe | 6 | 6 | |
| Translocation | 4 | 3 | |
| Unclassified | 6 | 4 | |
| ECOG performance status | 0 | 15 | 18 |
| 1 | 20 | 15 | |
| MSKCC risk group | Good | 4 | 4 |
| Intermediate | 29 | 29 | |
| Poor | 2 | 0 | |
| IMDC risk group | Good | 4 | 3 |
| Intermediate | 24 | 26 | |
| Poor | 7 | 4 | |
| No. of metastatic disease sites | 1 | 6 | 6 |
| ≥2 | 29 | 27 | |
| Metastatic disease sites | Lung | 17 | 16 |
| Bone | 7 | 11 | |
| Liver | 9 | 7 | |
| Supradiaphragmatic adenopathy | 14 | 15 | |
| Infradiaphragmatic adenopathy | 16 | 16 | |
| Pleural | 1 | 1 | |
| Kidney | 7 | 7 | |
| Renal fossa | 5 | 3 | |
| Pancreas | 0 | 1 | |
| Adrenal | 2 | 2 | |
| Skin | 1 | 0 | |
ECOG= Eastern Cooperative Oncology Group; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium; MSKCC = Memorial Sloan Kettering Cancer Center.
3.2. Efficacy
3.2.1. Progression-free survival and tumor response assessment in first-line therapy
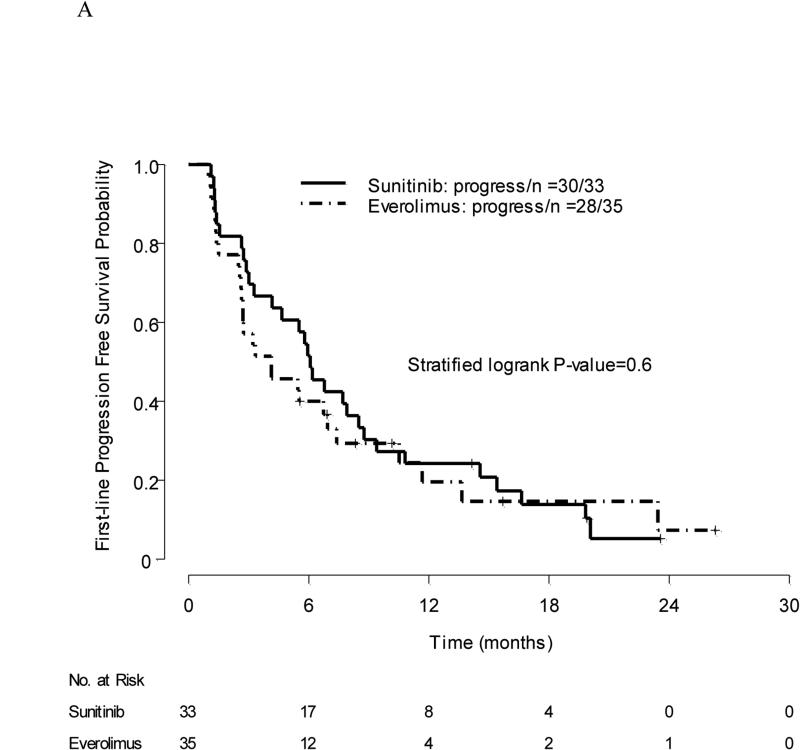
Among 33 patients who received first-line sunitinib, three (9%) had PR, 21 (64%) had stable disease (SD), and nine (27%) had PD, as best response. Among 35 patients who received first-line everolimus, one (3%) had PR, 26 (74%) had SD, and eight (23%) had PD, as best response. Median PFS was 6.1 mo with sunitinib (95% CI, 4.2–9.4) versus 4.1 mo with everolimus (95% CI, 2.7–10.5); stratified log-rank p value = 0.6 (Fig. 2a).
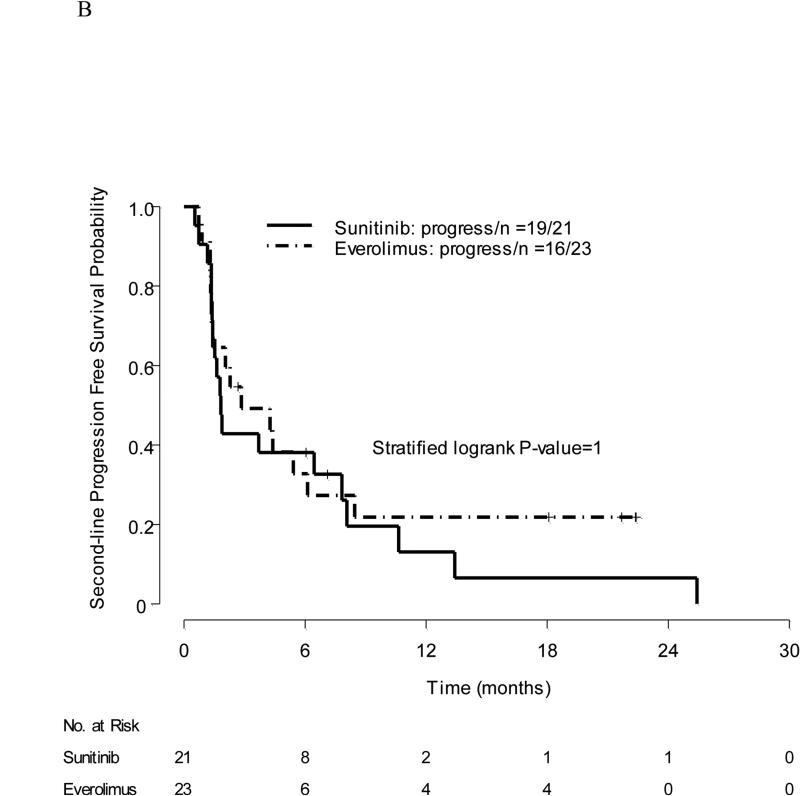
Fig. 2.
(a) Progression-free survival in first-line therapy: grouping by initial treatment; (b) progression-free survival in second-line therapy.
PFS = progression-free survival.
3.2.2. Progression-free survival and tumor response assessment in second-line therapy
Forty-four patients received second-line therapy. Twenty-three patients had a crossover from sunitinib to everolimus (2 had PR, 15 had SD, and 6 had PD with prior sunitinib). Twenty-one patients had a crossover from everolimus to sunitinib (13 had SD and 8 had PD with prior everolimus). Response to second-line everolimus was as follows: Two patients had PR, nine had SD, nine had PD, one came off protocol due to toxicity prior to imaging studies, one discontinued protocol treatment per physician's decision but had SD, and one withdrew consent. Response to second-line sunitinib was as follows: Two patients had PR, seven had SD, ten had PD, one came off protocol due to toxicity but had SD, and one patient died. Median PFS was 2.8 mo with everolimus (95% CI, 1.4–not available [NA]) and 1.8 mo with sunitinib (95% CI, 1.4–10.6); stratified log-rank p value = 0.6 (Fig. 2b).
3.2.3. Overall survival
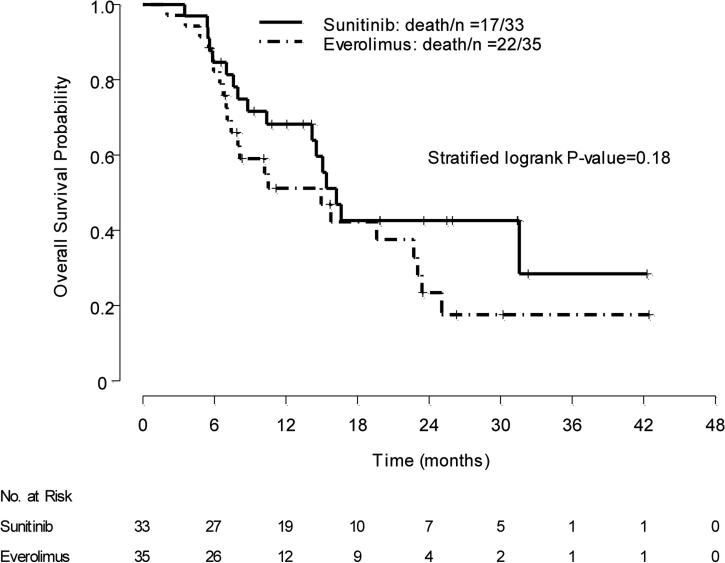
At interim analysis, a survival advantage was noted in patients who received sunitinib over those who received everolimus as first-line therapy: Median OS was not reached (95% CI, 14.6–NA) with sunitinib versus 10.5 mo with everolimus (95% CI, 7.4–NA); stratified log-rank p = 0.014. However, the final results for OS did not demonstrate a significant difference between the two arms. At final analysis, 17 of 33 patients who received first-line sunitinib and 22 of 35 patients who received first-line everolimus had died. Median OS was 16.2 mo in the first-line sunitinib group (95% CI, 14.2–NA), and 14.9 mo in the first-line everolimus group (95% CI, 8.0–23.4); stratified log-rank p = 0.18 (Fig. 3). Additional analyses suggest there are no significant differences, either by looking at crossover patients versus noncrossover patients overall or by looking at the groups divided by the first-line and second-line treatments that they received (Supplementary Table 1).
Fig. 3.
Overall survival: grouping by initial treatment.
3.3. Safety
Any grade 3 or 4 AE occurred in 29 of 33 patients (88%) who received first-line sunitinib and in 19 of 35 patients (54%) who received first-line everolimus. Common grade 3 or 4 sunitinib-associated treatment-emergent AEs included fatigue (36%), hypertension (18%), diarrhea (21%), neutropenia (27%), and hyponatremia (15%). Grade 3 anemia occurred in 11% of patients who received everolimus. Supplementary Table 2 presents the treatment-emergent AEs occurring in at least 5% of patients in each treatment arm.
A total of 72% (n = 24) and 31% (n = 11) of patients receiving first-line therapy with sunitinib or everolimus required drug interruption, respectively. Dose reductions were needed in 39% (n = 13) and 14% (n = 5) with first-line sunitinib or everolimus, respectively. In second-line therapy, sunitinib was interrupted in 38% (n = 8) and dose reduced in 19% (n = 4) of patients, whereas everolimus was interrupted in 17% (n = 4) and dose reduced in 4% of patients (n = 1).
3.4. Exploratory analyses
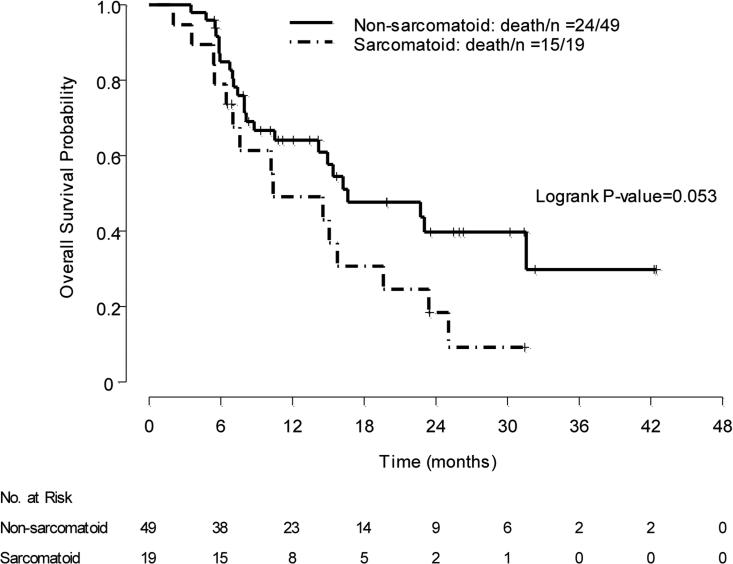
Table 2 shows OS by treatment arm according to histology. We explored the impact of sarcomatoid features on OS. Overall, 19 patients had sarcomatoid features in their tumors; these included 12 patients with ccRCC with >20% sarcomatoid features and 7 patients who had sarcomatoid features associated with other histology. Median OS was 16.6 mo in patients who had no sarcomatoid features in their tumors (n = 49; 95% CI, 14.2–NA), irrespective of the treatment they received, versus 10.4 mo in patients whose tumors had any sarcomatoid features (n = 19; 95% CI, 7–NA) (log-rank p = 0.053) (Fig. 4). Among the 49 patients whose tumors had no sarcomatoid features, median PFS with first-line sunitinib was 6.5 mo (95% CI, 5.8–15.4) versus 4.1 mo (95% CI, 2.7–13.7) with first-line everolimus (log-rank p = 0.35). In those 49 patients who had no sarcomatoid features in their tumors, median OS for patients receiving first-line sunitinib and first-line everolimus was 31.6 mo (95% CI, 15.4–NA), and 10.5 mo (95% CI, 7.4–NA), respectively (log-rank p =0.075).
Table 2.
Overall survival and first-line progression-free survival by treatment arm and histologic subtype
| Subtype | Everolimus | Sunitinib | ||||
|---|---|---|---|---|---|---|
| n | Median OS, mo (95% CI) | Median PFS, mo (95% CI) | n | Median OS, mo (95% CI) | Median PFS, mo (95% CI) | |
| Papillary | 13 | 14.9 (7.1–22.7) | 4.1 (1.5–7.4) | 14 | 16.6 (5.9–NA) | 5.7 (1.4–19.8) |
| Chromophobe | 6 | 25.1 (4.7–NA) | NA | 6 | 31.6 (14.2–NA) | 8.9 (2.9–20.1) |
| Unclassified | 6 | NA | 4.7 (2.6–NA) | 4 | 15.4 (NA) | 9.4 (3.3–15.4) |
| Translocation | 4 | 8.1 (5.5–23) | 3.0 (1.3–NA) | 3 | 16.2 (8.8–NA) | 6.1 (6.0–8.8) |
| Clear cell with >20% sarcomatoid features | 6 | 11.1 (2.0–NA) | 1.9 (1.0–23.4) | 6 | 7.0 (5.4–10.4) | 3.5 (1.3–7.7) |
CI = confidence interval; NA = not applicable; OS = overall survival; PFS = progression-free survival.
Fig. 4.
Exploratory analysis: overall survival according to the presence of sarcomatoid features regardless of treatment received in first-line therapy.
We performed genomic profiling of tumors obtained from three DFCI patients who achieved a PR. In one patient with chromophobe RCC who had a PR with first-line everolimus, the following somatic mutations were identified: TP53, PTEN, NF1, SETD2, and TSC2. Multiple copy number alterations were also found including losses of chromosomes 1, 2, 3, 10, 13, 17, and 21, a characteristic pattern for chromophobe RCC [20]. Tumors from two patients who achieved a PR with sunitinib did not have specific actionable gene mutations (Table 3).
Table 3.
Objective responders and gene mutations detected
| Objective response to first-line therapy | First-line everolimus (n = 35) | First-line sunitinib (n = 33) | ||
|---|---|---|---|---|
| n | 1 | 3 | ||
| Histologic subtype | Chromophobe, GA | Papillary, GA | Chromophobe* | Chromophobe |
| Response to first-line therapy (% tumor regression by RECIST) | PR (–58%) | PR (–58%) | PR (–56%) | PR (–56%) |
| Response to second-line therapy | NA | NA | PR with everolimus | SD with everolimus |
| Analysis of mutations | TP53, NFI, PTEN, AXL, MLL2, NOTCH1, SETD2, TSC2, ZRSR | No mutation identified | Not performed | Not performed |
| Objective response to second-line therapy | Second-line sunitinib (n = 21) | Second-line everolimus (n = 23) | ||
|---|---|---|---|---|
| n | 2 | 2 | ||
| Histologic subtype | Translocation carcinoma | Unclassified, GA | Chromophobe* | Papillary |
| Response to second-line therapy (% tumor regression by RECIST) | PR (–38%) | PR (–41%) | PR (–42%) | PR (–34%) |
| Analysis of mutations | Not performed | ATM, CDK6, ERCC4, GLI3 | Not performed | Not performed |
GA = genomic analysis performed; NA = not applicable because patient was still receiving first-line agent; PR = partial response; RECIST = Response Evaluation Criteria in Solid Tumors; SD = stable disease.
Same patient had PR with sunitinib as first-line therapy and with everolimus as second-line therapy.
4. Discussion
This is the first randomized phase 2 trial in metastatic non-ccRCC that compared two widely used targeted agents. The trial failed to demonstrate superior efficacy of everolimus over sunitinib as first-line therapy in non-ccRCC. Median PFS with first-line everolimus was numerically inferior to that with sunitinib, but no significant differences were observed between the two arms in OS, PFS, or ORR. The results of our randomized phase 2 trial address controversies surrounding the use of approved agents for the management of metastatic non-ccRCC. The rationale for sunitinib use came from retrospective studies [5] and expanded access programs [6,7] of sunitinib, showing ORRs of 3–11% and median PFS ranging between 6 and 11 mo in non-ccRCC. Several small single-arm phase 2 studies recently investigated sunitinib in non-ccRCC (Supplementary Table 3). Lee and colleagues reported a 36% ORR and a 6.4-mo median time to progression (95% CI, 4.2–8.6 mo) in 31 Korean patients (including 22 with papillary RCC) [9]. Molina and colleagues reported a 5.5-mo median PFS (95% CI, 2.5–7.1) but no responders in 23 patients [11]. In a phase 2 trial assessing sunitinib in 57 patients with non-ccRCC, we reported a 2.7-mo (95% CI, 1.4–5.4) median PFS, a 5% ORR, and a 16.8-mo (95% CI, 10.7–26.3) median OS [8]. These disappointing results have been partly attributed to the inclusion of patients with poor-risk disease, ECOG performance status 2, or prior systemic therapy. Although the 6.1-mo median PFS with sunitinib in our current trial is longer than we previously reported, the 6% ORR and 16-mo median OS are consistent with our previously reported single-arm study [8].
The rationale of targeting the mTOR pathway as an upfront therapeutic strategy was based on a subgroup analysis of 73 patients with poor-risk non-ccRCC treated with temsirolimus in a phase 3 trial, yielding a 7-mo median PFS (hazard ratio [HR]: 0.38; 95% CI, 0.23–0.62) and a 11.6-mo median OS (HR: 0.49; 95% CI, 0.29–0.85) [14,15]. A study from the everolimus expanded access program in 75 patients with advanced non-ccRCC reported SD as the best response in 37 patients (49.3%) and a PR in one patient [16]. In a single-arm phase 2 study, Koh and colleagues reported a 5.2-mo median PFS in 49 patients treated with everolimus, 46.9% of whom had received prior anti-VEGF agents (Supplementary Table 3) [10]. In a retrospective study, Voss and colleagues evaluated the role of mTOR inhibitors in 62 patients with non-ccRCC and 23 patients with ccRCC who had sarcomatoid features in their tumors. Median PFS was 2.9 mo and median OS was 8.7 mo in the overall cohort [21].
RECORD-3, a randomized phase 2 trial in metastatic RCC, recently reported the results of the sequence of sunitinib followed by everolimus versus the opposite sequence [22]. In a subgroup analysis of 66 patients who had non-ccRCC, everolimus did not yield better results than sunitinib as first-line therapy, with a median PFS of 5.0 and 7.2 mo, respectively (HR: 1.54; 95% CI, 0.86–2.75).
Although the numbers are small for each histologic subtype, our results are consistent with other reports on papillary and chromophobe RCC. Median OS with sunitinib for papillary RCC in our current study was 16.6 mo, consistent with the SUPAP trial results, showing median OS of 17.8 mo (95% CI, 5.7–26.1) and 12.4 mo (95% CI, 8.2–16) in papillary types I and II RCC, respectively (Table 3) [12]. In our trial, median OS with everolimus was 14.9 mo compared with 20.0-mo median OS (95% CI, 11.1–28.0) reported with first-line everolimus in the RAPTOR study (Table 3) [13]. Patients with chromophobe RCC had a favorable outcome with a median OS of 31.6 mo with sunitinib and 25.1 mo with everolimus, consistent with results of one study on 37 patients with chromophobe RCC reporting a median OS of 27.1 mo [5] and another study of 50 chromophobe RCC reporting a median OS of 30.0 mo with VEGF/VEGFR inhibitors [23]. Sarcomatoid features can be associated with any RCC subtype and portend a poor prognosis, as we observed in our cohort [24–26].
Our study has several strengths. It is the first randomized study in metastatic non-ccRCC comparing a VEGF-TKI with an mTOR inhibitor with a crossover design. It has established benchmarks for tumor response and survival outcomes of non-ccRCC patients who are treated with these agents. However, we recognize that the histologic heterogeneity and the small numbers of patients in each subgroup limit the interpretation of our findings.
At present, there are no predictive biomarkers of response to mTOR inhibitors or VEGF-directed therapies. Besides the role of serum lactate dehydrogenase [27], a recent report suggests that mutations in TSC1, TSC2, or MTOR may predict response to mTOR inhibitors in RCC [21]. In addition, a recent case report of initial sensitivity and further resistance to everolimus in another tumor model highlights the role of nonsense mutation in TSC2 [28]. The identification of a somatic TSC2 mutation in a chromophobe case with PR to everolimus in our trial adds to these observations.
5. Conclusions
In this trial, everolimus was not superior to sunitinib. Both agents demonstrated modest efficacy, underscoring the need for better therapies in non-ccRCC. We believe that patients with non-ccRCC or sarcomatoid features in their tumors should be enrolled in clinical trials testing novel agents. Future trials in non-ccRCC should investigate agents with mechanisms of action other than mTOR inhibition and VEGF blockade. Research efforts will unravel molecular targets that are relevant to each of the diverse subtypes of non-ccRCC. Some of these efforts are ongoing through The Cancer Genome Atlas projects, which recently reported the comprehensive molecular characterization of chromophobe RCC [29]. A similar approach is ongoing for papillary RCC. Future trials will elucidate the role of immune checkpoint inhibitors in nonccRCC.
Supplementary Material
Acknowledgments
The authors thank Charla McMichael, Lisa Smith, Kevin Winslow, Margarita Roserika Brooks, Racheal Garza, Maria Ramos, and Marla Polk for data collection and logistical help.
Funding/Support and role of the sponsor: The trial was funded by Novartis, supported by NIH/NCI award P30CA016672. The Genitourinary Cancers Program of the Cancer Center Support Grant Programs shared resources at M.D. Anderson Cancer Center and was funded in part by the Dana-Farber/Harvard Cancer Center Kidney Cancer Specialized Program of Research Excellence, the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research for Toni K. Choueiri. Novartis had no role in the study design, data collection, analysis, or interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Nizar M. Tannir had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tannir.
Acquisition of data: Tannir, Jonasch, Albiges, Matin, Lim, Karam, McDermott, Wood, Choueiri. Analysis and interpretation of data: Tannir, Jonasch, Albiges, Altinmakas, Ng, Matin, Wang, Qiao, Lim, Tamboli, Rao, Sircar, Karam, McDermott, Wood, Choueiri.
Drafting of the manuscript: Tannir, Albiges, Karam, Choueiri.
Critical revision of the manuscript for important intellectual content: Tannir.
Statistical analysis: Wang, Qiao.
Obtaining funding: Tannir, Choueiri.
Administrative, technical, or material support: Tannir.
Supervision: Tannir.
Other (specify): None.
Presented at the 50th Annual Meeting of the American Society of Clinical Oncology, May 30–June 3, 2014, in Chicago, Illinois.
Financial disclosures: Nizar M. Tannir certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Nizar M. Tannir receives honoraria from Pfizer, Novartis, GlaxoSmithKline, and Exelixis; is a consultant for Pfizer, Novartis, GlaxoSmithKline, and Exelixis; receives research funding from Pfizer, Novartis, Exelixis, Bristol-Myers Squibb, and GlaxoSmithKline; and receives travel, accommodations, and expenses from Pfizer, Novartis, and GlaxoSmithKline. Eric Jonasch is a consultant/adviser for GlaxoSmithKline, Pfizer, Novartis, and Genentech-Roche; receives research funding from Exelixis, Pfizer, Onyx, GlaxoSmithKline, and Novartis; and receives travel, accommodations, and expenses from GlaxoSmithKline, Pfizer, and Novartis. Laurence Albiges is a consultant/adviser for Pfizer, Novartis, Sanofi, and Amgen and receives research funding from Pfizer and Novartis. Chaan S. Ng receives research funding and travel, accommodations, and expenses from GE Healthcare. Jose A. Karam receives honoraria and serves as a consultant/adviser for Pfizer. David F. McDermott is a consultant/adviser for Roche-Genentech, Merck, Bristol-Myers Squibb, and Pfizer and receives research funding from Prometheus Labs. Christopher G. Wood is a consultant/adviser for Ono Pharmaceutical; receives research funding from Argos Therapeutics, Pfizer, GlaxoSmithKline, Boehringer Ingelheim, and Ono Pharmaceutical; and receives travel, accommodations, and expenses from Argos Therapeutics. Toni K. Choueiri receives honoraria from NCCN; is a consultant/adviser for Pfizer, GlaxoSmithKline, Novartis, Merck and Bayer, and AVEO; receives research funding from Pfizer, GlaxoSmithKline, Novartis, Bristol-Myers Squibb, Merck, Exelixis, Roche, Astra Zeneca, and Tracon; and receives travel, accommodations, and expenses from Pfizer, GlaxoSmithKline, Novartis, Bayer, and Bristol-Myers Squibb. The other authors have nothing to disclose.
References
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. 2009;16:432–43. doi: 10.1111/j.1442-2042.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 3.Kidney cancer, version 3. National Comprehensive Cancer Network Web site; 2014. [June 23, 2014]. NCCN clinical practice guidelines in oncology (NCCN Guidelines®). http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. [Google Scholar]
- 4.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49–56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 5.Kroeger N, Xie W, Lee J-L, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013;119:2999–3006. doi: 10.1002/cncr.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116:1272–80. doi: 10.1002/cncr.24864. [DOI] [PubMed] [Google Scholar]
- 7.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–63. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 8.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–9. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J-L, Ahn J-H, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. 2012;23:2108–14. doi: 10.1093/annonc/mdr586. [DOI] [PubMed] [Google Scholar]
- 10.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol. 2013;24:1026–31. doi: 10.1093/annonc/mds582. [DOI] [PubMed] [Google Scholar]
- 11.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30:335–40. doi: 10.1007/s10637-010-9491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG). Ann Oncol. 2015;26:1123–8. doi: 10.1093/annonc/mdv149. [DOI] [PubMed] [Google Scholar]
- 13.Escudier BJ, Bracarda S, Rey JPM, et al. Open-label, phase II raptor study of everolimus (EVE) for papillary mRCC: efficacy in type 1 and type 2 histology [abstract 410]. J Clin Oncol. 2014:32. [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 15.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–9. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 16.Grünwald V, Karakiewicz PI, Bavbek SE, et al. An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer. 2012;48:324–32. doi: 10.1016/j.ejca.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Albiges L, Molinie V, Escudier B. Non-clear cell renal cell carcinoma: does the mammalian target of rapamycin represent a rational therapeutic target? Oncologist. 2012;17:1051–62. doi: 10.1634/theoncologist.2012-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellmunt J, Dutcher J. Targeted therapies and the treatment of non-clear cell renal cell carcinoma. Ann Oncol. 2013;24:1730–40. doi: 10.1093/annonc/mdt152. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 20.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voss MH, Bastos DA, Karlo CA, et al. Treatment outcome with mTOR inhibitors for metastatic renal cell carcinoma with nonclear and sarcomatoid histologies. Ann Oncol. 2014;25:663–8. doi: 10.1093/annonc/mdt578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:2765–72. doi: 10.1200/JCO.2013.54.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colomba E, Albiges L, Le Teuff G, et al. Metastatic chromophobe renal cell carcinoma treated with targeted therapies: a Renal Cross Chanel Group (RCCG) study [abstract 4561]. J Clin Oncol. 2015;33(Suppl) [Google Scholar]
- 24.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27:235–41. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakopoulos CE, Chittoria N, Choueiri TK, et al. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer. 2015;13:e79–85. doi: 10.1016/j.clgc.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17:46–54. doi: 10.1634/theoncologist.2011-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. 2012;30:3402–7. doi: 10.1200/JCO.2011.40.9631. [DOI] [PubMed] [Google Scholar]
- 28.Wagle N, Grabiner BC, Van Allen EM, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371:1426–33. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–30. doi: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.