Abstract
Several experimental and observational studies have demonstrated the antiandrogenicity of several phthalates. However, there is limited evidence of an association between phthalate exposure in adult life and semen quality. The aim of this study was to examine phthalate exposure during adulthood in relation to semen quality in fertile US men. This multi-center cross-sectional study included 420 partners of pregnant women who attended a prenatal clinic in one of five U.S. cities during 1999–2001. Nine phthalate metabolites [mono (2-ethylhexyl) phthalate (MEHP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono (2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono (2-ethyl-5-carboxypentyl) phthalate (MECPP)], as well as mono-n-butyl phthalate (MBP) and mono-isobutyl phthalate (MiBP), mono (3 carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP) and monoethyl phthalate (MEP)] were measured in urine collected at the same time as the semen sample. We regressed natural log-transformed (ln) sperm concentration, ln(total sperm count), ln(total motile sperm count), percent motile sperm and percent sperm with normal morphology on each of the nine natural log-transformed metabolite concentrations and on the molar-weighted sum of DEHP metabolites in separate models. We fit unadjusted models and models that adjusted for confounders determined a priori. In unadjusted models, ln(MiBP) was significantly and positively associated with motility and ln(MBzP) significantly negatively associated with ln(total sperm count). In adjusted linear models, urinary metabolite concentrations of DEHP, DBP, DEP, and DOP were not associated with any semen parameter. We found an inverse association between ln(MBzP) concentrations and sperm motility (β = −1.47, 95% CI: −2.61, −0.33), adjusted for ln(creatinine concentration), geographic location, age, race, smoking status, stress, recent fever, time from sample collection and time to complete analysis. Several sensitivity analyses confirmed the robustness of these associations. This study and the available literature suggest that impacts of adult exposure to phthalates at environmental levels on classical sperm parameters are likely to be small.
Keywords: endocrine disruption, epidemiology, phthalates, reproduction, semen quality
Introduction
Phthalate esters are pervasive environmental chemicals present in food and many commonly used products (plastics, food contact applications, wall coverings, varnishes, lacquers or personal-care products) (ATSDR, 2002) and manufactured in such quantity that exposure is ubiquitous (CDC, 2015). Exposures through ingestion, dermal contact or inhalation are considered the main important routes of exposure (Meeker et al., 2009). Animal studies have shown that exposure to certain phthalates during fetal sexual differentiation reduces fetal testosterone (Welsh et al., 2008). As a result, male pups exhibit a cluster of altered androgen-dependent anatomical features that reflect disordered sex differentiation, including a reduced – that is, a less masculine – anogenital distance (AGD), impaired testicular descent, and reduced testicular size. This cluster of alterations has been referred to as the “phthalate syndrome”, which in male rodents, has been shown to have adverse consequences for later sexual development (Foster, 2006). In humans, an association between prenatal exposure to certain phthalates and a similar cluster of reproductive developmental outcomes, most notably a shorter AGD, has been reported in male infants (Swan et al., 2005; Swan, 2008, Swan et al., 2015). Most observational studies show limited or weak evidence of a relationship between impaired semen quality in adulthood and phthalate exposures, including dibutyl phthalates (DBPs), benzyl butyl phthalate (BzBP) and di(2-ethylhexyl) phthalate (DEHP) metabolites (Duty et al., 2003, 2005; Hauser et al., 2006; Han et al., 2014; Herr et al., 2009; Jonsson et al., 2005; Jurewicz et al., 2013; Lenters et al., 2015; Liu et al., 2012; Pant et al., 2014; Specht et al., 2014; Wirth et al., 2008).
In this analysis, we use linear regression to examine the associations between phthalate urinary metabolite concentration in adult men and four measures of semen quality. We use data collected from partners of pregnant women in the Study for Future Families, a large multi-center study designed to look at geographic variability in semen quality (Redmon et al., 2013). This population differs from those in most previous studies of phthalates exposure and semen quality in which participants were mainly recruited at infertility clinics (Duty et al., 2005; Hauser et al., 2006; Herr et al., 2009; Jonsson et al., 2005; Jurewicz et al., 2013; Pant et al., 2014; Wirth et al., 2008). To the best of our knowledge, these associations have previously been assessed in fertile men in only one European study (Lenters et al., 2015; Specht et al., 2014).
Material and Methods
Study Population
The Study for Future Families (SFF) is a multi-center study of partners of pregnant women recruited in Los Angeles, CA (Harbor-UCLA Medical Center and Cedars-Sinai Medical Center), Minneapolis, MN (University of Minnesota Medical Center), Columbia, MO (University Physicians), Iowa City, IA (University of Iowa), and New York City, NY in 1999–2001. Only couples whose pregnancies were conceived without medical intervention were eligible. Men were asked to give two semen samples and one blood and one urine sample. In this analysis we report on semen parameters from the first semen sample in 420 men and phthalates in urine samples provided on the same day.
Ethical approval
Human subject committees at all participating institutions approved SFF and all subjects signed informed consents. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research. Further details of the study design have been described previously (Swan et al., 2003, Stokes-Riner et al., 2007, Redmon et al., 2013).
Urine collection and phthalate metabolite analysis
Men were requested to give a urine sample at the first study visit. Urine samples were collected in phthalate-free polypropylene cups and all collection and storage materials were shown to be phthalate-free. Samples were stored at −20°C or −80°C before shipment to the Division of Laboratory Sciences, National Center for Environmental Health, CDC. Metabolites were measured at the Division of Laboratory Sciences, National Center for Environmental Health, CDC (Atlanta, GA), where laboratory staff had no access to participant data. The laboratory analysis involved the enzymatic deconjugation of the phthalate metabolites from their glucuronidated form, automated on-line solid-phase extraction coupled with separation with high-performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry. This high-throughput method allows for the concurrent quantification in human urine of the phthalate metabolites reported here. Further details of the analytical approach have been described previously (Silva et al., 2007). For this analysis we considered nine phthalate metabolites measured in SFF: four metabolites of DEHP [mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)], as well as mono-n-butyl phthalate (MBP), and mono-isobutyl phthalate (MiBP), metabolites of dibutyl phthalate (DBP); mono(3 carboxypropyl) phthalate (MCPP), a metabolite of di(n-octyl) phthalate (DOP), monobenzyl phthalate (MBzP), a metabolite of butylbenzyl phthalate (BBzP) and monoethyl phthalate (MEP), a metabolite of diethyl phthalate (DEP). We also created a molar-weighted sum of DEHP metabolites, where the weight for each of the four DEHP metabolites is the inverse of its molecular weight. Limits of detection (LOD) ranged from 0.2 to 1.2 ng/ml.
Semen collection and analysis
Men were requested to observe a two to five day abstinence period before providing a semen sample, and at the time of the visit the importance of accurately reporting the actual abstinence period was stressed. At the study visit, men collected semen samples by masturbation in the clinic, and on average the samples were analyzed within 27 minutes of collection (Stokes-Riner et al., 2007). We examined five semen quality parameters: sperm concentration, percent motile sperm, percent sperm with normal morphology, total sperm count (TSC) (specimen weight × sperm concentration), and total motile sperm count (TMSC) (specimen weight × sperm concentration × motility). Methods used for semen evaluation and quality control of laboratory methods have been described previously (Brazil et al., 2004). Briefly, ejaculate volumes were estimated by specimen weight, assuming a semen density of 1.0 g/mL and concentration assessments were evaluated manually using MicroCell counting chambers. The percent motile sperm was determined in a MicroCell (Conception Technologies, San Diego, California) chamber and refers to total motility: the percent of sperm with any flagellar movement, whether twitching or progressive (Overstreet and Brazil, 1997). For MicroCell counting, semen was diluted with equal parts of fixative to immobilize the sperm. The final concentration was the mean of the concentration values from the separate dilutions of 2 drops, when these differed by less than 10%. If the duplicate concentrations varied by more than 10%, a third dilution was prepared and counted and the median of the 3 values used as the estimate of concentration. On average, 200–300 sperm were counted for each dilution. Seminal smears were prepared at the clinical centers and shipped to the Andrology Coordinating Center at the University of California, Davis, for Papanicalou staining, analysis, and storage, where a single technician assessed sperm morphology using the strict morphology method, recommended by the World Health Organization (Guzick et al., 2001; WHO, 1999).
Statistical methods
Phthalate metabolites concentrations were natural log-transformed to normalize their distribution. Sperm concentration, TSC, and TMSC were also natural log-transformed in order to better satisfy model assumptions. Statistical programming was performed in SAS (SAS, Cary, N.C) and R 3.0.2 (R Foundation for Statistical Computing, www.r-project.org).
Each of the five semen parameters [ln(concentration), percent motile sperm, percent sperm with normal morphology ln(TSC), and ln(TMSC)] was regressed on each of the nine natural log-transformed metabolites concentrations [ln(MEHP), ln(MEHHP), ln(MEOHP), ln(MECPP), ln(MBP), ln(MiBP), ln(MCPP), ln(MBzP), ln(MEP)] and on the molar-weighted sum of DEHP metabolites in separate models; metabolite concentrations below the LOD were assigned the value LOD/sqrt(2) for data analysis (Hornung and Reed, 1990). With the exception of ln(MBP), all creatine-adjusted ln(phthalate metabolites) differed significantly across locations (data not shown). In addition, since sperm concentration and motility were determined locally and some differences remained despite tight quality control procedures (Brazil et al., 2004), we adjusted for geographic location (MO, CA, MN, NY, IA, coded as dummy variables) in all primary models. We also adjusted for age (years), and used binary variables for race (black/not black), current smoking status (yes/no), stress [number of stressful life events in 3 months prior to sample collection (< 2 vs. ≥ 2 events) (Gollenberg et al. 2010)] and recent fever (yes/no). The natural logarithm of creatinine concentration was used to control for urine sample dilution. Ln(creatinine) was used because the relationships between metabolites and creatinine were only linear when both were natural log-transformed, and this transformation is consistent with other papers (Mendiola et al., 2010). In addition, models for ln(sperm concentration), ln(TSC) and ln(TMSC) were adjusted for abstinence time (as a continuous variable); models for motility were adjusted for time from sample collection to analysis, and time to complete analysis because motility decreases with time from sample collection; and models for morphology were adjusted for time to complete analysis. Covariates were selected a priori to be consistent with previous decisions about covariate adjustment for these outcomes in the SFF cohort (Swan et al., 2003, Mendiola et al., 2010; Redmon et al., 2013); all covariates selected were included in the final models. Standard residual-based plots were examined to assess the regression modeling assumptions of constant residual variance, linearity between covariates and outcome and normality of the residuals. We also flagged extreme outliers and influential observations (as assessed by Cook’s distance), and if warranted, refit the model without these observations (Weisberg, 2005).
In order to assess the influence of several factors that might have affected results, we carried out four sensitivity analyses for the three primary outcomes ln(concentration), motility, and morphology. We fit models without adjusting for geographic location. Because only eight subjects had a fever, we also fit models that did not adjust for fever. A third sensitivity analysis excluded men whose creatinine concentration was below 30 or above 300 (WHO, 1996). Finally, to assess sensitivity to using a fixed value of metabolites below the LOD, we fit models that excluded subjects with values below the LOD for the metabolite in the model.
Results
Of the 950 men who participated in SFF, 805 gave at least one semen sample. Because urine was not collected until the second year of the study only 533 men provided urine samples. Phthalate metabolite measurements and semen quality were available for 441 of these 533 men. Of these, eight subjects who reported abstinence time greater than 240 hours were excluded. We excluded an additional 13 subjects who were missing data on one or more model covariates. Demographic characteristics and summaries of semen quality parameters of the 420 SFF participants included in this analysis are presented in Table 1. Summary statistics of urinary concentrations of phthalate metabolites are presented in Table 2. Correlations between the natural logarithm of the urinary concentrations of the four DEHP metabolites ranged from 0.84 to 0.99 and correlations between the logarithm of pairs of metabolites from different phthalates ranged from 0.23 to 0.77 for ln(MBP) and ln(MiBP). After adjusting for ln(creatinine), correlations between metabolites were somewhat lower, ranging from 0.84 to 0.95 for pairs of DEHP metabolites, and from −0.06 (for ln(MEP) and most other metabolites) to 0.53 for ln(MBP) and ln(MiBP).
Table 1.
Characteristics of study population, partners of pregnant women in five US cities (N = 420)
| Characteristics | Mean | SD | Min | Median | Max |
|---|---|---|---|---|---|
| Age (years) | 32 | 6 | 18 | 32 | 53 |
| Abstinence time (hours) | 77 | 30 | 9 | 72 | 227 |
| Time to start semen analysis (minutes) | 27 | 10 | 0 | 28 | 90 |
| Time to complete semen analysis (minutes) | 58 | 9 | 24 | 58 | 97 |
| Sperm concentration (106/ml) | 66 | 45 | 3 | 59 | 316 |
| Sperm motility (%) | 52 | 12 | 10 | 54 | 78 |
| Morphology (%) | 11 | 5 | 0.5 | 10 | 26 |
| Total sperm count (106) | 297 | 211 | 6 | 246 | 1218 |
| Total motile sperm count (106) | 134 | 103 | 0.5 | 114 | 627 |
| Creatinine concentration (mg/dl) | 143 | 81 | 7 | 138 | 509 |
| N | % | ||||
| WHO reference valuesa | |||||
| Sperm concentration (106/ml) <20×106/ml | 46 | 11 | |||
| Sperm motility (%) < 50% | 160 | 38 | |||
| Morphology (%) < 4% | 33 | 8 | |||
| Total sperm count (106) <40×106/ml | 27 | 6 | |||
| Center: CA | 114 | 27 | |||
| Center: MN | 127 | 30 | |||
| Center: MO | 88 | 21 | |||
| Center: NY | 18 | 4 | |||
| Center: IA | 73 | 17 | |||
| Race: Black | 35 | 8 | |||
| Current smoker | 91 | 22 | |||
| Stress | 100 | 24 | |||
| Recent fever | 8 | 2 |
SD: Standard deviation.
Number (%) of subjects below the WHO (1999) reference values for each semen parameter.
Table 2.
Summary statistics for the urinary phthalate metabolites (in ng/mL)
| Metabolite | Geometric mean | Min | Max | Limit of Detection (LOD) | %> LOD |
|---|---|---|---|---|---|
| MEHP | 3.61 | 0.85 | 403 | 1.20 | 77 |
| MEHHP | 22.87 | 0.49 | 2600 | 0.70 | 99 |
| MEOHP | 12.59 | 0.49 | 1320 | 0.70 | 97 |
| MECPP | 32.78 | 0.90 | 3250 | 0.60 | 100 |
| MBP | 15.28 | 0.42 | 388 | 0.60 | 98 |
| MiBP | 2.80 | 0.21 | 83 | 0.30 | 92 |
| MCPP | 2.09 | 0.14 | 86 | 0.20 | 96 |
| MBzP | 11.13 | 0.21 | 364 | 0.30 | 98 |
| MEP | 200.96 | 0.80 | 14700 | 0.80 | 100 |
Coefficients and corresponding 95% confidence intervals for the association between each semen parameter and each ln(metabolite) concentration, from separate models, are shown without adjustment for other variables in Table 3, and in models that adjusted for confounding variables in Table 4. In the unadjusted models, ln(MiBP) was positively associated with motility and ln(MBzP) was negatively associated with total sperm count. In adjusted models, we found no statistically significant associations between any of the semen parameters and any of the metabolites concentrations, with the exception of a inverse relationship between ln(MBzP) and sperm motility. Our checks for the regression assumptions showed that model assumptions were reasonably well met. There were no extreme outliers (all standardized residuals were less than 4 in absolute value) and no influential observations (Cook’s distances were all less than 0.11). Figure 1 shows the relationships between each ln(metabolite) and ln(concentration), morphology, and motility from the adjusted models, when all variables are put on a common scale by subtracting the variable-specific mean and dividing by the variable-specific standard deviation. Figure 2 shows the relationship between motility and ln(MBzP) with the superimposed regression line and 95% confidence interval from the covariate-adjusted regression.
Table 3.
Estimated coefficients (95% confidence intervals) from separate unadjusted linear regression models of each semen parameter on the natural logarithm of each phthalate metabolite concentration (N=420). Significant associations (p<0.05) are bolded.
| Metabolite | Log(Conc) | 95% CI | Motility | 95% CI | Morphology | 95% CI | Log(TSC) | 95% CI | Log(TMSC) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| MEHP | 0.02 | (−0.04, 0.08) | 0.48 | (−0.42, 1.38) | 0.18 | (−0.21, 0.57) | 0.00 | (−0.06, 0.06) | 0.01 | (−0.07, 0.09) |
| MEHHP | 0.01 | (−0.05, 0.07) | 0.67 | (−0.18, 1.52) | 0.18 | (−0.19, 0.55) | −0.01 | (−0.07, 0.05) | 0.01 | (−0.07, 0.09) |
| MEOHP | 0.02 | (−0.04, 0.08) | 0.77 | (−0.08, 1.62) | 0.18 | (−0.19, 0.55) | 0.00 | (−0.06, 0.06) | 0.02 | (−0.06, 0.10) |
| MECPP | 0.00 | (−0.06, 0.06) | 0.68 | (−0.24, 1.60) | 0.12 | (−0.29, 0.53) | −0.01 | (−0.09, 0.07) | 0.00 | (−0.08, 0.08) |
| Molar Sum DEHP | 0.01 | (−0.05, 0.07) | 0.72 | (−0.16, 1.60) | 0.17 | (−0.22, 0.56) | −0.01 | (−0.07, 0.05) | 0.01 | (−0.07, 0.09) |
| MBP | −0.01 | (−0.07, 0.05) | 0.35 | (−0.61, 1.31) | 0.13 | (−0.30, 0.56) | −0.04 | (−0.12, 0.04) | −0.03 | (−0.11, 0.05) |
| MiBP | 0.01 | (−0.05, 0.07) | 1.01 | (0.13, 1.89) | 0.17 | (−0.22, 0.56) | −0.02 | (−0.08, 0.04) | 0.00 | (−0.08, 0.08) |
| MCPP | −0.03 | (−0.11, 0.05) | −0.19 | (−1.25, 0.87) | 0.08 | (−0.39, 0.55) | −0.04 | (−0.12, 0.04) | −0.04 | (−0.14, 0.06) |
| MBzP | −0.04 | (−0.10, 0.02) | −0.50 | (−1.36, 0.36) | −0.07 | (−0.44, 0.30) | −0.07 | (−0.13, −0.01) | −0.07 | (−0.15, 0.01) |
| MEP | −0.03 | (−0.09, 0.03) | 0.31 | (−0.46, 1.08) | 0.17 | (−0.16, 0.50) | −0.05 | (−0.11, 0.01) | −0.03 | (−0.09, 0.03) |
Table 4.
Estimated coefficients (95% confidence intervals) from separate covariate-adjusted linear regression models of each semen parameter on the natural logarithm of each phthalate metabolite concentration (N=420)1.
| Metabolite | Log(Conc) | 95% CI | Motility | 95% CI | Morphology | 95% CI | Log(TSC) | 95% CI | Log(TMSC) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| MEHP | 0.05 | (−0.02, 0.11) | 0.08 | (−0.89, 1.05) | 0.16 | (−0.31, 0.63) | 0.04 | (−0.04, 0.11) | 0.04 | (−0.05, 0.13) |
| MEHHP | 0.03 | (−0.04, 0.10) | 0.27 | (−0.76, 1.31) | 0.25 | (−0.25, 0.74) | 0.03 | (−0.04, 0.11) | 0.04 | (−0.06, 0.13) |
| MEOHP | 0.04 | (−0.03, 0.12) | 0.41 | (−0.64, 1.46) | 0.24 | (−0.26, 0.74) | 0.05 | (−0.03, 0.13) | 0.06 | (−0.04, 0.15) |
| MECPP | 0.03 | (−0.05, 0.11) | 0.21 | (−0.92, 1.35) | 0.13 | (−0.41, 0.67) | 0.03 | (−0.05, 0.12) | 0.03 | (−0.07, 0.14) |
| Molar Sum DEHP | 0.04 | (−0.04, 0.11) | 0.30 | (−0.80, 1.41) | 0.21 | (−0.32, 0.74) | 0.04 | (−0.04, 0.12) | 0.04 | (−0.06, 0.14) |
| MBP | 0.01 | (−0.09, 0.11) | −0.58 | (−2.00, 0.84) | 0.22 | (−0.46, 0.91) | 0.00 | (−0.11, 0.11) | −0.02 | (−0.15, 0.11) |
| MiBP | 0.02 | (−0.06, 0.11) | 0.82 | (−0.31, 1.96) | 0.28 | (−0.27, 0.83) | 0.01 | (−0.07, 0.10) | 0.02 | (−0.08, 0.13) |
| MCPP | −0.01 | (−0.11, 0.08) | −0.92 | (−2.28, 0.43) | 0.15 | (−0.51, 0.80) | 0.01 | (−0.09, 0.11) | −0.03 | (−0.15, 0.10) |
| MBzP | −0.04 | (−0.12, 0.04) | −1.47 | (−2.61, −0.33) | −0.19 | (−0.75, 0.36) | −0.06 | (−0.14, 0.03) | −0.09 | (−0.19, 0.02) |
| MEP | −0.02 | (−0.07, 0.04) | −0.13 | (−0.95, 0.69) | 0.16 | (−0.24, 0.55) | 0.01 | (−0.05, 0.07) | 0.01 | (−0.07, 0.09) |
All models adjusted for the natural logarithm of creatinine concentration, geographic location, age, race, smoking status, stress and recent fever. Models for sperm concentration, total sperm count (TSC) and total motile sperm count (TMSC) also adjusted for abstinence time; models for motility also adjusted for time from sample collection to analysis and time to complete analysis; and models for morphology also adjusted for time to complete analysis. Phthalate metabolites and sperm counts were natural log-transformed. Significant associations (p<0.05) are bolded.
Figure 1.
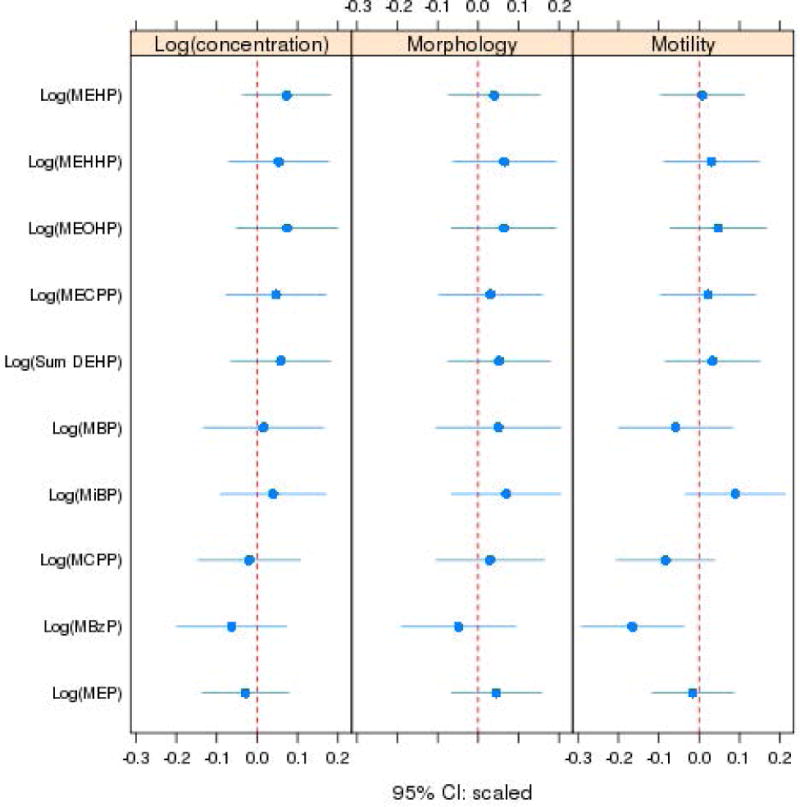
Point estimates and 95% confidence intervals for the relationship between the ln-metabolites and each semen quality outcome from adjusted regression models, with all variables centered and scaled.
Figure 2.
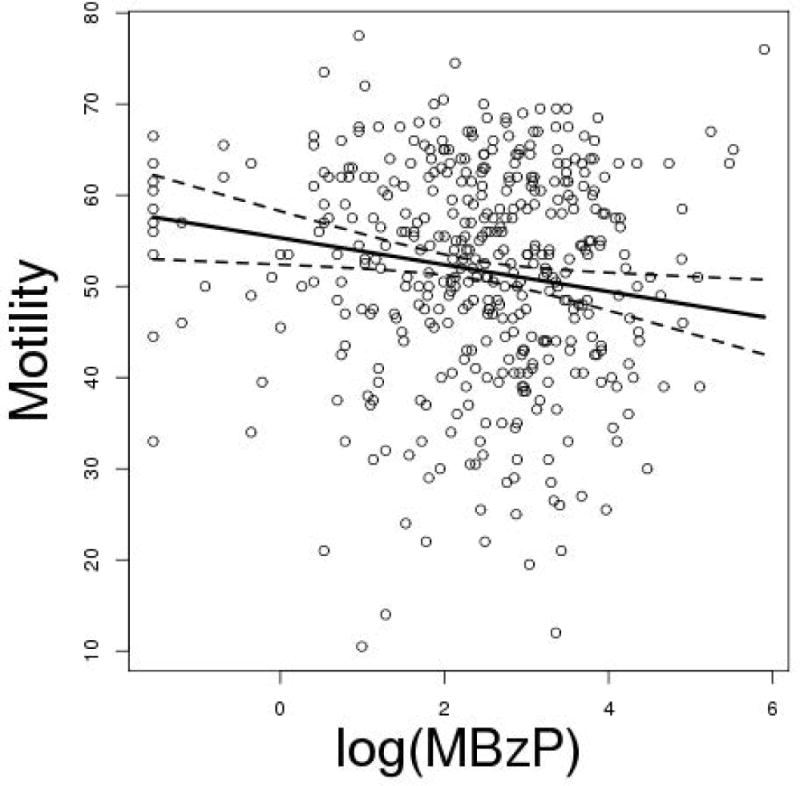
Scatterplot of motility versus ln(MBzP), with superimposed regression line and 95% confidence interval from the covariate-adjusted model.
In models using ln(molar-weighted DEHP metabolites), stress was associated with a lower ln(concentration) (slope: −0.20, 95% CI: −0.37, −0.02), fever was associated with a lower ln(concentration) (slope: −0.72, 95% CI: −1.26, −0.18), abstinence time was associated with a higher ln(concentration) (slope: 0.005, 95% CI: 0.002, 0.007), and time to complete the analysis was associated with a lower motility (slope: −0.38, 95% CI: −0.51, −0.26). Center was also a very significant predictor of sperm motility (p<0.0001 from the 4 degree of freedom test), with Missouri associated with much lower motility than the other centers. The covariate associations were similar when other metabolites were used in place of the molar-weighted sum of DEHP metabolites.
Results from the four sensitivity analyses showed that there were no significant associations between semen parameters and phthalate metabolites in any of the covariate-adjusted models considered other than the inverse relationship between ln(MBzP) and sperm motility. Ln(MBzP) was a significant predictor of motility in all sensitivity analyses.
Discussion
The present study did not find any association between urinary concentrations of phthalate metabolites and any classical semen quality parameters in multiple regression models, adjusted for appropriate covariates except for an inverse association between ln(MBzP) and sperm motility. While the robustness of this latter association was confirmed by several sensitivity analyses, based on the number of tested associations we cannot rule out the possibility that the observed significant association could be a chance finding. This is the first study to examine this association in a U.S. population of fertile men. Our results extend previous findings in selected populations, mainly of infertile men.
Several animal toxicology studies report that a number of phthalates are testicular toxicants and impair spermatogenesis (Aly et al., 2015; Uren-Webster et al., 2010). Based on results in several animal species DEHP and DBP metabolites appear to have the capacity to disrupt normal reproduction (semen quality, etc.) (Fabjan et al., 2006). However, previous results and ours show only weak or no associations between human adult exposure to phthalates and impaired semen quality. Several investigations found inconsistent or weak associations between sperm concentration, motility or morphology and urinary phthalate metabolites (mainly DEHP, DBPs or DEP) in young unselected men (Jonsson et al., 2005; Joensen et al., 2012), men from the general population (Han et al., 2014), fertile men (Lenters et al., 2015; Specht et al., 2014) or male partners of subfertile couples (Wirth et al., 2008; Liu et al., 2012). These results are consistent with Herr et al. (2009) who looked at this question in men referred for fertility work-up and found no association between the sum of four urinary DEHP metabolites (MEHP, MEHHP, MEOHP and MECPP) and any sperm parameter. In contrast, significant inverse relationships between exposure to DBPs and both sperm concentration and motility were seen in a study of men who are part of a couple seeking an infertility work-up (Hauser et al., 2006). Using data from an earlier sample of these men, Duty et al. (2003) described a dose-response relation between exposure to DBPs and sperm motility and concentration. In similar potentially infertile male populations, Jurewicz and collaborators (2013) and Pant and coworkers (2014) showed an inverse association between urinary DEHP metabolites and sperm motility. Finally, in a recent meta-analysis (14 studies) looking at the associations between phthalates or their metabolite levels and human semen quality, Cai and colleagues (2015) found some evidence of an inverse relationship between specific phthalate exposures and impaired semen quality (e.g. MBP, MBzP or MEHP), but also noted that there were several cases where associations between phthalates and semen quality were not found (e.g. MMP).
These differences across studies may reflect population differences, or chance. The SFF cohort only included partners of pregnant women, who have better than average semen quality, while other cohorts included men from couples presenting to infertility clinics, who tend to have poorer semen quality. These data, coupled with other published literature, suggest that phthalate exposure during adulthood is not a major effector of classical parameters of semen quality. However, phthalates might alter other measures related to sperm quality (e.g. measures of DNA damage, alteration of gene expression) (Spade et al., 2014).
We compared unadjusted urinary concentrations of the BBzP metabolite in our men to those from male participants in the 2001–2002 National Health and Nutrition Examination Survey (NHANES) (CDC, 2015). Geometric mean MBzP concentration was almost equal in both populations; 11.2 ng/mL compared with 11.1 ng/mL for male NHANES and our study population, respectively. Several previous studies have also suggested that exposure to phthalates may affect reproductive hormones. These results, observed across different designs and study populations (young, infertile or fertile men) indicate harmful effects of DEHP and diisononyl phthalate metabolites on the reproductive axis, in particular with regard to free and total testosterone and estradiol levels (Joensen et al., 2012; Meeker et al., 2009; Mendiola et al. 2011, 2012; Specht et al., 2014).
Our data were limited by the use of a single spot urine sample to assess phthalate exposure and a single semen sample, which does not capture the day-to-day variability within the same subject. Furthermore, the time when spermatogenesis occurred likely preceded the time urine was collected and exposure assessed. However, while urinary concentrations of phthalate metabolites are variable, because exposure is ubiquitous metabolite concentrations in multiple samples from the same subject tend to fall in the same quartile of their distribution (Hauser et al., 2004).
With regard to use of a single semen sample per man, there is evidence that as long as the model adjusts for important covariates, semen parameters from the first and the second sample are not significantly different from each other (Stokes-Riner et al. 2007). Future analyses could extend the modeling to accommodate repeated semen samples and phthalate metabolite concentrations from each participant.
In conclusion, we did not find associations between phthalate exposure in adulthood and classical semen quality parameters in fertile US men, with the exception of an association between MBzP and decreased motility. This study and the available literature suggest that impacts of adult exposure to phthalates at environmental levels on classical sperm parameters are likely to be small.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Antonia M. Calafat, M. Silva, E. Samandar, J. Preau and J. Reidy (Centers for Disease Control and Prevention, Atlanta, GA) for measuring the urinary concentrations of the phthalate metabolites and Charlene Brazil and James Overstreet (University of California Davis) for semen analyses. This work was supported by STAR grant RD83215 from US Environmental Protection agency; NIH grant M01RR00400 to the University of Minnesota General Clinical Research Center; NIEHS grant R01ES09916 to the University of Missouri; UCLA CTSI grant UL1TR000124 to Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, University of Iowa Center for Health Effects of Environmental Contamination cooperative project grant, and grants T32 ES007271 and P30 ES001247 from the NIEHS (NIH) to the University of Rochester. Hagai Levine gratefully acknowledges support by a post-doctoral fellowship from the Environment and Health Fund, Jerusalem, Israel.
Footnotes
Authors’ contribution section
SHS, JBR, AS, CW and ED were involved in study conception and design. JBR, AS, CW, and ED were involved in study execution and acquisition of data. SWT, JM, ARB, and HL contributed to data analysis and interpretation. SWT, JM, ARB, HL and SHS drafted the manuscript. All authors provided substantial intellectual contributions and approved the final version of the manuscript.
Conflict of interest
The authors declare they have no actual or potential competing financial interests.
References
- Aly HA, Hassan MH, El-Beshbishy HA, Alahdal AM, Osman AM. Dibutyl phthalate induces oxidative stress and impairs spermatogenesis in adult rat. Toxicol Ind Health. 2015:pii. doi: 10.1177/0748233714566877. in press. 0748233714566877. [DOI] [PubMed] [Google Scholar]
- , editor. ATSDR. Toxicological Profile for di-n-butyl Phthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry, Division of Toxicology; 2002. 2001. [PubMed] [Google Scholar]
- Brazil C, Swan SH, Tollner CR, Treece C, Drobnis EZ, Wang C, Redmon JB, Overstreet JW. Quality control of laboratory methods for semen evaluation in a multicenter research study. J Androl. 2004;25:645–656. doi: 10.1002/j.1939-4640.2004.tb02836.x. [DOI] [PubMed] [Google Scholar]
- Cai H, Zheng W, Zheng P, Wang S, Tan H, He G, Qu W. Human urinary/seminal phthalates or their metabolite levels and semen quality: A meta-analysis. Environ Res. 2015;142:486–494. doi: 10.1016/j.envres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- CDC. Updated Tables. Centers for Disease Control and Prevention; Washington, DC: 2015. Fourth National Report on Human Exposure to Environmental Chemicals. http://www.cdc.gov/exposurereport/ [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–277. [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Hum Reprod. 2005;20:604–610. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Fabjan E, Hulzebos E, Mennes W, Piersma AH. A category approach for reproductive effects of phthalates. Crit Rev Toxicol. 2006;36:695–726. doi: 10.1080/10408440600894914. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, Overstreet JW, Redmon JB, Sparks A, Wang C, Swan SH. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–1111. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, Xu D, Vogel DL. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Han X, Cui Z, Zhou N, Ma M, Li L, Li Y, Lin H, Ao L, Shu W, Liu J, Cao J. Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China. Int J Hyg Environ Health. 2014;217:271–278. doi: 10.1016/j.ijheh.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Herr C, zur Nieden A, Koch HM, Schuppe HC, Fieber C, Angerer J, Eikmann T, Stilianakis NI. Urinary di(2-ethylhexyl)phthalate (DEHP)-Metabolites and male human markers of reproductive function. Int J Hyg Environ Health. 2009;212:648–653. doi: 10.1016/j.ijheh.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Joensen UN, Frederiksen H, Jensen MB, Lauritsen MP, Olesen IA, Lassen TH, Andersson AM, Jørgensen N. Phthalate excretion pattern and testicular function: a study of 881 healthy Danish men. Environ Health Perspect. 2012;120:1397–1403. doi: 10.1289/ehp.1205113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Radwan M, Sobala W, Ligocka D, Radwan P, Bochenek M, Hawuła W, Jakubowski L, Hanke W. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod Toxicol. 2013;42:232–241. doi: 10.1016/j.reprotox.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Lenters V, Portengen L, Smit LA, Jönsson BA, Giwercman A, Rylander L, Lindh CH, Spanò M, Pedersen HS, Ludwicki JK, Chumak L, Piersma AH, Toft G, Bonde JP, Heederik D, Vermeulen R. Phthalates, perfluoroalkyl acids, metals and organochlorines and reproductive function: a multipollutant assessment in Greenlandic, Polish and Ukrainian men. Occup Environ Med. 2015;72:385–393. doi: 10.1136/oemed-2014-102264. [DOI] [PubMed] [Google Scholar]
- Liu L, Bao H, Liu F, Zhang J, Shen H. Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environ Int. 2012;42:78–83. doi: 10.1016/j.envint.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl. 2009;30:287–297. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Jørgensen N, Andersson AM, Calafat AM, Ye X, Redmon JB, Drobnis EZ, Wang C, Sparks A, Thurston SW, Swan SH. Are environmental levels of bisphenol a associated with reproductive function in fertile men? Environ Health Perspect. 2010;118:1286–1291. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Jørgensen N, Andersson AM, Calafat AM, Silva MJ, Redmon JB, Sparks A, Drobnis EZ, Wang C, Liu F, Swan SH. Associations between urinary metabolites of di(2-ethylhexyl) phthalate and reproductive hormones in fertile men. Int J Androl. 2011;34:369–378. doi: 10.1111/j.1365-2605.2010.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Meeker JD, Jørgensen N, Andersson AM, Liu F, Calafat AM, Redmon JB, Drobnis EZ, Sparks AE, Wang C, Hauser R, Swan SH. Urinary concentrations of di(2-ethylhexyl) phthalate metabolites and serum reproductive hormones: pooled analysis of fertile and infertile men. J Androl. 2012;33:488–498. doi: 10.2164/jandrol.111.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet JW, Brazil C. Semen analysis. In: Lipshultz LI, Howards SS, editors. Infertility in the Male. Mosby, St. Louis, MO: 1997. pp. 487–490. [Google Scholar]
- Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res Int. 2014;21:11066–11074. doi: 10.1007/s11356-014-2986-5. [DOI] [PubMed] [Google Scholar]
- Redmon JB, Thomas W, Ma W, Drobnis EZ, Sparks A, Wang C, Brazil C, Overstreet JW, Liu F, Swan SH. Semen parameters in fertile US men: the Study for Future Families. Andrology. 2013;1:806–814. doi: 10.1111/j.2047-2927.2013.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Spade DJ, Hall SJ, Saffarini CM, Huse SM, McDonnell EV, Boekelheide K. Differential response to abiraterone acetate and di-n-butyl phthalate in an androgen-sensitive human fetal testis xenograft bioassay. Toxicol Sci. 2014;138:148–160. doi: 10.1093/toxsci/kft266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht IO, Toft G, Hougaard KS, Lindh CH, Lenters V, Jönsson BA, Heederik D, Giwercman A, Bonde JP. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ Int. 2014;66:146–156. doi: 10.1016/j.envint.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH. One semen sample or 2? Insights from a study of fertile men. J Androl. 2007;28:638–643. doi: 10.2164/jandrol.107.002741. [DOI] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RHN, Redmon JB. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015;30:963–972. doi: 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren-Webster TM, Lewis C, Filby AL, Paull GC, Santos EM. Mechanisms of toxicity of di(2-ethylhexyl) phthalate on the reproductive health of male zebrafish. Aquat Toxicol. 2010;99:360–369. doi: 10.1016/j.aquatox.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Weisberg S. Applied Linear Regression. John Wiley and Sons; Hoboken, NJ, USA: 2005. [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth JJ, Rossano MG, Potter R, Puscheck E, Daly DC, Paneth N, Krawetz SA, Protas BM, Diamond MP. A pilot study associating urinary concentrations of phthalate metabolites and semen quality. Syst Biol Reprod Med. 2008;54:143–154. doi: 10.1080/19396360802055921. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization laboratory manual for the examination of human semen and semen-cervical mucus interactions. 4th. Cambridge University Press; UK/New York: 1999. [Google Scholar]
- WHO. Biological Monitoring of Chemical Exposure in the Workplace. Vol. 1. WHO; Geneva: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


