Abstract
Background
Central adiposity is a risk factor for Barrett's esophagus (BE). Serum levels of adiponectin and leptin are deregulated in obese states and implicated as putative mediators in the pathophysiology of esophageal columnar metaplasia.
Aim
Describe associations between serum adiponectin and leptin levels with BE.
Methods
Patients were recruited prospectively for a case-control study. Fasting serum levels of adiponectin and leptin were measured in 135 patients with BE and compared with two separate control groups: 133 subjects with gastroesophageal reflux disease (GERD) and 1157 colon screening controls.
Results
Multivariate analyses adjusted for age, race and waist to hip ratio show that patients in the highest tertile of serum adiponectin had decreased odds of BE compared with screening colonoscopy controls (odds ratio (OR)=0.42, 95% Confidence Interval (CI)=0.22, 0.80)). This effect was more pronounced in males (OR=0.35, 95% CI=0.17, 0.74) compared to females (OR=0.71, 95%CI=0.17, 3.03).
In comparisons of BE cases to GERD controls, subjects in the highest tertile of serum adiponectin showed decreased odds of BE (OR=0.65, 95% CI 0.31, 1.36), however, this was not statistically significant. Patients in the highest tertile of serum leptin did not have significantly increased risk of BE in comparison to GERD (OR=1.32, 95%CI 0.61, 2.88) or screening colonoscopy controls (OR=1.57, 95%CI=0.82, 3.04) in analyses including both sexes. Based on sex specific analyses, sex did not significantly alter association of leptin with odds of BE.
Conclusions
Serum adiponectin was inversely associated with BE and this effect was more pronounced in males, whereas serum leptin showed no evidence of association with BE in comparisons with multiple control groups. The exact mechanism, if any, by which these adipokines promote metaplasia in the esophagus needs to be further explored.
Keywords: adipokine, esophagus, epidemiology
Introduction
Obesity is a strong risk factor for Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC)[1-4]. Being obese increases the odds of esophageal cancer approximately 1.5 fold in both sexes[5]. Obesity is now well recognized as a state of low grade inflammation, and adipocyte dysfunction with increased secretion of inflammatory cytokines and adipokines has been associated with esophageal cancer and other cancers.
Leptin is an important hormone produced by adipocytes and its circulating levels are proportional to total amount of fat mass[6]. A variety of factors affect serum leptin levels including short term fasting, sleep deprivation, level of stress, degree of tissue inflammation, medications and physical exercise[7]. Obese individuals have markedly elevated levels of circulating leptin and they exhibit leptin resistance. Leptin binds the leptin receptor at the cell surface and the signal is transduced intracellularly through the Janus kinase 2 (Jak2)/Signal transducer and activator of transcription 3 (STAT3) pathway[8]. Once STAT3 is activated by phosphorylation, it is able to translocate itself to the nucleus where it can regulate gene expression[9, 10]. At the molecular level, leptin is pro-angiogenic, pro-inflammatory and mitogenic. Adiponectin is a protein encoded by the ADIPOQ gene and also secreted by the white adipose tissue. It circulates in three multimeric forms: low molecular weight (LMW), middle molecular weight (MMW) and high molecular weight (HMW) adiponectin. The adiponectin protein contains four major domains and the structure of its globular region closely resembles that of Tissue Necrosis Factor-α. Adiponectin transmits its signal through AdipoR1 and AdipoR2 receptors by threonine mediated activation of 5’adenosine monophosphate-activated protein kinase as well as peroxisome proliferator-activated receptor alpha mediated signaling[11, 12]. Adiponectin plays an important role in glucose flux and energy metabolism, upregulation of uncoupling proteins, and protection from endothelial dysfunction[13].
Studies on serum leptin and tissue leptin receptor expression have shown positive associations with BE[14, 15]. Levels of serum total adiponectin have either shown no evidence of association with BE[14], or demonstrated a protective effect for LMW adiponectin[16]. Other studies have suggested that the effect of adipokines on esophageal metaplasia may be sex specific[17]. We sought to further clarify these relationships in a case-control study comparing patients with BE to GERD controls and population controls. We hypothesized that serum leptin levels will be positively associated with increased risk of BE whereas increasing serum adiponectin will show an inverse association.
Methods
Study population
Patients were recruited from University Hospitals Case Medical Center and Cleveland Clinic Foundation between January 2005 and May 2009. Potential participants were recruited at the time of their endoscopy visit scheduled for management of refractory reflux, BE surveillance or colorectal cancer screening. The majority of BE cases were referred from outside providers. Criteria for recruitment of BE cases and both control groups have been described elsewhere[18]. Patients in the GERD control group were recruited from subjects with refractory GERD symptoms which were defined as report of persistent reflux not relieved by adequate dose of proton pump inhibitor warranting further endoscopic evaluation. Diagnosis of GERD was established based on the assessment of patient's treating physician. pH probe testing was not used to establish diagnosis of pathologic reflux. Patients in the screening colonoscopy group did not undergo routine upper endoscopy. Patients could not be included in the screening colonoscopy group if they had polyps on prior endoscopic exams, history of inflammatory bowel disease or colorectal cancer. In brief, we enrolled 135 patients with BE, 133 patients with GERD but no endoscopic or histological evidence of BE, and 1157 screening colonoscopy controls. This sample size was calculated based on assumption that at least 30% of cases will have elevated levels of serum adipokines, BMI, waist to hip ratio and other risk factors relevant to pathophysiology of BE and our goal to identify risk factors that increased odds of BE 2-4 fold. The study was approved by the Institutional Review Board of the Case Comprehensive Cancer Center and written informed consent was obtained on all participants.
Serum adiponectin and leptin
Fasting blood samples were taken from each subject prior to the endoscopic procedure and placed on ice for processing. Grossly lipemic and icteric specimen were excluded. Samples were centrifuged under refrigeration and separated serum was aliquoted into cryovials and stored at −70C. Serum leptin and total adiponectin concentrations were measured by enzyme-linked immunosorbent assay (Linco, St Charles, MO, USA) according to the manufacturer's instructions. All measurements of serum adipokine levels were performed in the Dahms Clinical Research Unit Laboratory of the Case Western Reserve University.
Statistical methods
Simple descriptive statistics were performed to describe the frequencies of risk factors among cases and controls. Univariate logistic regressions were completed to calculate ORs and the associated 95% CIs on all variables of interest. Multivariate regression analyses were conducted in order to adjust for baseline differences among study groups. OR estimates were adjusted for age, sex (male vs. female), race (white vs. non-white), and central adiposity (waist-to-hip ratio). For variables that were split into tertiles, ORs and the associated 95% CIs were calculated by comparing highest tertiles to the lowest. Cutoff points for tertile analysis of adiponectin and leptin were first determined separately for each sex for the entire study group. These sex-specific tertiles were then also used in the subsequent analysis of BE cases with screening colonoscopy as well as GERD controls. Multivariate sex specific regression models were adjusted for age, race, and waist to hip ratio. The Cochran Armitage test was applied in the analysis of trends. All tests of statistical significance were two-sided and p values less than 0.05 were considered significant. Statistical analyses were performed in Statistical Analysis Systems software package 9.3 (Cary, NC).
Results
Baseline characteristics
Cases with BE were older than subjects in both control groups. There were no significant differences in BMI between cases and both control groups. Waist to hip ratio of BE cases was significantly higher than that of screening colonoscopy controls. More female participants were recruited into both control groups compared to the BE case group. A higher proportion of non-white individuals were included among screening colonoscopy controls (see Table 1).
Table 1.
Demographic Variables by Study Group
| BE Cases | GERD Controls | Screening Colonoscopy Controls | p* | p** | |
|---|---|---|---|---|---|
| Age (mean, SD) | 63.7 (11.2) | 56.4 (11.1) | 54.6 (8.8) | <0.01 | <0.01 |
| Waist-to-hip ratio (mean, SD) | 0.98 (0.06) | 0.97 (0.07) | 0.91 (0.09) | 0.09 | <0.01 |
| Body Mass Index (mean, SD) | 30.8 (5.7) | 29.5 (5.6) | 29.3 (6.9) | 0.09 | 0.03 |
| Gender (% female) | 20.3% | 40.0% | 65.3% | <0.01 | <0.01 |
| Race | <0.01 | <0.01 | |||
| Caucasian | 93.23% | 82.96% | 61.28% | ||
| Other | 6.77% | 17.04% | 38.72% | ||
p-value of difference between BE and GERD controls
p-value of difference between BE and colonoscopy controls
Total Adiponectin and risk of BE
Mean total adiponectin levels were not significantly different between BE cases and GERD controls [8.39 μg/mL (SD 4.18) vs. 8.82 μg/mL (SD 5.07), p=0.45]. Mean levels of serum adiponectin were statistically significantly lower in BE cases compared to screening colonoscopy controls [8.39 (SD 4.18) vs. 10.36 (SD 6.25) μg/mL, p=<0.001.
In tertile analysis of total adiponectin among BE cases and GERD controls, subjects in the highest tertile of serum adiponectin levels showed no statistically significant association with BE case status (ORa=0.65 (95% CI=0.31, 1.36)). When BE cases were compared to screening colonoscopy controls, subjects in the highest tertile of serum adiponectin did show a significantly decreased odds of BE (ORa=0.42 (95% CI=0.22, 0.80)). Details of these analyses are summarized in Table 2. Effects of sex on risk of BE in relation to serum adiponectin were considered. Association between adiponectin and BE in comparisons with screening colonoscopy group was statistically significant in males but not in females (Table 3). Including the interaction term of sex and adiponectin in modeling odds of BE was not statistically significant (p=0.90). Adiponectin showed an inverse association with BE case status and the strength of this association increased with rising serum levels of adiponectin suggesting a dose response relationship. This held true in sex specific analysis of BE cases with GERD controls in males but not among female participants (Table 4).
Table 2.
Tertiles of Adiponectin and Leptin for BE cases compared to GERD Controls and Screening Colonoscopy Controls with adjustment for age, gender, race, and waist to hip ratio.
| Tertiles | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | p (trend) | |
|
BE cases vs. Colonoscopy controls
| ||||
| Adiponectin | ||||
| Range | ||||
| Men | <5.33 | 5.33-8.75 | >8.75 | |
| Women | <7.62 | 7.62-12.92 | >12.92 | |
| Cases | 36 | 52 | 45 | |
| Controls | 386 | 372 | 399 | |
| ORc95%CI)† | 1.0 (referent) | 1.49 (0.95, 2.35) | 1.20 (0.76, 1.92) | 0.45 |
| ORa (95%CI)‡ | 1.0 (referent) | 0.98 (0.55, 1.74) | 0.42 (0.22, 0.80) | 0.006 |
| Leptin | ||||
| Range | ||||
| Men | <5 | 5-12.24 | >12.24 | |
| Women | <13.65 | 13.65-30.72 | >30.72 | |
| Cases | 20 | 63 | 50 | |
| Controls | 408 | 366 | 383 | |
| ORc (95% CI) | 1.0 | 3.51 (2.08, 5.92) | 2.66 (1.55, 4.55) | 0.001 |
| ORa (95%CI) | 1.0 | 2.70 (1.48,4.94) | 1.57 (0.82, 3.04) | 0.22 |
|
BE cases vs. GERD controls | ||||
| Adiponectin | ||||
| Range | ||||
| Men | <5.33 | 5.33-8.75 | >8.75 | |
| Women | <7.62 | 7.62-12.92 | >12.92 | |
| Cases | 36 | 52 | 45 | |
| Controls | 48 | 46 | 41 | |
| ORc (95%CI) | 1.0 (referent) | 1.50 (0.84, 2.71) | 1.46 (0.80, 2.67) | 0.22 |
| ORa (95% CI) | 1.0 (referent) | 1.39 (0.70, 2.76) | 0.65 (0.31, 1.36) | 0.29 |
| Leptin | ||||
| Range | ||||
| Men | <5 | 5-12.24 | >12.24 | |
| Women | <13.65 | 13.65-30.72 | >30.72 | |
| Cases | 20 | 63 | 50 | |
| Controls | 39 | 46 | 50 | |
| ORc (95% CI) | 1.0 (referent) | 2.67(1.38, 5.16) | 1.94 (1.00, 3.79) | 0.12 |
| ORa (95% CI) | 1.0 (referent) | 2.72 (1.28, 5.74) | 1.32 (0.61, 2.88) | 0.66 |
ORc = crude odds ratio
ORa = adjusted odds ratio
Table 3.
Tertiles of Adiponectin and Leptin for BE cases compared to screening colonoscopy controls presented for each gender separately.
| Tertiles | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | p (trend) | |
|
Men (n=498)
| ||||
| Adiponectin | ||||
| Range | ||||
| Men | <5.33 | 5.33-8.75 | >8.75 | |
| Cases | 32 | 34 | 40 | |
| Controls | 136 | 131 | 125 | |
| ORc95%CI) | 1.0 (referent) | 1.10 (0.64, 1.89) | 1.36 (0.81, 2.30) | 0.25 |
| ORa (95%CI) | 1.0 (referent) | 0.70 (0.36, 1.38) | 0.35 (0.17, 0.74) | 0.005 |
| Leptin | ||||
| Range | ||||
| Men | <5 | 5-12.24 | >12.24 | |
| Cases | 18 | 45 | 43 | |
| Controls | 145 | 120 | 127 | |
| ORc (95% CI) | 1.0 | 3.02 (1.66, 5.49) | 2.72 (1.50, 4.97) | 0.001 |
| ORa (95%CI) | 1.0 | 2.21 (1.11, 4.40) | 1.26 (0.61, 2.64) | 0.62 |
|
Women (n=792) | ||||
| Adiponectin | ||||
| Range | ||||
| Women | <7.62 | 7.62-12.92 | >12.92 | |
| Cases | 4 | 18 | 5 | |
| Controls | 250 | 241 | 274 | |
| ORc (95%CI) | 1.0 (referent) | 4.66 (1.55, 14.0) | 1.14 (0.30, 4.29) | 0.97 |
| ORa (95% CI) | 1.0 (referent) | 2.56 (0.74, 8.85) | 0.71 (0.17, 3.03) | 0.43 |
| Leptin | ||||
| Range | ||||
| Women | <13.65 | 13.65-30.72 | >30.72 | |
| Cases | 2 | 19 | 9 | |
| Controls | 259 | 245 | 261 | |
| ORc (95% CI) | 1.0 (referent) | 8.45 (1.92, 37.2) | 4.46 (0.96, 20.86) | 0.11 |
| ORa (95% CI) | 1.0 (referent) | 6.44 (1.34, 31.1) | 5.21 (1.03, 26.18) | 0.05 |
†ORc = crude odds ratio
‡ORa = adjusted odds ratio
Table 4.
Tertiles of Adiponectin and Leptin for BE cases compared to GERD controls presented for each gender separately.
| Tertiles | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | p (trend) | |
|
Men (n=187)
| ||||
| Adiponectin | ||||
| Range | ||||
| Men | <5.33 | 5.33-8.75 | >8.75 | |
| Cases | 34 | 34 | 38 | |
| Controls | 27 | 28 | 26 | |
| ORc95%CI) | 1.0 (referent) | 0.93 (0.45, 1.97) | 0.90 (0.44, 1.82) | 0.76 |
| ORa (95%CI) | 1.0 (referent) | 0.92 (0.40, 2.10) | 0.45 (0.19, 1.07) | 0.07 |
| Leptin | ||||
| Range | ||||
| Men | <5 | 5-12.24 | >12.24 | |
| Cases | 18 | 47 | 41 | |
| Controls | 25 | 23 | 33 | |
| ORc (95% CI) | 1.0 | 2.84 (1.30, 6.22) | 1.72 (0.81, 3.69) | 0.30 |
| ORa (95%CI) | 1.0 | 2.18 (0.90, 5.26) | 0.88 (0.35, 2.21) | 0.58 |
|
Women (n=81) | ||||
| Adiponectin | ||||
| Range | ||||
| Women | <7.62 | 7.62-12.92 | >12.92 | |
| Cases | 3 | 9 | 15 | |
| Controls | 23 | 12 | 19 | |
| ORc (95%CI) | 1.0 (referent) | 5.62 (1.63, 19.3) | 3.47 (0.75, 15.9) | 0.04 |
| ORa (95% CI) | 1.0 (referent) | 3.89 (0.96, 15.7) | 1.93 (0.37, 9.97) | 0.34 |
| Leptin | ||||
| Range | ||||
| Women | <13.65 | 13.65-30.72 | >30.72 | |
| Cases | 2 | 16 | 9 | |
| Controls | 14 | 23 | 17 | |
| ORc (95% CI) | 1.0 (referent) | 4.87 (0.97, 24.4) | 3.70 (0.69, 20.02) | 0.22 |
| ORa (95% CI) | 1.0 (referent) | 5.76 (0.95, 35.0) | 4.42 (0.71, 27.43) | 0.17 |
†ORc = crude odds ratio
‡ORa = adjusted odds ratio
Leptin and risk of BE
Mean levels of serum leptin were lowest among BE cases and highest in screening colonoscopy controls [17.8 (SD 14.7) vs. 21.68 (SD 21.07) ng/mL, p=0.007].
In tertile comparisons of serum leptin among BE cases and GERD controls, subjects in the highest tertile of leptin did not show increased odds of BE (ORa=1.32, 95% CI=0.61, 2.88). In comparisons of BE cases with screening colonoscopy controls, subjects in the highest tertile of serum leptin did not have increased odds of BE (ORa=1.57, 95%CI=0.81, 3.04).
Sex specific effects of leptin were further explored. Serum leptin did not show significant association with BE case status in either sex in comparisons with screening colonoscopy controls of GERD controls (Tables 3 and 4). There was a trend toward positive association of serum leptin and BE case status in females in analysis with screening colonoscopy controls and males in comparisons with GERD controls, however, this association was not statistically significant. The number of BE cases for this analysis was small questioning the validity of this analysis. The p values reported in tables 3 and 4 correspond to the test for linear trend when models are adjusted for age, sex, and race. Changing the models further to include an interaction term for gender and leptin did not show a significant association with presence of BE (p=0.09).
DISCUSSION
To understand the putative role of central obesity and its associated hormonal derangements we assessed the relationships of two serum adipokines with BE compared to two control groups. Consistent with our hypothesis, rising serum adiponectin showed an inverse association with BE and this effect was more pronounced in males. The observed associations of adiponectin with BE remained significant after adjustment of potential confounders such as age, sex, race and waist to hip ratio suggesting that this adipokine is strongly associated with BE and may play a biological role in the pathogenesis of esophageal columnar metaplasia. Contrary to our hypothesis, rising levels of serum leptin did not increase the odds of BE.
The protective effect of adiponectin observed in this study is biologically plausible and consistent with the findings of several other epidemiological[19-21] and molecular studies[22]. The role of adiponectin as an anti-proliferative factor has been recently studied in OE33 ECA cell lines. Adiponectin reduces leptin stimulated JAK2 activation and STAT3 transcriptional activity and increases protein-tyrosine phosphatase 1B (PTP1B) protein expression and its activity. Activation of PTP1B by adiponectin appears to downregulate leptin induced signaling and its pro-carcinogenic potential[23]. Increased adiponectin receptor expression and higher leptin receptor protein levels have been measured in areas of intestinal metaplasia vs. that of normal esophagus[24]. These findings emphasize the interplay of both adiponectin and leptin as regulators of cellular proliferation.
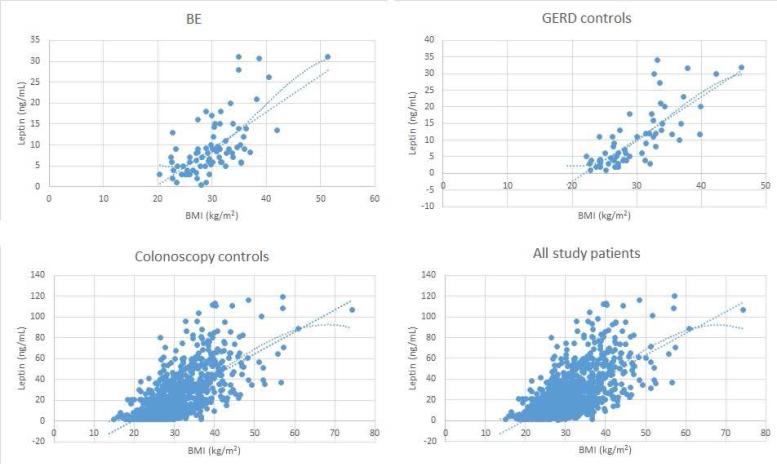
The lack of significant association of serum leptin with BE observed here was contrary to some reports published to date. This negative finding may reflect differences in case and control populations among various studies examining this subject. Recent analysis by Garcia et al[14] demonstrated that cases in the highest quintile of serum leptin had an 8 fold increased odds of BE (OR=8.02, 95% CI 2.79-23.07). In that study, analyses of leptin were also adjusted for medication use (PPI, NSAIDs) as well as Helicobacter pylori status. Rubenstein et al. described that leptin increased odds of BE approximately three fold in the highest tertile (OR= 3.25, 95% CI 1.29, 8.17)[25]. Both of these reports were from Veterans Affairs Medical Centers and the study populations were almost exclusively males. However, similarly to findings of this study, Thompson et al [17] found that males in the highest tertile of leptin had no increased risk of BE. The Seattle study was conducted among non-Veterans and the demographics of their study group may be more representative of the general U.S. population and similar to those subjects enrolled in our study possibly yielding similar estimates of effect. It is also important to note that in our study serum leptin appeared to have strong positive association with BE in second but not third tertiles in comparisons with both colonoscopy and GERD controls. This was also true for sex specific analyses. In fact, analysis of BE and screening colonoscopy controls showed that in the second tertile the odds of BE were increased in males 2-3 fold (ORc=3.02, 95% CI 1.66, 5.49; ORa=2.21, 95% 1.11, 4.40)) and for females they were increased six to eight fold (ORc=8.45, 95% CI 1.92, 37.2; ORa=6.44, 95% CI 1.34, 31.1); estimates quite similar to those published in other studies[14, 16]. The overall trend of these associations may represent a ceiling effect where distribution of the leptin is skewed. In our further analysis of association of leptin with BMI, leptin levels appeared to plateau at the highest levels of BMI (Figure 1). If indeed the association of Leptin and BMI is non-linear, it provides a possible explanation to why odds ratios peaked in the second tertile and then appeared to decline in the third tertile. An observation like this could lead us to an erroneous conclusion that there is no association of leptin and BE case status when in fact there is.
Figure 1.
Distribution of serum leptin in each study group and the entire study cohort. Two trend lines included are the linear trend line and polynomial trend line with 3 degrees of freedom.
Males have a higher distribution of metabolically active central fat compared to females and this may partly explain the higher prevalence of BE in males. Thus, our study as well as others[17] have performed subgroup analyses to explore sex specific associations of adipokines with BE. We did find that the protective effect of adiponectin was present in males but not in females, whereas there was no evidence of association with leptin in either subgroup. The number of females with BE in our study was small (n=27), leading to imprecise estimates of effect for both adipokines in this subgroup. In the only other study that included greater number of women[17] the authors did find a positive association between leptin and BE among females but did not find an association in males.
There are a few limitations to our study. A case-control design is susceptible to selection bias. This was partially controlled here by the prospective nature of this study. Selection bias was further minimized by recruitment of cases and both control groups from a similar target population. The BE case group included a small number of females, which did not allow for thorough examination of how sex mediated associations of interest. We were also unable to control for certain confounders that may contribute to risk of BE such as tobacco use, Helicobacter pylori status, dietary habits, presence of metabolic syndrome, diabetes and level of physical activity. Finally, the choice of our control groups could have introduced misclassification. Not all screening colonoscopy cases had an upper endoscopy performed and it is possible that there were undiagnosed BE cases among these controls. Prior studies have indicated that in individuals over 65 years of age, BE may be present in 16.7% of cases with 14.9% of those being asymptomatic[26]. However, misclassification would have attenuated our estimates only slightly given overall low prevalence of BE.
In summary, this study demonstrates that adiponectin has an inverse association with BE case status. The association was statistically significant in men when compared to colonoscopy controls but not GERD controls. Given that our results regarding the magnitude of associations for serum adiponectin and leptin differ from other studies, performing a high quality metanalysis would be useful in order to obtain a better understanding of the relationship between these serum adipokines and BE case status. Further molecular and translational research will be needed to understand the specific role of adipokine signaling in BE. Future research efforts will need to determine whether interception of adipokine signaling pathways by upregulation of signaling or receptor activity could provide a therapeutic means of halting the metaplasia-dysplasia-carcinoma sequence or novel treatment targets for management of EAC.
Acknowledgments
Grant support: This work was supported by the U.S. Public Health Service research grants R21 CA135692, U54 CA163060, and P50 CA150964 from the National Cancer Institute.
Abbreviations
- BE
Barrett's esophagus
- GERD
gastroesophageal reflux disease
- OR
odds ratio
- CI
confidence interval
- EAC
esophageal adenocarcinoma
- Jak 2
Janus kinase 2
- STAT3
Signal transducer and activator of transcription 3
- LMW
low molecular weight
- MMW
middle molecular weight
- HMW
high molecular weight
- PTP1B
protein-tyrosine phosphatase 1B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Authors have no conflict of interest to disclose relevant to this work.
Author contributions: Dr. Katarina B. Greer collected data, performed statistical analyses and is the primary author on this manuscript. Dr. Amitabh Chak was responsible for study concept and analysis. All authors participated in the preparation of the manuscript.
CONFLICT OF INTEREST: None
Guarantor of the article: A. Chak, MD
We would also like to express special appreciation to Mrs. Karen Stear from Dahms Clinical Research Unit for help in organization and processing of blood samples.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19(6):1468–70. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett's esophagus. Am J Gastroenterol. 2008;103(2):292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 7.Janeckova R. The role of leptin in human physiology and pathophysiology. Physiol Res. 2001;50(5):443–59. [PubMed] [Google Scholar]
- 8.Clemons N, Phillips W, Lord RV. Signaling pathways in the molecular pathogenesis of adenocarcinomas of the esophagus and gastresophageal junction. Cancer Biol Ther. 2013;14(9) doi: 10.4161/cbt.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks AS, Davis SM, Bates SH, et al. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275(19):14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 10.Bates SH, Stearns WH, Dundon TA, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 13.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148(3):293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JM, Splenser AE, Kramer J, et al. Circulating Inflammatory Cytokines and Adipokines Are Associated With Increased Risk of Barrett's Esophagus: A Case-Control Study. Clin Gastroenterol Hepatol. 2014;12(2):229–238. e3. doi: 10.1016/j.cgh.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francois F, Rope J, Goodman AJ, et al. The association of gastric leptin with oesophageal inflammation and metaplasia. Gut. 2008;57(1):16–24. doi: 10.1136/gut.2007.131672. [DOI] [PubMed] [Google Scholar]
- 16.Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett's oesophagus. Gut. 2009;58(12):1583–9. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett's esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010;18(11):2204–11. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer KB, Kresak A, Bednarchik B, et al. Association of insulin and insulin-like growth factors with Barrett's oesophagus. Gut. 2012;61(5):665–72. doi: 10.1136/gutjnl-2011-300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokrowiecka A, Daniel P, Jasinska A, et al. Serum adiponectin, resistin, leptin concentration and central adiposity parameters in Barrett's esophagus patients with and without intestinal metaplasia in comparison to healthy controls and patients with GERD. Hepatogastroenterology. 2012;59(120):2395–9. doi: 10.5754/hge12587. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AM, Healy LA, Power DG, et al. Barrett esophagus: prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann Surg. 2008;247(6):909–15. doi: 10.1097/SLA.0b013e3181612cac. [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein JH, Dahlkemper A, Kao JY, et al. A pilot study of the association of low plasma adiponectin and Barrett's esophagus. Am J Gastroenterol. 2008;103(6):1358–64. doi: 10.1111/j.1572-0241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 22.Konturek PC, Burnat G, Rau T, et al. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig Dis Sci. 2008;53(3):597–605. doi: 10.1007/s10620-007-9922-1. [DOI] [PubMed] [Google Scholar]
- 23.Beales IL, Garcia-Morales C, Ogunwobi OO, et al. Adiponectin inhibits leptin-induced oncogenic signalling in oesophageal cancer cells by activation of PTP1B. Mol Cell Endocrinol. 2014;382(1):150–8. doi: 10.1016/j.mce.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Mokrowiecka A, Sokolowska M, Luczak E, et al. Adiponectin and leptin receptors expression in Barrett's esophagus and normal squamous epithelium in relation to central obesity status. J Physiol Pharmacol. 2013;64(2):193–9. [PubMed] [Google Scholar]
- 25.Rubenstein JH, Morgenstern H, McConnel D, et al. Associations of diabetes mellitus, insulin, leptin, and ghrelin with gastroesophageal reflux and Barrett's esophagus. Gastroenterology. 2013;145(6):1237–44. e1–5. doi: 10.1053/j.gastro.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward EM, Wolfsen HC, Achem SR, et al. Barrett's esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol. 2006;101(1):12–7. doi: 10.1111/j.1572-0241.2006.00379.x. [DOI] [PubMed] [Google Scholar]