Abstract
Cervical spondylosis is the most common cause of nontraumatic spinal cord injury and is the most common cause of spinal cord dysfunction in the elderly. Magnetic resonance imaging (MRI) is an invaluable tool for the diagnosis and assessment of cervical spondylosis due to its sensitivity to soft tissues; however, standard MR techniques have some limitations in predicting neurological impairment and response to intervention. Therefore, there is great interest in novel MR techniques including diffusion tensor imaging (DTI) and MR spectroscopy (MRS) as imaging biomarkers for neurological impairment and tools for understanding spinal cord physiology. This review outlines the pathogenesis of cervical spondylotic myelopathy (CSM), the correlative abnormalities observed on standard MRI, the biological implications and current status of DTI and MRS as clinical tools, and future directions of MR technology in the management of CSM patients.
Keywords: cervical, spinal cord, DTI, MRI, myelopathy
Introduction
Since its advent, Magnetic Resonance Imaging (MRI) has played an indispensible role in the management of patients with Cervical Spondylotic Myelopathy (CSM). There has been tremendous advancement in MR technology over the past several decades, resulting in enhanced resolution and image quality that is manifold greater than previous generations. As the image quality has increased, the application of MRI to CSM has progressed in parallel, evolving from purely a diagnostic modality to a non-invasive tool that can be used to potentially predict neurological outcome and response to intervention.
In addition to conventional MRI, recent application of novel imaging techniques such as diffusion tensor imaging (DTI) [1–5] and magnetic resonance spectroscopy (MRS) [6] to CSM further highlights the potential impact of MR technology on this debilitating disease process. By providing pertinent information regarding the spinal cord microstructure and metabolism, these novel techniques provide increased sensitivity to the spinal cord injury and derangement of cellular function that ubiquitously occurs during CSM pathogenesis.
This manuscript seeks to examine the application of MR technology to the management of CSM patients. Recent and future advances in both conventional and novel MR techniques will be discussed.
CSM Pathophysiology
In order to fully appreciate the importance of MRI in the management of CSM, as well as the significance of its radiographical findings, an understanding of the complex CSM pathophysiology is necessary. CSM is caused by progressive degenerative vertebral column abnormalities that result in spinal cord damage related to both primary mechanical and secondary biological injury. Primary mechanical spinal cord injury can be initiated by static and dynamic forces including compression, distraction, and shear [7] (Fig. 1). Direct spinal cord compression is the most frequently encountered mechanism, and it has been theorized that myelopathic symptoms appear after the spinal cord has been reduced in size by 30% or to a transverse area of less than 60 square millimeters [8]. Secondary spinal cord injury in CSM is likely related to a variety of mechanisms such as glutamergic toxicity, free radical-mediated cell injury, cationic-mediated cell injury, and apoptosis [9–11]. Ischemia is considered to be a significant contributor to the pathophysiology of CSM. Ventral compression impairs perfusion via the transverse arterioles originiating from the anterior sulcal arteries, whereas posterior vascular compromise can occur in the intramedullary branches of the central grey matter [12].
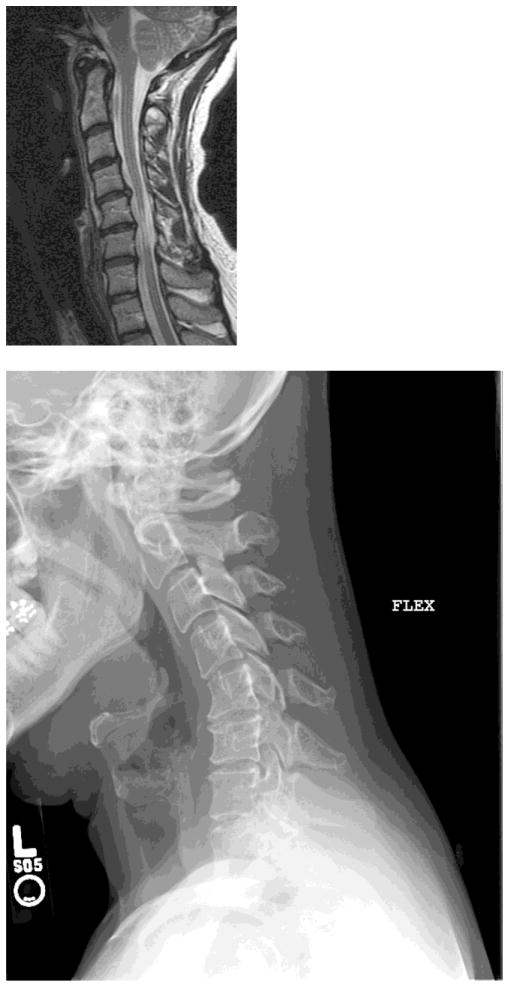
Figure 1. Cervical Spondylotic Myelopathy.
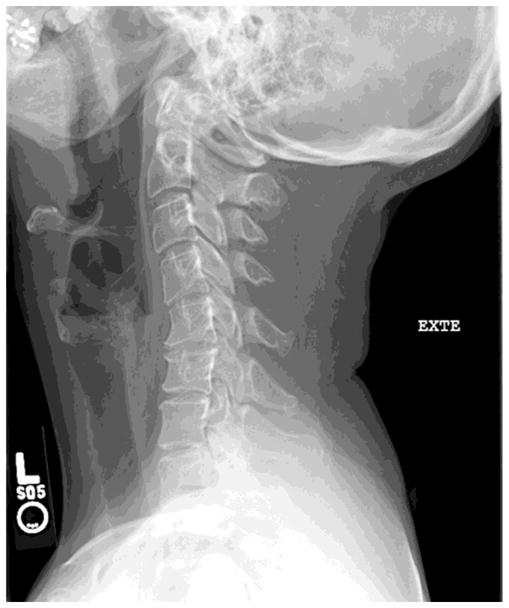
A) Cervical spine MRI of a 45 year old woman with CSM demonstrating extensive spinal cord atrophy and T2 weighted signal change at the C5 and C6 levels despite the absence of frank spinal cord compression. This spinal cord damage was caused by repetitive shear injury across the ventral disc bulges during flexion (B) and extension (C).
Conventional MRI
Prior to the development of MRI, computed tomography (CT) with or without myelography was the mainstay of the radiographical evaluation of CSM patients. Although these modalities offered useful anatomical information regarding the osseous vertebral column and degree of spinal canal stenosis, only limited information could be directly ascertained about the condition of the spinal cord. As such, there were definite limitations in the ability for imaging to adequately assess spinal cord damage in this patient population.
Through its ability to provide high resolution imaging of soft tissue anatomy, MRI bridged this knowledge gap, providing excellent anatomical detail of the spinal cord macrostructure. MRI has granted insight into the aforementioned structural histopathological changes that occur during CSM pathogenesis. The elucidation of T2 and/or T1 weighted signal change by MRI technology has spawned a plethora of clinical research studies that have intensively investigated their significance. These findings are attributed to myelomalacia, edema, gliosis, and ischemic white matter changes. Some authors suggested these observations result in irreversible spinal cord injury [13], whereas others feel that these regions represent a wide spectrum of recuperative potential [14]. There have even been several grading systems proposed to classify the spinal cord signal change subtypes [15, 16]; however, this remains one of the most controversial and actively debated topics in the area of degenerative spine disease.
There is great interest in assessing the utility of MRI to predict neurological outcome following decompression surgery for CSM. The reasons for this are many. Operative management may result in clinical improvement, worsened clinical function, or preservation of function without clear improvement or decline. Patients and their families commonly inquire about whether surgery will alleviate their symptoms and/or return lost neurological function. Surgery can involve technically challenging decompression and stabilization procedures, and the complication rate is not insignificant, particularly in the elderly. This, combined with the fact that a subset of CSM patients are likely to stabilize clinically without surgery [17], increases the importance of prospectively determining which patients are most likely to benefit from surgical intervention.
An expert group representing the Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and Congress of Neurological Surgeons published the Guidelines for the Surgical Management of Cervical Degenerative Disease in 2009 [18]. One of the highlights of this seminal work was their findings regarding the relationship between spinal cord signal change and clinical outcome in CSM patients. Based on their thorough and exhaustive literature review, they concluded that multilevel T2 hyperintensity, T1 focal hypointensity combined with T2 focal hyperintensity, and spinal cord atrophy each convey a poor prognosis following surgical intervention.
In addition to these factors, there is also some evidence that regression of T2 signal change following surgical intervention is associated with a better prognosis [19, 20]. From a theoretical standpoint this makes sense, as this phenomenon implies a healing of the injured spinal cord. This concept is at least in part supported by histopathological studies in which denuded axons and numerous thin myelinated fibers have been found in the damaged spinal cord segments in patients with CSM, suggesting that focal demyelination and remyelination can occur within the white matter [21]. It has been postulated that this process may be an important mechanism in the recovery of lost neurological function following therapeutic management of this disorder.
Diffusion Tensor Imaging (DTI)
Diffusion-weighted imaging (DWI) is a magnetic resonance imaging (MRI) technique that can be used to measure microscopic random movement of water molecules (i.e. diffusion). Specifically, the same magnetic field gradients used to create the MR images can be implemented in such a way that water molecules are “tagged” (or “dephased”) at one time point, then “untagged” (or “rephased”) at a slightly later time point (Fig. 2A). The random movement of water molecules during the time of tagging and untagging, termed the “diffusion time”, results in signal attenuation in the MR image that is proportional to the apparent diffusion coefficient (ADC), a measure of water diffusion magnitude or simply the “rate” of water diffusion. In other words, the faster water molecules are diffusing within a tissue the larger the signal attenuation in DWIs, and the larger the ADC. Hence, tissues with high water mobility and few boundaries to water motion, such as regions containing cerebrospinal fluid (CSF) and vasogenic edema, have high ADC values whereas tissues with a high degree of complexity and boundaries to diffusion, such as white matter fiber bundles or tumors, have a relatively lower ADC (Fig. 2B). It is the sensitivity to microstructural complexity below the resolution of traditional MRI techniques that makes diffusion MRI particularly attractive for interrogating the health of the spinal cord in patients with cervical myelopathy.
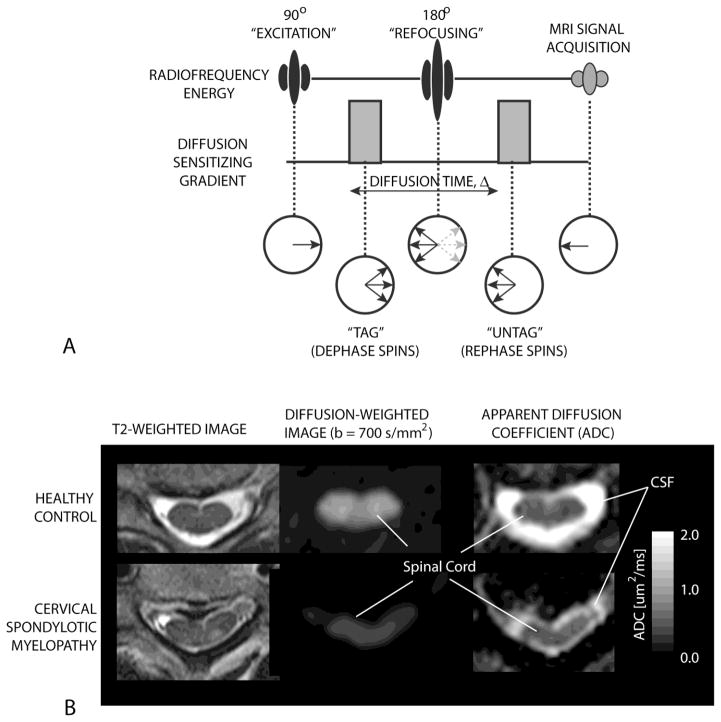
Figure 2. Diffusion weighted imaging (DWI) of the spinal cord.
A) The pulse sequence diagram (top) and water hydrogen spin dynamics (bottom) during a one-dimensional DI experiment. The use of pulsed “diffusion sensitizing” magnetic field gradients tags the water hydrogens by dephasing them at one time point and then untags them by rephrasing them at a later time point. Water hydrogens that are stationary will completely rephase and lead to maximum signal intensity on DWIs. Water hydrogens that are moving (i.e. diffusion) will have incomplete rephasing resulting in loss of signal amplitude by S=S0 e−bADC, where S is the MRI signal amplitude (image intensity) with diffusion weighting, S0 is the MRI signal amplitude (image intensity) without diffusion weighting, b is the “b-value” or the level of diffusion weighting (dependent on the gradient amplitude, duration, etc.), and ADC is the apparent diffusion coefficient.
B) Example T2-weighted (left), diffusion weighted (middle), and ADC maps (right) for a healthy control volunteer (top) and patient with cervical spondylotic myelopathy (bottom). Note that at the site of chronic compression the signal intensity on DWIs is decreased and ADC is increased relative to the normal spinal cord.
The standard DWI experiment involves measuring the ADC in a single direction (e.g. from left-to-right or from anterior-to-posterior), or averaging ADC measured in three orthogonal directions. In tissues with a preferred orientation, such as muscle or nerves where fibers are oriented in a similar direction, ADC varies according to the direction of measurement. In the central nervous system (CNS), diffusion measurements perpendicular or transverse to the direction of white matter tracts is lower than diffusion measurements parallel to the orientation of white matter tracts, partially due to a larger number of boundaries to diffusion in the transverse orientation including myelin, the axon membrane, neurofilaments, and microtubules (Fig. 3). Additionally, extracellular water is also confined to diffusing parallel to the orientation of the fiber bundles. In order to describe the diffusion characteristics of directionally dependent tissues, diffusion tensor imaging (DTI) was developed. DTI is a diffusion MRI technique that can be used to quantify the magnitude and direction of water diffusion with respect to a pre-defined coordinate system [22, 23] (Table 1). More specifically, if diffusion MRI measurements are made in various directions (i.e. more than 6 directions), then a mathematical “diffusion tensor” can be constructed, which consists of a diffusion matrix defining the tensor field of ADC values. The preferred magnitude and direction of diffusion are then found from decomposing the diffusion tensor into its eigenvalues and eigenvectors, respectively. The largest eigenvalue, termed the “primary eigenvalue”, “longitudinal ADC”, lADC, λ1, or λ||, forms the long axis of a “diffusion ellipsoid” used to model the diffusivity and is typically oriented parallel to the direction of white matter fibers (Fig. 3). The two smallest eigenvalues, termed the “secondary and tertiary eigenvalues”, “transverse ADC”, tADC, or λ⊥ form the short axes of the “diffusion ellipsoid” and are typically oriented perpendicular to the direction of white matter fibers (Fig. 3). Thus, the degree of difference between longitudinal and transverse ADC measurements determines the degree of diffusion anisotropy within an image voxel or tissue (e.g. CSF has isotropic diffusivity, thus lADC = tADC; whereas white matter has a high degree of diffusion anisotropy thus lADC > tADC). It is these features of the diffusion tensor that allow inference as to the microstructural integrity of various tissues [24, 25].
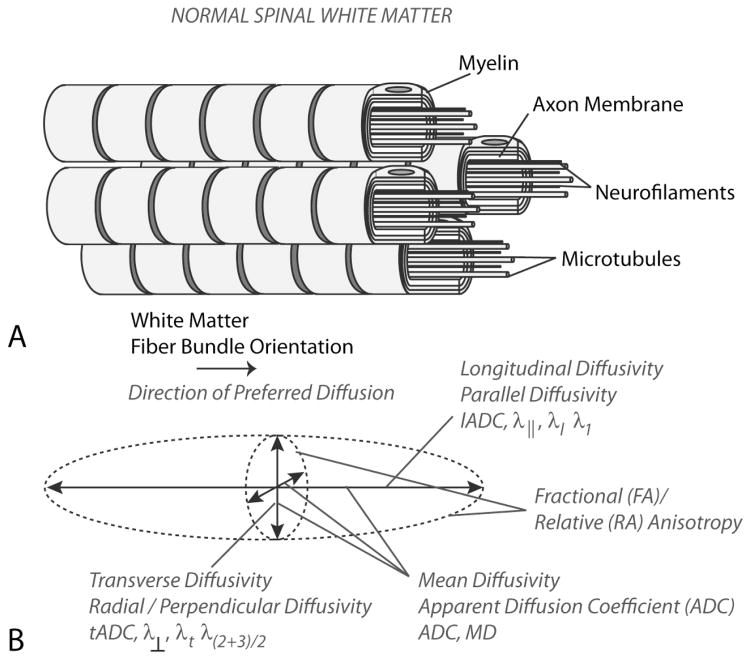
Figure 3. Microstructure and Diffusion Characteristics of Healthy Spinal White Matter.
A) Model of axon bundles showing boundaries to diffusion in the transverse orientation, including myelin, axon membranes, neurofilaments, and microtubules. B) The diffusion ellipsoid model derived from the traditional diffusion tensor shows anisotropic diffusion with preferred diffusion in the direction of white matter fiber bundles, with lower ADC in the transverse orientation and higher ADC in the longitudinal orientation.
Table 1.
| DTI Metric | What it Measures/Clinical Interpretation |
|---|---|
| ADC (Apparent Diffusion Coefficient), MD (Mean Diffusivity) |
|
| FA (Fractional Anisotropy) |
|
| tADC (Transverse ADC), λ⊥, RD (Radial Diffusivity) |
|
| lADC (Longitudinal ADC), λ||, AD (Axial Diffusivity) |
|
| RA (Relative Anisotropy) VR (Volume Ratio) MA (Measured Anisotropy) |
|
Various diffusion tensor “stains”, or image contrasts, have been developed in order to simplify the information present in the diffusion tensor. The “fractional anisotropy”, or FA, ranges from 0 (isotropic) to approximately 1 (anisotropic) and is the most common diffusion tensor index used for quantifying the degree of diffusion anisotropy [26]. Other lesser used anisotropy metrics include the relative anisotropy (RA), volume ratio (VR), measured anisotropy (MA) [27], along with circular or spherical invariants [26, 28]. Thus, changes in overall water diffusivity (e.g. mean ADC or mean diffusivity, MD), changes in directionally dependent ADC measurement (e.g. lADC and tADC), and/or changes in water diffusion anisotropy (e.g. FA) all provide information regarding microstructural integrity within the spinal cord [24, 25].
DTI in Cervical Spondylotic Myelopathy
Cervical spondylotic myelopathy (CSM) is a chronic progressive disorder of the spinal cord with a relatively ill-defined onset of pathogenesis compared with other spinal disorders. Although little is known about the diffusion characteristics within human spinal cord during the early phases of spinal cord compression prior to progressive myelopathy, the literature suggests acute compression of spinal cord tissue may result in a focal decrease in ADC as well as a focal increase in FA (Fig. 4). Assuming spinal cord axons can be modeled as infinite cylinders, diffusion MR simulations by Ford et al. [29, 30] suggest compression axon fibers will result in a decrease in transverse ADC, which may lead to a slight increase in FA. Additional diffusion MR simulations by Nilsson et al. [31] clearly demonstrate decreased mean ADC with increasing compression of spinal cord white matter. Facon et al. [3] explored acute spinal cord compression in two patients and noted a slightly elevated FA at the level of compression compared to normal controls (0.80–0.83 vs. 0.75 in healthy volunteers). Similarly, Hernandez et al. [4] examined 12 patients with CSM and noted a focal increase in FA and decrease in ADC at the site of compression, which was also observed in a pilot study by Benae et al. [32] and attributed to compression of spinal tissue resulting in a focal increase in the boundaries to diffusion in the transverse direction. In perhaps one of the largest studies of DTI in CSM, Mamata et al. [33] described a subset of CSM patients (46%) having either normal to decreased ADC, or normal to elevated FA, at the site of compression and described these patients as having few clinical symptoms other than arm or neck pain, likely related to focal compression of the spinal cord.
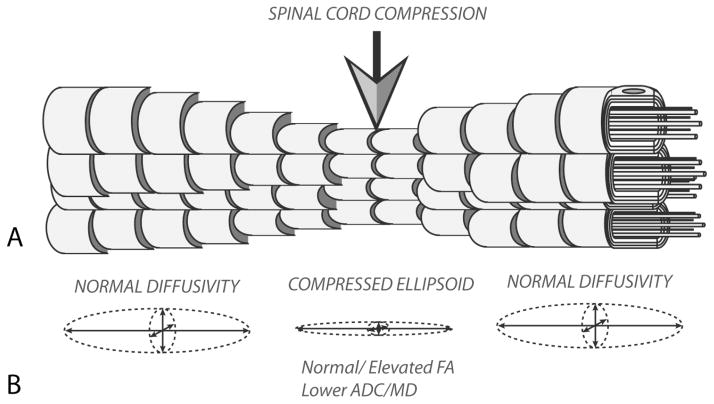
Figure 4. Microstructure and Diffusion Characteristics of Acute Spinal Cord Compression.
A) During acute compression of spinal cord white matter fibers are compressed and blood flow to these regions is reduced, resulting in transient ischemia. B) Transient, acute compression of the spinal cord results in compression of the diffusion ellipsoid, resulting in lower ADC and, in some cases, localized increases in FA values relative to adjacent spinal segments.
Progressive, chronic compression of the spinal cord results in many pathological changes that are detectable using diffusion MRI (Fig. 5). Specifically, clinical studies have clearly documented a significant increase in ADC and decrease in FA occur the late stages of chronic compression of the spinal cord [1–3, 33–46]. These changes have also been verified using an animal model of chronic compression [47], illustrating the characteristic increase in ADC and decrease in FA as late as 9 months after the start of compression. The specific changes in diffusion MR characteristics observed at this alter stage of injury closely resemble those observed in chronic spinal cord injury (SCI) from trauma, albeit to a lesser degree [48, 49]. Specifically, the changes in diffusion characteristics are likely to be a result of chronic, repeated ischemic insults to the spinal cord leading to downstream histopathological changes (Fig. 5) including gliosis, loss of motoneuron function, vasogenic edema, and ultimately necrosis and cavitation [50–55]. These pathological changes, together, result in elevation of ADC due to the increase in extracellular water and suppression of FA due to lack of directional organization within the cord.
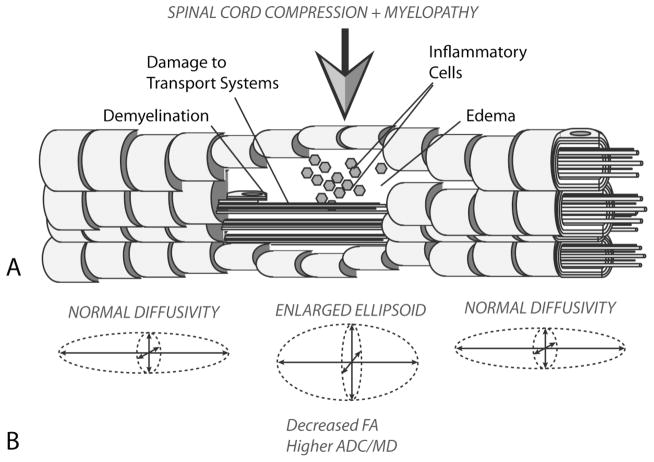
Figure 5. Pathological Changes and Diffusion Characteristics in Cervical Spondylotic Myelopathy.
A) After chronic compression of the spinal cord resulting in sustained ischemia, white matter tracks eventually degenerate, resulting in demyelination, damage to axon transport systems, the influx of inflammatory cells, and vasogenic edema, which eventually leads to functional impairment. B) ADC increases and FA decreases in the site of chronic compression due to the increased extracellular water concentration and decreased fiber tract density, respectively.
An increase mean ADC typically accompanies a decrease in FA at the site of chronic stenosis, however, empirical evidence suggests mean ADC and FA may vary in their clinical and biological significance. For example, Uda et al. [34] implemented receiver-operator characteristic (ROC) analysis and determined the mean ADC as the best predictor of myelopathy when compared with FA (ROC area under the curve, AUC = 0.903 for mean ADC and AUC = 0.760 for FA). Similarly, data from Demir et al. [2] suggested nearly an 80% sensitivity and 53% specificity for detecting myelopathy in patients with spinal cord compression. On the other hand, FA appears to be significantly correlated with specific clinical outcomes, including a positive linear correlation with the modified Japanese Orthopedic Association score (mJOA) [5] and a clinical pain assessment via self questionnaire [38], and a negative linear correlation with Nurick score [5]. A recent study by Jones et al. [5, 56] also showed that patients with a higher pre-operative FA at the site of compression appeared to have better functional recovery compared to patients with lower pre-operative FA measurements, further implicating mean FA at the site of compression as being a potential biomarker for determining favorable surgical candidates. Consistent with this hypothesis, other studies have demonstrated a significantly higher ADC and significantly lower FA occur in symptomatic compared to asymptomatic patients with spinal cord compression [37, 39]. Comparatively, the presence and degree of T2 hyperintensity observed within the spinal cord was not shown to be predictive of neurological impairment [5, 38], demonstrating a mere 60% sensitivity but more than 90% specificity for detecting myelopathy in patients with spinal cord compression [2].
Deflection of the primary eigenvector orientation derived from DTI data has also been examined as a potential biomarker for clinical impairment. A study by Song et al. [1] illustrated that nearly 75% of patients with CSM had disruption in the orientation of the primary eigenvalue as illustrated with FA color maps (Fig. 6). Similarly, Cui et al.[57] noted an increase in primary eigenvector orientational entropy, or randomness, in patients with CSM compared with healthy controls. Consistent with this observation, DTI tractography, a method that uses the direction of the primary eigenvector to create “pseudo”-axonal tracts [58, 59], CSM clearly defines the site of compression in patients with CSM (Fig. 6).
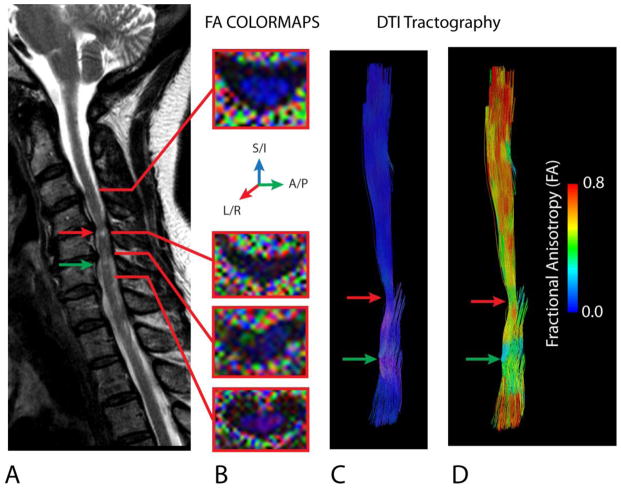
Figure 6. Disruption of Fiber Orientation in CSM.
A) Sagittal T2-weighted image of a 65 year old female patient with CSM showing T2 hyperintensity at the site of most severe spinal cord compression. B) FA colormaps showing the orientation of the primary eigenvector (blue = superior/inferior; green = anterior/posterior; red = left/right) in the normal-appearing spinal cord (top image at C3–4) and regions of compression (bottom images). Note that in areas of compression that FA is low (dark) and the orientation of the primary eigenvalue is deflected from the superior/inferior (blue) orientation, resulting in other colors appearing within the spinal cord. C) DTI tractography labeled (colored) with respect to the primary eigenvalue orientation shows deflection (pink regions) through the area of compression. D) DTI tractography labeled (colored) with respect to fractional anisotropy (FA) shows a decrease in FA at the site of primary eigenvalue deflection. Red arrows = area of significant compression resulting in T2 hyperintensity. Green arrow = second area of compression caudal from the first lesion.
MR Spectroscopy
In comparison to DTI, the application of MRS to CSM has been significantly understudied, but has some intriguing potential. MRS offers metabolic information regarding cellular biochemistry and function of the neural structures within the cervical spine. MRS can be used to assay a number of pertinent biochemical markers, including N- acetyl aspartate (NAA), lactate, choline (CHO), and creatine (Cr). Of particular interest are NAA and lactate. NAA is found almost exclusively in axons and neurons, and is considered an indicator of axonal integrity. Although not completely understood, lactate is considered to play a central role in metabolic dysfunction after central nervous system injury, presumably related to ischemia and mitochondrial dysfunction.
Presently, spinal cord MRS is most frequently used to investigate multiple sclerosis (MS) lesions. These studies have demonstrated suppressed levels of NAA in MS patients compared with normal volunteers [60, 61] and some correlation betweel NAA levels and clinical status. Although less studied, cervical spine MRS has recently been applied to CSM. In a cohort of 22 CSM patients, Holly et al [6] found that the NAA/Cr ratio was significantly lower in CSM patients than normal volunteers (Fig. 7), suggesting increased axonal and neuronal injury in these patients. An abnormal lactate signal was present in nearly one-third CSM patients and none of the control subjects, further supporting the role of ischemia in the pathogenesis of CSM.
Figure 7.
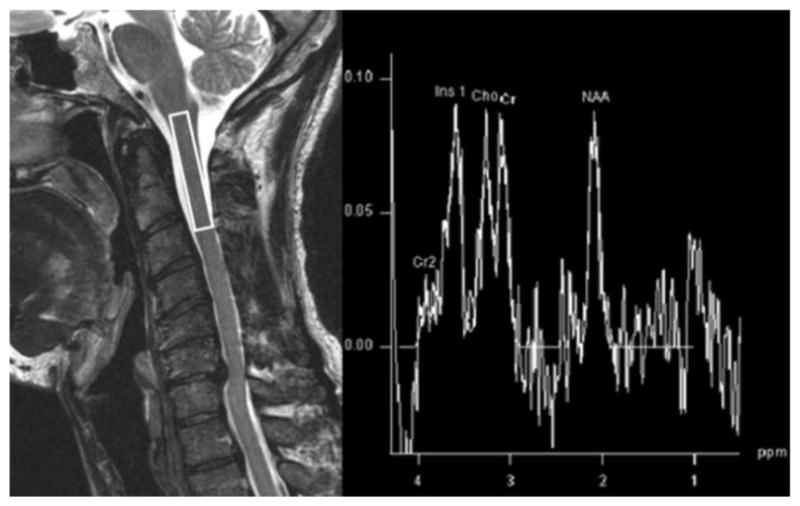
MRS obtained with voxel at the C2 level in a 60 year old gentleman with CSM. The MRS spectra demonstrate a low NAA/Cr ratio indicative of axonal and neuronal injury.
Limitations of DTI and MRS
Although these advanced imaging techniques offer novel insights into CSM, there are some limitations. The spinal cord is relatively small, and differences in magnetic susceptibility of adjacent tissues can cause contamination. The cross sectional area of the spinal cord is only slightly larger than the minimum voxel size commonly used for MRS, and a suboptimally placed voxel can cause a significant decrease in signal to noise ratio [62]. MRS and DTI are very sensitive to patient or structural movement during scan acquisition. The physiological rostral-caudal movement of the spinal cord in response to cardiac pulsations and the respiratory cycle is fairly significant, and even more marked than in the brain [62]. Thus, cardiac gating, specialized radiofrequency coils, and MR signal suppression bands are frequently used to enhance the quality of both MRS and DTI in the spinal cord.
Future directions
In addition to predicting outcome following surgical intervention, there are several other potential future applications for MR technology in the management of CSM patients. The medical literature is clear in that some patients with mild CSM can be successfully treated nonoperatively. Advanced MRI techniques such as DTI or MRS could potentially serve as a non-invasive method to monitor asymptomatic or mildly affected patients treated nonoperatively for impending neurological deterioration. Such an early warning system does not currently exist, as serial standard MR imaging is likely to demonstrate a stable radiographical appearance despite the progression of cellular spinal cord injury and subsequent neurological symptomatology. As such, in conjunction with other nonoperative treatment techniques such as physical therapy and cervical collar placement, asymptomatic and mildly affected CSM patients under observation could undergo serial DTI and/or MRS to assess for subclinical disease progression.
There has been an increasing interest in the investigation of novel molecular and biochemical therapies to treat the secondary biological injury that occurs during CSM pathogenesis. Some of the laboratory investigations include the inhibition of cell apoptosis with a Fas ligand blocking antibody [63], the administration of neurotropins either through genetically altered fibroblasts [64] or adenovirus mediated retrograde spinal cord delivery [65], and dietary therapy to repair injured plasma membranes and cellular oxidative damage [66]. Once translated to clinical use, irrespective of the exact neural repair strategy, these therapies will require a non-invasive modality to ascertain cellular response to intervention. DTI and/or MRS could potentially serve such a modality, as both techniques can identify subtle changes in spinal cord microarchitecture and biochemistry, unlike conventional MRI. In fact, DTI has been used to assess spinal cord regeneration following epidermal neural crest stem cell grafting in an SCI model [67]. In this study DTI demonstrated decreased mean diffusivity and increased diffusion anisotropy in study animals, indicating functional and structural improvement of the spinal cord.
In addition to DTI and MRS, other novel MR technologies may provide further insight into spinal cord health. Techniques such as q-space imaging, chemical exchange saturation transfer (CEST) imaging and magnetization transfer imaging (MTI), dynamic susceptibility contrast (DSC) perfusion MRI, dynamic contrast enhanced (DCE) perfusion MRI, arterial spin labeling (ASL) and spinal cord fMRI all hold promise for providing important physiological information about the status of the spinal cord. Q-space imaging, a diffusion MRI technique that provides quantitative information about the physical size of microstructural compartments, has been successfully performed ex vivo in spinal cord tissues to study the evolution of traumatic injury [68–71] and recently been explored in human CSM patients [35]. CEST, amide proton transfer (APT), and MT imaging are quantitative spectroscopic molecular imaging technique that can be used to study the exchange rates between various biochemical species [72]. Specifically, CEST involves quantification of biochemical species by taking advantage of off-resonance excitation of hydrogen atoms on exchangeable proteins or lipids. Only a few studies have been performed using CEST or APT within the spinal cord [73], but MTI has been very useful for studying MS and amyotrophic lateral sclerosis (ALS) since it can be optimized to quantify myelin integrity [74, 75]. Perfusion imaging techniques such as DSC, DCE, and ASL have yet to gain traction as a tool for assessing spinal cord health; however, they are likely to play an important role in assessing CSM in the future, since spinal cord ischemia and hypoxia is thought to exacerbate spinal cord injury. Additionally, future studies will likely also focus on quantification of neuronal function using spinal cord fMRI to both detect functional impairment and localize regions of injury [76]. This technique, however, has yet to be tested in CSM patients.
Conclusion
MRI plays an integral part in the management of CSM patients, and has evolved from primarily a diagnostic modality to one that can help prognosticate patient outcome following surgical intervention. Advanced imaging techniques such as DTI and MRS provide novel information regarding the spinal cord microstructure and biochemistry, which have further enhanced our knowledge base regarding the pathogenesis and cellular derangement encountered in CSM. It is likely that in the future these MR techniques and others will play an expanded role in the management of CSM patients treated with and without surgery.
Acknowledgments
Research Support: NIH 1R21NS065419-01A1 and 1R01NS078494-01A1
References
- 1.Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, Dong TF, Li TS. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20:422–428. doi: 10.1007/s00586-010-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demir A, Ries M, Moonen CT, Vital JM, Dehais J, Arne P, Caille JM, Dousset V. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229:37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 3.Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26:1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez E, Mackay AL, MacMillan EL, Madler B, Li DK, Dvorak MF, Cordova T, Ramirez-Manzanares A, Laule C. Diffusion tensor imaging of subjects with Cervical Spondylotic Myelopathy: use of the eigenvalues as indicators of spinal stenosis. Proc Intl Soc Mag Reson Med. 2009;17:1309. [Google Scholar]
- 5.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion Tensor Imaging Correlates with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy and Predicts Outcome following Surgery. AJNR Am J Neuroradiol. 2012 doi: 10.3174/ajnr.A3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10:194–200. doi: 10.3171/2008.12.SPINE08367. [DOI] [PubMed] [Google Scholar]
- 7.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976) 1998;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara K, Yonenobu K, Ebara S, Yamashita K, Ono K. The prognosis of surgery for cervical compression myelopathy. An analysis of the factors involved. J Bone Joint Surg Br. 1989;71:393–398. doi: 10.1302/0301-620X.71B3.2722928. [DOI] [PubMed] [Google Scholar]
- 9.Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005;56:1101–1113. discussion 1101–1113. [PubMed] [Google Scholar]
- 10.Kim DH, Vaccaro AR, Henderson FC, Benzel EC. Molecular biology of cervical myelopathy and spinal cord injury: role of oligodendrocyte apoptosis. Spine J. 2003;3:510–519. doi: 10.1016/s1529-9430(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 11.Yamaura I, Yone K, Nakahara S, Nagamine T, Baba H, Uchida K, Komiya S. Mechanism of destructive pathologic changes in the spinal cord under chronic mechanical compression. Spine (Phila Pa 1976) 2002;27:21–26. doi: 10.1097/00007632-200201010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6:190S–197S. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Wada E, Yonenobu K, Suzuki S, Kanazawa A, Ochi T. Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy? Spine (Phila Pa 1976) 1999;24:455–461. doi: 10.1097/00007632-199903010-00009. discussion 462. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, Matsuo M. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg. 1991;74:887–892. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 15.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26:217–226. doi: 10.1097/00006123-199002000-00006. discussion 226–217. [DOI] [PubMed] [Google Scholar]
- 16.Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K. MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine (Phila Pa 1976) 2007;32:1675–1678. doi: 10.1097/BRS.0b013e318074d62e. discussion 1679. [DOI] [PubMed] [Google Scholar]
- 17.Kadanka Z, Bednarik J, Vohanka S, Vlach O, Stejskal L, Chaloupka R, Filipovicova D, Surelova D, Adamova B, Novotny O, Nemec M, Smrcka V, Urbanek I. Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur Spine J. 2000;9:538–544. doi: 10.1007/s005860000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mummaneni PV, Kaiser MG, Matz PG, Anderson PA, Groff M, Heary R, Holly L, Ryken T, Choudhri T, Vresilovic E, Resnick D. Preoperative patient selection with magnetic resonance imaging, computed tomography, and electroencephalography: does the test predict outcome after cervical surgery? J Neurosurg Spine. 2009;11:119–129. doi: 10.3171/2009.3.SPINE08717. [DOI] [PubMed] [Google Scholar]
- 19.Mastronardi L, Elsawaf A, Roperto R, Bozzao A, Caroli M, Ferrante M, Ferrante L. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7:615–622. doi: 10.3171/SPI-07/12/615. [DOI] [PubMed] [Google Scholar]
- 20.Park YS, Nakase H, Kawaguchi S, Sakaki T, Nikaido Y, Morimoto T. Predictors of outcome of surgery for cervical compressive myelopathy: retrospective analysis and prospective study. Neurol Med Chir (Tokyo) 2006;46:231–239. doi: 10.2176/nmc.46.231. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Oyanagi K, Takahashi H, Takahashi HE, Ikuta F. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine (Phila Pa 1976) 1996;21:827–833. doi: 10.1097/00007632-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 22.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 25.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 26.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 27.Ellingson BM, Ulmer JL, Schmit BD. Gray and white matter delineation in the human spinal cord using diffusion tensor imaging and fuzzy logic. Acad Radiol. 2007;14:847–858. doi: 10.1016/j.acra.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med. 2006;55:136–146. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- 29.Ford JC, Hackney DB. Numerical model for calculation of apparent diffusion coefficients (ADC) in permeable cylinders--comparison with measured ADC in spinal cord white matter. Magn Reson Med. 1997;37:387–394. doi: 10.1002/mrm.1910370315. [DOI] [PubMed] [Google Scholar]
- 30.Ford JC, Hackney DB, Lavi E, Phillips M, Patel U. Dependence of apparent diffusion coefficients on axonal spacing, membrane permeability, and diffusion time in spinal cord white matter. J Magn Reson Imaging. 1998;8:775–782. doi: 10.1002/jmri.1880080405. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson M, Latt J, Stahlberg F, van Westen D, Hagslatt H. The importance of axonal undulation in diffusion MR measurements: a Monte Carlo simulation study. NMR Biomed. 2012;25:795–805. doi: 10.1002/nbm.1795. [DOI] [PubMed] [Google Scholar]
- 32.Benae JL, Ellingson BM, Kurpad SN, Kocak M, Wang MC. Diffusion tensor MR imaging in degenerative cervical stenosis. Congress of Neurological Surgeons Annual Meeting; New Orleans, LA. 2009. [Google Scholar]
- 33.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22:38–43. doi: 10.1002/jmri.20357. [DOI] [PubMed] [Google Scholar]
- 34.Uda T, Takami T, Tsuyuguchi N, Sakamoto S, Yamagata T, Ikeda H, Nagata T, Ohata K. Assessment of Cervical Spondylotic Myelopathy using Diffusion Tensor MRI Parameter at 3.0 Tesla. Spine (Phila Pa 1976) 2012 doi: 10.1097/BRS.0b013e31826f25a3. [DOI] [PubMed] [Google Scholar]
- 35.Hori M, Fukunaga I, Masutani Y, Nakanishi A, Shimoji K, Kamagata K, Asahi K, Hamasaki N, Suzuki Y, Aoki S. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur Radiol. 2012;22:1797–1802. doi: 10.1007/s00330-012-2410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, Mutlu A, Ornek I, Sirvanci M, Hamzaoglu A. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011;53:609–616. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- 37.Kerkovsky M, Bednarik J, Dusek L, Sprlakova-Pukova A, Urbanek I, Mechl M, Valek V, Kadanka Z. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 2012;37:48–56. doi: 10.1097/BRS.0b013e31820e6c35. [DOI] [PubMed] [Google Scholar]
- 38.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21:426–433. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- 39.Hori M, Okubo T, Aoki S, Kumagai H, Araki T. Line scan diffusion tensor MRI at low magnetic field strength: feasibility study of cervical spondylotic myelopathy in an early clinical stage. J Magn Reson Imaging. 2006;23:183–188. doi: 10.1002/jmri.20488. [DOI] [PubMed] [Google Scholar]
- 40.Lindberg PG, Feydy A, Sanchez K, Rannou F, Maier MA. Measures of spinal canal stenosis and relationship to spinal cord structure in patients with cervical spondylosis. J Neuroradiol. 2011 doi: 10.1016/j.neurad.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Lee JW, Kim JH, Park JB, Park KW, Yeom JS, Lee GY, Kang HS. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol. 2011;40:1543–1551. doi: 10.1007/s00256-011-1161-z. [DOI] [PubMed] [Google Scholar]
- 42.Xiangshui M, Xiangjun C, Xiaoming Z, Qingshi Z, Yi C, Chuanqiang Q, Xiangxing M, Chuanfu L, Jinwen H. 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol. 2010;65:465–473. doi: 10.1016/j.crad.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Sato T, Horikoshi T, Watanabe A, Uchida M, Ishigame K, Araki T, Kinouchi H. Evaluation of cervical myelopathy using apparent diffusion coefficient measured by diffusion-weighted imaging. AJNR Am J Neuroradiol. 2012;33:388–392. doi: 10.3174/ajnr.A2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aota Y, Niwa T, Uesugi M, Yamashita T, Inoue T, Saito T. The correlation of diffusion-weighted magnetic resonance imaging in cervical compression myelopathy with neurologic and radiologic severity. Spine (Phila Pa 1976) 2008;33:814–820. doi: 10.1097/BRS.0b013e318169505e. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchiya K, Katase S, Fujikawa A, Hachiya J, Kanazawa H, Yodo K. Diffusion-weighted MRI of the cervical spinal cord using a single-shot fast spin-echo technique: findings in normal subjects and in myelomalacia. Neuroradiology. 2003;45:90–94. doi: 10.1007/s00234-002-0898-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Qin W, Hao N, Wang Y, Zong G. Diffusion tensor imaging in spinal cord compression. Acta Radiol. 2012 doi: 10.1258/ar.2012.120271. [DOI] [PubMed] [Google Scholar]
- 47.Cheung MM, Li DT, Hui ES, Fan S, Ding AY, Hu Y, Wu EX. In vivo diffusion tensor imaging of chronic spinal cord compression in rat model. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2715–2718. doi: 10.1109/IEMBS.2009.5333389. [DOI] [PubMed] [Google Scholar]
- 48.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008;29:1976–1982. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deo AA, Grill RJ, Hasan KM, Narayana PA. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J Neurosci Res. 2006;83:801–810. doi: 10.1002/jnr.20783. [DOI] [PubMed] [Google Scholar]
- 50.Harkey HL, al-Mefty O, Marawi I, Peeler DF, Haines DE, Alexander LF. Experimental chronic compressive cervical myelopathy: effects of decompression. J Neurosurg. 1995;83:336–341. doi: 10.3171/jns.1995.83.2.0336. [DOI] [PubMed] [Google Scholar]
- 51.Bennett MH, McCallum JE. Experimental decompression of spinal cord. Surg Neurol. 1977;8:63–67. [PubMed] [Google Scholar]
- 52.DeGirolami U, Zivin JA. Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol. 1982;41:129–149. doi: 10.1097/00005072-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy. Effects of ischemia and compression of the canine cervical spinal cord. J Neurosurg. 1975;43:9–17. doi: 10.3171/jns.1975.43.1.0009. [DOI] [PubMed] [Google Scholar]
- 54.Hukuda S, Wilson CB. Experimental cervical myelopathy: effects of compression and ischemia on the canine cervical cord. J Neurosurg. 1972;37:631–652. doi: 10.3171/jns.1972.37.6.0631. [DOI] [PubMed] [Google Scholar]
- 55.Wilson CB, Bertan V, Norrell HA, Jr, Hukuda S. Experimental cervical myelopathy. II. Acute ischemic myelopathy. Arch Neurol. 1969;21:571–589. doi: 10.1001/archneur.1969.00480180027002. [DOI] [PubMed] [Google Scholar]
- 56.Jones J, Lerner A, Kim PE, Law M, Hsieh PC. Diffusion tensor imaging in the assessment of ossification of the posterior longitudinal ligament: a report on preliminary results in 3 cases and review of the literature. Neurosurg Focus. 2011;30:E14. doi: 10.3171/2011.1.FOCUS10262. [DOI] [PubMed] [Google Scholar]
- 57.Cui JL, Wen CY, Hu Y, Mak KC, Mak KH, Luk KD. Orientation entropy analysis of diffusion tensor in healthy and myelopathic spinal cord. Neuroimage. 2011;58:1028–1033. doi: 10.1016/j.neuroimage.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 58.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 59.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Blamire AM, Cader S, Lee M, Palace J, Matthews PM. Axonal damage in the spinal cord of multiple sclerosis patients detected by magnetic resonance spectroscopy. Magn Reson Med. 2007;58:880–885. doi: 10.1002/mrm.21382. [DOI] [PubMed] [Google Scholar]
- 61.Kendi AT, Tan FU, Kendi M, Yilmaz S, Huvaj S, Tellioglu S. MR spectroscopy of cervical spinal cord in patients with multiple sclerosis. Neuroradiology. 2004;46:764–769. doi: 10.1007/s00234-004-1231-1. [DOI] [PubMed] [Google Scholar]
- 62.Cooke FJ, Blamire AM, Manners DN, Styles P, Rajagopalan B. Quantitative proton magnetic resonance spectroscopy of the cervical spinal cord. Magn Reson Med. 2004;51:1122–1128. doi: 10.1002/mrm.20084. [DOI] [PubMed] [Google Scholar]
- 63.Yu WR, Baptiste DC, Liu T, Odrobina E, Stanisz GJ, Fehlings MG. Molecular mechanisms of spinal cord dysfunction and cell death in the spinal hyperostotic mouse: implications for the pathophysiology of human cervical spondylotic myelopathy. Neurobiol Dis. 2009;33:149–163. doi: 10.1016/j.nbd.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 64.Tobias CA, Han SS, Shumsky JS, Kim D, Tumolo M, Dhoot NO, Wheatley MA, Fischer I, Tessler A, Murray M. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 2005;22:138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- 65.Xu K, Uchida K, Nakajima H, Kobayashi S, Baba H. Targeted retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene prevents loss of mouse (twy/twy) anterior horn neurons in vivo sustaining mechanical compression. Spine (Phila Pa 1976) 2006;31:1867–1874. doi: 10.1097/01.brs.0000228772.53598.cc. [DOI] [PubMed] [Google Scholar]
- 66.Holly LT, Blaskiewicz D, Wu A, Feng C, Ying Z, Gomez-Pinilla F. Dietary therapy to promote neuroprotection in chronic spinal cord injury. J Neurosurg Spine. 2012;17:134–140. doi: 10.3171/2012.5.SPINE1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellingson BM, Schmit BD, Gourab K, Sieber-Blum M, Hu YF, Schmainda KM. Diffusion heterogeneity tensor MRI (Alpha-DTI): mathematics and initial applications in spinal cord regeneration after trauma. Biomed Sci Instrum. 2009;45:167–172. [PubMed] [Google Scholar]
- 68.Nossin-Manor R, Duvdevani R, Cohen Y. q-Space high b value diffusion MRI of hemi-crush in rat spinal cord: evidence for spontaneous regeneration. Magn Reson Imaging. 2002;20:231–241. doi: 10.1016/s0730-725x(02)00470-8. [DOI] [PubMed] [Google Scholar]
- 69.Assaf Y, Mayk A, Eliash S, Speiser Z, Cohen Y. Hypertension and neuronal degeneration in excised rat spinal cord studied by high-b value q-space diffusion magnetic resonance imaging. Exp Neurol. 2003;184:726–736. doi: 10.1016/S0014-4886(03)00274-7. [DOI] [PubMed] [Google Scholar]
- 70.Biton IE, Mayk A, Assaf Y, Cohen Y. Structural changes in glutamate cell swelling followed by multiparametric q-space diffusion MR of excised rat spinal cord. Magn Reson Imaging. 2004;22:661–672. doi: 10.1016/j.mri.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 71.Nossin-Manor R, Duvdevani R, Cohen Y. Spatial and temporal damage evolution after hemi-crush injury in rat spinal cord obtained by high b-value q-space diffusion magnetic resonance imaging. J Neurotrauma. 2007;24:481–491. doi: 10.1089/neu.2006.0158. [DOI] [PubMed] [Google Scholar]
- 72.van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65:927–948. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng MC, Hua J, Hu Y, Luk KD, Lam EY. Magnetization transfer (MT) asymmetry around the water resonance in human cervical spinal cord. J Magn Reson Imaging. 2009;29:523–528. doi: 10.1002/jmri.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci U S A. 2012;109:9605–9610. doi: 10.1073/pnas.1115107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, Benali H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55:1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- 76.Stroman PW. Magnetic resonance imaging of neuronal function in the spinal cord: spinal FMRI. Clin Med Res. 2005;3:146–156. doi: 10.3121/cmr.3.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]