Abstract
Background
Previously, we showed that fragmented Lactobacillus amylovorus CP1563 (CP1563) functions as a dual agonist of peroxisome proliferator-activated receptor α and γ in vitro and in vivo.
Objective
Here, we examined the safety and effect of CP1563 ingestion on body fat in obese class I participants in a double-blinded, placebo-controlled, randomized clinical trial (RCT).
Design
In the RCT, 200 participants with a body mass index (BMI) of 25–30 kg/m2 consumed test beverages with or without 200 mg of CP1563 daily for 12 weeks. In total, 197 subjects completed the study without any adverse effects.
Results
Body fat percentage, whole body fat, and visceral fat were significantly decreased in the test group compared with the placebo group (p<0.001, p<0.001, and p<0.001, respectively). Triglycerides, total cholesterol, LDL-cholesterol, and diastolic blood pressure showed significant reductions in the test group compared with the placebo group (p<0.001, p<0.001, p<0.001, and p<0.001, respectively). Additionally, significant differences in the changes in blood glucose, insulin, homeostasis model assessment-insulin resistance (HOMA-IR), and uric acid were observed between the two groups (p<0.001, p=0.004, p<0.001, and p<0.001, respectively). Improvements in anthropometric measurements and markers were observed in obese class I subjects in the test group.
Conclusions
Daily consumption of beverages containing fragmented CP1563 for 12 weeks by obese class I subjects improved anthropometric measurements and markers related to lipid and glucose metabolism without any adverse effects. These results suggest that the consumption of foods containing fragmented CP1563 reduces body fat and prevents metabolic syndrome.
Keywords: PPARα/γ dual agonist, fragmented lactic acid bacteria, visceral fat, paraprobiotics, biogenics
Obesity causes lifestyle-related metabolic disorders, including hypertension, dyslipidemia, and diabetes, which lead to advanced atherosclerosis or cardiovascular diseases. Chronic low-grade adipose tissue inflammation and insulin resistance are closely associated with the development of metabolic dysfunction (1–3). Lifestyle modifications are effective for the control of overweight and obesity; specifically, healthy dietary modifications and regular physical activity play key roles in preventing non-communicable diseases (4).
Several dietary ingredients can substantially modulate overweight, obesity, obesity-related inflammation, obesity-related metabolic aberration, and cardiovascular risk (5). The use of probiotics is one approach used to improve these abnormalities. Previous studies have reported that certain probiotic strains reduce visceral fat mass. This effect might reflect the fact that living beneficial bacteria produce short-chain fatty acids, including conjugated linoleic acid, within the gut tract (6–8). Probiotics modulate the gut microbiome by promoting competition among the constituents. These changes might cause a favorable environmental change, resulting in obesity suppression.
In a previous study, we screened bacterial strains belonging to 5 genera and 32 species, including 6 Bifidobacterium and 19 Lactobacillus species, for agonistic activities on peroxisome proliferator-activated receptor (PPAR) α and γ (9). The results revealed that substances derived from Lactobacillus amylovorus CP1563 (CP1563) showed the highest dual agonistic activity for PPARα and γ in vitro among the strains screened. Feed-induced obese mice administered fragmented CP1563 showed reduced visceral fat mass and serum and liver triglyceride levels, and increased high-density lipoprotein (HDL) cholesterol. PPARα/γ are transcription factors that are primarily expressed in the liver, skeletal muscle, and white and brown adipose tissues, and these proteins are master regulators of lipid catabolism and homeostasis, glucose homeostasis, and lipid storage. PPARα regulates triglyceride-reducing steps and fatty acid oxidation. PPARα pharmacological agonists (fibrates) reduce the concentration of triglycerides and increase the serum concentration of HDL-cholesterol. PPARγ is highly expressed in adipocytes and regulates adipocyte differentiation, lipid storage, and glucose metabolism. Pharmacological PPARγ agonists (glitazones) improve insulin resistance and are widely used in the treatment of dyslipidemia. Dual-PPAR (PPARα/γ) pharmacological agonists (glitazars) exert positive effects on both lipid and glucose metabolism and have been recently developed as promising agents for the treatment of type 2 diabetes mellitus with dyslipidemia (10, 11). Anti-dyslipidemic and body fat–reducing effects were observed in fragmented CP1563-fed mice compared with mice-fed unprocessed bacterial cells (9). The PPARα activation of fragmented CP1563 was also significantly higher than that of intact cells and was inversely correlated with the shredding size, that is, the length of the fragments. These observations suggested that the physical ‘fragmentation’ of bacteria is a key step in inducing the high agonistic activity of PPARα/γ. This process might expose the agonistic constituents of CP1563 cells.
In the present study, we examined the safety and efficacy of the oral administration of fragmented CP1563 on the depletion of body fat in a double-blinded, parallel group randomized clinical trial involving overweight and pre-obese subjects with a body mass index (BMI) ranging 25 or more but less than 30 kg/m2 (12, 13). Improvements in anthropometric measurements and markers were observed in obese class I subjects in the test group. This result suggests that the consumption of foods containing CP1563 reduces body fat and prevents metabolic syndrome.
Materials and methods
Preparation of CP1563 powder
L. amylovorus CP1563 was isolated from human fecal specimens and stocked in our culture collection library. It was screened as a strain with high potential by luciferase assays for PPAR ligand activity (9). Here, the strain was cultured in an original food-grade medium (Table 1) for 24 h at 37°C under a partially anaerobic atmosphere. At the start of the culture, the pH of the medium was adjusted to 6.8, and thereafter, the pH was controlled at 5.2 by the addition of sodium hydroxide. The resulting bacterial pellet was harvested by filtration with a ceramic filter, washed with sterilized water, and then filtered again. The washed bacterial pellet was then heat-inactivated and lyophilized. The resulting bacterial powder was fragmented by a FS-4 jet mill (Seishin Enterprise Co., Ltd., Tokyo, Japan) under the pressure of 0.64 MPa at a feed speed of 0.5 kg/h. The degree of fragmentation was evaluated as the ratio of the average long dimension of the fragmented bacterial cell preparation against that of the original bacterial cells. The quality of the fragmented bacterial preparation was checked by standard analysis methods (Table 2).
Table 1.
Composition of the medium for CP1563 culture
| Ingredients | Combination ratio (%) |
|---|---|
| Glucose | 8.438 |
| Fish peptone | 3 |
| Yeast peptone | 4.5 |
| Yeast extract | 0.25 |
| Sodium acetate | 0.5 |
| Dipotassium hydrogen phosphate | 0.445 |
| Magnesium sulfate | 0.1 |
| Oleate glycerol ester | 0.3 |
| Fermented barley lees | 3.5 |
| Water | 78.97 |
The pH value of the medium was adjusted to 6.8 before sterilization, and after inoculation, the pH was controlled to approximately 5.2. All ingredients met the Japanese standards for food additives.
Table 2.
Results of general analysis of fragmented CP1563 powder
| Analytical items | Content | Detection limit | Analytical method |
|---|---|---|---|
| Water | 3.3 g/100 g | – | Ambient-pressure heat method |
| Protein | 81.9 g/100 g | – | Kjeldahl method |
| Lipid | 5.8 g/100 g | – | Acid digestion method |
| Ash | 5.9 g/100 g | – | Direct carburization method |
| Carbohydrate | 3.1 g/100 g | – | – |
| Energy | 392 kcal/100 g | – | – |
| Sodium | 0.441 g/100 g | – | Atomic absorption method |
| Arsenicum | 0.5 ppm | – | Atomic absorption method |
| Heavy metals | 5 ppm | – | Sodium sulfide method |
| Lead | N.D. | 0.05 ppm | Atomic absorption method |
| Cadmium | 0.09 ppm | – | Atomic absorption method |
| Mercury | N.D. | 0.01 ppm | Reduction vaporization atomic absorption method |
| Standard plate count bacteria | Negative | 300/g | Standard agar plate culture method |
| Coliform bacteria | Negative | 1/2.22 g | BGLB method |
| Fungus | Negative | 10/g | Potato dextrose agar plate culture method |
All analyses were performed at General Foundation Japan Food Research Laboratories (Tokyo, Japan).
Supplementary beverages
Test beverages were prepared by blending powdered skim milk, citrate, flavors, sweeteners, soybean polysaccharide, food emulsifier, and 200 mg of the fragmented CP1563 powder in water, followed by carbonation, pasteurization, and packaging into 500 mL bottles. The placebo beverage was prepared using the same formula and procedure as for the test beverage, but it did not contain CP1563 powder.
The nutritional contents of the test and placebo beverages are as follows. The test beverage provided a total of 83.7 kJ (20 kcal/day), 1.0 g of protein, 4.5 g of carbohydrates, 0 g of fat, and 128 mg of sodium, whereas the placebo beverage provided a total of 83.7 kJ (20.0 kcal), 1.0 g of protein, 4.5 g of carbohydrates, 0 g of fat, and 128 mg of sodium. The supplementary beverages were obtained from Asahi Soft Drinks Co., Ltd. (Tokyo, Japan).
Subjects
The subjects were recruited from a pool of volunteers from the Clinical Support Corporation (Sapporo, Japan). The subjects were all healthy adult women and men who underwent a medical examination within a month before the trial at the Medical Corporation Hokubukai Utsukushigaoka Hospital, which is affiliated with the Clinical Support Corporation. The selection criteria included healthy men and women classified as having obesity class I according to the guidelines of the Japanese Society for the Study of Obesity, having a BMI (in kg/m2) of 25 or more but less than 30, and not receiving treatment for any lifestyle-related diseases (14). The exclusion criteria included the following: allergy to cow's milk and soy bean; use of medications and health foods affecting lipid metabolism; designation of unsuitable by the medical doctor in charge of the study; a history of severe disorders; a history of gastrointestinal tract surgery; pregnancy or breastfeeding; withdrawal of more than 400 mL of whole blood or blood components within the 4 months prior to the study and/or more than 200 mL of whole blood within the 2 months prior to this study; extremely irregular dietary habits; alternative work schedule or employment in the midnight shift; and smoking and high alcohol intake. Two hundred subjects who fulfilled the eligibility criteria agreed to participate in the study. All subjects were enrolled in the study prior to random allocation. Allocation to the test or placebo group was concealed from the investigator who enrolled the subjects, the nurses, and the medical doctor in charge of the study.
This study was approved by the Institutional Review Board of the Medical Corporation Hokubukai Utsukushigaoka Hospital according to ethical principles and an experimental plan based on the Declaration of Helsinki. Prior to the trial, the medical doctor (KT) provided the subjects with a full explanation of the purpose and methodology of the study. All subjects who agreed to participate in the study provided fully informed consent.
Trial design
This study followed a randomized, double-blinded, placebo-controlled design, and the experimental periods were divided into 2 weeks of observation before treatment, 12 weeks of treatment, and 4 weeks of observation after treatment. A concealed study coordinator (IT) randomly and blindly assigned the 200 subjects into two groups of 100 that were matched according to age, gender, BMI, and abdominal fat area. Volunteers in one group (n=100) received the test beverages (a 500 mL bottle of active beverage per volunteer per day: test group), and those in the other group (n=100) received one placebo beverage per day (a 500 mL bottle of the beverage per volunteer per day: placebo group). The subjects were instructed to ingest one bottle daily. The subjects were also instructed to assess their health condition and maintain healthy living practices, including diet and exercise.
The sample size was set to 100 individuals per group to detect changes in the whole body fat area at week 12 of the treatment using a CT scanner between the test and placebo groups at a p-value of 0.05, with 80% power using analysis of covariance (ANCOVA) based on information from a preliminary dose-finding study (unpublished information). A dropout rate of 10% was expected; thus, 200 participants were recruited.
Measurements
At baseline (week 0), every 4 weeks (weeks 4 and 8), at the end of the intervention (week 12), and at the end of the post-observation period (week 16), we performed the following anthropometric measurements at the hospital: body weight, BMI, hip and waist circumferences, body fat percentage, systolic and diastolic blood pressures, pulse rate, body temperature, subcutaneous fat thickness (arm and back), blood sampling, and urinalysis. Abdominal computed tomography (Somatic Emotion 16 Excel, Siemens Japan K.K., Tokyo, Japan) scans and dual bioelectrical impedance analysis (HDS2000, Omron Healthcare Co., Ltd., Kyoto, Japan) were conducted to measure the abdominal visceral and subcutaneous fat areas at weeks 0, 4, 8, 12, and 16 and at weeks 0 and 12, respectively. Height was only measured at the screening examination, and the BMI was calculated based on this measurement.
Each participant maintained a daily record of test or placebo beverage consumption and diet, exercise, and physical condition, including the presence of any subjective symptoms during the trial. The participants also maintained a detailed record of diet and pedometer measurements for 3 consecutive days before each visit: at the start of treatment (week 0) and at weeks 4, 8, 12, and 16. A managerial dietitian analyzed the dietary records to determine the intake of total energy, protein, carbohydrate, fat, total fiber, magnesium, calcium, potassium, and sodium using Excel Eiyokun ver. 6.0 (Kenpakusha Co., Ltd., Tokyo, Japan).
Blood examination and urinalysis were performed at Sapporo Clinical Laboratory Inc. (Sapporo, Japan). The following biochemical and hematological parameters were measured: total protein, albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LD), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride (TG), uric acid, blood urea nitrogen (BUN), creatinine, sodium, potassium, chloride, calcium, glucose, adiponectin, white blood cells, red blood cells, hemoglobin, hematocrit, and platelets. Protein, glucose, urobilinogen, bilirubin, ketone bodies, occult blood, pH, and density were determined through urinalysis. The participants were required to fast for at least 12 h prior to the test dates.
Medical examinations and inquiries were performed, and a medical doctor (KT) assessed the subjective symptoms at each measurement. All measurements were performed under the supervision of a doctor.
Statistical analysis
Statistical analyses were performed using JMP software (version 11; SAS Institute Japan Ltd., Tokyo, Japan) and SPSS software (version 20; IBM SPSS Japan, Tokyo, Japan). For comparisons between the groups, ANCOVA for repeated measures was primarily used to assess the time course of treatment. Because of the wide ranges of the initial values of these parameters, ANCOVA for repeated measures was the first choice for analyzing the data to not only minimize type II errors but also ensure that the overall probability of type I errors was less than 0.05. Multivariate analysis of variance (MANOVA) for repeated measures was also applied to compare the variation of the mean values with time, where necessary, in cases for which the mean value was considered to be the representative value. ANCOVA with baseline values as the covariate and Student's t-test were used as post hoc tests. P<0.05 for a two-tailed test was considered statistically significant.
Per protocol analysis included only the data from participants who completed the study. Compliance with the test or placebo beverage was assessed, and participants with ≥80% consumption of the beverages were considered eligible.
Results
Approximately 21 kg of dried CP1563 cells were harvested from the culture medium of 4,000 L. The fragmentation process was successfully completed as indicated by an average long dimension of less than 70% of that of the native bacteria (9). The results of a standard method analysis of the fragmented bacterial preparation are shown in Table 2. The quality was satisfactory for a food material for the test beverages.
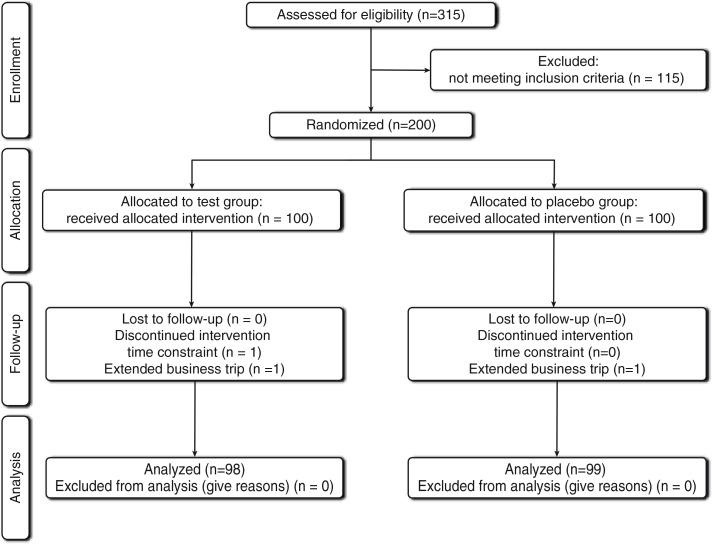
In total, 200 subjects were enrolled, and 197 participants completed the present study. These individuals were randomly assigned to fragmented CP1563 or control treatments. The progress through the phases of the trial is shown in Fig. 1. Three individuals discontinued participation in the study because of an inability to comply with the time constraint (n=1) or an extended business trip (n=2). During the study, adverse effects, such as abnormal values of hepatic or renal function, cutaneous symptoms, nausea, abdominal discomfort, and edema caused by PPARα or PPARγ synthetic agonists, were not observed in the test group. Although transient diarrhea (four subjects in the placebo group and two subjects in the test group) and constipation (two subjects in the placebo group and one subject in the test group) were observed, the doctor determined that the symptoms were not associated with the intake of the supplementary beverages. No signs of imbalance in the occurrence rates of unwellness between the test and placebo groups were recorded.
Fig. 1.
Flow diagram of the trial.
The characteristics of the subjects at baseline are shown in Table 3. No significant differences were observed between the test and placebo groups regarding gender, age, BMI, or abdominal fat area (p>0.05). This study achieved relatively high compliance: the frequencies of test or placebo beverage consumption were 99.85±0.59 and 99.84±0.59, respectively.
Table 3.
Baseline characteristics of the participantsa
| Placebo group | CP1563 group | ||
|---|---|---|---|
| (n=100) | (n=100) | ||
| Male | 50 | 50 | |
| Female | 50 | 50 | |
| Age | (y) | 46.6±11.9 | 46.2±12.0 |
| Body weight | (kg) | 76.4±9.5 | 76.7±9.6 |
| Height | (cm) | 163.6±9.3 | 164.3±9.4 |
| Body mass index | (kg/m2) | 28.3±1.6 | 28.5±1.6 |
| Waist circumference | (cm) | 97.6±4.8 | 98.0±4.8 |
| Hip circumference | (cm) | 100.4±4.0 | 101.5±4.1 |
| Visceral fat area | (cm2) | 109.4±35.6 | 106.9±35.9 |
| Subcutaneous fat area | (cm2) | 277.5±63.0 | 285.9±63.6 |
| Whole body fat area | (cm2) | 386.8±59.1 | 392.8±59.8 |
| Systolic blood pressure | (mm Hg) | 124.4±17.7 | 125.5±17.9 |
| Diastolic blood pressure | (mm Hg) | 73.6±13.2 | 75.0±13.3 |
| Pulse rate | (beats/min) | 69.3±9.8 | 69.6±9.9 |
×±SD.
No significant difference was observed between groups.
BMI, body fat percentage, abdominal fat area, and whole body fat area
The baseline demographic, anthropometric, and clinical characteristics of the participants were comparable in both groups (Table 3). Significant differences were not observed between the two groups. Daily records indicated that the daily physical activity was constant during the study period. Table 4 shows the daily energy, protein, fat, and carbohydrate intake based on body weight. No significant changes were observed in either group.
Table 4.
Daily energy, protein, fat, and carbohydrate intake based on weight measured every 4 weeksa
| Treatment period | |||||
|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Week 12 | ||
| Energy intake (kJ/day/kg) | Placebo | 332.6±72.8 | 320.9±72.4 | 297.5±71.9 | 321.3±72.8 |
| CP1563 | 306.3±73.6 | 315.5±73.2 | 319.2±72.8 | 297.1±73.6 | |
| Protein intake (g/day/kg) | Placebo | 3.0±0.9 | 2.8±0.8 | 2.5±0.7 | 2.8±0.8 |
| CP1563 | 2.8±0.9 | 2.7±0.8 | 2.8±0.7 | 2.6±0.8 | |
| Fat intake (g/day/kg) | Placebo | 2.6±0.8 | 2.6±0.9 | 2.4±0.8 | 2.6±0.9 |
| CP1563 | 2.3±0.8 | 2.6±0.9 | 2.6±0.8 | 2.4±0.9 | |
| Carbohydrate intake (g/day/kg) | Placebo | 10.5±2.6 | 10.0±2.4 | 9.3±2.6 | 9.9±2.5 |
| CP1563 | 9.9±2.6 | 9.8±2.4 | 10.0±2.5 | 9.5±2.6 | |
×± SD.
No significant difference was observed between groups (MANOVA was used for repeated measures analysis).
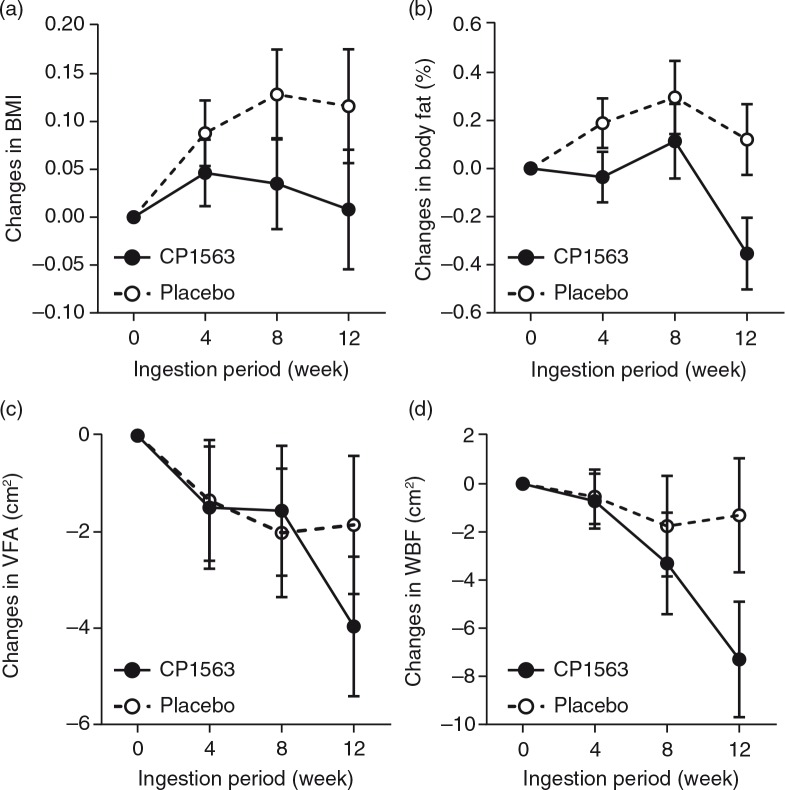
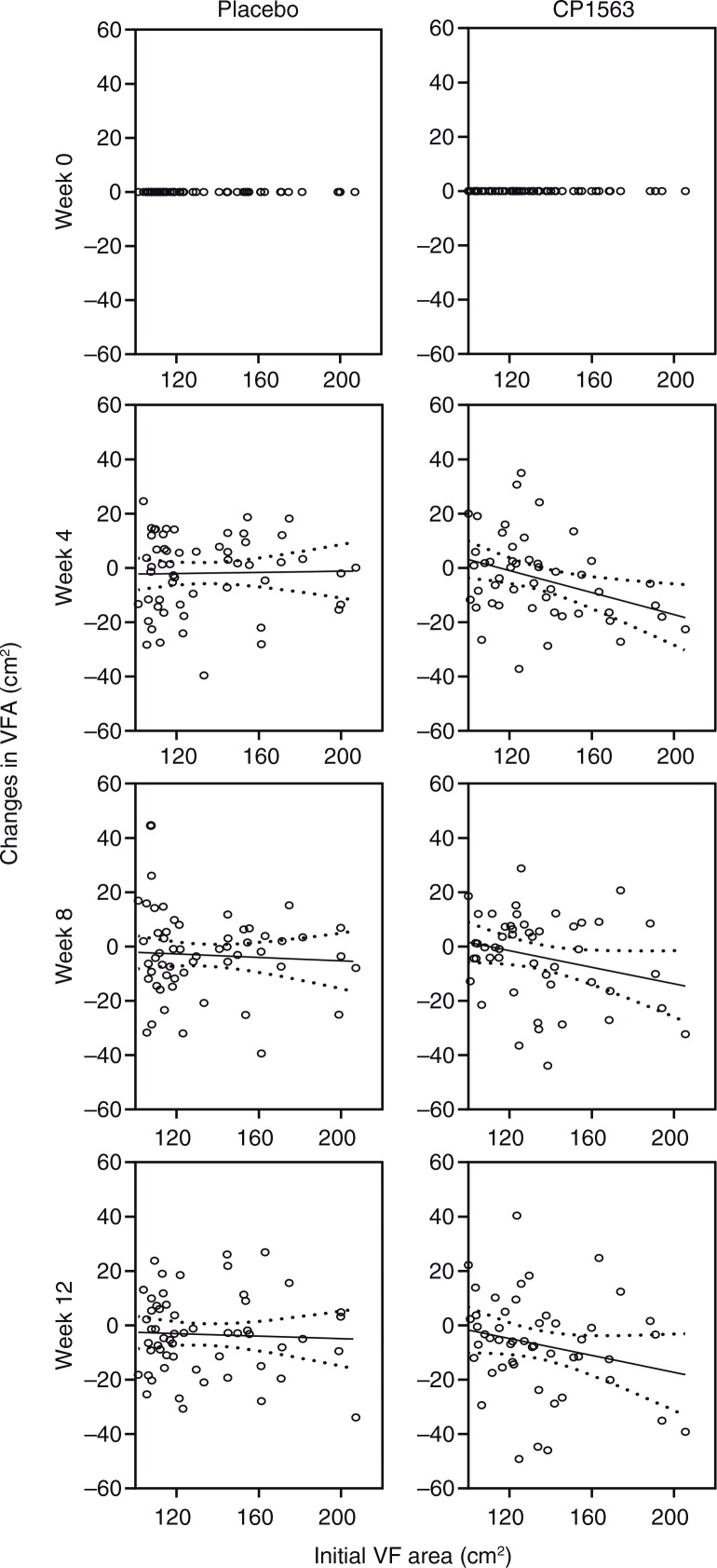
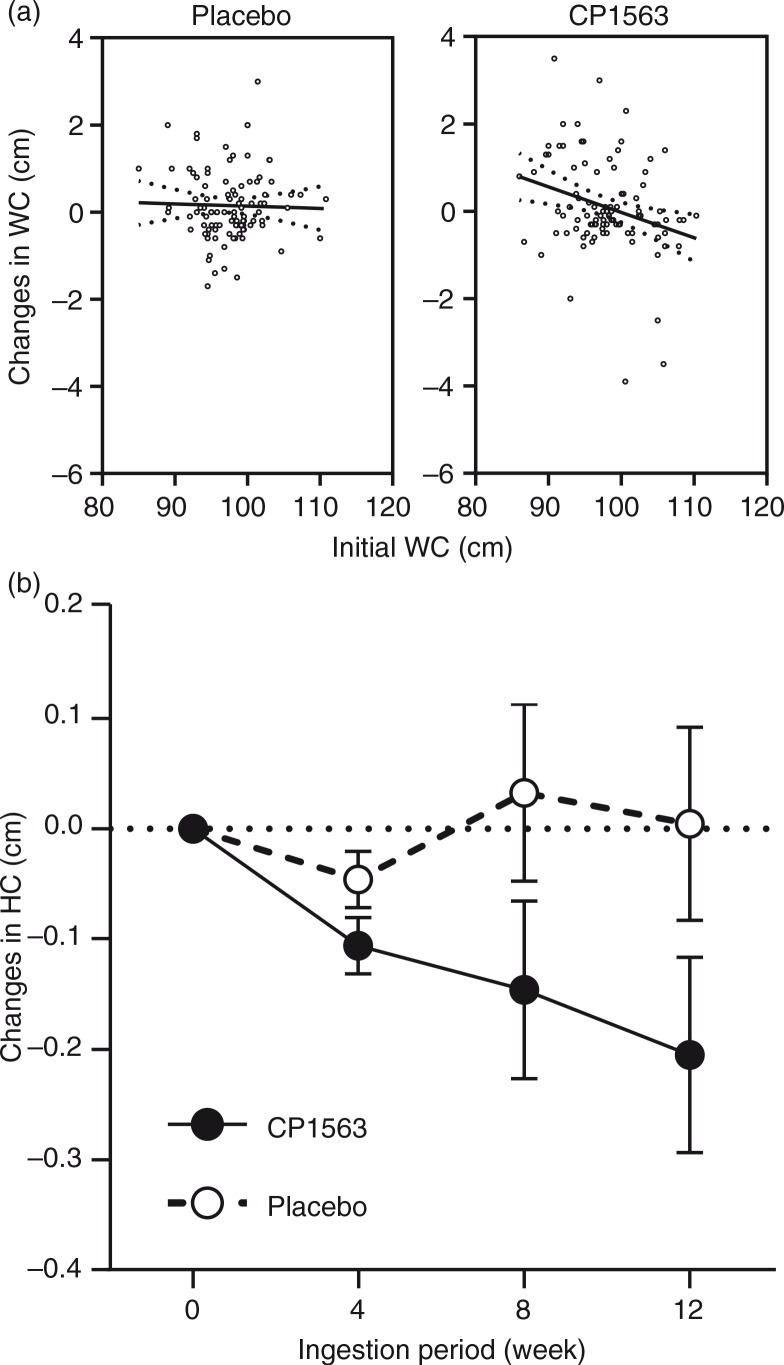
Time-dependent changes in BMI, body fat percentage, visceral fat area, and whole body fat area are shown in Fig. 2 (p=0.524, p<0.001, p<0.001, and p<0.001, respectively). In stratified analyses, the subjects whose visceral fat exceeded 100 cm2 showed a significantly larger reduction in the test group than in the placebo group in whole body fat area (p<0.001, data not shown). However, the participants in the other subgroup with a low baseline value (visceral fat<100 cm2) also showed a significant reduction in the body fat area in the test group (p<0.05 [test for efficacy ratio], p=0.031; 390.19±4.75 cm2 at the start [mean±SEM] to 381.89±4.70 cm2 at 12 weeks on CP1563 [mean±SEM]), but those in the placebo group did not. Another stratified analysis of the participants in the subgroup with a high baseline value (visceral fat ≥100 cm2) exhibited a larger reduction in visceral fat area (Fig. 3; p<0.001). Regarding body weight, a significant difference was observed between the placebo and test groups in men (Table 5; p=0.035). In women, a reduction in the body weight was observed in the test group (Table 5; p=0.051). Regarding waist circumference, a significant interaction between the factor group and time was observed (p<0.001), and the graph showed a significant difference between the placebo and test groups at 12 weeks in the measurement item, as shown in Fig. 4a. Similarly, in terms of hip circumference, a significant difference between the placebo and test groups was noted (Fig. 4b; p=0.015).
Fig. 2.
Changes in anthropometric measurements. (a) BMI, (b) body fat percentage, (c) visceral fat area, and (d) whole body fat area. Changes in BMI and fat area based on CT scans from baseline in obese class I participants during the 12-week period of consumption in the test group (closed circles; n=100) and the placebo group (open circles; n=100). The participants ingested one bottle (500 mL) of carbonated beverage containing fragmented CP1563 or the same amount of carbonated placebo beverage daily for 12 weeks. After the treatment was initiated, the whole and visceral fat areas were measured at weeks 4, 8, 12 and 16. Subgroup analysis was performed after the groups were divided into two subgroups using 100 cm2 of visceral fat area as the cutoff value. The data are presented as the means±SEM. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA for repeated measures.
Fig. 3.
Time-dependent changes in visceral fat area (cm2) in subjects with higher values (≥100 cm2) at baseline. Visceral fat areas were measured at weeks 4, 8, 12 and 16 in subjects with higher initial values at baseline. The solid line is the regression line, and the dotted line shows the 95% confidence intervals of the population mean values. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA for repeated measures.
Table 5.
Changes in anthropometric measurements during the course of the study
| Factor | Probability | Regression | ||
|---|---|---|---|---|
| Body weight (kg) | Whole model | Group | p=0.505 | CP1563: δBW=0.496−0.006×Init. BW |
| Group×Init. BW | p=0.202 | Placebo: δBW=0.950−0.010×Init. BW | ||
| Female | Group | p=0.051 | CP1563: δBW=1.591−0.021×Init. BW | |
| Group×Init. BW | p=0.059 | Placebo: δBW=−0.551+0.012×Init. BW | ||
| Male | Group | p=0.035 | CP1563: δBW=−1.284+0.153×Init. BW | |
| Group×Init. BW | p=0.104 | Placebo: δBW=2.414−0.027×Init. BW | ||
| BMI (kg/m2) | Whole model | Group | p=0.524 | CP1563: δBMI=0.025−3.550×10–5× Init. BMI |
| Group×Init. BMI | p=0.757 | Placebo: δBMI=0.368−0.010×Init. BMI | ||
| Female | Group | p=0.111 | CP1563: δBMI=0.737−0.024×Init. BMI | |
| Group×Init. BMI | p=0.195 | Placebo: δBMI=−0.280+0.014×Init. BMI | ||
| Male | Group | p=0.026 | CP1563: δBMI=−1.076+0.038×Init. BMI | |
| Group×Init. BMI | p=0.094 | Placebo: δBMI=0.942−0.031×Init. BMI | ||
| Body fat percentage (%) | Whole model | Group | p<0.001 | CP1563: δBFP=−0.882+0.024×Init. BFP |
| Group×Init. BFP | p<0.001 | Placebo: δBFP=1.167−0.029×Init. BFP | ||
| Female | Group | p=0.911 | CP1563: δBFP=1.517−0.034×Init. BFP | |
| Group×Init. BFP | p=0.547 | Placebo: δBFP=1.251−0.029×Init. BFP | ||
| Male | Group | p<0.001 | CP1563: δBFP=0.247−0.018×Init. BFP | |
| Group×Init. BFP | p<0.001 | Placebo: δBFP=7.829−0.269×Init. BFP | ||
| Waist circumference (cm) | Whole model | Group | p=0.233 | CP1563: δWC=2.413−0.024×Init. WC |
| Group×Init. WC | p<0.001 | Placebo: δWC=1.228−0.012×Init. WC | ||
| Female | Group | p=0.010 | CP1563: δWC=3.330−0.032×Init. WC | |
| Group×Init. WC | p=0.003 | Placebo: δWC=−0.812+0.011×Init. WC | ||
| Male | Group | p=0.082 | CP1563: δWC=−0.072+4.382×10–6×Init. WC | |
| Group×Init. WC | p=0.039 | Placebo: δWC=1.840−0.020×Init. WC | ||
| Hip circumference (cm) | Whole model | Group | p=0.089 | CP1563: δHC=2.173−0.022×Init. HC |
| Group×Init. HC | p=0.015 | Placebo: δHC=0.329−0.004×Init. HC | ||
| Female | Group | p=0.338 | CP1563: δHC=3.114−0.0301×Init. HC | |
| Group×Init. HC | p=0.063 | Placebo: δHC=1.130−0.012×Init. HC | ||
| Male | Group | p=0.077 | CP1563: δHC=1.269−0.013×Init. HC | |
| Group×Init. HC | p=0.123 | Placebo: δHC=−0.265+0.002×Init. HC | ||
| Visceral fat area (cm2) | Whole model | Group | p=0.637 | CP1563: δVF=3.443−0.041×Init. VF |
| Group×Init. VF | p<0.001 | Placebo: δVF=4.682−0.062×Init. VF | ||
| Female | Group | p=0.575 | CP1563: δVF=4.182+0.602×Init. VF | |
| Group×Init. VF | p<0.001 | Placebo: δVF=6.045−0.083×Init. VF | ||
| Male | Group | p=0.935 | CP1563: δVF=5.321−0.049×Init. VF | |
| Group×Init. VF | p=0.014 | Placebo: δVF=4.946−0.058×Init. VF | ||
| Subcutaneous fat area (cm2) | Whole model | Group | p=0.078 | CP1563: δSCF=−4.385+0.011×Init. SCF |
| Group×Init. SCF | p=0.343 | Placebo: δSCF=2.503−0.009×Init. SCF | ||
| Female | Group | p=0.368 | CP1563: δSCF=−3.432−0.006×Init. SCF | |
| Group×Init. SCF | p=0.823 | Placebo: δSCF=2.968−0.008×Init. SCF | ||
| Male | Group | p=0.022 | CP1563: δSCF=−6.776+0.022×Init. SCF | |
| Group×Init. SCF | p=0.088 | Placebo: δSCF=5.296−0.023×Init. SCF | ||
| Whole body fat area (cm2) | Whole model | Group | p=0.013 | CP1563: δWBF=1.812−0.012×Init. WBF |
| Group×Init. WBF | p<0.001 | Placebo: δWBF=21.74−0.059×Init. WBF | ||
| Female | Group | p=0.192 | CP1563: δWBF=9.647−0.031×Init. WBF | |
| Group×Init. WBF | p=0.003 | Placebo: δWBF=24.333−0.063×Init. WBF | ||
| Male | Group | p=0.009 | CP1563: δWBF=–11.707+0.025×Init. WBF | |
| Group×Init. WBF | p=0.008 | Placebo: δWBF=21.625−0.061×Init. WBF | ||
| Systolic blood pressure (mmHg) | Whole model | Group | p=0.291 | CP1563: δSBP=1.338−0.012×Init. SBP |
| Group×Init. SBP | p=0.176 | Placebo: δSBP=2.614−0.019×Init. SBP | ||
| Female | Group | p=0.319 | CP1563: δSBP=0.443−0.004×Init. SBP | |
| Group×Init. SBP | p=0.613 | Placebo: δSBP=2.202−0.017×Init. SBP | ||
| Male | Group | p=0.634 | CP1563: δSBP=2.220−0.020×Init. SBP | |
| Group×Init. SBP | p=0.218 | Placebo: δSBP=3.013−0.022×Init. SBP | ||
| Diastolic blood pressure (mmHg) | Whole model | Group | p=0.432 | CP1563: δDBP=9.562−0.122×Init. DBP |
| Group×Init. DBP | p<0.001 | Placebo: δDBP=11.657−0.138×Init. DBP | ||
| Female | Group | p=0.016 | CP1563: δDBP=7.552−0.104×Init. DBP | |
| Group×Init. DBP | p<0.001 | Placebo: δDBP=17.0818−0.205×Init. DBP | ||
| Male | Group | p=0.173 | CP1563: δDBP=12.015−0.147×Init. DBP | |
| Group×Init. DBP | p<0.001 | Placebo: δDBP=7.1040.083×Init. DBP |
BMI, body mass index; ANCOVA for repeated measures was used to analyze the changes in these measures during the course of the study.
Fig. 4.
Changes in (a) waist, and (b) hip circumferences at week 12. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA.
Systolic blood pressure did not show a significant change; however, diastolic blood pressure was affected by the constant ingestion of fragmented CP1563 (Table 5; p<0.001).
Parameters relevant to lipid, glucose, and liver metabolism
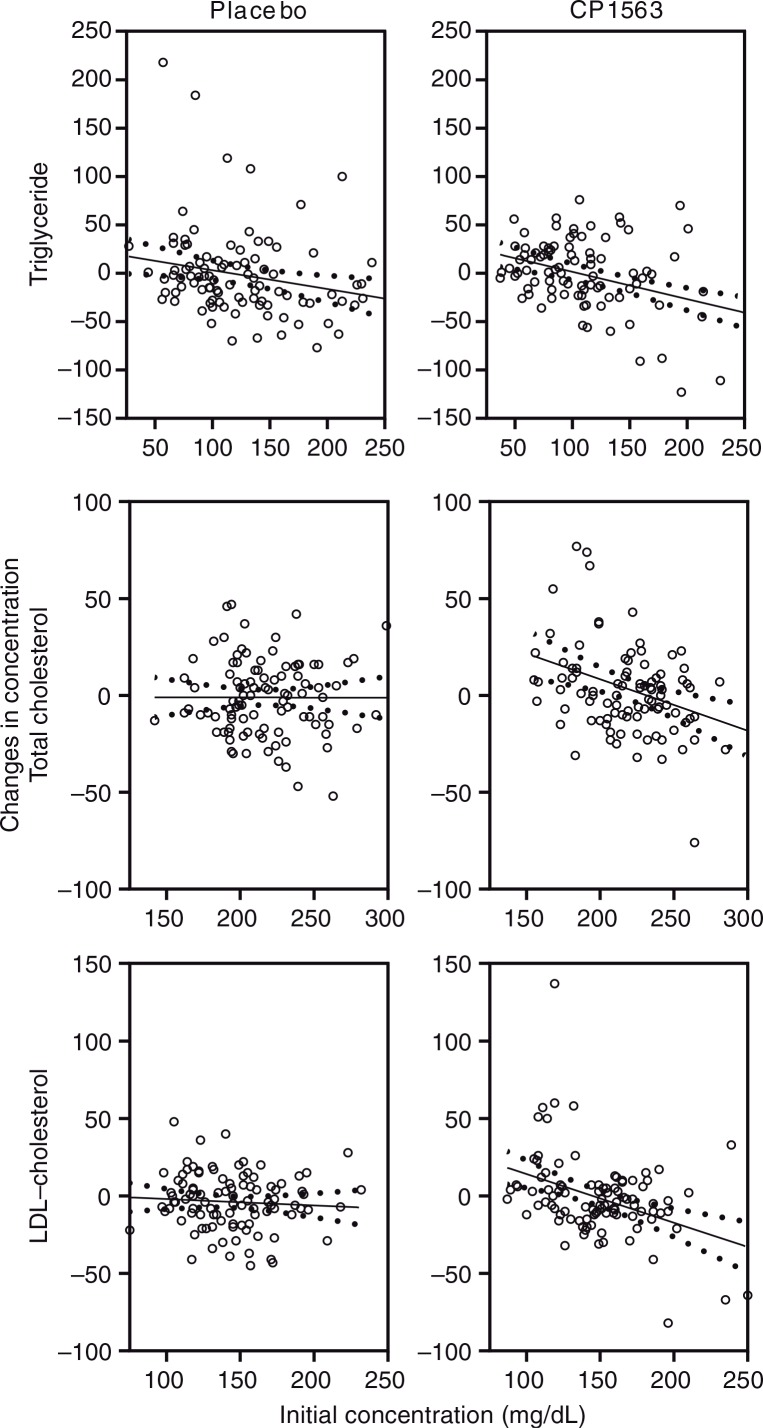
Changes in lipid and glucose metabolism are shown in Table 6. The regular intake of fragmented CP1563 significantly affected the triglyceride and total and LDL-cholesterol levels in the plasma samples (Fig. 5; p<0.001, p<0.001, and p<0.001, respectively). Larger reductions of these lipid compositions were observed in the test group than in the placebo group at week 12, particularly in men (Table 6). There was no major change in the HDL-cholesterol level, but a significant difference between the placebo and test groups was observed, particularly in men.
Table 6.
Changes in serum metabolic variables during the course of the study
| Factor | Probability | Regression | ||
|---|---|---|---|---|
| Total cholesterol (mg/dL) | Whole model | Group | p=0.063 | CP1563: δT-C=30.0878−0.132×Init. T-C |
| Group×Init. T-C | p<0.001 | Placebo: δT-C=14.012−0.068×Init. T-C | ||
| Female | Group | p=0.884 | CP1563: δT-C=19.889−0.084×Init. T-C | |
| Group×Init. T-C | p=0.006 | Placebo: δT-C=18.237−0.079×Init. T-C | ||
| Male | Group | p=0.029 | CP1563: δT-C=43.043−0.192×Init. T-C | |
| Group×Init. T-C | p<0.001 | Placebo: δT-C=13.360−0.073×Init. T-C | ||
| HDL-cholesterol (mg/dL) | Whole model | Group | p=0.574 | CP1563: δHDL-C=3.462−0.054×Init. HDL-C |
| Group×Init. HDL-C | p=0.004 | Placebo: δHDL-C=2.498−0.042×Init. HDL-C | ||
| Female | Group | p=0.631 | CP1563: δHDL-C=3.527−0.055×Init. HDL-C | |
| Group×Init. HDL-C | p=0.244 | Placebo: δHDL-C=2.016−0.016×Init. HDL-C | ||
| Male | Group | p=0.413 | CP1563: δHDL-C=3.439−0.054×Init. HDL-C | |
| Group×Init. HDL-C | p<0.001 | Placebo: δHDL-C=5.118−0.112×Init. HDL-C | ||
| LDL-cholesterol (mg/dL) | Whole model | Group | p=0.014 | CP1563: δLDL-C=23.956−0.170×Init. LDL-C |
| Group×Init. LDL-C | p<0.001 | Placebo: δLDL-C=10.863−0.083×Init. LDL-C | ||
| Female | Group | p=0.299 | CP1563: δLDL-C=19.785−0.141×Init. LDL-C | |
| Group×Init. LDL-C | p<0.001 | Placebo: δLDL-C=12.117−0.089×Init. LDL-C | ||
| Male | Group | p=0.019 | CP1563: δLDL-C=28.728−0.202×Init. LDL-C | |
| Group×Init. LDL-C | p<0.001 | Placebo: δLDL-C=8.559−0.080×Init. LDL-C | ||
| Triglyceride (mg/dL) | Whole model | Group | p=0.950 | CP1563: δTG=21.142−0.176×Init. TG |
| Group×Init. TG | p<0.001 | Placebo: δTG=20.767−0.168×Init. TG | ||
| Female | Group | p=0.216 | CP1563: δTG=37.363−0.144×Init. TG | |
| Group×Init. TG | p<0.001 | Placebo: δTG=27.526−0.267×Init. TG | ||
| Male | Group | p=0.604 | CP1563: δTG=23.7438−0.194×Init. TG | |
| Group×Init. TG | p<0.001 | Placebo: δTG=18.927−0.118×Init. TG | ||
| Fasting glucose (mg/dL) | Whole model | Group | p<0.001 | CP1563: δGLC=15.101−0.161×Init. GLC |
| Group×Init. GLC | p<0.001 | Placebo: δGLC=−5.624+0.071×Init. GLC | ||
| Female | Group | p=0.001 | CP1563: δGLC=23.229−0.250×Init. GLC | |
| Group×Init. GLC | p<0.001 | Placebo: δGLC=6.949−0.086×Init. GLC | ||
| Male | Group | p=0.004 | CP1563: δGLC=2.886−0.024×Init. GLC | |
| Group×Init. GLC | p<0.001 | Placebo: δGLC=−13.855+0.179×Init. GLC | ||
| Fasting insulin (mU/mL) | Whole model | Group | p=0.418 | Incomputable |
| Group×Init. Ins. | p=0.004 | |||
| HOMA-IR | Whole model | Group | p=0.598 | Incomputable |
| Group×Init. HOMA-IR | p<0.001 | |||
| Uric acid (mg/dL) | Whole model | Group | p=0.022 | CP1563: δUA=0.822−0.144×Init. UA |
| Group×Init. UA | p<0.001 | Placebo: δUA=0.436+0.087×Init. UA | ||
| Female | Group | p=0.029 | CP1563: δUA=0.603−0.104×Init. UA | |
| Group×Init. UA | p=0.005 | Placebo: δUA=0.067+0.020×Init. UA | ||
| Male | Group | p=0.870 | CP1563: δUA=1.070−0.180×Init. UA | |
| Group×Init. UA | p<0.001 | Placebo: δUA=1.019+0.169×Init. UA |
T-C, total cholesterol; Init., initial; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; TG, triglyceride; GLC, glucose; Ins, insulin; HOMA-IR, homeostasis model assessment-insulin resistance; UA, uric acid.
Statistical analysis with ANCOVA was used for repeated measures, except for the items ‘fasting insulin’ and ‘HOMA-IR’.
The fasting insulin concentration was measured at only 2 time points: before and after ingestion; therefore, ANCOVA was used for these variables.
Fig. 5.
Changes in biological markers in the blood after 12 weeks of ingestion: triglycerides, total cholesterol, and LDL-cholesterol. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA for repeated measures.
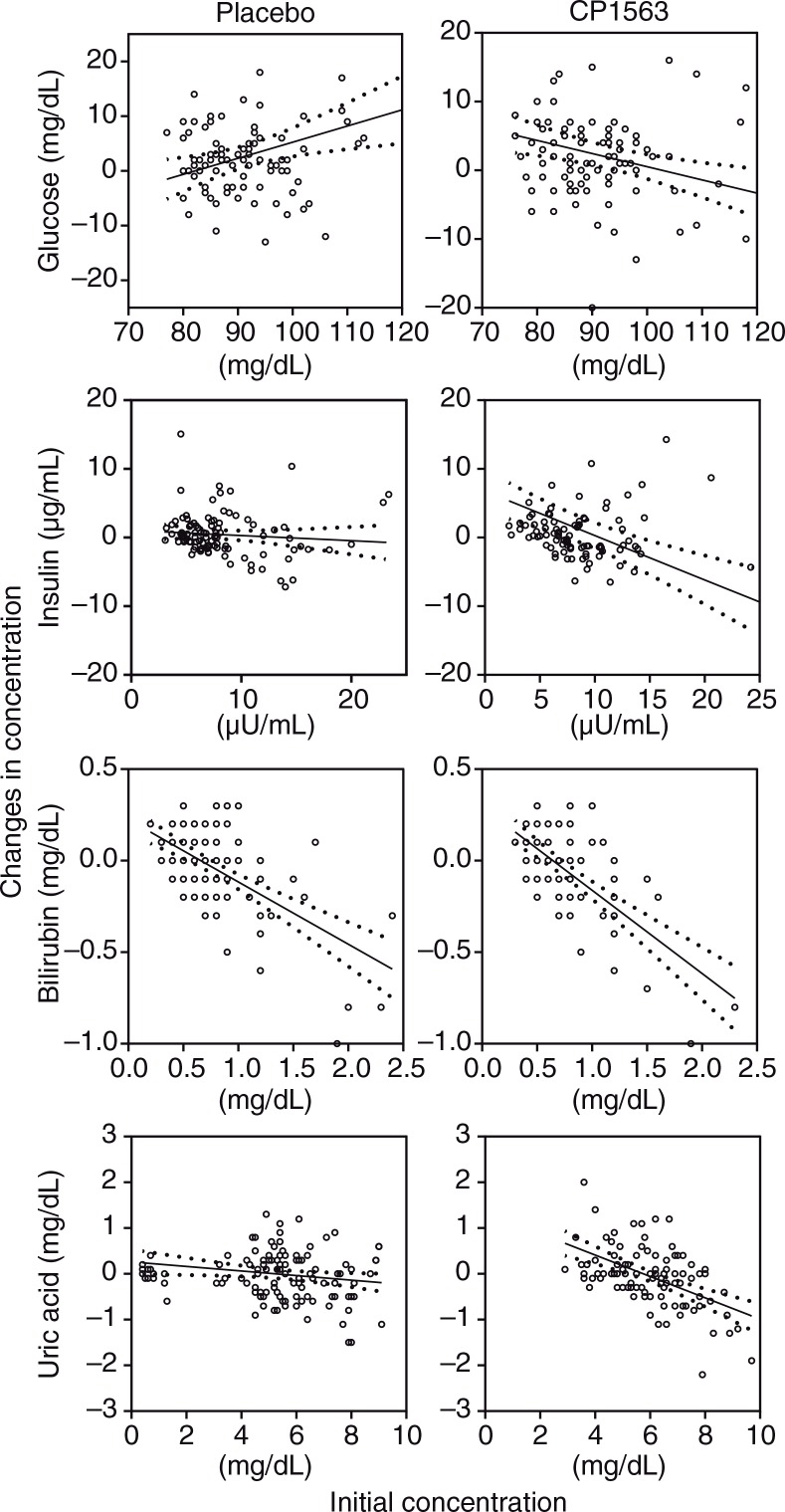
Blood glucose, insulin, and homeostasis model assessment-insulin resistance (HOMA-IR) were affected by the ingestion of fragmented CP1563 (Fig. 6, Table 6), and significant differences between the placebo and test groups were observed in these parameters (p<0.001, p=0.004, and p<0.001, respectively). However, glycosylated hemoglobin (HbA1c) levels were not significantly different between the two groups (data not shown). Total bilirubin was significantly decreased in the test group during the early stage of the time course, especially in women (Table 7; p<0.001). Uric acid was also significantly reduced in the test group (Fig. 6; p<0.001). These differences were relatively well shown in the participants who had high baseline values for these parameters.
Fig. 6.
Changes in the concentrations of chemical substance in the blood after 12 weeks of ingestion: glucose, insulin, bilirubin and uric acid. The solid line is the regression line, and the dotted line shows the 95% confidence intervals of the population mean values. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA for repeated measures.
Table 7.
Changes in hematological and serum biochemical variables during the course of the study
| Factor | Probability | Regression | |
|---|---|---|---|
| WBC (×1000/µL) | Group | p=0.017 | CP1563: δWBC=0.528−0.073×Init. WBC |
| Group×Init. WBC | p<0.001 | Placebo: δWBC=1.163−0.162×Init. WBC | |
| RBC (×10,000/µL) | Group | p=0.249 | CP1563: δRBC=43.179−0.088×Init. RBC |
| Group×Init. RBC | p<0.001 | Placebo: δRBC=29.004−0.062×Init. RBC | |
| Hemoglobin (g/dL) | Group | p=0.155 | CP1563: δHb=1.005−0.070×Init. Hb |
| Group×Init. Hb | p<0.001 | Placebo: δHb=0.513−0.041×Init. Hb | |
| Hematocrit (%) | Group | p=0.106 | CP1563: δHt=3.441−0.079×Init. Ht |
| Group×Init. Ht | p<0.001 | Placebo: δHt=1.488−0.039×Init. Ht | |
| Platelet (×10,000/µL) | Group | p=0.441 | CP1563: δPLT=2.006−0.571×Init. PLT |
| Group×Init. PLT | p=0.001 | Placebo: δPLT=2.006−0.055×Init. PLT | |
| ALT (IU/L) | Group | p=0.003 | CP1563: δALT=5.373−0.181×Init. ALT |
| Group×Init. ALT | p<0.001 | Placebo: δALT=1.105−0.039×Init. ALT | |
| AST (IU/L) | Group | p<0.001 | CP1563: δAST=5.666−0.149×Init. AST |
| Group×Init. AST | p<0.001 | Placebo: δAST=–2.302+0.193×Init. AST | |
| γ-GTP (IU/L) | Group | p=0.005 | CP1563: δγGTP=−2.351+0.080×Init. γGTP |
| Group×Init. γ-GTP | p<0.001 | Placebo: δγGTP=4.093−0.128×Init. γGTP | |
| LD (IU/L) | Group | p<0.001 | CP1563: δLD=74.931−0.388×Init. LD |
| Group×Init. LD | p<0.001 | Placebo: δLD=25.641−0.138×Init. LD | |
| TP (g/dL) | Group | p=0.011 | CP1563: δTP=2.241−0.305×Init. TP |
| Group×Init. TP | p<0.001 | Placebo: δTP=1.316−0.183×Init. TP | |
| BUN (mg/dL) | Group | p=0.030 | CP1563: δBUN=1.710−0.127×Init. BUN |
| Group×Init. BUN | p<0.001 | Placebo: δBUN=3.404−0.262×Init. BUN | |
| Uric acid (mg/dL) | Group | p=0.022 | CP1563: δUA=0.822−0.144×Init.UA |
| Group×Init. UA | p<0.001 | Placebo: δUA=0.436+0.087×Init.UA | |
| Creatinine (mg/dL) | Group | p=0.428 | CP1563: δCRE=0.046−0.062×Init. CRE |
| Group×Init. CRE | p<0.001 | Placebo: δCRE=0.033−0.040×Init. CRE | |
| Bilirubin (mg/dL) | Group | p=0.426 | CP1563: δT-Bil=0.161−0.266×Init. T-Bil |
| Group×Init. T-Bil | p<0.001 | Placebo: δT-Bil=0.137−0.223×Init. T-Bil | |
| Sodium (mEq/L) | Group | p=0.283 | CP1563: δNa=46.984−0.330×Init. Na |
| Group×Init. Na | p<0.001 | Placebo: δNa=55.558−0.391×Init. Na | |
| Potassium (mEq/L) | Group | p=0.351 | CP1563: δK=1.486−0.368×Init. K |
| Group×Init. K | p<0.001 | Placebo: δK=1.297−0.321×Init. K | |
| Chloride (mEq/L) | Group | p=0.048 | CP1563: δCL=26.277−0.257×Init. Cl |
| Group×Init. Cl | p<0.001 | Placebo: δCL=37.498−0.366×Init. Cl | |
WBC, white blood cell; RBC, red blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-glutamyl transpeptidase; LD, lactate dehydrogenase; TP, total protein; BUN, blood urea nitrogen; UA, uric acid; CRE, creatinine; T-Bil, total bilirubin; Na, sodium; K, potassium; Cl, chloride.
Statistical significance was evaluated using ANCOVA for repeated measures.
Blood test valuables and urinalysis
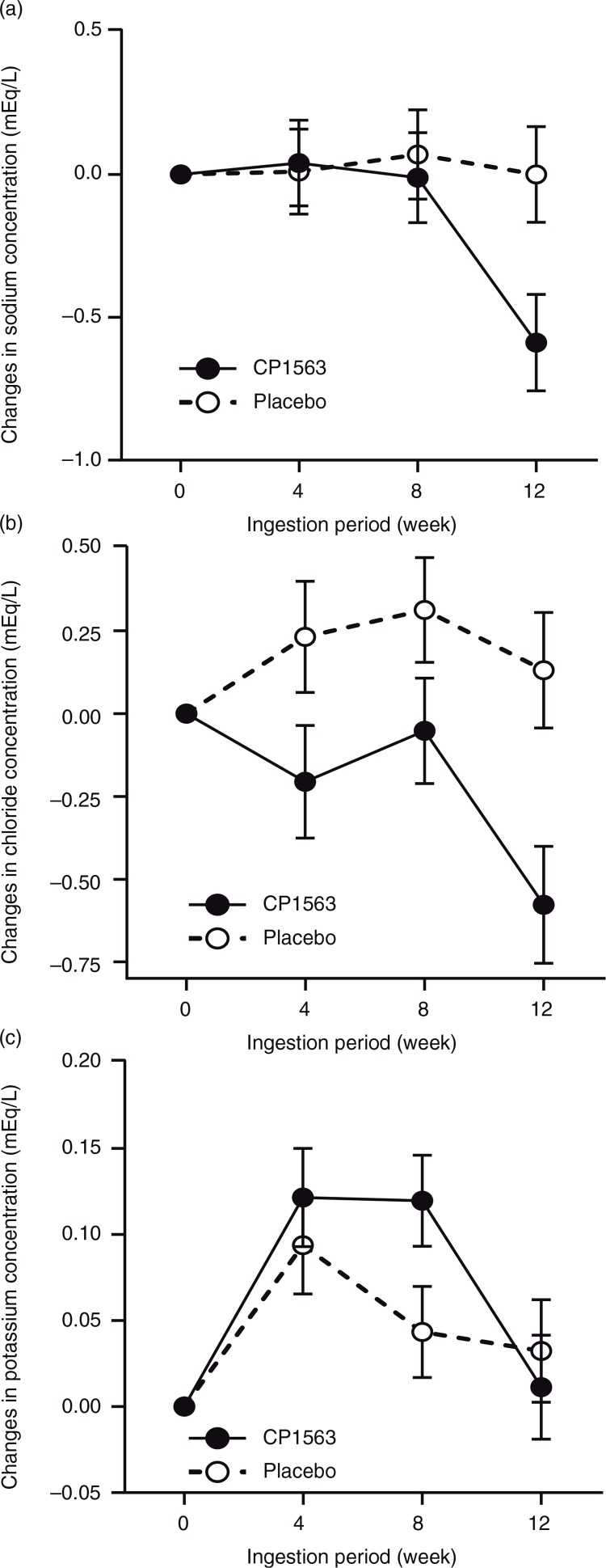
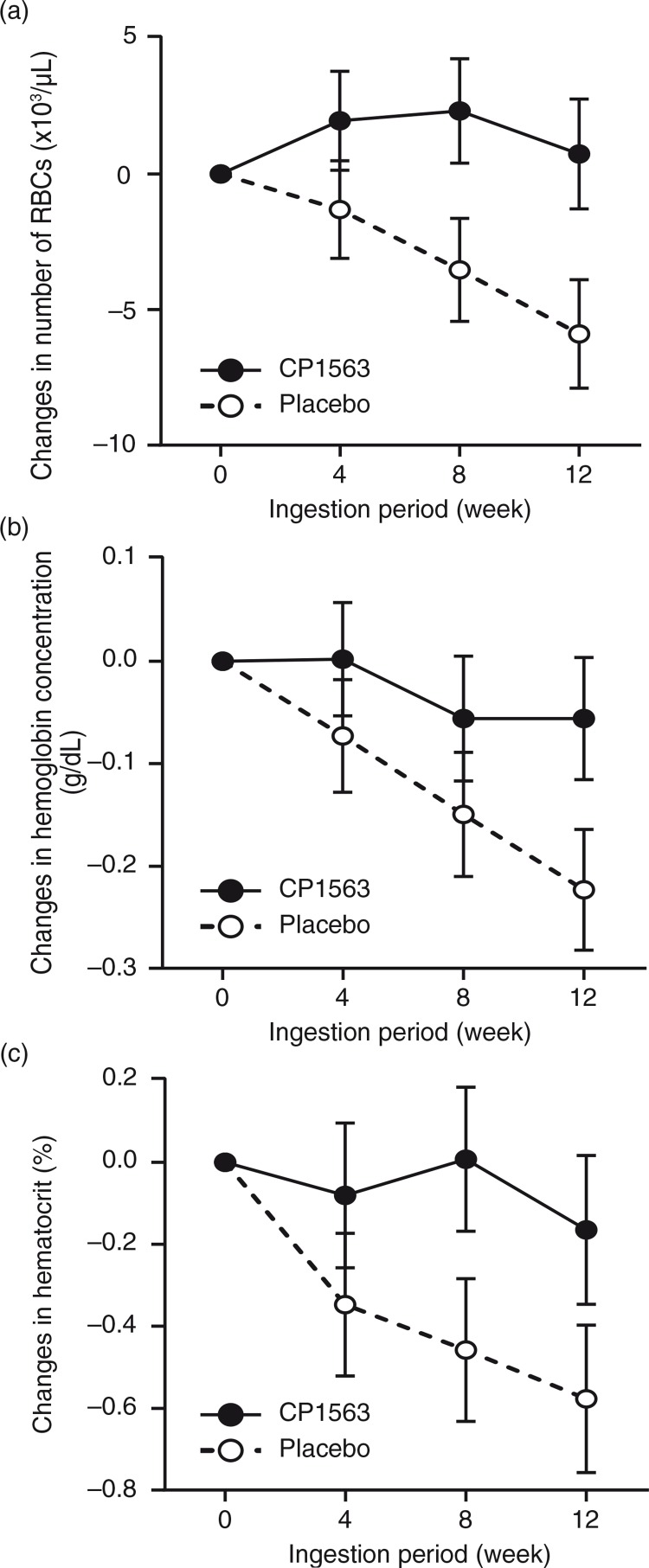
BUN, sodium, and chloride exhibited significant reductions in the test group compared with the placebo group (Table 7, Fig. 7a and b; p<0.001, p<0.001, and p<0.001, respectively). In contrast, potassium was increased in the test group (Fig. 7c; p<0.001). Red blood cells, hemoglobin, and hematocrit showed significant increases in the test group compared with the placebo group (Table 7 and Fig. 8; p<0.001, p<0.001, and p<0.001, respectively). In contrast, the white blood cell count was significantly lower in the test group than in placebo group (Table 7; p<0.001). ALT, AST, and LD exhibited significant reductions in the test group compared with those in the placebo group (Table 7; p<0.001, p<0.001, and p<0.001, respectively). In contrast, γ-GTP was increased to a greater extent in the test group than in the placebo group (Table 7; p<0.001). These changes in blood test values were, however, within the normal ranges, and even the results obtained for individual subjects failed to reveal changes that might develop into abnormal ranges (data not shown).
Fig. 7.
Changes in the plasma concentrations of chemical elements throughout the ingestion period: (a) sodium, (b) chloride and (c) potassium. Closed circles indicate the test group, and open circles indicate the placebo group. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA for repeated measures.
Fig. 8.
Changes in red blood cell counts throughout the ingestion period: (a) erythrocyte, (b) hemoglobin concentration and (c) hematocrit concentration. Closed circles indicate the test group, and open circles indicate the placebo group. Statistical significance was determined according to the interaction of the factor group×time using ANCOVA for repeated measures.
After CP1563 treatment, decreases in the number of white blood cells (WBCs) and the concentrations of AST, ALT, LD, BUN, uric acid, creatinine, sodium, and chloride; and increases in γ-GTP and potassium were observed (Table 7). However, these changes were within the normal ranges. In addition, no statistically significant changes in any of the other markers, except those mentioned above, were recorded. No abnormal changes were observed in urinalysis during or after intervention in the test and placebo groups (data not shown). Although two subjects were positive for uric protein and uric glucose during the pre-intake period, no abnormal changes were observed between the intake and post-intake periods.
Discussion
In the present study, we demonstrated that non-viable, that is, paraprobiotic, lactic acid bacteria consumption has a beneficial body fat-lowering effect on humans and affects lipid, glucose, and uric acid metabolism. Regarding the relationship between fat metabolism and the administration of lactic acid-producing bacteria, the effects of Bifidobacterium breve B-3 (15), Lactobacillus gasseri SBT2055 (16), and other bacteria on abdominal adiposity have been determined in clinical trials. Our previous study has evaluated the PPARα/γ activation potential of organic extracts from various lactic acid bacteria in vitro, and the highest activity was observed in L. amylovorus CP1563 (9). Furthermore, the anti-dyslipidemic and anti-obesity effects of fragmented CP1563 have been recorded in high-fat diet-induced obese mice, and the fragmentation of CP1563 is required for its PPARα-agonistic activity in vivo. In the present study, we demonstrated the effect of 12 weeks of ingestion of fragmented CP1563 on lipid metabolism, including decreased body fat, plasma triglycerides, and LDL-cholesterol in pre-obese and mildly obese participants. All 200 participants were confirmed to meet the criteria for investigating food for specified health use (FOSHU) for somatic fat reduction, as verified by the control doctor (KT). Significant reductions in plasma glucose, insulin, HOMA-IR, and uric acid were observed in the test groups (Fig. 6 and Table 6). The parameters used to diagnose metabolic syndrome included waist circumstance, which represents visceral fat area; insulin resistance; serum/plasma triglycerides and HDL-cholesterol; blood pressure; and fasting blood glucose. In the present study, the body fat and visceral fat areas; insulin resistance; and levels of plasma triglycerides, HDL-cholesterol, insulin, HOMA-IR, and fasting blood glucose were significantly improved in the test group compared with the placebo group (Tables 5 and 6). The results also demonstrated that a longer ingestion period is desirable to obtain health-promoting benefits. The expected changes only begin to occur during ingestion periods of at least 3 months. Regarding the average initial visceral fat area, the initial value obtained in the present study was lower than those in other studies with similar study designs: 108.1±35.8 (cm2) in the present study compared with 114–118 (cm2) in other studies (16, 17). In addition, the initial range of visceral fat area values of the participants was wider than previously reported in other studies. The initial value of the visceral fat area was inversely correlated with the reduction in visceral fat area after 12 weeks of intervention in the test group compared with the placebo group (Fig. 3), suggesting that a reduction in visceral fat area is expected when the initial value falls within a higher range. However, the whole body fat area in the participants with low visceral fat (<100 cm2) was also significantly reduced in the test group but not in the same subgroup of the placebo group. This fact may suggest that fragmented CP1563 cells work not only in the patients classified as obese class I but also in individuals with moderate levels of fat. These results suggest that the constant intake of beverages containing fragmented CP1563 could improve metabolic syndrome, although further clinical studies are needed to determine the effects of longer ingestion periods.
These findings indicate that fragmented L. amylovorus CP1563 has the same benefits as synthetic PPARα, PPARγ, or PPARα/γ agonists. Furthermore, uric acid was significantly decreased in the test group compared with the placebo group (Fig. 6). The synthetic PPARα agonist, fenofibrate, was shown to significantly decrease the concentration of serum uric acid in healthy male subjects (18). The PPARα agonist property of fragmented CP1563 likely decreased the plasma uric acid levels in the present study. The serum level of uric acid is positively correlated with the frequency of metabolic syndrome, and metabolic syndrome is associated with a high incidence of hyperuricemia (19). However, the synthetic agonists exhibit some adverse effects. Indeed, the use of glitazones leads to weight gain, edema, bone fractures, and heart failure, which limits the use of these drugs in diabetic patients with high lipid levels (10). In the present study, no adverse effects attributed to the administration of the fragmented CP1563 were observed (data not shown). Food-derived PPAR agonists, such as fragmented CP1563, are expected to be relatively safe compared with PPAR agonist drugs; and dietary approaches utilizing food ingredients with PPARα and PPARγ dual-activating properties have become important for preventing metabolic disorders.
In addition, the average reduction in visceral fat after intervention in the present study was relatively small compared with those observed in previous studies (16, 17). One limitation of this study is the seasonal variation in fat metabolism. The test beverages were consumed from September to December (autumn to winter), a period when people tend to gain weight easily. It has been reported that body weight tends to decrease in summer and increase in winter (20, 21); therefore, in the present study, the visceral fat in the test group was relatively unlikely to decrease (Fig. 2c). Another limitation is the lack of information concerning the daily food consumption and calorie intake. We did not ensure strict dietary regulations or supply the participants with study diets in the present study; instead, the participants maintained a detailed record of diet and pedometer measurements for 3 consecutive days prior to each visit. Analysis of the records revealed that the calorie intake and nutrient composition were similar between the placebo and test groups (Table 4). However, other detailed information concerning daily dietary records was absent, and the daily calorie intake and nutrition might be altered during the intervention period, although the participants were instructed to maintain healthy living habits, including diet and exercise. Furthermore, to some extent, ‘misreporting’ might have occurred. Indeed, approximately one-fifth of all study participants have been shown to engage in ‘underreporting’, whereas half of the study participants have been shown to demonstrate ‘over reporting’, which is a common phenomenon in the assessment of habitual dietary intake (22, 23).
The results of the present study suggest that fragmented CP1563 is a novel foodstuff that regulates lipid and glucose metabolism. Several food-derived natural compounds with PPARα and/or PPARγ dual-activating properties have been reported (24). For example, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are PPARα agonists, and they are representative of foods that affect lipid metabolism, as supported by abundant evidence from clinical studies (25). The dosages used in relevant studies range from 0.185 to 9 g/day and are much higher than the dosage of fragmented CP1563 used in the present study, suggesting that lower dosages of CP1563 could exhibit PPAR agonist activity equivalent to the DHA/EPA activity. Alternatively, PPARα and PPARγ dual activation could be the reason underlying the lower effective dosage of fragmented CP1563 (9). We isolated PPARα/γ agonists from CP1563 to clarify the significance of the ligands’ roles in metabolism. The exact working mechanisms of the fragmented CP1563 are now under investigation. The series of information of the working materials, their working mechanisms, their absorbency indices, and their structures will soon be published.
In lipid metabolism, a close relationship between the composition of gut microbiota and obesity might exist, although conflicting research findings have been reported (26). In the present study, we did not investigate changes in the gut microbiota during the intervention period according to CP1563 treatment, although much attention has been paid to gut microbes. If changes in gut microbiota are related to obesity, then non-viable fragmented CP1563 might affect the composition. Indeed, in the case of L. gasseri CP2305, the consumption of beverages containing this strain led to some changes in the gut microbiota (27, 28). Thus, an undefined component(s) of CP1563 might directly or indirectly influence the composition of the gut microbiota and subsequently improve glucose and fat metabolism. This possibility remains to be investigated.
In conclusion, the daily consumption of beverages containing fragmented CP1563 for 12 weeks by obese class I subjects improves anthropometric measurements and markers related to lipid and glucose metabolism without any adverse effects. Although further clinical trials and investigations of the mechanisms of action are needed, the results of the present study suggest that the consumption of foods containing fragmented CP1563 ameliorates obesity and prevents metabolic syndrome and complications.
Acknowledgements
The authors would like to thank the volunteers who participated in this study, Toshimasa Higuchi and Yusuke Shibata of Asahi Soft Drinks Co., Ltd., for producing the placebo and test beverages, and Dr. Izumi Yoshida and Dr. Yukiko Aoyama of Tempstaff Co., Ltd., for substantially contributing to the preparation of the manuscript.
Author contributions
FN, YI, KA, DS, NA, TS, and YA performed experiments and analyzed the data. IT was the allocator of the trial. KT was the control doctor for the trial. KA prepared the draft. SF designed the trial and wrote the manuscript.
Conflict of interest and funding
This research received financial support from Asahi Group Holdings, Ltd. (Tokyo, Japan). FN, YI, KA, DS, NA, TS, YA, and SF are employees of Asahi Group Holdings, Ltd. None of the other authors have any conflicts of interest.
References
- 1.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO global strategy on diet, physical activity and health. Geneva: World Health Organization; 2004. [Google Scholar]
- 5.Labonté MÈ, Couture P, Richard C, Desroches S, Lamarche B. Impact of dairy products on biomarkers of inflammation: a systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am J Clin Nutr. 2013;97:706–17. doi: 10.3945/ajcn.112.052217. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–28. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, et al. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr. 2010;140:671S–6S. doi: 10.3945/jn.109.113779. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura F, Ishida Y, Sawada D, Ashida N, Sugawara T, Sakai M, et al. Fragmented lactic Acid bacterial cells activate peroxisome proliferator-activated receptors and ameliorate Dyslipidemia in obese mice. J Agric Food Chem. 2016;64:2549–59. doi: 10.1021/acs.jafc.5b05827. doi: http://dx.doi.org/10.1021/acs.jafc.5b05827. [DOI] [PubMed] [Google Scholar]
- 10.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications – A review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–9. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajima N, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, et al. Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int. 2015;6:151–87. doi: http://dx.doi.org/10.1007/s13340-015-0206-2:1-37. [Google Scholar]
- 13.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894 i–xii, 1–253. [PubMed] [Google Scholar]
- 14.Miyazaki S. Review of clinical guidelines for the treatment of obesity (Himan - shou) Jpn J Nutr Diet. 2007;65:1–10. [Google Scholar]
- 15.Minami J, Kondo S, Yanagisawa N, Odamaki T, Xiao JZ, Abe F, et al. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci. 2015;4:e17. doi: 10.1017/jns.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, et al. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr. 2013;110:1696–703. doi: 10.1017/S0007114513001037. [DOI] [PubMed] [Google Scholar]
- 17.Ono T, Murakoshi M, Suzuki N, Iida N, Ohdera M, Iigo M, et al. Potent anti-obesity effect of enteric-coated lactoferrin: decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br J Nutr. 2010;104:1688–95. doi: 10.1017/S0007114510002734. [DOI] [PubMed] [Google Scholar]
- 18.Uetake D, Ohno I, Ichida K, Yamaguchi Y, Saikawa H, Endou H, et al. Effect of fenofibrate on uric acid metabolism and urate transporter 1. Intern Med. 2010;49:89–94. doi: 10.2169/internalmedicine.49.2597. [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–7. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Gordon DJ, Trost DC, Hyde J, Whaley FS, Hannan PJ, Jacobs DR, Jr, et al. Seasonal cholesterol cycles: the Lipid Research Clinics Coronary Primary Prevention Trial placebo group. Circulation. 1987;76:1224–31. doi: 10.1161/01.cir.76.6.1224. [DOI] [PubMed] [Google Scholar]
- 21.Kajimoto O, Kajimoto Y, Yabune M, Nakamura T, Kotani K, Suzuki Y, et al. Tea catechins with a Galloyl moiety reduce body weight and fat. J Health Sci. 2005;51:161–71. [Google Scholar]
- 22.Castro-Quezada I, Ruano-Rodríguez C, Ribas-Barba L, Serra-Majem L. Misreporting in nutritional surveys: methodological implications. Nutr Hosp. 2015;31(Suppl 3):119–27. doi: 10.3305/nh.2015.31.sup3.8760. [DOI] [PubMed] [Google Scholar]
- 23.Murakami K, Sasaki S, Okubo H, Freshmen in Dietetic Courses Study II Group Characteristics of under- and over-reporters of energy intake among young Japanese women. J Nutr Sci Vitaminol (Tokyo) 2012;58:253–62. [PubMed] [Google Scholar]
- 24.Huang TH, Kota BP, Razmovski V, Roufogalis BD. Herbal or natural medicines as modulators of peroxisome proliferator-activated receptors and related nuclear receptors for therapy of metabolic syndrome. Basic Clin Pharmacol Toxicol. 2005;96:3–14. doi: 10.1111/j.1742-7843.2005.pto960102.x. [DOI] [PubMed] [Google Scholar]
- 25.Leslie MA, Cohen DJ, Liddle DM, Robinson LE, Ma DW. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015;14:53. doi: 10.1186/s12944-015-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20:16079–94. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawada D, Sugawara T, Ishida Y, Aihara K, Aoki Y, Takehara I, et al. Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res Int. 2016;79:33–9. [Google Scholar]
- 28.Sugawara T, Sawada D, Ishida Y, Aihara K, Aoki Y, Takehara I, et al. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb Ecol Health Dis. 2016;27 doi: 10.3402/mehd.v27.30259. 30259. doi: http://dx.doi.org/10.3402/mehd.v27.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]