Abstract
The human body hosts an enormous abundance and diversity of microbes, which perform a range of essential and beneficial functions. Our appreciation of the importance of these microbial communities to many aspects of human physiology has grown dramatically in recent years. We know, for example, that animals raised in a germ-free environment exhibit substantially altered immune and metabolic function, while the disruption of commensal microbiota in humans is associated with the development of a growing number of diseases. Evidence is now emerging that, through interactions with the gut–brain axis, the bidirectional communication system between the central nervous system and the gastrointestinal tract, the gut microbiome can also influence neural development, cognition and behaviour, with recent evidence that changes in behaviour alter gut microbiota composition, while modifications of the microbiome can induce depressive-like behaviours. Although an association between enteropathy and certain psychiatric conditions has long been recognized, it now appears that gut microbes represent direct mediators of psychopathology. Here, we examine roles of gut microbiome in shaping brain development and neurological function, and the mechanisms by which it can contribute to mental illness. Further, we discuss how the insight provided by this new and exciting field of research can inform care and provide a basis for the design of novel, microbiota-targeted, therapies.
Introduction
The disruption of the microbes that are resident in our gastrointestinal tract has long been implicated in the development or exacerbation of mental disorders. There is, for example, a long history of anecdotal reports of psychiatric side-effects of antibiotics, even in those without a premorbid psychiatric history.1 There have also been attempts to influence the composition of the gut microbiota to achieve clinical benefit. For example, in the first decades of the twentieth century, probiotic preparations containing Lactobacillus strains were marketed widely as a means to improve mental health or treat psychiatric disorders.2 These approaches fell from favour in the 1920s because of a lack of mechanistic understanding and their link to the increasingly unfashionable ‘autointoxication' model. However, the interest in the role of gut microbes in mental health, and our ability to improve psychiatric wellbeing through their manipulation, is resurgent.2, 3
In this review, we consider the potential of dysbiosis to contribute to psychopathology and the evidence linking disruption of gut microbiota with specific psychiatric disorders. We examine the role of the microbiome in neurological development and regulation, and consider its contribution to aging-related morbidity. Finally, we discuss the potential for modification of the gut microbiome to provide clinical benefit in the context of altered brain function.
Regulation of neurological function by the gut microbiome
The potential contribution of bidirectional communication between the gut and central nervous system (CNS) is suggested by high rates of comorbidity between gastrointestinal and psychiatric illnesses.4, 5 For example, mood disorders affect more than half of all patients with irritable bowel syndrome,6 with antidepressants being one of the most common pharmaceutical interventions for irritable bowel syndrome.4 The gut–brain axis consists of a bidirectional communication network that monitors and integrates gut functions and link them to cognitive and emotional centres of the brain. It encompasses the central, autonomic and enteric nervous systems, as well as the neuroendocrine, enteroendocrine and neuroimmune systems.7, 8 It mediates the effects of both genetic and environmental factors on brain development and function, and has been implicated in the aetiology of a number of psychiatric disorders.9, 10, 11, 12
In recent years, we have increasingly understood the contribution made by the gut microbiome not only in the regulation of host physiology, particularly metabolism and immunity,13, 14, 15, 16, 17 but also the CNS and brain function.11, 18, 19 Given mounting evidence that the microbiome has a key role in influencing the development and function of the nervous system through its interaction with the gut–brain axis, it has been suggested that a ‘microbiome–gut–brain axis' may be a more appropriate model.19, 20, 21, 22
The delicate balance between the human microbiome and the development of psychopathologies is particularly interesting given the ease with which the microbiome can be altered by external factors, such as diet,23 exposure to antimicrobials24, 25 or disrupted sleep patterns.26 For example, a link between antibiotic exposure and altered brain function is well evidenced by the psychiatric side-effects of antibiotics, which range from anxiety and panic to major depression, psychosis and delirium.1 A recent large population study reported that treatment with a single antibiotic course was associated with an increased risk for depression and anxiety, rising with multiple exposures.27 Bercik et al.28 showed that oral administration of non-absorbable antimicrobials transiently altered the composition of the gut microbiota in adult mice and increased exploratory behaviour and hippocampal expression of brain-derived neurotrophic factor (BDNF), while intraperitoneal administration had no effect on behaviour. Alteration of brain function may therefore add to the many reasons that inappropriate antibiotic use should be avoided. It should be noted though that unchecked bacterial infection also represents an acute stressor, and has been shown to be associated with memory dysfunction in mice.29
Diet is another important determinant of gut microbiota composition and function that is strongly linked with psychopathological outcomes. For example, consumption of high fat diet (HFD) is associated with altered microbial diversity and reduced synaptic plasticity,30, 31 with increased vulnerability to anxiety-like behaviour in mice,32 while altered microbial diversity upon consumption of a diet high in sucrose results in significantly impaired development of a spatial bias for long-term memory, short-term memory and reversal training.33 In contrast, adolescent rats fed a low-calorie diet show augmented neurogenesis and BDNF levels, and improved cognition in adulthood,34 and a diet that increases microbiota diversity is associated with improved cognitive ability.35 Although human data have shown reduced microbial diversity in individuals is linked with increased adiposity, insulin resistance, dyslipidaemia and more pronounced inflammatory phenotype,36, 37 strong evidence of a direct microbiome effect comes from studies using conventionally housed mice subjected to a microbiome depletion and/or transplantation paradigm using microbiota isolated from donors on either an HFD or control diet. Following re-colonization, mice given the HFD exposed microbiota showed significant and selective disruptions in exploratory, cognitive, and stereotypical behaviour.38 Although it is not possible to exclude the direct effect of host metabolism on brain function, such findings do suggest that diet-induced changes in the intestinal microbiome substantially influence brain function.
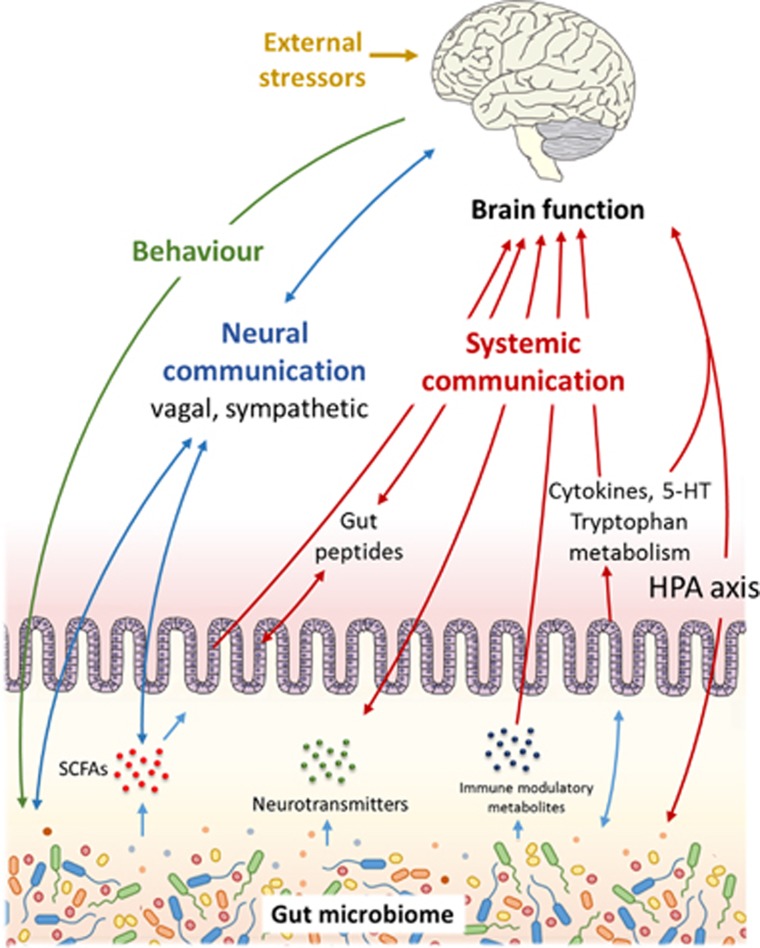
Diet and antibiotic exposure are only two factors that potentially influence brain function through shaping the gut microbiome (Figure 1). An array of common variables may be equally important. For example, alcohol consumption,39, 40 smoking habits41 and disruption of diurnal rhythm,26 have all been shown to substantially affect microbiota composition. As such, how wider influences on the microbiome contribute to dysregulation of brain function is an area of growing interest.
Figure 1.
Communication pathways linking the gut microbiome with brain function.
The microbiome in specific psychiatric conditions
While the links between the microbiome and specific psychiatric conditions have been reviewed elsewhere,18, 42, 43, 44, 45 a brief examination of the contribution of inter-kingdom interactions to two particularly distressing neuropsychiatric disorders provides a useful illustration.
Major depressive disorder (MDD) is typified by markers known to be influenced by the microbiome. For example, depression-associated changes seen in the hypothalamic-pituitary-adrenal (HPA) stress response,46 and altered levels of depression-associated monoamines (or their receptors) in corticolimbic regions of the brain, have both been demonstrated in germ-free (GF) mice.28, 47, 48, 49, 50 The increased concentrations of pro-inflammatory cytokines seen in MDD46 may also result from interactions with gut microbes. Levels of serum antibodies against lipopolysaccharide from gram-negative enterobacteria are higher in patients with MDD than in controls,51 and cause stress-associated with increased gut permeability and bacterial translocation in animal models.22, 52 Evidence also exists that depression alters the gut microbiota, as demonstrated in mice in which chronic depression- and anxiety-like behaviours has been induced by olfactory bulbectomy,53 suggesting a feedback loop between depressive states and dysbiosis. A reflection of the importance of this circular relationship may be the existence of host mechanisms that regulate microbiota composition.54, 55
Similar parallels between dysbiosis and psychopathogenesis exist in schizophrenia (reviewed by Dinan et al.56 and Nemani et al.44). Many of the strongest associations identified between genetic risk and schizophrenia relate to genes involved in immunity,57, 58 paralleling clinical studies that report an upregulated immune and inflammatory status in schizophrenia patients.59, 60, 61, 62, 63, 64, 65, 66, 67, 68 Serological markers of bacterial translocation are also substantially elevated in schizophrenia subjects and significantly correlated with systemic inflammatory markers.69 In turn, cytokine levels are correlated with the severity of clinical symptoms,59, 70, 71 and it has been suggested that the resulting neuroinflammation is involved directly in schizophrenia pathogenesis.72, 73, 74
As described later, the microbiota also modulate a range of neurotrophins and proteins involved in brain development and plasticity.48, 49, 75 There is evidence that such alterations are central to the pathophysiology of schizophrenia. For example, BDNF expression is believed to have a role in the molecular mechanism underlying altered cognition,76 and through its influence on brain plasticity, may contribute to the N-methyl-d-aspartate receptor dysfunction seen in schizophrenia.77
Treatment interactions with the microbiome in mental illness
In addition to influencing psychopathogenesis directly, the gut microbiome makes an important contribution to drug metabolism, and potentially explains much of the inter-individual variability in treatment efficacy and side-effects.78, 79 For example, the gut microbiota has been implicated in the reductive metabolism of psychotropic medications, including benzodiazepine clonazepam,80 risperidone81 and levodopa.82 In addition, the gut microbiome is also able to influence the gene expression of hepatic enzymes that aid in the metabolism and detoxification of drugs outside of the gut.83, 84
A reciprocal interaction also exists, with drugs used to target psychiatric or neurological disorders having the potential to affect the composition and function of the gut microbiome. For example, the atypical antipsychotic olazapine has been shown to affect microbiota composition in rats, as well as triggering inflammatory effects and weight gain,85, 86 with the co-administration of antibiotics shown to attenuate these physiological effects.87 The impact of atypical antipsychotics on the gut microbiota may therefore explain to some extent the increased levels of cardiac and metabolic disease in patients receiving these medications.88, 89, 90
The clinical implications of these pathways remain poorly understood, but suggest the utility of a precision approach to therapy, as has been advocated in psychiatry91, 92 and other disease contexts.93
The role of the microbiome in brain development
Prenatal neurodevelopment
Brain development spans the prenatal period to post adolescence and involves the interplay of genetic and environmental factors.94 Disruption of these interactions can alter normal developmental trajectories and contribute substantially to neuropsychiatric outcomes in later in life.95, 96
Neural development begins early in embryonic life with a number of important stages occurring before birth.94 Areas of the brain undergoing these events exhibit greater fragility97 and the significant impact of insults that occur during gestation is increasingly recognized.98 During this period, maternal immunity and metabolism represents a link between neurodevelopment in the womb and the external environment. Challenges to maternal homoeostasis, such as infection, poor nutrition or prenatal stress (PNS), are associated with neurodevelopmental disorders, including anxiety, autism, attention deficit hyperactivity disorder, depression and schizophrenia.99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109 Disruption of the maternal microbiome, or ‘dysbiosis', appears to act as a link between external stressors and fetal development, either by altering normal developmental cues, or through the presentation of inappropriate developmental stimuli.
The precise nature of relationships between maternal microbiome interactions, altered neurodevelopment and subsequent psychopathologies, remain poorly defined. To a large extent, this is due to the challenge of determining the relative contribution of parallel and overlapping pathways that link multiple interacting systems. Even in animal models, it is extremely difficult to identify the relative contribution of pathways by which a single factor can lead to an array of behavioural disorders. As an illustration, the consumption of a HFD during pregnancy is associated with subsequent behavioural disorders.110, 111 However, HFD has been shown to influence multiple regulatory pathways in the immune,112, 113 metabolic114 and neuroendocrine110 systems, through both microbiome dependent and independent mechanisms, as well as resulting in the vertical transmission of the associated dysbiosis.25 Further, the impact of an insult such as HFD consumption depends on the developmental stage at which it occurs, with similar adverse events during early or late periods associated with different outcomes.105, 115, 116
One important contributor to aberrant neurodevelopment appears to be the disruption of the immuno-regulatory role of the gut microbiome, resulting in a pro-inflammatory maternal state. Increased levels of circulating cytokines during pregnancy have been shown to negatively affect neural development 110 and could act by altering the fetal immune milieu (reviewed in detail elsewhere94, 117, 118, 119).
Immune-dysregulation could result from factors that ablate the normal microbiota, such as antibiotics, thereby suppressing microbial interactions with toll-like receptors and Treg cells in the gut120, 121, 122 or the production of immuno-regulatory metabolites, such as short-chain fatty acids (SCFAs).122, 123, 124 Alternatively, factors that trigger dysbiosis, such as high fat consumption, could act by promoting the production of pro-inflammatory bacterial metabolites.125 In addition, the dysbiotic changes in the gut microbiota could influence inflammation and CNS function through changes in activation of vagal and/or spinal nerve pathways.22, 108, 126, 127 The contribution of such a microbiota-immune interaction to stress-associated pathologies is supported by the observation that exposure to repeated stress affects the gut microbiota in a manner that correlates with changes in levels of pro-inflammatory cytokines.128
The maternal HPA axis is likely to represent another important link between prenatal insults and developmental abnormalities. The HPA axis is affected by factors such as PNS129, 130 and infection,131 which are risk factors for a wide range of neurodevelopmental disorders.132, 133, 134, 135, 136 In animal models of early-life postnatal stress, hyper-responsiveness of the HPA axis is coupled with altered visceral pain sensitivity and impaired intestinal barrier function,137, 138 while aberrant dietary protein:carbohydrate ratios during gestation have moderate long-term effects on the function of the HPA and sympatho-adrenomedullary axes in offspring.139 It is useful to note direct responses to in utero stressors such as hypoxia also involve the adrenal system140, 141 and are essential to fetal survival and neurodevelopment.142 Whether the maternal microbiome can influence these pathways remains unknown.
The manner in which a hyperactive maternal HPA stress response influences fetal development is unclear; however, an emerging hypothesis involves maternal cortisol crossing the placenta in a quantity sufficient to affect gene expression in fetal brain cells.143 This model is supported by in vitro analysis of human fetal brain aggregates144 and the observation that the effects of PNS on offspring can be partially mimicked by giving pregnant animals a synthetic glucocorticoid or adrenocorticotropic hormone.130, 145 However, the interaction of the HPA axis with the maternal microbiome is likely to be complex. In addition to affecting fetal neurodevelopment directly, stress-induced alterations to the HPA axis trigger maternal gut dysbiosis.146 These changes in the gut microbiota could further influence HPA axis dysfunction through altered tryptophan metabolism, as well as contributing to other dysbiosis-associated dysregulatory pathways.94 In addition, there is evidence that the gut microbiome influences the function of the placenta via the HPA axis, thereby altering fetal exposure to specific compounds in maternal circulation.147, 148, 149, 150
The maternal gut microbiota could also affect fetal neurodevelopment by influencing levels of circulating 5-hydroxytryptamine (5-HT). The gut microbiome regulates 5-HT biosynthesis by enterochromaffin (EC) cells in the gut.13 In turn, 5-HT regulates fetal neuronal cell division, differentiation and synaptogenesis151 and its depletion results in altered brain development.152 Furthermore, maternal plasma serotonin is required for proper neuronal morphogenesis during developmental stages that precede the appearance of serotonergic neurons, with embryos depending more on maternal plasma serotonin than their own during in utero development.153 Maternal gut dysbiosis is also likely to influence blood–brain barrier (BBB) formation, a critical component in CNS development, ensuring an optimal microenvironment for neuronal growth and specification.154 This is suggested by analysis of the embryos of GF mice, where the BBB has been shown to be substantially compromised.155
Postnatal neurodevelopment
Neurodevelopment continues outside the womb with the neonatal period characterized by substantial neurological development, including morphological changes, cell differentiation and acquisition of function.156, 157 Synaptogenesis begins shortly after birth and reaches maximum levels by around 2 years of age, before a process of synaptic refinement and elimination reduces the number of synapses in the postnatal brain to adult levels by mid-adolescence.158 Remodelling continues well into the third decade of life,159 providing a lengthy window of vulnerability to external perturbations. This critical period of neurodevelopment parallels the establishment and maturation of the microbiome, a process now known to be essential for the establishment of normal immune function,160, 161, 162, 163, 164 the neuroendocrine system165 and metabolic regulation.166, 167 Disruption of the microbiome in early life therefore has the potential to influence neurodevelopment and long-term mental health outcomes, particularly through its interaction with the immune system and the gut–brain axis.
Gnotobiotic animal models have been important in demonstrating the contribution of the developing microbiome to early-life neurodevelopment and the establishment of appropriate stress responses. For example, GF mice have an exaggerated hypothalamic-pituitary response to mild restraint stress, with elevated plasma adrenocorticotropic hormone and corticosterone and reduced BDNF expression levels in the cortex and hippocampus.49 Furthermore, mice that develop in the absence of microbes exhibit increased motor activity and reduced anxiety, associated with differential expression of synaptophysin and PSD-95, proteins that are specifically involved in synaptogenesis pathways.48 Microbial colonization is also required for programming and presentation of normal social behaviours, and is important for the regulation of repetitive behaviours,168 the development of non-spatial memory,29 and the development of pain signalling from the body.169 It is important to note that the absence of appropriate microbial developmental cues in early-life can result in aberrant mental development that is not corrected by later microbial exposure (Neufeld et al.).170
It is clear from these and other GF animal studies that the absence of a commensal microbiota during early-life substantially affects both neurophysiology and the risk of abnormal behaviour development. However, while a useful tool for highlighting mechanistic pathways, the GF animal poorly reflects the types of microbiome disruption that may occur in humans. As such, other investigations have attempted to recreate real-world early-life insults in the controlled context of animal models. For example, while associations between caesarean-section delivery, altered early life microbial colonization171, 172 and the incidence of behavioural disorders and abnormal cognitive development in humans173, 174, 175 have been known for some time, the extent to which a direct causal relationship exists is difficult to discern, given the number of other potentially contributing variables. However, when vaginally delivered mouse pups are compared with those delivered via caesarean section they show an altered gut microbiome and increased anxiety, social deficits and repetitive behaviours reminiscent of autism spectrum disorder-like behaviours in humans.176
Even in animal models though, the line between pre- and post-delivery periods is blurred by factors such as the vertical transmission of microbiota, the influence of the maternal microbiome of milk composition,177 and the continuation of stressors in the external environment. An example of this complexity is the impact of PNS on neurodevelopment. PNS has been shown to alter the composition of the gut178 and maternal vaginal microbiota in mice,98, 179 thereby altering the pool of microbes that can be passed to the neonate (an analogous situation has been described in humans, where PNS has been shown to affect the composition of the human infant gut microbiota over the first 110 days after birth180). As above although PNS also alters prenatal development, and therefore the nature of interactions between the neonate and microbes in early life. Determining the relative contribution and timing of contributory pathways to long-term psychopathological outcomes is therefore challenging.
The lasting impact of antibiotic exposure on the microbiome, whether during pregnancy,181, 182 intrapartum183 or in the neonatal period24, 184 is an example of a further complex factor. There is clear potential for antibiotic dysbiosis to contribute to maternally mediated antenatal neurodevelopment, while antibiotic dysbiosis is also heritable.25 Early-life exposure to antibiotics has been shown to result in long-term immune dysregulation185 and visceral hypersensitivity.186 Further, the developmental impact of antibiotic dysbiosis is not limited to the neonatal period, with adolescent rats exhibiting an altered tryptophan metabolic pathway, reduced anxiety and cognitive defects.187
Diet-induced maternal dysbiosis may also affect early-life neurodevelopment through milk composition. For example, the offspring of mice fed an HFD during lactation show developmental and neurobehavioral changes that suggest possible disruption of physical and sensory-motor maturation, and increased susceptibility to depressive and aggressive-like behaviour.188 These observations suggests further work in in relation to dietary inputs will be important in understanding brain function determinants in humans.
Mechanisms of interaction
Activation of inflammatory pathways appears to be a particularly important link between the microbiome and neonatal neurodevelopment. The gut microbiota can affect the immune system directly via activation of the vagus nerve,22, 126, 189, 190, 191 in turn triggering bidirectional communication with the CNS.192 In addition, indirect effects of the gut microbiota on the innate immune system can result in alterations in the circulating levels of pro- and anti-inflammatory cytokines that directly affect brain function.
Bacterial metabolites from the gut have a substantial influence on the regulation of the gut–brain axis and local and systemic immunity. SCFAs, produced by the bacterial fermentation of dietary carbohydrates, have immunomodulatory properties121, 123, 124, 193 and can interact with nerve cells by stimulating the sympathetic and autonomic nervous system via G-protein-coupled (GPR) receptor 41 (GPR41)194 and GPR43.195 In addition, they can cross the BBB, modulate brain development and behaviour196, 197, 198 and have been implicated in the development of autism.199 Further, gut microbiota derived SCFAs have been shown to regulate microglia homoeostasis,200 necessary for proper brain development and brain tissue homoeostasis.201, 202, 203 GF mice display global defects in microglia with altered cell proportions and an immature phenotype, leading to impaired innate immune responses in the CNS.200 SCFAs also regulate the release of gut peptides from enteroendocrine cells,204 which in turn affect gut–brain hormonal communication.205, 206 SCFAs have recently been shown to regulate the synthesis of gut-derived 5-HT from EC cells.13 The gut provides ~95% of total body 5-HT,207 most of which exists in plasma. Although this source of 5-HT has intrinsic roles within the gut208, 209 and peripherally in metabolic control,210 EC cell 5-HT can activate afferent nerve endings to signal to the CNS.211 Furthermore, this source of 5-HT has significant links to psychiatric disorders with the most commonly used antidepressant, fluoxetine, blocking the transport of gut 5-HT into plasma, while elevated plasma serotonin is observed in 25–50% of children with autism212, 213, 214, 215 and an inverse correlation between high plasma serotonin and low serotonergic neurotransmission has been demonstrated in young male adults with autism spectrum disorder.216 In addition to SCFAs, gut bacteria are also capable of producing an array of other neuroactive and immunomodulatory compounds, including dopamine,217 γ-aminobutyric acid,218 histamine219 and acetylcholine,220 while the gut microbiome is an important regulator of bile acid pool size and composition,221 and, in turn, BBB integrity and HPA function.222
The gut microbiota could also contribute to the regulation of brain function by influencing tryptophan metabolism (reviewed by O'Mahony and colleagues95). Tryptophan is an essential, diet-derived, amino acid,223 required for serotonin synthesis in the CNS.224 Once absorbed from the gut, tryptophan can cross the BBB and participate in serotonin synthesis.224 However, there are many other pathways through which tryptophan can be metabolized,224 including the largely hepatic kynurenine pathway225 and the major serotonin synthesis pathway in gut EC cells.226, 227, 228
The availability of tryptophan is heavily influenced by the gut microbiota. GF mice have been shown to have increased plasma tryptophan concentrations,47, 48 which can be normalized following post-weaning colonization.47 Resident gut bacteria can utilize tryptophan for growth229 and in some cases, production of indole,230, 231 or serotonin (reviewed by O'Mahony and colleagues95), while the microbiota might also affect tryptophan availability by influencing host enzymes responsible for its degradation.47 By limiting the availability of tryptophan for serotonin production in the CNS (EC-derived serotonin does not cross the BBB), the gut microbiota could influence serotonergic neurotransmission.95 In vulnerable populations, reducing the circulating concentrations of tryptophan has been shown to affect mood, and to reinstate depressive symptoms in patients who have successfully responded to selective serotonin reuptake inhibitors.232, 233 The gut microbiota could also influence the production of both neuroprotective and neurotoxic components of the kynurenine pathway.224
Other pathways by which the gut microbiota could influence the development and activity of brain tissue include regulation of the release of gut peptides from enteroendocrine cells,204 which in turn affect gut–brain hormonal communication,205, 206 and, as described above, the regulation of microglia homoeostasis.
Two recent, related papers by Wong et al. and Zheng et al. indicate that the microbiota–gut–brain axis functions in a bidirectional manner in the regulation of depressive-like behaviours. Data in the paper by Wong et al.234 demonstrate that changes in behaviour caused by increased stress levels, knockout of caspase 1 leading to decreased inflammasome function, or pharmacological treatments result in changes in the gut microbiome. The paper by Zheng et al. shows three key findings: (i) the absence of gut microbiota in GF mice resulted in decreased immobility time in the forced swimming test relative to conventionally-raised healthy control mice. (ii) From clinical sampling, the gut microbiotic compositions of MDD patients and healthy controls were significantly different from that of MDD patients. (iii) Faecal microbiota transplantation of GF mice with ‘depression microbiota' derived from MDD patients resulted in depression-like behaviours compared with colonization with ‘healthy microbiota' derived from healthy control individuals. Moreover, the concerned authors showed that mice harbouring ‘depression microbiota' primarily exhibited disturbances of microbial genes and host metabolites involved in carbohydrate and amino acid metabolism, indicating that the development of depressive-like behaviours is mediated through the host's metabolism.235 The combined findings of these two papers suggest that the microbiota–gut–brain axis is fully bidirectional, functioning in a manner through which changes in microbiota affect behaviour, while conversely, changes in behaviour brought about by chronic stress, genetic manipulation, or pharmacological intervention, result in alterations in microbiota composition. Novel approaches to target this bidirectional interface of gut microbiota and depressive-like behaviour may offer novel approaches for the treatment of major depression.
The role of the microbiome in age-related cognitive decline
Despite fluctuating in response to external influences, the gut microbiota is thought to remain relatively stable during adulthood.236 However, just as the microbiome has a critical role in the development of the nervous system in the neonate, it also appears to have a substantial influence on CNS degeneration in old age. Aging affects the brain on both cellular and functional levels, and is associated with decline in sensory, motor and higher cognitive functions.237, 238, 239 This period of life is also associated with marked changes in the microbiome.240, 241 In keeping with dysbiosis arising from a range of insults, age-related changes in gut microbiota composition appear to involve a reduction in microbial diversity, with an increased relative abundance of Proteobacteria and a reduction in bifidobacteria species, and reduced SCFA production.239
It has been suggested that the processes of age-related dysbiosis and neurological decline are linked through the former mediating chronic low-grade inflammation as a common basis for a broad spectrum of age-related pathologies, or so-called ‘inflamm-aging'.242 Inflammation has a substantial role in cognitive decline, not only in the context of normal aging but also in neurological disorders and sporadic Alzheimer's disease.243 There are a number of ways in which gut dysbiosis could contribute to this process, including direct inflammatory stimulation, the production of pro-inflammatory metabolites, and the loss of immune-regulatory function. In addition, the gut microbiome is essential to the bioavailability of polyphenols, unsaturated fats and antioxidants, all of which may help protect against neuronal and cell aging role under normal circumstances (reviewed by Caraccciolo et al.239). Notably, dysbiosis-associated inflammation is also strongly implicated in obesity and diabetes, both of which have been shown to exacerbate normal cognitive decline.244, 245, 246, 247
Age-related changes in the brain are most pronounced in the amygdala, hippocampus and frontal cortex,248 whose function is heavily dependent on serotonergic neurotransmission,249 potentially implicating microbiome-influenced changes in tryptophan metabolism. Further, altered serotonin systems could represent a common link with changes in sleep, sexual behaviour and mood in the elderly, as well as disorders such as diabetes, faecal incontinence and cardiovascular diseases.94, 250
An association between loss of microbiome function, specifically genes that encode SCFAs, and increased levels of circulating pro-inflammatory cytokines, has been shown in healthy elderly people.251 Further, markers of microbiome change are significantly correlated with diet, and with indices of frailty and poor health among long-term institutionalized people,251 while feeding cognitively healthy elderly individuals a diet low in meat and meat products is associated with subsequent increases in brain volume and cognitive function.252 Interestingly, in mice, the same HFD predisposes to physiological and anxiety-like effects in adults, while aged mice display deficits in spatial cognition,253 suggesting the effect of stressors changes during the aging process.
With a growing appreciation of the healthcare implications of an aging global population254, 255, 256 obtaining a better understanding of how the bidirectional interaction between the microbiome and gut–brain axis that influences age-related changes in brain function, must be a priority.
Modification of the gut microbiota to affect therapeutic change
As described above, studies in mice have shown that alteration of the microbial composition of the gut can induce changes in behaviour, raising the possibility of therapeutic manipulation of the microbiome. What approach might be appropriate depends on the specific role of the microbiome in pathogenesis.
In instances where the absence of particular bacterial species is linked to altered brain function, the addition of discrete microbes may be clinically effective. For example, in rats deprived of maternal contact at an early age, treatment with Bifidobacterium infantis results in normalization of the immune response, reversal of behavioural deficits, and restoration of basal noradrenaline concentrations in the brainstem,257 while in a mouse model of gastrointestinal inflammation and infection, exposure to B. longum normalizes anxiety-like behaviour.258, 259 The effects of psychosocial stress are also reversed in mice following probiotic treatments.260, 261 Such effects are not limited to rodent models; in healthy women, a probiotic cocktail alters activity of brain regions that control central processing of emotion and sensation.262 Broadly, such probiotic effects appear to mediate behavioural changes through stimulation of the vagus nerve22, 191, 258 or through modulation of cytokine production.263
Probiotic therapies have limitations, including a poor ability to establish a stable population within the recipient. Further, in many instances, pathogenesis may be contributed to by broad functions conserved across many different species, such as the ability to produce metabolites that are immunomodulatory, or that directly influence brain activity.264, 265 Here, it may be the absence of suitable drivers of beneficial behaviour that is limiting, rather than the absence of microbes capable of exhibiting them. In such instances, the broad-scale alteration of the microbiome using selective dietary microbial growth substrates, or prebiotics, may be more appropriate and result in longer lasting change. For example, consumption of fructooligosaccharides or a non-digestible galactooligosaccharide formulation (BGOS) elevates BDNF levels and NMDAR subunit expression in rats.266 BGOS consumption also reduces anxiety in mice injected with lipopolysaccharide to induce sickness behaviour, an effect that appears to be related to the modulation of cortical interleukin-1β and 5-HT2A receptor expression.267 In humans, daily consumption of BGOS for 3 weeks results in a significantly lower salivary cortisol awakening response compared with placebo and a decreased attentional vigilance to negative versus positive information.268 Pusceddu et al.269 showed that long-term supplementation with n-3 polyunsaturated fatty acids corrected dysbiosis seen in maternally separated female rats, and was associated with an attenuation of the corticosterone response to acute stress. Interestingly, while the supporting evidence for the efficacy or such approaches is only now emerging, the consumption of wholegrain and high fibre foods, essentially prebiotics, is already recommended to patients.270
Demonstrations of the transmissibility of behavioural traits between animals by faecal microbiota transfer are also intriguing. Faecal microbiota transfer is employed increasingly widely in the treatment of conditions such as recurrent Clostridium difficile infection.271 Its ability to influence behaviour suggests that it might also have a role in the treatment of psychopathology (reviewed by Collins et al.272). It is important to note, however, that these observations also raise important questions about current approaches to donor screening for therapeutic faecal microbiota transfer.
Future directions
The advances in our understanding of the role of the microbiome in neurodevelopment and mental health, particularly in the past 5 years, have been remarkable. The implications of this new insight are only beginning to become apparent; however, the potential value of microbiome analyses in revealing mechanisms that underpin altered brain development and mental illness is hugely exciting. There is now a need to close the gap between practice, including the increasing use of pro- and prebiotics, and the supporting science. The importance of achieving this is reflected in the substantial investments made to ‘microbiome–gut–brain axis' research by both the US government and the European Union.273
Achieving a better understanding of the contribution of the microbiome to mental health will require further development of analytical approaches. Studies based on reductive animal models, particularly those involving GF animals, have been important in identifying underlying mechanisms; however, they exclude the complexity of real-world interactions. The rapidly falling costs of ‘omics' approaches to microbiome analysis now allow them to be applied to large human cohorts within life-course studies, with data generated assessed in the context of detailed genetic, epigenetic, demographic and clinical assessments. Exploiting these opportunities will result in substantial improvement in our understanding of altered brain function and mental illness in the relative near-term.
In addition to changing analytical strategies, the conceptual framework within which these data are assessed must also continue to develop. A ‘three-hit' model of vulnerability and resilience to mental health issues, based on genetic predisposition, the prenatal environment, and later life experiences, has been proposed.274 However, just as the gut–brain axis might be extended to include the microbiome, such developmental pathways must also take into consideration points of interaction with our resident microbiota. Refining these models based on empirical data now represents a key challenge in understanding the processes behind altered brain function and mental illness.
The authors declare no conflict of interest.
References
- Sternbach H, State R. Antibiotics: neuropsychiatric effects and psychotropic interactions. Harv Rev Psychiatry 1997; 5: 214–226. [DOI] [PubMed] [Google Scholar]
- Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part I - autointoxication revisited. Gut Pathog 2013; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part II - contemporary contextual research. Gut Pathog 2013; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KA, Foster JA. Effects of gut microbiota on the brain: implications for psychiatry. J Psychiatry Neurosci 2009; 34: 230–231. [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol 2014; 20: 14105–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002; 122: 1140–1156. [DOI] [PubMed] [Google Scholar]
- Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol 2011; 2: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011; 62: 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13: 701–712. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10: 735–742. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013; 36: 305–312. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Doré J, Emmanuel A, Guarner F, Quigley EM. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil 2013; 25: 4–15. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015; 161: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med 2015; 21: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res 2015; 117: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini M, Piermattei A, Di Sante G, Migliara G, Delogu G, Ria F. Immunomodulation by gut microbiota: role of Toll-like receptor expressed by T cells. J Immunol Res 2014; 2014: 586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorini C, Falcone M. Shaping the (auto)immune response in the gut: the role of intestinal immune regulation in the prevention of type 1 diabetes. Am J Clin Exp Immunol 2013; 2: 156–171. [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil 2013; 25: 713–719. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 2011; 23: 187–192. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009; 136: 2003–2014. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009; 6: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Julio-Pieper M, Forsythe P, Kunze W, Dinan TG, Bienenstock J et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol 2012; 12: 667–672. [DOI] [PubMed] [Google Scholar]
- Gohir W, Whelan FJ, Surette MG, Moore C, Schertzer JD, Sloboda DM. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother's periconceptional diet. Gut Microbes 2015; 6: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012; 13: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 2014; 5: 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014; 159: 514–529. [DOI] [PubMed] [Google Scholar]
- Lurie I, Yang YX, Haynes K, Mamtani R, Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry 2015; 76: 1522–1528. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141: 599–609.e1–3. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011; 60: 307–317. [DOI] [PubMed] [Google Scholar]
- Liu Z, Patil IY, Jiang T, Sancheti H, Walsh JP, Stiles BL et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One 2015; 10: e0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Moghaddas Gholami A, Berry D, Desmarchelier C, Hahne H, Loh G et al. High-fat diet alters gut microbiota physiology in mice. ISME J 2014; 8: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes (Lond) 2013; 37: 1183–1191. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R et al. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015; 300: 128–140. [DOI] [PubMed] [Google Scholar]
- Kaptan Z, Akgün-Dar K, Kapucu A, Dedeakayoğulları H, Batu Ş, Üzüm G. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res 2015; 1618: 194–204. [DOI] [PubMed] [Google Scholar]
- Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav 2009; 96: 557–567. [DOI] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500: 541–546. [DOI] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E et al. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500: 585–588. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E 4th, Taylor CM, Welsh DA et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 2015; 77: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res 2015; 37: 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013; 8: e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One 2013; 8: e59260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N et al. The "psychomicrobiotic": targeting microbiota in major psychiatric disorders: a systematic review. Pathol Biol (Paris) 2015; 63: 35–42. [DOI] [PubMed] [Google Scholar]
- Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabuncyan S, Severance EG, Dickerson FB et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 2015; 3: e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuropsychopharmacol Biol Psychiatry 2015; 56: 155–160. [DOI] [PubMed] [Google Scholar]
- Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci 2015; 13: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol 2004; 19: 397–403. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2013; 18: 666–673. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 2011; 108: 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004; 558: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 2011; 23: 255–264, e119. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 2008; 29: 117–124. [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 2005; 113: 141–147. [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil 2013; 25: 733–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep 2015; 5: 12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A et al. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J 2013; 7: 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry 2014; 19: 1252–1257. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. Common variants conferring risk of schizophrenia. Nature 2009; 460: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016; 530: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert Rev Neurother 2007; 7: 789–796. [DOI] [PubMed] [Google Scholar]
- Song X, Fan X, Song X, Zhang J, Zhang W, Li X et al. Elevated levels of adiponectin and other cytokines in drug naïve, first episode schizophrenia patients with normal weight. Schizophr Res 2013; 150: 269–273. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 2009; 206: 587–602. [DOI] [PubMed] [Google Scholar]
- Müller N, Myint AM, Schwarz MJ. Inflammation in schizophrenia. Adv Protein Chem Struct Biol 2012; 88: 49–68. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008; 63: 801–808. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol 2011; 14: 746–755. [DOI] [PubMed] [Google Scholar]
- Francesconi LP, Ceresér KM, Mascarenhas R, Stertz L, Gama CS, Belmonte-de-Abreu P. Increased annexin-V and decreased TNF-α serum levels in chronic-medicated patients with schizophrenia. Neurosci Lett 2011; 502: 143–146. [DOI] [PubMed] [Google Scholar]
- Kunz M, Ceresér KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS et al. Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr 2011; 33: 268–274. [DOI] [PubMed] [Google Scholar]
- Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res 2012; 46: 819–824. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res 2013; 148: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liu EY, Freudenreich O, Park JH, Liu D, Wang J et al. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res 2010; 118: 211–217. [DOI] [PubMed] [Google Scholar]
- Hope S, Ueland T, Steen NE, Dieset I, Lorentzen S, Berg AO et al. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr Res 2013; 145: 36–42. [DOI] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48: 277–286. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Santos A, Lucio Teixeira A, Salgado JV. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol 2014; 12: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res 2012; 138: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr 2013; 167: 374–379. [DOI] [PubMed] [Google Scholar]
- Nieto R, Kukuljan M, Silva H. BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry 2013; 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr Bull 2012; 38: 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA. Predictors of response to antidepressants general principles and clinical implications. Psychiatr Clin North Am 2003; 26: 345–352, viii. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Malhotra AK. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin Drug Metab Toxicol 2011; 7: 9–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GW, Remmel RP. Role of the intestinal microflora in clonazepam metabolism in the rat. Xenobiotica 1984; 14: 829–840. [DOI] [PubMed] [Google Scholar]
- Taylor K, Elliott S. An unusual case of risperidone instability in a fatality presenting an analytical and interpretative challenge. Drug Test Anal 2013; 5: 748–752. [DOI] [PubMed] [Google Scholar]
- Fiddian-Green RG. Helicobacter pylori eradication and L-dopa absorption in patients with PD and motor fluctuations. Neurology 2007; 68: 1085. [DOI] [PubMed] [Google Scholar]
- Meinl W, Sczesny S, Brigelius-Flohé R, Blaut M, Glatt H. Impact of gut microbiota on intestinal and hepatic levels of phase 2 xenobiotic-metabolizing enzymes in the rat. Drug Metab Dispos 2009; 37: 1179–1186. [DOI] [PubMed] [Google Scholar]
- Claus SP, Ellero SL, Berger B, Krause L, Bruttin A, Molina J et al. Colonization-induced host-gut microbial metabolic interaction. MBio 2011; 2: e00271–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey KJ, O'Mahony SM, Schellekens H, O'Sullivan O, Bienenstock J, Cotter PD et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 2012; 221: 155–169. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Davey KJ, Cotter PD, O'Sullivan O, Crispie F, Dinan TG, Cryan JF et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry 2013; 3: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005; 80: 19–32. [DOI] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, Sparén P. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ 2000; 321: 483–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emul M, Kalelioglu T. Etiology of cardiovascular disease in patients with schizophrenia: current perspectives. Neuropsychiatr Dis Treat 2015; 11: 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Fox JC, Ng MY, Lee CM. Toward personalized medicine in the neuropsychiatric field. Int Rev Neurobiol 2011; 101: 329–349. [DOI] [PubMed] [Google Scholar]
- Alhajji L, Nemeroff CB. Personalized medicine and mood disorders. Psychiatr Clin North Am 2015; 38: 395–403. [DOI] [PubMed] [Google Scholar]
- Rogers GB, Wesselingh S. Precision respiratory medicine and the microbiome. Lancet Respir Med 2016; 4: 73–82. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 2015; 277: 32–48. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Neuropaediatric and neuroarchaeology: understanding development to correct brain disorders. Acta Paediatr 2013; 102: 331–334. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry 2012; 17: 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res 1998; 22: 304–312. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol Stress 2015; 1: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull 2008; 34: 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout RJ, Taylor VH, Boyle MH. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes Rev 2011; 12: e548–e559. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Irrsack E, Osorio C, Koch M. Prenatal LPS-exposure—a neurodevelopmental rat model of schizophrenia—differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuropsychopharmacol Biol Psychiatry 2015; 57: 17–30. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 2009; 35: 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe 2011; 17: 367–368. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol 2001; 184: 637–642. [DOI] [PubMed] [Google Scholar]
- Brown PL, Shepard PD, Elmer GI, Stockman S, McFarland R, Mayo CL et al. Altered spatial learning, cortical plasticity and hippocampal anatomy in a neurodevelopmental model of schizophrenia-related endophenotypes. Eur J Neurosci 2012; 36: 2773–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Bale TL. Prenatal programing: at the intersection of maternal stress and immune activation. Horm Behav 2012; 62: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AH, O'Connor TG, Roth C, Susser E, Bjørke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci 2013; 7: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun 2008; 22: 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci 2005; 119: 293–301. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Riper KM, Lockard R, Valleau JC. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm Behav 2015; 76: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan PO. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 2014; 272: 92–101. [DOI] [PubMed] [Google Scholar]
- Strandberg L, Verdrengh M, Enge M, Andersson N, Amu S, Onnheim K et al. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One 2009; 4: e7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwaerde C, Delanoye A, Macia L, Tailleux A, Wolowczuk I. Influence of high-fat feeding on both naive and antigen-experienced T-cell immune response in DO10.11 mice. Scand J Immunol 2006; 64: 457–466. [DOI] [PubMed] [Google Scholar]
- Choi MS, Kim YJ, Kwon EY, Ryoo JY, Kim SR, Jung UJ. High-fat diet decreases energy expenditure and expression of genes controlling lipid metabolism, mitochondrial function and skeletal system development in the adipose tissue, along with increased expression of extracellular matrix remodelling- and inflammation-related genes. Br J Nutr 2015; 113: 867–877. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect 2000; 108: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 2013; 43: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P, Nanan R. Foetal immune programming: hormones, cytokines, microbes and regulatory T cells. J Reprod Immunol 2014; 104-105: 2–7. [DOI] [PubMed] [Google Scholar]
- Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep 2015; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms. Adv Neurobiol 2015; 10: 269–283. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500: 232–236. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BO, Kim SH, Kong GY, Kim DH, Kwon MS, Lee SU et al. Selective novel inverse agonists for human GPR43 augment GLP-1 secretion. Eur J Pharmacol 2015; 771: 1–9. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 2011; 13: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev 2013; 35: 51–65. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421: 384–388. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav 2011; 105: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011; 25: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev 2010; 35: 17–22. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 2008; 32: 1073–1086. [DOI] [PubMed] [Google Scholar]
- McCann SM, Antunes-Rodrigues J, Franci CR, Anselmo-Franci JA, Karanth S, Rettori V. Role of the hypothalamic pituitary adrenal axis in the control of the response to stress and infection. Braz J Med Biol Res 2000; 33: 1121–1131. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Moghadam AA, Cordner ZA, Tamashiro KL. Prenatal stress and stress coping style interact to predict metabolic risk in male rats. Endocrinology 2014; 155: 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJ. Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front Psychol 2011; 1: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med 2014; 44: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry 2002; 180: 502–508. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 2003; 44: 810–818. [DOI] [PubMed] [Google Scholar]
- Dumont TM, Rughani AI, Penar PL, Horgan MA, Tranmer BI, Jewell RP. Increased rate of complications on a neurological surgery service after implementation of the Accreditation Council for Graduate Medical Education work-hour restriction. J Neurosurg 2012; 116: 483–486. [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol 2002; 283: G1257–G1263. [DOI] [PubMed] [Google Scholar]
- Otten W, Kanitz E, Tuchscherer M, Gräbner M, Nürnberg G, Bellmann O et al. Effects of low and high protein:carbohydrate ratios in the diet of pregnant gilts on maternal cortisol concentrations and the adrenocortical and sympathoadrenal reactivity in their offspring. 2013. J Anim Sci 2013; 91: 2680–2692. [DOI] [PubMed] [Google Scholar]
- Keating DJ, Rychkov GY, Adams MB, Holgert H, McMillen IC, Roberts ML. Opioid receptor stimulation suppresses the adrenal medulla hypoxic response in sheep by actions on Ca(2+) and K(+) channels. J Physiol 2004; 555: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating DJ, Rychkov GY, Roberts ML. Oxygen sensitivity in the sheep adrenal medulla: role of SK channels. Am J Physiol Cell Physiol 2001; 281: C1434–C1441. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL Do catecholamines contribute to the effects of neonatal hypoxia on development of brain and heart? Influence of concurrent alpha-adrenergic blockade on ornithine decarboxylase activity. Int J Dev Neurosci 1987; 5: 135–143.. [DOI] [PubMed]
- Glover V. Prenatal stress and child outcomes. In: Antonelli MC (ed). Advances in Neurobiology Perinatal Programming of Neurodevelopment. Springer: New York, 2015, pp 269–283. [Google Scholar]
- Salaria S, Chana G, Caldara F, Feltrin E, Altieri M, Faggioni F et al. Microarray analysis of cultured human brain aggregates following cortisol exposure: implications for cellular functions relevant to mood disorders. Neurobiol Dis 2006; 23: 630–636. [DOI] [PubMed] [Google Scholar]
- Crudo A, Suderman M, Moisiadis VG, Petropoulos S, Kostaki A, Hallett M et al. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology 2013; 154: 1168–1180. [DOI] [PubMed] [Google Scholar]
- Golubeva AV, Crampton S, Desbonnet L, Edge D, O'Sullivan O, Lomasney KW et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015; 60: 58–74. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007; 113: 1–13. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Bréant B, Hahn T, Darnaudéry M et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab 2007; 292: E1526–E1533. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol 2005; 186: R7–R12. [DOI] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, O'Connor TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology 2009; 34: 430–435. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 2003; 4: 1002–1012. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res 1997; 760: 68–73. [DOI] [PubMed] [Google Scholar]
- Côté F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci USA 2007; 104: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res 2003; 314: 119–129. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014; 6263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM. Genetics of childhood disorders: XLV. Autism, part 4: serotonin in autism. J Am Acad Child Adolesc Psychiatry 2002; 41: 1513–1516. [DOI] [PubMed] [Google Scholar]
- Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 2014; 20: 509–518. [DOI] [PubMed] [Google Scholar]
- Herschkowitz N, Kagan J, Zilles K. Neurobiological bases of behavioral development in the first year. Neuropediatrics 1997; 28: 296–306. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 2011; 108: 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar D, Rock KL. Microbiota signalling through MyD88 is necessary for a systemic neutrophilic inflammatory response. Immunology 2013; 140: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012; 37: 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008; 8: 411–420. [DOI] [PubMed] [Google Scholar]
- Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res 2011; 69: 465–472. [DOI] [PubMed] [Google Scholar]
- Clarke G, O'Mahony SM, Dinan TG, Cryan JF. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr 2014; 103: 812–819. [DOI] [PubMed] [Google Scholar]
- Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev 2015; 73: 32–40. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol 2014; 5: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry 2014; 19: 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA 2008; 105: 2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol 2011; 4: 492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010; 107: 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 2010; 86: 13–15. [DOI] [PubMed] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry 2004; 61: 618–627. [DOI] [PubMed] [Google Scholar]
- Li HT, Ye R, Achenbach TM, Ren A, Pei L, Zheng X et al. Caesarean delivery on maternal request and childhood psychopathology: a retrospective cohort study in China. BJOG 2011; 118: 42–48. [DOI] [PubMed] [Google Scholar]
- Al Khalaf SY, O'Neill SM, O'Keeffe LM, Henriksen TB, Kenny LC, Cryan JF et al. The impact of obstetric mode of delivery on childhood behavior. Soc Psychiatry Psychiatr Epidemiol 2015; 50: 1557–1567. [DOI] [PubMed] [Google Scholar]
- Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol 2014; 817: 373–403. [DOI] [PubMed] [Google Scholar]
- Thum C, Cookson AL, Otter DE, McNabb WC, Hodgkinson AJ, Dyer J et al. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J Nutr 2012; 142: 1921–1928. [DOI] [PubMed] [Google Scholar]
- Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun 1974; 9: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015; 156: 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015; 53: 233–245. [DOI] [PubMed] [Google Scholar]
- Fåk F, Ahrné S, Molin G, Jeppsson B, Weström B. Microbial manipulation of the rat dam changes bacterial colonization and alters properties of the gut in her offspring. Am J Physiol Gastrointest Liver Physiol 2008; 294: G148–G154. [DOI] [PubMed] [Google Scholar]
- Tormo-Badia N, Håkansson Å, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol 2014; 80: 250–260. [DOI] [PubMed] [Google Scholar]
- Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG; doi: 10.1111/1471-0528.13601; e-pub ahead of print. [DOI] [PubMed]
- Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol 2009; 56: 80–87. [DOI] [PubMed] [Google Scholar]
- Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 2014; 20: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014; 277: 885–901. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 2015; 48: 165–173. [DOI] [PubMed] [Google Scholar]
- Giriko CÁ, Andreoli CA, Mennitti LV, Hosoume LF, Souto Tdos S, Silva AV et al. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int J Dev Neurosci 2013; 31: 731–739. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 2006; 89: 350–357. [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol 2013; 304: G211–G220. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 2009; 29: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 2003; 62: 67–72. [DOI] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 2011; 108: 8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013; 154: 3552–3564. [DOI] [PubMed] [Google Scholar]
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res 2007; 176: 149–169. [DOI] [PubMed] [Google Scholar]
- MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res 2011; 217: 47–54. [DOI] [PubMed] [Google Scholar]
- Macfabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis 2012; 23; doi: 10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 2014; 37: 197–206. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015; 18: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009; 27: 119–145. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014; 15: 300–312. [DOI] [PubMed] [Google Scholar]
- Schéle E, Grahnemo L, Anesten F, Hallén A, Bäckhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 2013; 154: 3643–3651. [DOI] [PubMed] [Google Scholar]
- Cameron J, Doucet E. Getting to the bottom of feeding behaviour: who's on top? Appl Physiol Nutr Metab 2007; 32: 177–189. [DOI] [PubMed] [Google Scholar]
- Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology 2007; 132: 2116–2130. [DOI] [PubMed] [Google Scholar]
- Erspamer V. Pharmacology of indole-alkylamines. Pharmacol Rev 1954; 6: 425–487. [PubMed] [Google Scholar]
- Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 2010; 138: 659–670.e1–2. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ et al. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 2011; 301: G519–G527. [DOI] [PubMed] [Google Scholar]
- Young RL, Lumsden AL, Keating DJ. Gut serotonin is a regulator of obesity and metabolism. Gastroenterology 2015; 149: 253–255. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol 1997; 273: G422–G435. [DOI] [PubMed] [Google Scholar]
- Cook EH Jr, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX. Autistic children and their first-degree relatives: relationships between serotonin and norepinephrine levels and intelligence. J Neuropsychiatry Clin Neurosci 1990; 2: 268–274. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F et al. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biol Psychiatry 1999; 45: 158–163. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Bujas-Petkovic Z, Vragovic R, Vuk T, Hock K, Jernej B. Hyperserotonemia in adults with autistic disorder. J Autism Dev Disord 2007; 37: 1934–1940. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry 2004; 43: 491–499. [DOI] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ et al. Serotonergic responsivity in male young adults with autistic disorder. Results of a pilot study. Arch Gen Psychiatry 1989; 46: 213–221. [DOI] [PubMed] [Google Scholar]
- Tsavkelova EA, Botvinko IV, Kudrin VS, Oleskin AV. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem 2000; 372: 115–117. [PubMed] [Google Scholar]
- Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 2012; 113: 411–417. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012; 7: e31951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M, Rowatt E. The production of acetylcholine by a strain of Lactobacillus plantarum. J Gen Microbiol 1947; 1: 279–298. [DOI] [PubMed] [Google Scholar]
- Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014; 30: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin M, Frampton G, Quinn M, Divan A, Grant S, Patel N et al. Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol Endocrinol 2015; 29: 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc'h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011; 41: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 2006; 8: 1–27. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012; 13: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013; 10: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller R. Serotonin and GI clinical disorders. Neuropharmacology 2008; 55: 1072–1080. [DOI] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 2013; 20: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan TW, Doran TI, Straus DC, Mattingly SJ. Growth and amino acid requirements of various strains of group B streptococci. J Clin Microbiol 1978; 7: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Young KD. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 2013; 159: 402–410. [DOI] [PubMed] [Google Scholar]
- Lee Y, Yeom J, Kim J, Jung J, Jeon CO, Park W. Phenotypic and physiological alterations by heterologous acylhomoserine lactone synthase expression in Pseudomonas putida. Microbiology 2010; 156: 3762–3772. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 1990; 47: 411–418. [DOI] [PubMed] [Google Scholar]
- Hood SD, Bell CJ, Nutt DJ. Acute tryptophan depletion. Part I: rationale and methodology. Aust N Z J Psychiatry 2005; 39: 558–564. [DOI] [PubMed] [Google Scholar]
- Wong M-L, Inserra A, Lewis MD, Mastronardi CA, Kentish S, Leong L et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 2016. (in press). [DOI] [PMC free article] [PubMed]
- Zheng P, Zeng B, Zhou C, Liu M, Xu X, Zeng L et al. Microbiome remodeling induces depression-like behaviors in a pathway that is mediated through the host's metabolism. Mol Psychiatry 2016; doi: 10.1038/mp.2016.44 (in press). [DOI] [PubMed]
- Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 2011; 140: 1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. J Int Neuropsychol Soc 2009; 15: 650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer S, Asseburg H, Kuntz S, Muller WE, Eckert GP. Effects of polyphenols on brain ageing and Alzheimer's disease: focus on mitochondria. Mol Neurobiol 2012; 46: 161–178. [DOI] [PubMed] [Google Scholar]
- Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev 2014; 136-137: 59–69. [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 2010; 5: e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 2011; 108: 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908: 244–254. [DOI] [PubMed] [Google Scholar]
- Griffin WS. Neuroinflammatory cytokine signaling and Alzheimer's disease. N Engl J Med 2013; 368: 770–771. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes (Lond) 2009; 33: 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005; 62: 1556–1560. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Chiu MJ, Wang JH, Liu HC, Shyu YI, Huang GH et al. Cognitive stimulation during hospitalization improves global cognition of older Taiwanese undergoing elective total knee and hip replacement surgery. J Adv Nurs 2012; 68: 1322–1329. [DOI] [PubMed] [Google Scholar]
- Exalto LG, Whitmer RA, Kappele LJ, Biessels GJ. An update on type 2 diabetes, vascular dementia and Alzheimer's disease. Exp Gerontol 2012; 47: 858–864. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10: 434–445. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 2009; 60: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo AR. Experimental insights into age-exacerbated cognitive dysfunction after peripheral surgery. Aging Cell 2013; 12: 523–524. [DOI] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488: 178–184. [DOI] [PubMed] [Google Scholar]
- Titova OE, Ax E, Brooks SJ, Sjögren P, Cederholm T, Kilander L et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol 2013; 48: 1443–1448. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM et al. Spatial cognition in adult and aged mice exposed to high-fat diet. PLoS One 2015; 10: e0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Simpkins JW, Ji X, Leis M, Stambler I. The critical need to promote research of aging and aging-related diseases to improve health and longevity of the elderly population. Aging Dis 2014; 6: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world—what do we know? Lancet 2015; 385: 484–486. [DOI] [PubMed] [Google Scholar]
- Chatterji S, Byles J, Cutler D, Seeman T, Verdes E. Health, functioning, and disability in older adults—present status and future implications. Lancet 2015; 385: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010; 170: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010; 139: 2102–2112.e1. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 2011; 23: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006; 55: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012; 37: 1885–1895. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144: 1394–401.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 2008; 43: 164–174. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009; 106: 3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstock TL, Mallet PE, Clayton EH. Increased plasma d-lactic acid associated with impaired memory in rats. Physiol Behav 2010; 101: 653–659. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Corona G, Mills H, Chen L, Spencer JP, Tzortzis G et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int 2013; 63: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Couch Y, Stratford M, Bannerman DM, Tzortzis G, Anthony DC et al. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav Immun 2015; 52: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 2015; 232: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu MM, El Aidy S, Crispie F, O'Sullivan O, Cotter P, Stanton C et al. N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One 2015; 10: e0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mental Health Foundation, 2007. Available at: http://www.mentalhealth.org.uk/help-information/mental-health-a-z/D/diet/ (accessed on January 2016).
- Rogers GB, Bruce KD. Challenges and opportunities for faecal microbiota transplantation therapy. Epidemiol Infect 2013; 141: 2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol 2013; 16: 240–245. [DOI] [PubMed] [Google Scholar]
- Smith PA. The tantalizing links between gut microbes and the brain. Nature 2015; 526: 312–314. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013; 38: 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]