Abstract
The inflammasome is hypothesized to be a key mediator of the response to physiological and psychological stressors, and its dysregulation may be implicated in major depressive disorder. Inflammasome activation causes the maturation of caspase-1 and activation of interleukin (IL)-1β and IL-18, two proinflammatory cytokines involved in neuroimmunomodulation, neuroinflammation and neurodegeneration. In this study, C57BL/6 mice with genetic deficiency or pharmacological inhibition of caspase-1 were screened for anxiety- and depressive-like behaviors, and locomotion at baseline and after chronic stress. We found that genetic deficiency of caspase-1 decreased depressive- and anxiety-like behaviors, and conversely increased locomotor activity and skills. Caspase-1 deficiency also prevented the exacerbation of depressive-like behaviors following chronic stress. Furthermore, pharmacological caspase-1 antagonism with minocycline ameliorated stress-induced depressive-like behavior in wild-type mice. Interestingly, chronic stress or pharmacological inhibition of caspase-1 per se altered the fecal microbiome in a very similar manner. When stressed mice were treated with minocycline, the observed gut microbiota changes included increase in relative abundance of Akkermansia spp. and Blautia spp., which are compatible with beneficial effects of attenuated inflammation and rebalance of gut microbiota, respectively, and the increment in Lachnospiracea abundance was consistent with microbiota changes of caspase-1 deficiency. Our results suggest that the protective effect of caspase-1 inhibition involves the modulation of the relationship between stress and gut microbiota composition, and establishes the basis for a gut microbiota–inflammasome–brain axis, whereby the gut microbiota via inflammasome signaling modulate pathways that will alter brain function, and affect depressive- and anxiety-like behaviors. Our data also suggest that further elucidation of the gut microbiota–inflammasome–brain axis may offer novel therapeutic targets for psychiatric disorders.
Introduction
Increasing evidence suggests an involvement of neuroinflammatory pathways in the etiopathophysiology of major depressive disorder (MDD) and antidepressant response.1, 2 Depressive symptoms are underlined by increased levels of proinflammatory cytokines (that is, interleukin (IL)-1β and IL-6), decreased levels of anti-inflammatory cytokines (that is, IL-4 and IL-10) and are associated with polymorphisms in inflammation-related genes.3, 4, 5 IL-1 receptor type-I and its ligands are expressed in brain areas relevant to stress response,6, 7, 8 and IL-1β signaling is fundamental in mediating the deleterious neurobehavioral and neuroendocrine responses to stress and adaptation.9, 10 Chronic stress or IL-1β administration triggers depressive-like behavior.11
A variety of stressors activate the inflammasome through the NLRP3 or P2X7 receptors, resulting in caspase-1 maturation that processes and releases bioactive IL-1β and IL-18.12, 13 Caspase-1 and NLRP3 mRNA are increased in blood cells of depressed patients,14 suggesting that the inflammasome is a key mediator by which physical and psychological stressors contribute to the development of depression, leading to the ‘inflammasome hypothesis' of depression.15 If that proves to be correct, caspase-1 inhibiting compounds may have antidepressant effects. Minocycline is a semisynthetic tetracycline antibiotic that inhibits caspase-1 and caspase-3 transcription and has anti-apoptotic, anti-inflammatory and neuroprotective properties as well as acute antidepressant-like effects.16, 17, 18, 19, 20, 21, 22
Caspase-1 knockout (casp1−/−) mice are overtly normal, despite having undetectable IL-1β and low IL-1α levels.23 They have decreased systemic inflammatory response and increased survival to lethal endotoxin doses when compared with wild-type (wt) mice.23, 24 This is underlined by reduced inflammation-induced brain transcription, decreased inflammasome assembly and consequently decreased circulating IL-1β and IL-18.23, 24
The microbiota–gut–brain axis is a complex multiorgan bidirectional signaling system between the microbiota and the brain that plays a fundamental role in host physiology, homeostasis, development and metabolism.25 Growing evidence shows reproducible and consistent effects of microbial states on mouse behavior, supporting a role for microbiota in modulating behavior.26, 27, 28 Differences in anxiety-related behaviors are commonly reported in mice with altered gut microbiomes, implicating the role of gut microbiota in stress and depression.29, 30 Casp1−/− mice display depressive-like behavior and anorexia after peripheral but not central LPS administration and differ in gut microbiota composition compared with wt mice.31, 32, 33 Therefore, our primary and secondary hypotheses were, respectively, (1) that decreased caspase-1 activity would result in decreased depressive-like behavior and (2) that caspase-1 inhibition using intraperitoneal minocycline administration and chronic restraint stress would result in changes in the gut microbiome. The null hypothesis was that there would be no difference in these parameters between casp1−/−, wt and minocycline-treated mice.
Materials and methods
Procedures were approved by the Animal Ethics Committees of the Australian National University and the South Australian Health and Medical Research and are in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition, 2013). Male mice (C57BL/6J background, wt, n=81; casp1−/−, n=20) aged 60–90 days were obtained from the Australian Phenomics (Canberra, ACT, Australia) or the Bioresources Facilities (Adelaide, SA, Australia). Genetic caspase-1 deficiency was confirmed by genotyping in experimental mice (Supplementary Figure S1). Littermates were group housed (Green Line IVC Sealsafe PLUS mouse, Tecniplast, Varese, Italy) in a temperature-specific (22C±1 °C) and light-specific (12 h cycles, lights on at 0700 h) pathogen-free room with water and standard regular chow ad libitum. Animals were assigned and randomized as described in the Supplementary Materials and Methods. The investigators were not blinded to group assignment. Behavioral phenotyping was performed between 0900 h and 1600 h. Animals were given 30 min of habituation to the behavioral testing room. Tests were performed from the least to the most invasive to minimize the influence of prior test history (in order: rotarod, elevated plus maze, marble burying test, open field test, sucrose preference test, novelty suppressed feeding and forced swim test; see Supplementary Materials and Methods for details).34 Following chronic restraint stress this order was reversed for a bell-shaped stress exposure (Supplementary Figure S2).
Chronic restraint stress
After baseline behavioral testing, animals were submitted to restraint stress for 21 days. Every day, mice were placed in a horizontal resting position inside a well-ventilated (12 holes, 0.5 mm diameter) 50 ml falcon tube at 1000 h and after 4–6 h they were unrestrained.
Pharmacological caspase-1 inhibition with minocycline
The wt animals were treated with either saline (0.2 ml, intraperitoneally, n=27) or minocycline (LKT Laboratories, St Paul, MN, USA; 5 mg kg−1 per day in 10 ml kg−1 saline, intraperitoneally, n=27). Treatment lasted for the same duration of the restraint procedure (21 days).
Respirometry
Minocycline- or saline-treated restrained animals were individually housed in the Promethion Metabolic Monitoring System (Sable Systems International, Las Vegas, NV, USA) for 48 h to assess the effects of minocycline on exploratory behavior, food intake, energy expenditure and volume of oxygen inhaled and of carbon dioxide exhaled at baseline and after chronic restraint.
16S rRNA analysis
Please see Supplementary Materials and Methods for a detailed explanation of the methods used for the 16S rRNA analysis. Briefly, fecal pellets were collected with autoclaved toothpicks, placed in 1.5 ml tubes, snap-frozen on dry ice and stored at −80 °C. Following DNA extraction, fecal microbiota profiling was performed by paired-end 16S rRNA gene amplicon sequencing, based on the Illumina MiSeq platform (Australia and New Zealand, VIC, Australia) to a depth of ∼40 000 reads per sample. Sequence data processing was performed as previously described.35
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 22.0 for Windows (SPSS, Chicago, IL, USA) using a general linear model for repeated measures. The effects of genotype, stress, treatment and their interaction were explored and the significance set at P<0.05. Sphericity of the variances of the groups was assessed with Mauchly's sphericity test. If the assumption of sphericity was violated, the Greenhouse–Geisser correction was generated. Effect size was reported as partial eta-squared (η2p). Significant stress × genotype or stress × treatment interaction was unpacked as described previously.36 Comparison of microbiota composition between groups (β-diversity) was performed using Bray–Curtis similarity matrices in PRIMER (v6, PRIMER-E, Plymouth, UK). Matrices were generated from sample-normalized, square-root transformed, relative operational taxonomic unit abundance. Community-level changes were assessed for significance using one-way permutational multivariate analyses of variance (PERMANOVA) tests with 9999 random permutations and at a significance threshold of P<0.01. The contribution of individual taxa to between-group variation was assessed by similarity percentage analysis, as previously reported.37 Where specific bacterial taxa were identified as contributing to change in microbiota composition, variation in their relative abundance was further assessed through Mann–Whitney U-tests between groups. Differences of median relative abundance between groups were assessed using Hodges–Lehmann estimator.
Results
Our primary outcome measure was the assessment of depressive-like behavior in the forced swim test. Secondary outcome measures included anxiety-like behavior, changes in the sucrose preference test, locomotor activity, gut microbiome and respirometry. Analyses and results of behavioral tests results are available in Supplementary Tables S1–S3.
Caspase-1 deficiency decreases depressive and anxiety-like behaviors
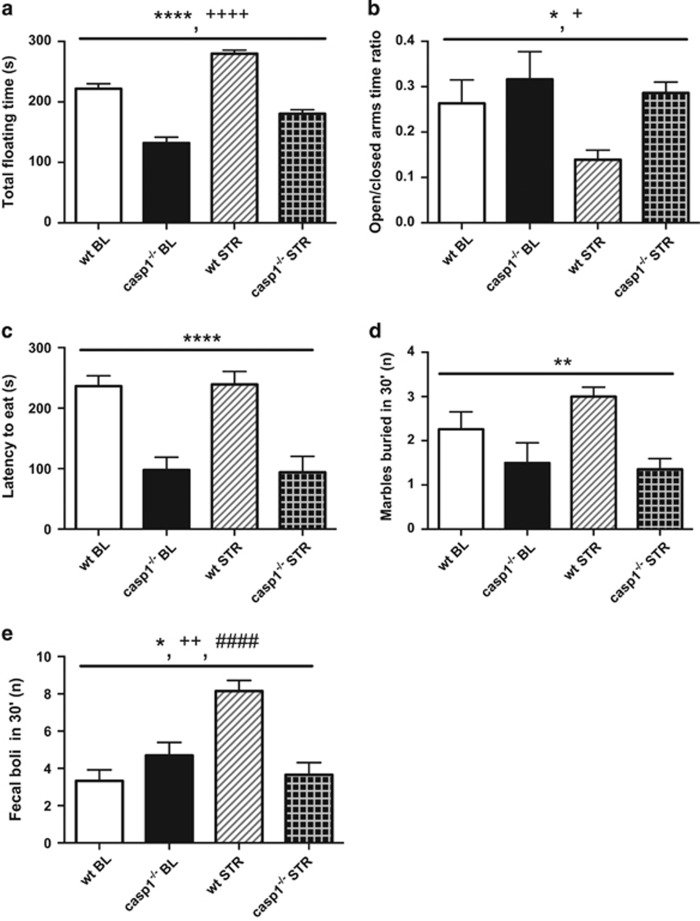
Our results showed that caspase-1 deficiency decreased depressive- and anxiety-like behaviors. In the forced swim test, the total floating time was lower in casp1−/− compared with wt mice (F1, 45=117.04, P<0.0001, Figure 1a and Supplementary Table S1). At the same time, swimming and climbing behaviors were higher in casp1−/− mice compared with wt (respectively F1, 45=117.10, P<0.0001, and F1, 45=38.69, P<0.0001). Anxiety-like behaviors had a significant main effect of genotype in 4 tests: (1) elevated plus maze, (2) novelty suppressed feeding, (3) marble burying and (4) open field tests. We found a significant main effect of genotype in the elevated plus maze open to closed arms time ratio (F1, 45=4.16, P=0.047, Figure 1b), indicating an anxiolytic phenotype in casp1−/− mice. Accordingly, in the novelty suppressed feeding, casp1−/− mice showed decreased latency to eat in a novel environment following fasting (F1, 43=32.17, P<0.0001, Figure 1c). In the marble burying test, which is considered predictive of anxiolytic compounds,38 we observed a decreased number of marbles buried by casp1−/− mice (F1, 45=11.55, P=0.001, Figure 1d). Moreover, casp1−/− mice displayed a decreased number of fecal boli during the open field test (F1, 45=4.72, P=0.035, Figure 1e), whereas no differences were observed for the time spent in the center area of the arena, another measure of anxiety-related behavior (F1, 45=0.05, P=0.826). In the sucrose preference test, casp1−/− mice displayed an increased preference for a 1% sucrose solution (F1, 33=5.52, P=0.025, Supplementary Table S1), suggesting greater hedonic behavior.
Figure 1.
Caspase-1 (casp1) deficiency decreases anxiety-like and depressive like behavior and affects chronic restraint stress response. (a) Casp1 knockout (casp1−/−) mice displayed decreased floating time in the forced swim test in comparison with wild-type (wt) mice and (b) displayed decreased anxiety-like behavior as measured by the open/closed arms time ratio in the elevated plus maze. (c) In the novelty suppressed feeding test, casp1−/− mice showed significantly decreased latency to feed following 16 h of fasting but not water deprivation. (d) Moreover, casp1 deficiency resulted in less marbles buried in the marble burying test. (e) In the open field test, we observed a decreased number of fecal boli as a result of casp1 deficiency as well as a different response to chronic restraint stress. Data are presented as mean±s.e.m. Genotype effect: *P<0.05, **P<0.01, ****P<0.0001; stress effect: +P<0.05, ++P<0.01, ++++P<0.0001; genotype × stress effect: ####P<0.0001. BL, baseline; STR, after chronic restraint stress paradigm; wt, wild type.
Caspase-1 deficiency affects chronic restraint stress response
Our results suggest that casp-1−/− mice had an attenuated response to chronic stress. We found a significant (genotype × stress) interaction for swimming and climbing time in the forced swim test (respectively F1, 45=7.02, P=0.011, and F1, 45=8.60, P=0.005). The wt mice showed a greater decrease in swimming time (70%, F1, 45=45.48, P<0.0001) than casp1−/− mice (14%, F1, 45=5.33, P=0.026) following stress. Accordingly, wt animals displayed a greater reduction in climbing time (91%, F1, 45=33.33, P<0.0001) compared with casp1−/− mice (64%, F1, 45=78.13, P<0.0001) following restraint (Figure 1a). We found a significant (genotype × stress) interaction for body weight changes (F1, 45=6.06, P=0.018) that decreased in wt mice following restraint (F1, 45=14.24, P<0.0001, average Δ body weight=−1.3 g) but remained unchanged in casp1−/− mice (F1, 45=0, P=1, average Δ body weight= 0 g). Furthermore, we found a significant (genotype × stress) interaction in the number of defecations in the open field test (F1, 45=30.93, P<0.0001, Figure 1d); casp1−/− mice did not show an increase in this parameter following restraint (F1, 45=1.73, P=0.196) whereas wt mice did (F1, 45=48.98, P<0.0001).
Caspase-1 deficiency increases locomotion and locomotor skills
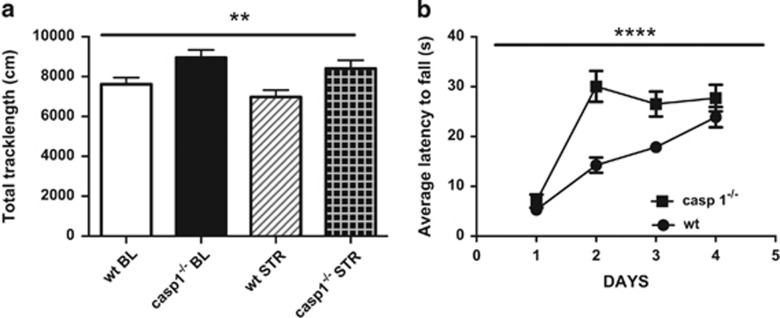
We found that caspase-1 deficiency increases locomotor activity in the open field test (F1, 45=10.54, P=0.002, Figure 2a). Moreover, casp1−/− mice acquired skills more quickly than wt mice to perform in the accelerating rotarod test (F1, 45=15.35, P<0.0001, Figure 2b and Supplementary Table S2).
Figure 2.
Caspase-1 (casp1) deficiency increases spontaneous locomotion and locomotory skills. (a) Casp1 knockout (casp1−/−) mice had increased locomotor activity in the open field test when compared with wild-type (wt) mice and (b) acquired quicker the skills to perform the rotarod test. Data are mean±s.e.m. Genotype effect: P<0.05; **P<0.01; ****P<0.0001. BL, baseline; STR, after chronic restraint stress paradigm.
Chronic restraint stress increases anxiety-like and depressive-like behaviors
Chronic restraint stress (4–6 h per day for 21 days) increased the floating time in the forced swim test (F1, 45=66.92, P<0.0001, Figure 1a), whereas it decreased swimming (F1, 45=37.80, P<0.0001) and climbing behavior (F1, 45=109.52, P<0.0001). It also increased anxiety-like behavior in the elevated plus maze test, decreasing the time spent in the open arms (F1, 45=5.65, P=0.022) and the open to closed arms time ratio (F1, 45=4.55, P=0.038, Figure 1b), as well as in the open field test, increasing the number of defecations (F1, 45=12.74, P=0.001, Figure 1e). Furthermore, restraint decreased body weight gain (F1, 45=6.06, P=0.018) and food intake (F1, 43=5.75, P=0.021). Nevertheless, restrained mice showed an increase in ratio quotient (F1, 28=4.79, P=0.037). Following restraint, no changes were observed in the sucrose preference test (F1, 33=0.05, P=0.817, Supplementary Table S1) or in locomotor activity in the open field test (F1, 45=3.64, P=0.063, Figure 2a).
Minocycline treatment affects stress response and metabolic parameters
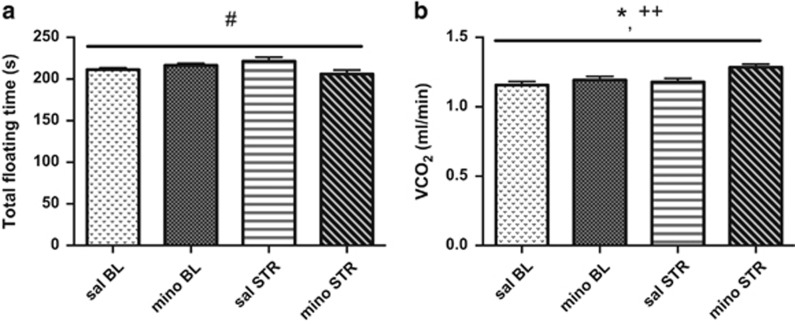
We found a significant (treatment × stress) interaction in the floating time in the forced swim test (F1, 28=6.67, P=0.015, Figure 3a and Supplementary Table S3). Saline- and minocycline-treated animals displayed similar floating times at baseline (F1, 28=2.35, P=0.137); however, minocycline-treated mice were less immobile than saline-treated mice following restraint (F1, 28=5.25, P=0.030). No differences were observed between restrained mice receiving saline or minocycline in locomotion, food intake, energy expenditure, body mass and volume of oxygen inhaled (not shown). We found a significant effect of treatment and stress on the volume of carbon dioxide exhaled (respectively F1, 28=5.64, P=0.025 and F1, 28=8.13, P=0.008, Figure 3b).
Figure 3.
Caspase-1 antagonism affects chronic restraint stress response. (a) Minocycline treatment (mino) in wild-type (wt) animals during chronic restraint stress (STR) prevented stress-induced increased floating time in the forced swim test. (b) Respirometry measurement for volume of CO2 exhaled revealed a significant effect of stress as well as treatment. Data are mean±s.e.m. Treatment effect: *P<0.05; stress effect: ++P<0.01; treatment × stress effect: #P<0.05. BL, baseline.
Chronic restraint stress affects the gut microbiome
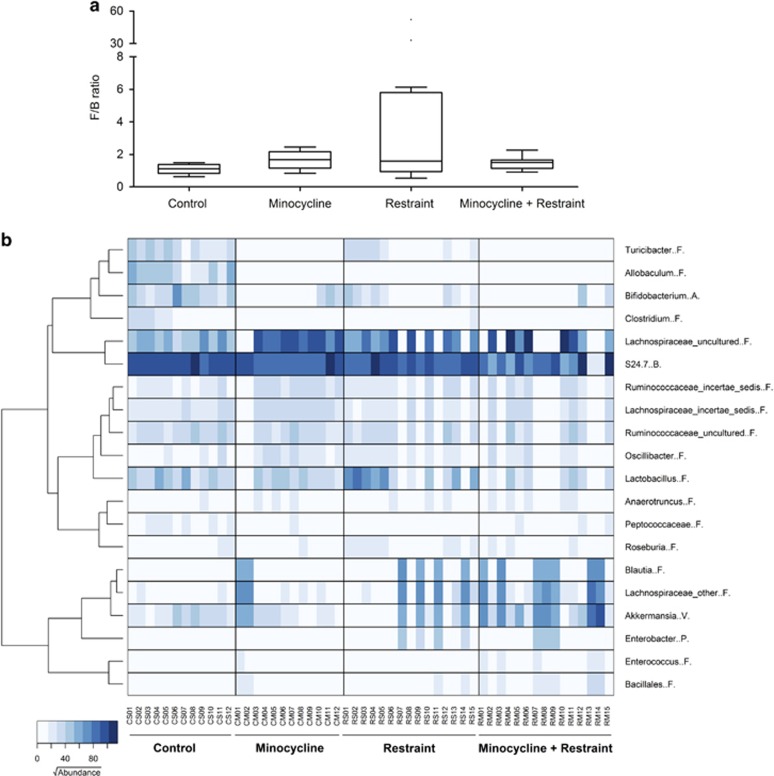
Chronic restraint stress (4–6 h per day for 21 days) affected the gut microbiome compared with nonstressed animals (PERMANOVA P=0.0027, t=2.3492). Although the shallowest level of classification (that is, phylum level) only revealed a nonsignificant trend toward an increased ratio of Firmicutes to Bacteroidetes (Figure 4a), the deeper analysis did identify clear differences between the animal groups. In particular, the relative abundances of the genera Allobaculum (difference in median relative abundance −7.8%, P<0.0001 Mann–Whitney U-test), Bifidobacterium (−4.6%, P=0.0002), Turicibacter (−3.4%, P<0.0007), Clostridium (−0.7%, P<0.0001) and the family S24-7 (−5.8%, P=0.0021) were all reduced in restrained animals, and the relative abundance of the family Lachnospiraceae was increased (+0.3%, P=0.0244). Variance in the relative abundance of these taxa accounted for >40% of intergroup variance.
Figure 4.
Minocycline treatment and chronic restraint stress affect the gut microbiome and chronic restraint stress changes the gut Firmicutes/Bacteroidetes (F/B) ratio. (a) Box and whiskers plot displayed the analysis of the differences of the main composition of the microbiota (Firmicutes to Bacteroidetes). Upper and lower quartiles defined the box with median midline, and the whiskers were assessed using Tukey's method. (b) Microbiota distribution at species level of taxon contributing to 97.5% of sample variations. Heatmap shows square root-transformed read counts for the 20 taxa determined by similarity percentage analysis. The dendrogram shows the clustering of genera based on Ward's hierarchical clustering method. Phyla are abbreviated as follows: Actinobacteria (A), Bacteroidetes (B), Firmicutes (F), Proteobacteria (P) and Verrocomicrobia (V).
Minocycline affects the gut microbiome
Minocycline treatment (5 mg kg−1 per day for 21 days) also affected microbiota composition compared with saline-treated controls (PERMANOVA P=0.0001, t=3.0947, Figure 4b) and, interestingly, in a manner very similar to that observed for restrained animals. In particular, minocycline-treated animals were also found to possess lower relative abundances of the genera Allobaculum (difference in median relative abundance −7.8%, P<0.0001 Mann–Whitney U-test), Bifidobacterium (−5.8%, P<0.001), Turicibacter (−4.2%, P<0.0001), Clostridium (−0.7%, P<0.0001) and the family S24-7 (−7.4%, P=0.003), and significantly high relative abundances of the family Lachnospiraceae (+25.3%, P=0.005) and Ruminococcaceae incertae sedis (+2.4%, P=0.024). Variance in the relative abundance of these taxa accounted for >67% of intergroup variance.
Effect of chronic restraint stress on the gut microbiome in combination with minocycline
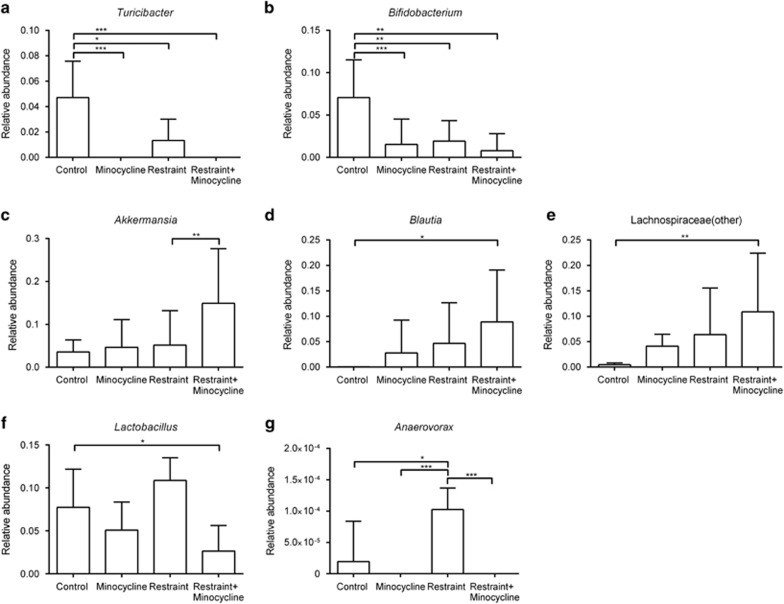
Combining chronic restraint with minocycline treatment resulted in a microbiota composition that was different to that of nonrestrained saline-treated controls (PERMANOVA P=0.0002, t=3.4593, Figure 4b). There were also no significant differences in the shallow, phylum-level profiles produced from mice receiving each treatment alone or in combination (using a PERMANOVA threshold of P<0.01), and at deeper levels of analysis, the significant reductions in the relative abundances of both Turicibacter and Bifidobacterium spp. were also observed in mice receiving both treatments (Figures 5a and b), as was the increase in members of the Lachnospiraceae (Figure 5e). Additional changes in the microbiome profiles not observed with either treatment alone were found when restraint and minocycline were used together. For example, the relative abundances of Akkermansia spp. and Blautia spp. were increased (Figures 5d and e). Minocycline also appears to have a stronger effect on the relative abundances of Lactobacillus spp. and Anaerovorax spp., with relatively greater abundances of these genera observed in the restrained animals, but reductions in their relative abundances when minocycline was also administered (Figures 5f and g).
Figure 5.
The effect of minocycline treatment, chronic restraint stress and their combination assessed at the level of individual taxa. Individual minocycline effect on the (a) Turicibacter and (b) Bifidobacterium populations; synergistic effect of minocycline and chronic restraint stress on the (c) Akkermansia, (d) Blautia and (e) Lachnospiraceae populations; and antagonistic effect of minocycline and chronic restraint stress on the (f) Lactobacillus and (g) Anaerovorax populations. Significant difference between treatment groups: *P<0.05, **P<0.01, ***P<0.001.
Discussion
Caspase-1 is a cysteine protease that cleaves pro-IL-1β and pro-IL-18 into their mature isoforms in the NLRP3 inflammasome in response to stressful stimuli such as psychosocial and microbial stress, adenosine triphosphate, toxins and particulate matter.13, 39 Because casp1−/− mice lack caspase-1 mRNA and its mature protein product, they have decreased inflammasome bioactivity and inflammasome-driven IL-1β and IL-18 production, and could be helpful in identifying the role of caspase-1 in behavior, via either innate or after stress-induced inflammasome activation.23 Our data highlight a role for caspase-1 in the modulation of innate behavior as well as in the response to chronic stress, as caspase-1 modulation decreased baseline anxiety- and depressive-like behaviors, as well as the exacerbation of depressive-like behaviors following chronic restraint stress. Our results are in line with studies reporting that modulation of the IL-1β axis is a potential approach to attenuate the behavioral and molecular effects of stress-induced inflammation.40, 41 Our findings strengthen the role of caspase-1 as a potential therapeutic target aiming at modulating inflammasome-mediated pathways in psychiatric disorders.
Minocycline exerts anti-inflammatory and neuroprotective effects in animal models of neurodegenerative disorders, neurotoxicity and brain injury as well as presents acute antidepressant-like effects in the forced swim test by increasing climbing, potentially through interaction with glutamatergic and/or noradrenergic systems.17, 19, 42, 43 Its antidepressant-like effects might be related to the protection of serotonergic and dopaminergic circuitries.44, 45 Consistent with this literature, we found that minocycline prevented the exacerbation of depressive-like behavior in the forced swim test following chronic restraint stress. Given this finding, we suggest that minocycline may be valuable in the treatment of MDD and other psychiatric disorders. Indeed, two clinical trials investigating minocycline as a stand-alone or adjuvant treatment in psychotic depression and schizophrenia yielded promising results.46, 47, 48, 49 Two studies have planned to investigate the efficacy of minocycline in MDD and bipolar disorder (Clinical Trials.gov identifier NCT01574742 and NCT01403662); yet, no recruitment data have been available.
Our findings show that the gut microbiota composition of mice subjected to chronic restraint stress is significantly altered when compared with control mice. Subtle alterations in the Firmicutes/Bacteroidetes ratio have already been described in studies of irritable bowel syndrome patients (and correlated with clinical depression and anxiety) and in animal models of hypertension,50, 51 and these two conditions are associated with chronic low-grade inflammation.52, 53 The deeper levels of comparison were more informative, such as the levels of Bifidobacterium spp., a genus associated with the suppression of inflammation through inhibition of the nuclear factor-κB pathway,54 being significantly reduced in animals undergoing restraint. Such findings are consistent with the notion that nuclear factor-κB is increased in response to stress, and is a critical mediator of stress-induced depressive-like behavior and stress-impaired neurogenesis.55 Furthermore, Allobaculum spp. were absent in restrained animals, despite representing a substantial component of the microbiota in control animals. Members of the genus Allobaculum include mucin degraders, and their relative abundance is already known to be inversely correlated with dietary-induced inflammation markers, including leptin and IL-22.56, 57 Chronic restraint also led to an increase in the relative abundance of Lactobacillus spp., members of which have been implicated in inflammasome activation through stimulation of caspase-1-dependent IL-1β production by macrophages.58 As such, it is interesting to speculate that the increases in Lactobacillus spp. observed here when either restraint or minocycline were used alone could be a contributing factor to the increased IL-1β levels observed in depression and in animal models of stress.1, 59, 60
Minocycline treatment also significantly altered microbiota composition, and the changes observed following minocycline or restraint separately were not significantly different. However, when restrained mice were also treated with minocycline, evidence of both synergistic and antagonistic effects on microbiota composition was observed. For example, Akkermansia, Blautia and an uncultured member of the Lachnospiraceae family were significantly increased. This effect is notable given that Akkermansia attenuates inflammation in adipose tissue through induction of Foxp3 regulatory T cells, and suppression of IL-6 and IL-1β.61, 62, 63 Moreover, a similar increase in Akkermansia was reported in a study in which minocycline rebalanced the gut microbiota in a rat model of hypertension.50 Lachnospiraceae is one of the most abundant families of Firmicutes and is most often associated with beneficial production of short-chain fatty acids from complex polysaccharides.64 An increase in Lachnospiraceae relative abundance in minocycline-restraint animals is consistent with changes in the gut microbiota of casp-1−/− mice.33
This study has several limitations. In this study, we chose minocycline as a caspase-1 inhibiting compound to further explore the relationship between the microbiota and host behavior.27, 28 Minocycline is an antibiotic and therefore it would likely affect the gut microbiome regardless of caspase-1 inhibition.65 As minocycline has antidepressant effects, its influence on the gut microbiome could be at least partially responsible for such effects.21, 44, 45 Further work is required to elucidate the exact mechanisms of gut microbiota–host interactions, including the analysis of short-chain fatty acid production. The altered microbiome profiles we observed are likely to translate into alterations in both metabolic profiles and host–microbe crosstalk, and to investigate this is beyond the scope of the work presented here. It could be argued that the withdrawal of food and water during the restraint period might impact the microbiome.66 However, because food was only withheld for short periods (4–6 h), we contend that this will have had minimal impacts on the fecal microbiome profiles. Mice were submitted twice to a battery of behavioral test; exposure to one test could impact subsequent test performance.34, 67 However, we tried to minimize bias by performing the tests from the least to the most invasive and by giving the animals recovery time in between tests.34, 67 Our chronic restraint stress paradigm did not decrease sucrose preference, which replicates published findings.68 Although this test is considered a model of clinical anhedonia, it has highly variable outcomes, even within the same facility.69
In a companion paper,70 we have shown three findings that are highly complementary to the results reported here. (1) The absence of gut microbiota in germ-free mice resulted in decreased immobility time in the forced swimming test relative to conventionally raised healthy control mice. (2) From clinical sampling, the gut microbiotic compositions of healthy controls were significantly different from those of MDD patients. (3) Fecal microbiota transplantation of germ-free mice with ‘depression microbiota' derived from MDD patients resulted in depression-like behaviors compared with colonization with ‘healthy microbiota' derived from healthy control individuals. Moreover, we showed that mice harboring ‘depression microbiota' primarily exhibited disturbances of microbial genes and host metabolites involved in carbohydrate and amino acid metabolism, indicating that the development of depressive-like behaviors is mediated through the host's metabolism.70 Based on those findings, in conjunction with the results presented here, we propose that the microbiota–gut–brain axis is fully bidirectional, functioning in a manner through which changes in microbiota affect behavior as shown in our companion paper, whereas, as demonstrated here, changes in behavior brought about (1) by chronic stress, (2) genetic manipulation or (3) pharmacological intervention result in changes in the gut microbiota.
In summary, our findings suggest that caspase-1 inhibition has a protective effect on the stress response by modulating the interface between stress and the gut microbiota. This supports the concept of a microbiota–gut–inflammasome–brain axis in which the gut microbiota exert some of its effects on brain function via the inflammasome signaling platform that modulates inflammatory pathways which will in turn alter brain function and affect depressive- and anxiety-like behaviors. Taken together, a reduction of inflammasome bioactivity may represent a feasible and direct therapeutic strategy in the treatment of MDD and other neuropsychiatric disorders with inflammatory components. Moreover, direct modification of the gut microbiota may offer new opportunities to further mitigate these disorders, via indirect modulation of inflammasome signaling. Future studies should address the tolerability, safety and long-term effects of inflammasome modulation for the treatment of major depression.
Acknowledgments
This work was supported by funding from The National Health and Medical Research Council, Australia, APP1070935, and The Australian National University (Canberra, Australia) and The South Australian Health and Medical Research Institute (SAHMRI, Adelaide, Australia). We acknowledge the support of Neil Dear, Sian Dear and their team at SAHMRI Research and Biomedical Services in the conduct of the animal experiments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012; 36: 764–785. [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry 1999; 4: 317–327. [DOI] [PubMed] [Google Scholar]
- Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol 2001; 11: 203–208. [DOI] [PubMed] [Google Scholar]
- Kokai M, Kashiwamura S, Okamura H, Ohara K, Morita Y. Plasma interleukin-18 levels in patients with psychiatric disorders. J Immunother 2002; 25(Suppl 1): S68–S71. [DOI] [PubMed] [Google Scholar]
- Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry 2008; 13: 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Localization of interleukin 1 type I receptor mRNA in rat brain. Neuroimmunomodulation 1994; 1: 110–115. [DOI] [PubMed] [Google Scholar]
- Wong ML, Bongiorno PB, Rettori V, McCann SM, Licinio J. Interleukin (IL) 1beta, IL-1 receptor antagonist, IL-10, and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications. Proc Natl Acad Sci USA 1997; 94: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Wong ML, Gold PW. Localization of interleukin-1 receptor antagonist mRNA in rat brain. Endocrinology 1991; 129: 562–564. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 2009; 30: 30–45. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008; 13: 717–728. [DOI] [PubMed] [Google Scholar]
- Udina M, Moreno-Espana J, Capuron L, Navines R, Farre M, Vieta E et al. Cytokine-induced depression: current status and novel targets for depression therapy. CNS Neurol Disord Drug Targets 2014; 13: 1066–1074. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev 2011; 243: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcocer-Gomez E, de Miguel M, Casas-Barquero N, Nunez-Vasco J, Sanchez-Alcazar JA, Fernandez-Rodriguez A et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun 2014; 36: 111–117. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 2013; 31: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res 2013; 67: 18–30. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 380–386. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 2000; 6: 797–801. [DOI] [PubMed] [Google Scholar]
- Ahuja M, Bishnoi M, Chopra K. Protective effect of minocycline, a semi-synthetic second-generation tetracycline against 3-nitropropionic acid (3-NP)-induced neurotoxicity. Toxicology 2008; 244: 111–122. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA 2001; 98: 14669–14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Desipramine or glutamate antagonists synergized the antidepressant-like actions of intra-nucleus accumbens infusions of minocycline in male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1660–1666. [DOI] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol 2004; 3: 744–751. [DOI] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 1995; 80: 401–411. [DOI] [PubMed] [Google Scholar]
- Mastronardi C, Whelan F, Yildiz OA, Hannestad J, Elashoff D, McCann SM et al. Caspase 1 deficiency reduces inflammation-induced brain transcription. Proc Natl Acad Sci USA 2007; 104: 7205–7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013; 11: 227–238. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144: 1394–1401, e1391-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13: 701–712. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013; 36: 305–312. [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil 2013; 25: 733–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun 2015; 48: 165–173. [DOI] [PubMed] [Google Scholar]
- Lawson MA, McCusker RH, Kelley KW. Interleukin-1 beta converting enzyme is necessary for development of depression-like behavior following intracerebroventricular administration of lipopolysaccharide to mice. J Neuroinflammation 2013; 10: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W, Gheusi G, Yao J, Johnson RW, Dantzer R, Kelley KW. Interleukin-1beta-converting enzyme-deficient mice resist central but not systemic endotoxin-induced anorexia. Am J Physiol 1998; 274(6 Pt 2): R1829–R1833. [DOI] [PubMed] [Google Scholar]
- Brinkman BM, Hildebrand F, Kubica M, Goosens D, Del Favero J, Declercq W et al. Caspase deficiency alters the murine gut microbiome. Cell Death Dis 2011; 2: e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav 2001; 73: 705–717. [DOI] [PubMed] [Google Scholar]
- Jervis-Bardy J, Leong LE, Marri S, Smith RJ, Choo JM, Smith-Vaughan HC et al. Deriving accurate microbiota profiles from human samples with low bacterial content through post-sequencing processing of Illumina MiSeq data. Microbiome 2015; 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear PR, Gray CD. PASW Statistics 17 Made Simple (Replaces SPSS Statistics 17) 1st edn Psychology Press: East Sussex, 2010. [Google Scholar]
- Rogers GB, Shaw D, Marsh RL, Carroll MP, Serisier DJ, Bruce KD. Respiratory microbiota: addressing clinical questions, informing clinical practice. Thorax 2015; 70: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 2006; 1: 122–124. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996; 87: 2095–2147. [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 2008; 105: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett 2009; 456: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi S, Federico F, Croci N, Palmier B, Plotkine M, Marchand-Leroux C et al. Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res 2009; 1291: 122–132. [DOI] [PubMed] [Google Scholar]
- Kim SS, Kong PJ, Kim BS, Sheen DH, Nam SY, Chun W. Inhibitory action of minocycline on lipopolysaccharide-induced release of nitric oxide and prostaglandin E2 in BV2 microglial cells. Arch Pharm Res 2004; 27: 314–318. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shirayama Y, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on 3,4-methylenedioxymethamphetamine-induced neurotoxicity in serotonergic and dopaminergic neurons of mouse brain. Eur J Pharmacol 2006; 544: 1–9. [DOI] [PubMed] [Google Scholar]
- Arakawa S, Shirayama Y, Fujita Y, Ishima T, Horio M, Muneoka K et al. Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol Biochem Behav 2012; 100: 601–606. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry 2012; 37: 222–226. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol 2008; 31: 287–292. [DOI] [PubMed] [Google Scholar]
- Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 2012; 26: 1185–1193. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo X, Wu R, Ou J, Zheng Y, Zhang B et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophr Res 2014; 153: 169–176. [DOI] [PubMed] [Google Scholar]
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM et al. Gut dysbiosis is linked to hypertension. Hypertension 2015; 65: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery IB, O'Toole PW, Ohman L, Claesson MJ, Deane J, Quigley EM et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012; 61: 997–1006. [DOI] [PubMed] [Google Scholar]
- Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc 2014; 125: 130–138, discussion 138-140. [PMC free article] [PubMed] [Google Scholar]
- Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol 2010; 1: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel CU, Foata F, Philippe D, Adolfsson O, Eikmanns BJ, Blum S. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappaB activation. World J Gastroenterol 2006; 12: 3729–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA 2010; 107: 2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012; 20: 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol 2013; 190: 5306–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M, Pietila TE, Kekkonen RA, Kankainen M, Latvala S, Pirhonen J et al. Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes 2012; 3: 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des 2005; 11: 963–972. [DOI] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M et al. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci 1995; 15(3 Pt 1): 1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015; 64: 872–883. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 2013; 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014; 63: 727–735. [DOI] [PubMed] [Google Scholar]
- Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013; 5: 627–640. [Google Scholar]
- Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest 2014; 124: 4212–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr 2015; 34: 341–349. [DOI] [PubMed] [Google Scholar]
- Hanell A, Marklund N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front Behav Neurosci 2014; 8: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Shi R, Wang J, Wang JF, Li XM. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014; 25: 1151–1155. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Couch Y, Kholod N, Boyks M, Malin D, Leprince P et al. Update in the methodology of the chronic stress paradigm: internal control matters. Behav Brain Funct 2011; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Xu X, Zeng L et al. Altered gut microbiome induces depression-like behaviors in a pathway that is mediated through the host's metabolism. Mol Psychiatry 2016; doi:10.1038/mp.2016.44 (in press). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.