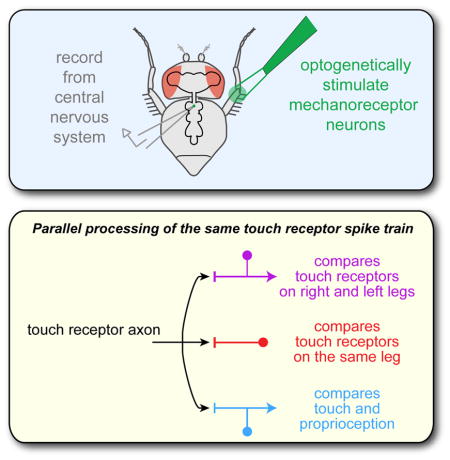
SUMMARY
To distinguish between complex somatosensory stimuli, central circuits must combine signals from multiple peripheral mechanoreceptor types, as well as mechanoreceptors at different sites in the body. Here, we investigate the first stages of somatosensory integration in Drosophila using in vivo recordings from genetically labeled central neurons, in combination with mechanical and optogenetic stimulation of specific mechanoreceptor types. We identify three classes of central neurons that process touch: one compares touch signals on different parts of the same limb, one compares touch signals on right and left limbs, and the third compares touch and proprioceptive signals. Each class encodes distinct features of somatosensory stimuli. The axon of an individual touch receptor neuron can diverge to synapse onto all three classes, meaning that these computations occur in parallel, not hierarchically. Representing a stimulus as a set of parallel comparisons is a fast and efficient way to deliver somatosensory signals to motor circuits.
Graphical Abstract
INTRODUCTION
A tactile stimulus may recruit multiple mechanoreceptor types that encode different stimulus features (e.g., vibration versus pressure). Moreover, a stimulus may recruit neurons that encode the same stimulus feature at different locations on the body (e.g., vibration at two fingertips). Thus, downstream circuits must often integrate signals from multiple mechanoreceptor neurons in order to elicit appropriate behavioral responses. It is therefore fundamental to understand how signals from different mechanoreceptors are integrated in the central nervous system (CNS).
An important constraint on somatosensory integration is processing speed. Fast mechanosensory reflexes are an integral part of many motor behaviors (Burrows, 1996; Lundberg, 1979). For example, an insect can react to a mechanical stimulus within 20–30 ms (Jindrich and Full, 2002; Schaefer et al., 1994). A large fraction of this latency is due to mechanosensory transduction and axonal conduction (6–8 ms; Holtje and Hustert, 2003; Ridgel et al., 2001), as well as the kinetics of muscle force production (10 ms; Ahn et al., 2006). Thus, the central circuits that transform sensory signals into motor commands are subject to tight constraints on speed.
In vertebrates, somatosensory integration may begin within a few synapses from the periphery. Indeed, some spinal cord projection neurons show evidence of both spatial pooling and cross-talk between different mechanoreceptor types (Brown and Franz, 1969; Wall, 1967). However, it has been difficult to identify the specific sites and mechanisms of somatosensory processing in vertebrates, partly due to the complications involved in recording from the spinal cord.
Here, we investigate the early stages of somatosensory processing in the fruit fly, Drosophila, which allowed us to combine in vivo single-cell electrophysiological recordings with genetic tools for labeling and manipulating specific neurons. A fly’s sense of touch is mediated mainly by bristles that cover its body surface. Touching a bristle can evoke postural adjustment and grooming (Corfas and Dudai, 1989; Seeds et al., 2014; Vandervorst and Ghysen, 1980). Bristles may also contribute to tactile exploration (Pick and Strauss, 2005) and social interaction (Ramdya et al., 2015). However, nothing is known about how signals from bristle neurons are processed in the adult fly.
In this study, we show how signals from bristle neurons on the Drosophila leg are integrated and transformed in second-order somatosensory neurons of the ventral nerve cord (VNC), a region analogous to the vertebrate spinal cord. We identified three classes of second-order neurons that receive direct input from bristle touch receptors. One class compares touch signals from different locations on the same limb, whereas another performs bilateral comparisons across limbs, and another compares touch and proprioceptive signals. Notably, these different computations operate in parallel: we find that a single touch receptor axon can diverge to synapse onto all three cell classes. This parallel processing scheme may reflect an adaptation for speed.
RESULTS
Genetic driver lines for leg mechanoreceptor neurons
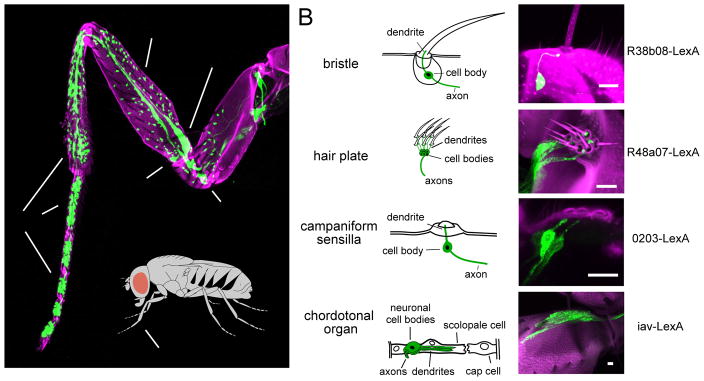
Adult Drosophila possess four basic types of peripheral mechanoreceptor organs: bristles, hair plates, campaniform sensilla, and chordotonal organs (Figure 1A). Bristle neurons are the main touch receptors, and in this study we focus on these neurons and their downstream targets. The other three organs are thought to serve mainly proprioceptive functions, based on studies in other insects (Burrows, 1996; Zill et al., 2004).
Figure 1. Genetic tools for targeting mechanoreceptor neurons of the Drosophila leg.
(A) Projection of a confocal stack through the prothoracic leg. GFP (green) is expressed in sensory neurons (under the control of ChAT-Gal4). Magenta shows cuticle autofluorescence.
(B) Schematic diagrams of each mechanoreceptor type. Associated mechanoreceptor neurons are in green.
(C) Projections of confocal stacks showing sensory neurons within each mechanoreceptor type. GFP (green) is expressed under the control of LexA. Magenta shows cuticle autofluorescence. Scale bar in each image is 10 Im. See also, S1 and S2. See Supplemental Experimental Procedures for all genotypes.
The axons of leg bristle neurons form a topographic map within the most ventral layer of the VNC neuropil. These axons are largely segregated from the axons of other mechanoreceptor types, which project to more dorsal regions of the neuropil (Merritt and Murphey, 1992; Murphey et al., 1989a; Murphey et al., 1989b; Smith and Shepherd, 1996). We took advantage of these distinct axonal arborization patterns to visually screen existing genetic libraries (Gohl et al., 2011; Jenett et al., 2012) for LexA driver lines that labeled each mechanoreceptor type. We then further refined this screen by imaging GFP expression in the front (prothoracic) leg. In this way, we identified four LexA lines that delineate the four major mechanoreceptor types on the leg (Figure 1; see Figures S1 and S2, and Supplemental Experimental Procedures for details on each line). This LexA toolkit provides independent genetic access to neurons within each of the basic peripheral mechanoreceptor types.
Propagation of touch signals in the fly ventral nerve cord
A single neuron resides at the base of each bristle. Mechanical stimulation of the bristle can evoke intense spiking activity within the bristle neuron (Figure 2A). Spiking activity in bristle neurons can also be driven optogenetically, by expressing the ChR2 variant Chrimson (Klapoetke et al., 2014) under the control of our LexA line specific for bristle neurons (R38b08-LexA), and illuminating the leg with green light (Figure 2A). These signals are recorded by clipping the bristle hair and placing a recording electrode over the tip so that it makes electrical contact with the hair shaft; mounting the electrode on a piezoelectric actuator allows us to deliver calibrated mechanical stimuli to the bristle by moving the electrode itself (Corfas and Dudai, 1990).
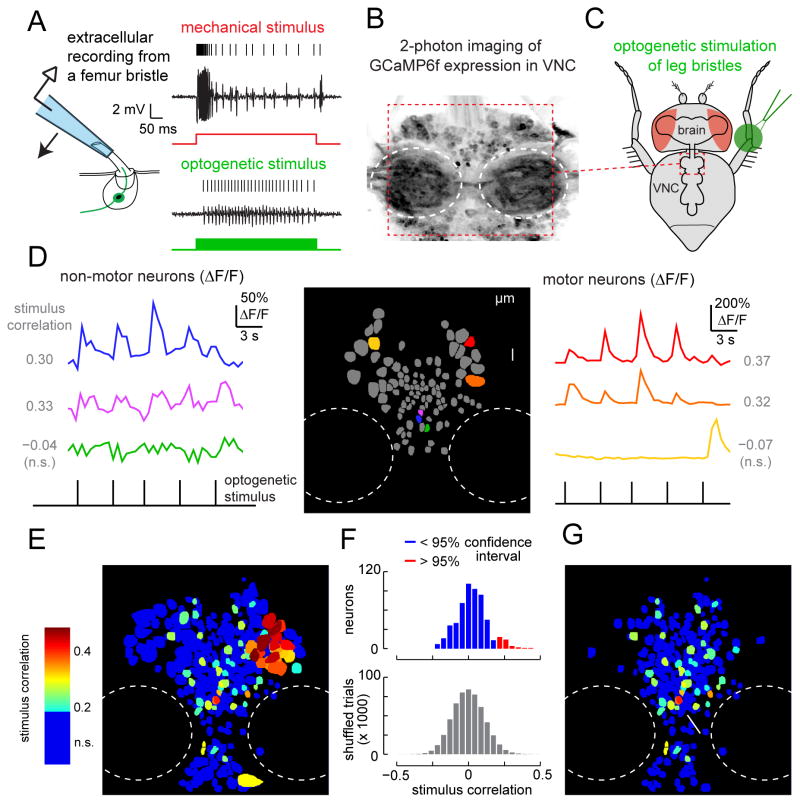
Figure 2. Propagation of touch signals in the fly ventral nerve cord (VNC).
(A) Left: Schematic of bristle recording configuration. Right: Responses of a bristle neuron to mechanical (red) and optogenetic (green) stimuli. Signals are band-pass filtered to facilitate spike identification (see Supplemental Experimental Procedures). All bristle neuron recordings are made from the same bristle on the prothoracic leg, near the femur-tibia joint (Figure S5A).
(B) Projection of a coronal stack through a region (~180 μm x 180 μm) of the prothoracic VNC showing resting GCaMP6f fluorescence. GCaMP6f is expressed pan-neuronally and imaged with a two-photon microscope. Outlined in white dashed lines are the neuropil regions (neuromeres); these regions do not contain neuronal cell bodies.
(C) Schematic of optogenetic bristle stimulation for calcium imaging experiments. The fly is positioned ventral side up. Light is directed at the femur/tibia joint of a prothoracic leg. The imaged region of the VNC is outlined in red.
(D) GCaMP signals recorded during periodic optogenetic stimulation of leg bristles. The left and right panels show color-coded ΔF/F responses of example neurons from a single imaging plane, illustrated in the center panel. Cross-correlation values, computed between each neuron’s ΔF/F signal and the stimulus waveform, are indicated alongside each trace.
(E) Map of all 699 neurons in the prothoracic region of a typical VNC, with individual neurons color-coded by their correlation value. In this projection, neurons with higher correlation are displayed on top. Neurons with correlation values below the threshold for statistical significance (0.19) are blue (n.s.).
(F) Top: distribution of correlation values between calcium signals (ΔF/F) and the stimulus waveform for all 699 neurons. Bottom: correlation values after shuffling the stimulus waveform; the 95th percentile of this distribution was taken as the threshold for significance.
(G) Correlation map of VNC neurons (same as panel E) but excluding motor neurons. The arrow points to the cluster of neurons that we identified for further scrutiny.
To assess the number and location of central neurons that respond to bristle stimulation, we expressed the genetically-encoded calcium indicator GCaMP6f (Chen et al., 2013) pan-neuronally, and we imaged the VNC with a 2-photon microscope (Figure 2B). Meanwhile, we optogenetically stimulated bristle neurons at the femur-tibia joint of the fly’s left prothoracic leg (Figure 2C). We found that many VNC neurons displayed responses that were correlated with bristle neuron stimulation (Figure 2D–E). Specifically, in one representative fly, 69 of the 699 identifiable neuronal somata in the anterior portion of the VNC showed calcium bursts that were significantly correlated with stimulus pulses (Figure 2E–F). We interpret this number as a lower bound on the number of responsive VNC neurons, due to the limited sensitivity of calcium imaging.
The VNC contains motor neurons in addition to the circuits that process sensory information. Motor neuron cell bodies are identifiable by their distinctively large size and characteristic position (Baek and Mann, 2009; Brierley et al., 2012). We tentatively classified VNC neurons as either motor or non-motor based on their cell body size. Some putative motor neurons exhibited robust calcium signals correlated with bristle neuron stimulation (Figure 2D,E), which may be related to the initiation of motor reflexes (Harris et al., 2015; Vandervorst and Ghysen, 1980). Because we were primarily interested in sensory processing, we excluded these putative motor neurons from our analysis. Of the remaining 43 neurons whose responses were significantly correlated with the stimulus, most were localized in a ventral cluster along the midline (Figure 2G). Based on these results, we focused our efforts on obtaining genetic access to neurons in this cluster.
Three cell classes that receive direct and divergent synaptic input from the same bristle axon
To identify neurons postsynaptic to leg bristle neurons, we conducted another visual screen of genetic driver lines, this time focusing on the central nervous system. Because bristle neuron axons terminate in the ventral layer of the VNC neuropil (Murphey et al., 1989b), we looked for neurons having dendrites in this zone. Guided by our calcium imaging results, we focused on neurons with somata in the ventral cluster along the midline. Candidate lines that emerged from this visual screen were then re-screened electrophysiologically: we labeled neurons with GFP, and we made visually targeted in vivo whole-cell recordings from neuronal somata while deflecting bristles at the femur-tibia joint of the fly’s left prothoracic leg.
We identified three neuron classes that reliably responded to this mechanical stimulus (Figures 3 and S3), each labeled by a distinct Gal4 line. The first of these lines (R13d11-Gal4) labeled intersegmental neurons with axons that ascended through the neck connective to the brain. The second line (R69c05-Gal4) labeled midline local neurons that arborized within a single segment (neuromere) of the VNC. The third line (R18g08-Gal4) labeled midline projection neurons that arborized mainly in the ipsilateral neuromere, but also sent a projection to the contralateral neuromere. Within each neuron class, all recorded cells exhibited consistent morphological and intrinsic electrophysiological properties. Antibody staining against candidate neurotransmitters indicated that the midline local neurons and midline projection neurons are likely glutamatergic, while at least some of the intersegmental neurons are likely cholinergic (Figure S4).
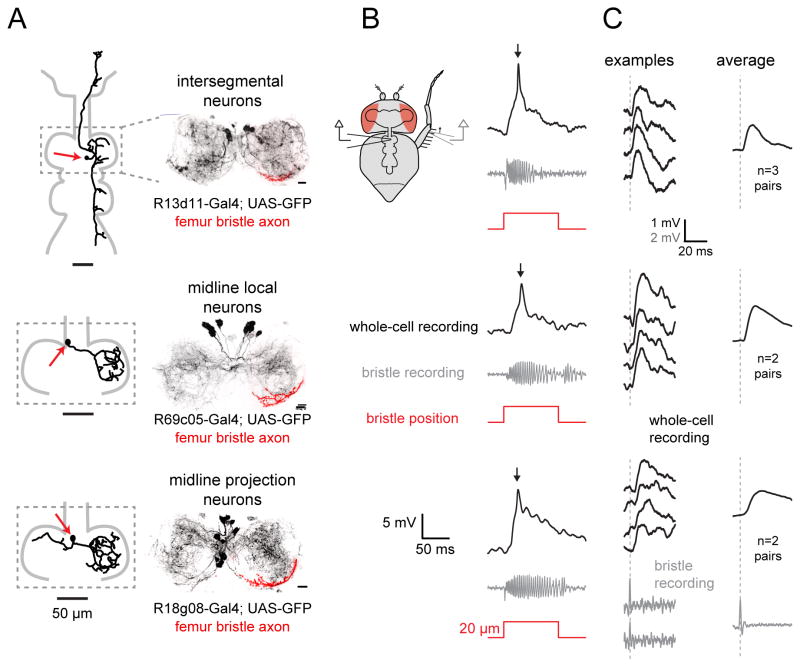
Figure 3. Three classes of VNC neurons that receive direct synaptic input from the same femur bristle.
(A) Left: morphology of a biocytin-filled neuron which expressed GFP in the indicated genotype. Red arrows indicate cell body position. Right: maximum intensity projection of GFP expression within the prothoracic neuromere of the VNC (black), co-labeled with the axonal arbor of a single femur bristle neuron filled with DiI (red); we always targeted the same femur bristle (Figure S5A). All three central neuron classes overlap with this bristle neuron axon. Scale bars, 10 Im. See also Figures S3–S5.
(B) Each row shows a typical in vivo whole-cell current-clamp recording from a central neuron and the simultaneously-recorded signal from a bristle neuron. As before, we targeted the same bristle on the femur, near the femur-tibia joint (Figure S5A).
(C) Single spikes in this bristle neuron reliably trigger excitatory postsynaptic potentials (EPSPs) in each class of central neuron. As before, we targeted the same bristle on the posterior femur, near the femur-tibia joint (Figure S5A). Examples of bristle neuron spikes are shown at bottom. The left column shows representative single-trial EPSPs recorded from each corresponding central neuron class. At right are spike-triggered-averages of the postsynaptic voltage, averaged across all paired recordings from the same central neuron class where a connection was detected.
In total, the three neuron classes we identified contained ~14 cells on the left side of the prothoracic VNC and the same number on the right. Recall that, in a typical fly, 43 putative non-motor neurons showed significant calcium responses to touching the fly’s left prothoracic leg. The cell classes we have identified represent a sizeable fraction of that number (14/43). Thus, these neurons likely represent a substantial component of the VNC neurons that respond to touch on this region of the leg.
All three classes of VNC neurons are anatomically positioned to receive direct synaptic input from bristle neurons. Evidence for this came from filling an identified femur bristle neuron (indicated in Figure S5A) with a fluorescent dye. The dendrites of all three central neuron classes roughly overlapped with the bristle neuron axon terminal, indicating the potential for direct connectivity (Figure 3A and S5B).
We confirmed that some neurons within each class are postsynaptic to this same femur bristle neuron by making paired electrophysiological recordings from the bristle neuron and individual central neurons. Movement of this bristle elicited a train of spikes in the bristle neuron, and it evoked a large depolarization and a single spike in most of the central neurons we recorded from. This was true of all intersegmental neurons (8 of 8), most midline local neurons (6 of 7), and most midline projection neurons (5 of 8) (Figure 3B). All central neuron responses to bristle stimulation were completely blocked by bath application of the nicotinic acetylcholine receptor antagonist methyllycaconitine (MLA, 1 μM; data not shown), indicating that bristle neurons release acetylcholine.
In a subset of the recordings where we found a connection between a bristle neuron and a central neuron, we were able to evoke single spikes in the presynaptic bristle neuron by making a very small movement of the bristle (< 10 μm). In every one of these cases, and for all cell classes, we found that a single bristle spike produced a reliable excitatory postsynaptic potential (EPSP) in the central neuron (Figure 3C). The trial-averaged latency of these responses was similar across all three cell classes (~3 msec, Figure S6A). The trial-to-trial standard deviation in the latency of these responses was relatively small (typically <1 msec). Because a single presynaptic spike is sufficient to elicit an EPSP, and because the EPSP has a consistent latency of about 3 msec, we can conclude that this is a monosynaptic connection.
Together, these results indicate that some neurons within all three classes are directly postsynaptic to bristle neurons. Moreover, because all these experiments targeted one specific femur bristle, we can conclude that some neurons within all three classes share at least one bristle afferent in common.
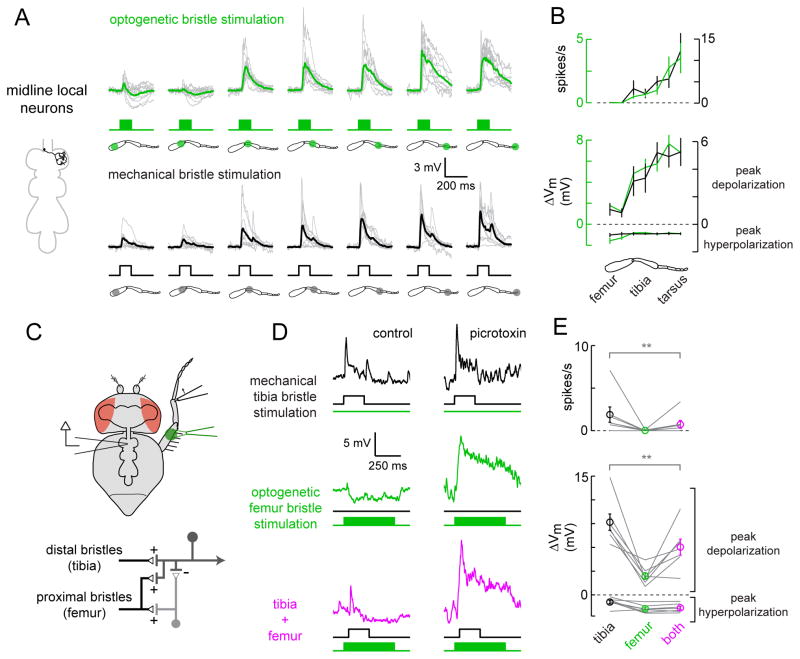
Intersegmental neurons compare touch and proprioceptive inputs
We next turned our attention to the central integration of touch signals, considering each central neuron class in turn. We began with the intersegmental neurons. R13d11-Gal4 labels two pairs of intersegmental neurons in each prothoracic neuromere, each of which extends an anterior process that ascends to the brain, and a posterior process that innervates the meso- and metathoracic neuromeres (Figure 4A). Biocytin fills revealed that, within each pair, one intersegmental neuron projects to the ipsilateral side of the brain, the other to the contralateral side, where they both arborize in a brain region called the vest (Figure S3B and S5C). The dendrites of every intersegmental neuron branch throughout the ventral prothoracic neuromere, in the same region where leg bristle axons terminate.
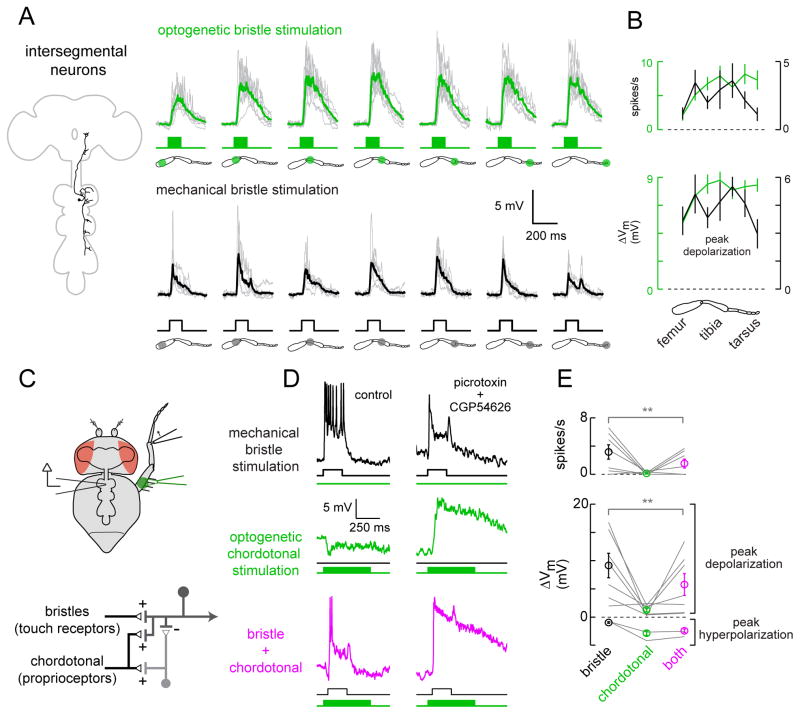
Figure 4. Intersegmental neurons compare touch and proprioceptive inputs.
(A) Top: membrane potential responses of intersegmental neurons to optogenetic stimulation of bristle neurons (individual cells in gray, average in green, n = 9). Bottom: responses of a subset of the same neurons to mechanical stimulation of small numbers of bristles (individual cells in gray, average in black, n = 6).
(B) Average spike rates and peak voltage changes for the cells shown in (A), mean ± SEM across cells, plotted versus stimulus location. Optogenetic responses are in green, mechanical responses in black.
(C) Top: bristle neurons on the distal tibia are stimulated mechanically, and the femoral chordotonal organ is stimulated optogenetically. Bottom: proposed circuit diagram for sensory inputs converging onto intersegmental neurons, with proprioceptive inhibition routed via an interposed inhibitory interneuron. Chordotonal neurons also drive excitation (directly or indirectly), but this is normally masked by inhibition.
(D) Inhibitory input driven by chordotonal neurons suppresses excitatory input from leg bristle neurons. The top and middle rows show responses of a typical intersegmental neuron to stimulation of bristle neurons or chordotonal neurons alone. In the bottom row, the two stimuli are delivered together. The optogenetic stimulus precedes the mechanical stimulus, and is more prolonged, in order to increase the effect of inhibition. Antagonists of synaptic inhibition (100 μM picrotoxin and 50 μM CGP54626) block the suppressive effect of chordotonal neuron stimulation, revealing underlying excitation; similar effects were seen in a total of 6 experiments (data not shown). The postsynaptic neuron is not spiking in the presence of antagonists because the neuron has been depolarized to the point where it cannot initiate spikes.
(E) Average spike rates and peak voltage changes, ± SEM across cells, for all experiments like that shown in the left column of (D). Inhibition driven by chordotonal neurons significantly suppressed responses to bristle neuron stimulation (n = 9 cells, **, P = 0.03 for depolarization and P=0.03 for spikes, Wilcoxon signed rank test).
We mapped the touch receptive fields of intersegmental neurons using both mechanical and optogenetic stimulation of leg bristles. For mechanical stimulation, we used a probe mounted on a piezoelectric actuator to deflect one or two bristles, taking care not to touch the cuticle to avoid stimulating proprioceptors. For optogenetic stimulation, we expressed Chrimson in bristle neurons and illuminated small regions of the leg (~200 μm in diameter). Mechanical stimuli allowed us to investigate how central neurons respond to activation of one or two bristle neurons, while optogenetic stimuli revealed how downstream neurons respond to activation of many bristle neurons.
We found that intersegmental neurons receive excitatory synaptic input from bristles along the entire length of the leg (Figure 4A,B). Mechanical and optogenetic mapping methods always produced similar results. Optogenetic stimulation of the distal leg produced somewhat larger responses than did stimulation of the proximal leg; this is likely because bristles are most heavily concentrated on the distal leg (Figure 1A).
Touch receptors may be activated during self-movement – for example, when the leg touches an obstacle during walking. Comparing activity in touch receptors and proprioceptors might allow downstream neurons to contextualize touch signals during self-generated movement. Therefore, we investigated whether these central neurons integrate signals from leg proprioceptors. In separate experiments, we drove expression of Chrimson in each of the three major proprioceptor types (Figure 1) and optogenetically stimulated mechanoreceptors on the leg while recording from GFP-labeled intersegmental neurons in the VNC. We discovered that optogenetic stimulation of leg chordotonal neurons hyperpolarized the intersegmental neurons (Figure 4C–E). This effect was specific to chordotonal neurons, because we found no response to stimulation of the other two proprioceptor types, hair plate neurons or campaniform sensilla (Table S1).
To assess the functional consequences of proprioceptive inhibition, we combined optogenetic activation of chordotonal neurons with mechanical stimulation of bristles on the tibia. Inhibition driven by chordotonal neurons reduced touch-evoked excitation, as measured by either peak membrane depolarization or spike rate (Figure 4D,E). Thus, proprioceptive inhibition from chordotonal neurons can suppress excitatory touch signals in these central neurons.
Antagonists of inhibitory neurotransmitter receptors (100 μM picrotoxin and 50 μM CGP54626) blocked inhibition driven by chordotonal neurons, and revealed underlying excitation from chordotonal neurons (Figure 4D). Inhibition driven by chordotonal neurons was also blocked by an antagonist of nicotinic receptors (MLA, 1 μM; n =2, data not shown), as we would expect if chordotonal neurons were cholinergic and excited inhibitory interneurons via nicotinic synapses.
These data are most consistent with a circuit model in which intersegmental neurons receive indirect inhibitory input from chordotonal neurons via interposed inhibitory interneurons (Figure 4C). Excitation from chordotonal neurons (which may be direct or indirect) is normally masked by this inhibitory input.
Overall, these data demonstrate that the touch processing system integrates signals from distinct mechanoreceptor types at the first stage of central circuitry – i.e., in neurons directly postsynaptic to peripheral afferents. This cross-modal comparison could allow intersegmental neurons to encode specific conjunctions of touch and proprioceptor signals.
Midline local neurons compare proximal and distal touch within a leg
In contrast to the long-range projections of the intersegmental neurons, the arbors of midline local neurons are limited to a single neuromere. R69c05-Gal4 labels 12–16 midline local neurons within the prothoracic region of the VNC, and each neuron exclusively innervates either the left or right neuromere (Figure 5A). Every neuron we filled within this class had a similar morphology. Their processes were restricted to the ventral edge of the neuropil, in the same region where leg bristle axons terminate (Fig. S3).
Figure 5. Midline local neurons compare proximal and distal touch within a leg.
(A) Responses of midline local neurons, as in Figure 4 (n = 10 for optogenetic stimuli, n = 9 for mechanical stimuli).
(B) Average spike rates and peak voltage changes for the cells shown in (A), mean ± SEM across cells.
(C) Top: to elicit combined distal and proximal touch inputs, bristle neurons on the distal tibia are stimulated mechanically, and bristle neurons on the femur are stimulated optogenetically. Bottom: proposed circuit diagram for sensory inputs converging onto local neurons, with proximal inhibition routed via an interposed inhibitory interneuron.
(D) Inhibitory input driven by proximal femur bristle neurons suppresses excitatory input from distal tibia bristle neurons. Top and middle: responses of a midline local neuron to stimulation of femur or tibia bristle neurons alone. Bottom: the two stimuli are delivered together. Picrotoxin (10 μM) blocks the suppressive effects of femur bristle stimulation, revealing underlying excitation; similar effects were seen in a total of 4 experiments.
(E) Average spike rates and peak voltage changes, ± SEM across cells, for all experiments like those shown in the left column of (D). Inhibition from femur bristles significantly suppressed excitatory signals from tibia bristles (n = 7 cells, **, P = 0.02 for depolarization and P=0.03 for spikes, Wilcoxon signed rank test).
The spatial receptive fields of midline local neurons were more sharply tuned than those of the intersegmental neurons. Only the distal leg produced strong excitation. The proximal leg produced weak excitation if the stimulus was mechanical, and weak excitation followed by inhibition if the stimulus was optogenetic (Figure 5A,B). The difference between optogenetic and mechanical stimulation likely reflects the fact that the optogenetic stimulus recruits dozens of bristle neurons, whereas the mechanical stimulus stimulates only one or two (Figure 2A). The difference in the postsynaptic response suggests a nonlinearity in the recruitment of inhibition: stimulation of a few bristles on the proximal leg is not sufficient to recruit inhibition, but co-stimulation of many bristles produces a net inhibitory effect, suggesting that a threshold for recruiting inhibition has been crossed. Midline local neurons did not respond to input from chordotonal neurons, campaniform sensilla, or hair plates (Table S1). Thus, unlike the intersegmental neurons, they specifically receive input from a single mechanoreceptor type.
We investigated the integration of excitatory and inhibitory touch signals through combined activation of proximal and distal bristles (Figure 5C–E). We found that optogenetic stimulation of proximal (femur) bristles produced inhibition that suppressed excitatory responses to mechanical stimulation of distal (tibia) bristles (Figure 5D,E). Picrotoxin abolished this inhibition, and unmasked an excitatory response to femur bristle stimulation (Figure 5D). All postsynaptic responses were eliminated by blocking nicotinic receptors with 1 μM MLA (n=3 cells, data not shown).
These data suggest that inhibition to midline local neurons is supplied by an inhibitory interneuron that receives excitatory input from femur bristles (Figure 5C). The nonlinearity in the recruitment of inhibition might reside, for example, in the spike threshold of this interneuron. Excitation from femur bristles is normally masked by inhibition. The dominant excitatory input to the midline local neurons is from the bristle neurons of the distal leg.
Thus, in contrast to the intersegmental neurons – which compare touch and proprioceptive inputs – we find that the midline local neurons compare touch inputs from the proximal and distal regions of the leg. This local computation should allow the midline local neurons to respond preferentially to objects that touch the distal leg, regardless of the size of the object or the magnitude of the force it exerts. It may not be a coincidence that the distal leg has the highest density of bristles, and it is most likely to contact objects during walking and tactile exploration.
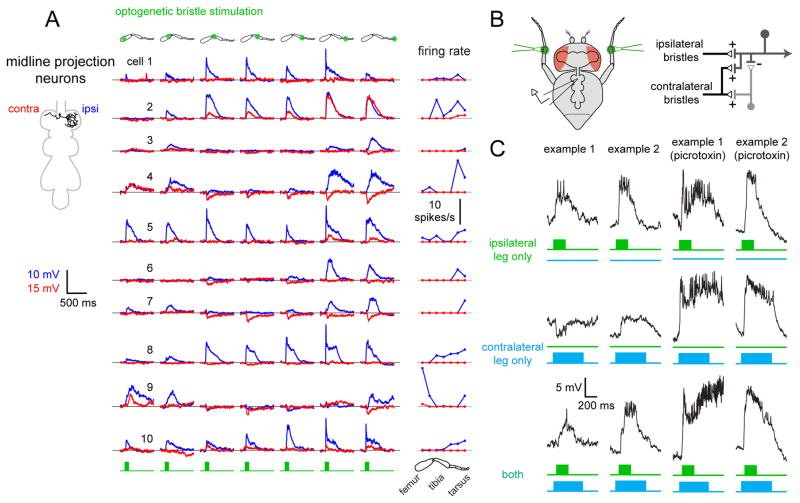
Midline projection neurons compare touch stimuli across contralateral legs
The final cell class we studied consisted of the midline projection neurons. R18g08-Gal4 labels a group of 10–14 neurons with a distinctive bilateral morphology. Each neuron arborizes in both the left and right neuromeres, and one arborization is larger than the other (Figure 6A and S3). The cell body is typically located contralateral to its principal arbor.
Figure 6. Midline projection neurons compare touch stimuli across the body midline.
(A) Trial-averaged responses of 10 midline projection neurons to optogenetic stimulation of bristle neurons. For each neuron, responses were measured for both the ipsilateral (blue) and contralateral (red) prothoracic legs. Ipsilateral is defined as the side containing the reconstructed neuron’s primary arborization (schematic at left). The right column shows trial-averaged spike rates versus stimulus location.
(B) Left: schematic of the recording configuration. Bristles on the contralateral and ipsilateral tibia are stimulated optogenetically. Right: proposed circuit diagram for those midline projection neurons that combine ipsilateral excitation with contralateral inhibition.
(C) Integration of touch signals across legs. Top and middle: responses of local neurons to independent stimulation of ipsilateral and contralateral bristles. Bottom: the two stimuli are delivered together. Picrotoxin (10 μM) blocks inhibition from contralateral bristles, revealing excitation (Figure S7B). Data for all experiments of this type are shown in Figure S7.
These neurons responded exclusively to bristle stimulation. They were unresponsive to stimulation of chordotonal neurons, campaniform sensilla, or hair plates (Table S1). Thus, like the midline local neurons, and unlike the intersegmental neurons, they specifically receive input from a single mechanoreceptor type.
As suggested by their morphology, we discovered that each of the midline projection neurons integrates touch input from both legs. We first used optogenetic stimuli to map bristle inputs from both the ipsilateral and contralateral legs (relative to the principal arbor). Each neuron was dye-filled and anatomically reconstructed to verify its orientation. Every recorded neuron had a similar morphology.
In general, we found that optogenetic stimulation of bristle neurons evoked a mix of excitation and inhibition (Figure 6A). Ipsilateral inputs were typically stronger and more likely to be excitatory, although we found a few ipsilateral inhibitory responses as well. By comparison, contralateral inputs were weaker and more often inhibitory. As a group, the midline projection neurons had relatively diverse receptive field structures, although all were bilateral, and most combined excitation and inhibition. The most common pattern was ipsilateral excitation and contralateral inhibition. Other cells received net excitation from both the ipsilateral and contralateral legs. Mechanical stimulation of one or two bristles produced exclusively excitatory responses, which were largest for distal leg regions (Figure S7A). Again, we attribute differences between optogenetic and mechanical receptive fields to the fact that optogenetic stimuli drive activity in dozens of bristle neurons, whereas mechanical stimuli are confined to one or two bristles. As inhibition is only recruited by optogenetic stimulation, it likely requires co-activation of many bristle neurons.
To examine bilateral integration of touch signals in these neurons, we optogenetically stimulated bristle neurons on the tibia of the right and left prothoracic legs (Figure 6B). Two example experiments of this type are shown in Figure 6C. In the first example, inhibition from the contralateral leg reduced excitation from the ipsilateral leg. In essence, a neuron like this is comparing input to the right and left legs. This should allow such a neuron to respond preferentially to an object touching the ipsilateral leg only. In the second example, ipsi- and contralateral excitation combined additively. This neuron should respond best to an object that is touching both legs. Across the population, combining ipsilateral and contralateral stimulation evoked a variety of results (Figure S7C). In all cases, picrotoxin eliminated inhibition evoked by optogenetic bristle neuron stimulation (Figure 6C) and uncovered latent excitatory inputs along the entire length of both legs (Figure S7B). This result suggests that midline projection neurons receive broad excitatory input from leg bristles, which is suppressed by concomitant inhibition in some regions of the leg. Thus, as with the midline local neurons, excitation appears to be spatially broader than inhibition. Although these receptive fields were diverse, the most common pattern appears to be ipsilateral excitation combined with contralateral inhibition, with inhibition likely relayed through interposed inhibitory neurons (Figure 6B). As a population, these neurons may play a role in behaviors that involve right-left coordination based on touch cues, such as during walking, grooming, or courtship.
Parallel somatosensory pathways encode distinct features of complex movements
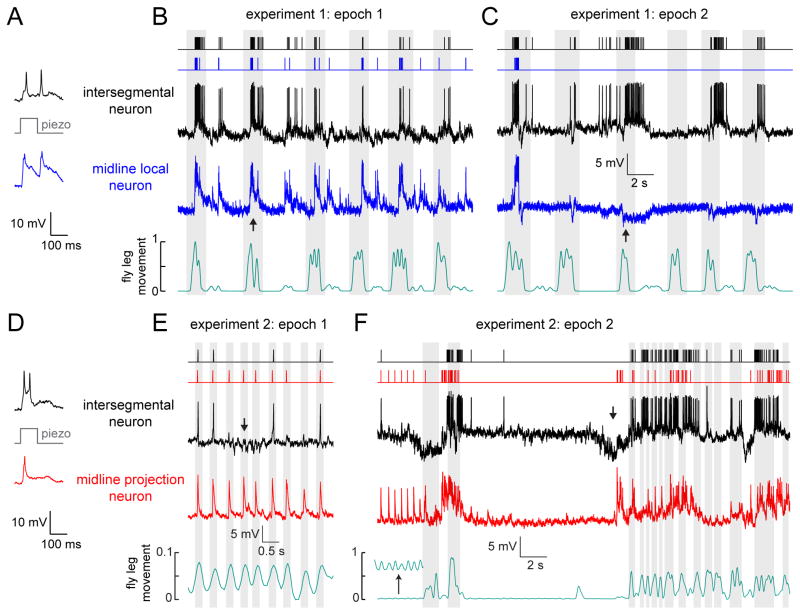
Up to this point, we have shown that three classes of VNC neurons perform distinct computations on the same touch input. However, all of these experiments used a very restricted range of mechanical stimuli. Normally, the fly would be able to stimulate leg bristles and proprioceptors through self-movement, and the mechanical forces impinging on the leg would be more complex. Given what we know about these cell classes, we would expect them to encode distinct but overlapping features of complex stimuli.
To explore this idea, we allowed the fly to freely move its legs while we performed simultaneous whole-cell recordings from pairs of VNC neurons. During these recordings, the prothoracic leg frequently collided with the other legs or with the recording platform, likely stimulating touch and proprioceptive mechanoreceptor neurons on the legs. Two representative recordings are shown in Figure 7. In these experiments, we first used the piezoelectric actuator to mechanically stimulate a femur bristle and confirm that both central neurons responded (Figure 7A, D), as expected from our previous results (Figure 3). After we confirmed responses to controlled mechanical stimuli, we provoked the fly with a flash of light, causing it to initiate complex movements. Epochs of large struggling movements alternated with epochs of low-amplitude squirming. During different epochs, the leg was in a different part of its movement range, and different parts of the leg were touched. As the fly transitioned between epochs, we observed changes in the patterns of correlation among different VNC neurons.
Figure 7. Parallel coding of complex stimuli in simultaneously-recorded central neuron pairs.
(A) Paired whole-cell recording from an intersegmental neuron and a midline local neuron. Example traces show the simultaneous responses of the two neurons to mechanical stimulation of a femur bristle. These responses confirm that this particular pair of neurons share input from some of the same bristles (Figure 3).
(B) During this epoch of the experiment, the fly makes large movements which cause both neurons to depolarize and fire correlated bursts of spikes (e.g., at the arrow). Fly movement is quantified in arbitrary units (see Supplemental Experimental Procedures). Movement bouts are shaded in gray. Spike times are represented with rasters above the raw voltage traces.
(C) During a later epoch of the same experiment, the midline local neuron stops being excited during movement bouts, and is instead inhibited by movement (e.g., at the arrow). This change corresponds to a switch between large movements of multiple legs, to smaller movements of the prothoracic leg.
(D) Same as (A) but for a simultaneously-recorded intersegmental and a midline projection neuron.
(E) The same pair of neurons as in (D), but now responding to spontaneous movement. Small periodic twitching movements of the fly’s leg (gray shading) evoke reliable responses in the midline projection neuron, but not in the intersegmental neuron. The periodic responses of the intersegmental neuron are interrupted by barrages of inhibitory postsynaptic potentials (e.g., at the arrow). Note the expanded vertical scale of the movement measurements.
(F) During a later epoch of the same experiment, experiment, the fly spontaneously switches from twitching to making larger movements of the entire leg. The midline projection neuron is depolarized during large movement bouts, while the intersegmental neuron responds with sequences of inhibition and excitation at movement onset (e.g., at the arrow). The inset in the movement trace (bottom) shows periodic movement on a 10-fold expanded vertical scale.
In the first experiment illustrated here, the fly initially made large movements of multiple legs. During this epoch, the responses of an intersegmental neuron and a midline local neuron were tightly correlated with each other, and also correlated with leg movement (Figure 7B). Later, the fly switched to making smaller amplitude movements of the prothoracic leg. Now the activity of the two neurons became anti-correlated: movement produced excitation in the intersegmental neuron and inhibition in the midline local neuron (Figure 7C). Recall that midline local neurons receive substantial inhibition driven by bristle neurons, while bristle neuron input to intersegmental neurons is mainly excitatory; this finding may explain why these neurons can be anti-correlated during certain epochs.
In the second experiment illustrated here, we also observed periods of correlated and anti-correlated activity (Figure 7D–F). Here, the fly initially made small periodic twitching movements, during which midline projection neuron spiked on each movement cycle, while the simultaneously-recorded intersegmental neuron showed more irregular activity, and was occasionally hyperpolarized during movement (Figure 7E). Later, the fly switched to making large movements of the entire leg. Now the activity of the two neurons became anti-correlated: the midline projection neuron was depolarized during each movement bout, whereas the intersegmental neuron responded with an inhibition-excitation sequence (Figure 7F). Recall that the intersegmental neurons receive inhibition driven by proprioceptors (Figure 4), whereas the midline projection neurons do not receive proprioceptive input (Table 1). This finding may explain why these two cell classes can be anti-correlated during certain epochs.
These complex mechanical stimuli revealed relationships among VNC neurons that were not observed in previous experiments with simpler stimuli. Pairs of VNC neurons were positively correlated during some epochs, but uncorrelated or even anti-correlated during other epochs. This makes sense if each cell encodes a distinct feature of the mechanical forces acting on the body. When these features are temporally correlated, these fire together; when these features are anti-correlated, these cells become opponent to each other.
DISCUSSION
In this study, we used somatosensory circuits in the Drosophila VNC to investigate the neural computations that occur at the first stages of touch processing. Our results suggest a conceptual framework for the central integration of peripheral touch signals. First, signals from peripheral touch receptors are directly transmitted to multiple, parallel processing channels. Within these channels, spatial selectivity is achieved through integration of excitatory and inhibitory inputs from touch receptors in different locations. In parallel, contextual selectivity is achieved by integrating touch signals with information from proprioceptors.
Comparisons as the building blocks of efficient codes
One idea unites the three CNS cell classes we describe here. Namely, all three classes are performing a comparison – within a limb, across limbs, or between different mechanoreceptor types (Figures 4–6). Each class of second-order neurons is encoding the difference between mechanical stimuli of different types, and/or mechanical stimuli at different sites on the body. In general terms, any neuron with an inhibitory receptive field component is encoding a comparison. What is notable in our results is the observation that different central neurons directly postsynaptic to the same afferent axon are performing a variety of different comparisons. At the very first synapse of the somatosensory system, excitation from a given afferent is being integrated with inhibition from several different sources, with each type of comparison occurring in a distinct parallel processing channel. Collectively, these comparisons span a wide range of spatial scales, even though they are all being performed one synapse from the periphery.
Encoding sensory information via comparisons brings several advantages. When a neuron computes the difference between two input signals, the shared component of those input signals is suppressed. This arrangement can allow neurons to transmit finer spatial or temporal features of a stimulus (Srinivasan et al., 1982) and reduce redundancy among the spike trains of different neurons (Barlow, 1961), thereby increasing metabolic efficiency (Niven and Laughlin, 2008). This strategy may be particularly useful in a system facing an information bottleneck. In this case, the relevant bottleneck is the neck of the fly, which contains only about 3600 axons (Hengstenberg, 1973). Among the cell classes we study here, one projects directly to the brain (the intersegmental neurons), while the others may relay information indirectly to the brain, as well as participating in local VNC reflex circuits.
Parallel processing as an adaptation for speed
Here we show that an individual touch receptor axon diverges to directly contact multiple postsynaptic cell classes, each performing a different parallel computation. Why perform these computations in parallel, rather than hierarchically? One important consideration is the necessity for speed. Speed may be a particularly important constraint in somatosensory processing, because the site of sensory transduction (e.g., the foot) can be relatively distant from the CNS. Because Drosophila axons are unmyelinated and usually narrow, axonal conduction is likely to be slow. Indeed, we measured a consistent delay of about 3 msec from the time of a femur bristle neuron spike in the periphery to the onset of an EPSP in the VNC (Figure S6A). This delay is presumably even longer for mechanosensory signals arising from the distal leg, since the axons of tarsus bristle neurons can be over twice the length of femur bristle neurons.
Bilateral comparison across limbs
Some of the central neurons we describe here – the midline projection neurons – integrate information from the right and left legs. Although we observed considerable receptive field diversity within this neural population, the general receptive field structure consisted of ipsilateral excitation, together with mixed excitation and inhibition from the contralateral leg (Figure 6). This organization is similar to that of some neurons in vertebrate spinal cord (Brown and Franz, 1969) and somatosensory cortex (Iwamura, 2000), which integrate excitatory input from one side of the body with mixed excitation and inhibition from the opposite side.
Bilateral tactile integration is clearly important to some behaviors. Rats can distinguish the relative distance of two walls using their whiskers, a behavior which requires activity in somatosensory cortices of both hemispheres (Shuler et al., 2002). In a similar manner, integrating touch signals from the two legs may allow the fly to compare bilateral tactile features. For example, when faced with a small gap, flies reach forward across the void with their front legs, and attempt to cross only when both legs have contacted the opposite side (Pick and Strauss, 2005). Comparison of bilateral somatosensory signals is also critical for the refinement of rhythmic motor behaviors, such as crawling in Drosophila larvae (Heckscher et al., 2015).
Integration of touch and proprioception
In vertebrates, different types of peripheral mechanoreceptors have been traditionally considered to be functionally segregated pathways. Different mechanoreceptor types have been thought to independently mediate the perception of specific somatosensory “submodalities”, such as vibration, stretch, and texture (Johnson, 2001). However, mounting evidence suggests that signals from distinct somatosensory submodalities are in fact combined in the CNS, and that most tactile percepts rely on multiple submodalities (Saal and Bensmaia, 2014). For example, a recent study found that all areas of somatosensory cortex receive input from both touch and proprioceptive neurons (Kim et al., 2015).
Where in the somatosensory processing hierarchy are signals from different mechanoreceptor types first integrated? There is some anatomical evidence that this type of integration begins within the dorsal horn of the spinal cord (Abraira and Ginty, 2013; Maxwell et al., 1985). There is also functional evidence of early submodality integration—for example, some neurons in the cat spinal cord respond to both skin touch and joint movement (Wall, 1967), while neurons in the cuneate nucleus of the brainstem exhibit tactile feature selectivity that is indicative of submodality integration (Jorntell et al., 2014). In the mouse brainstem, there are neurons that receive direct convergent projections from different mechanoreceptor types that innervate the same whisker on the face (Sakurai et al., 2013). However, despite these examples, little is known about the specific sites and mechanisms of submodality integration in vertebrate somatosensation.
Our results provide an example of submodality integration at the very first stage of somatosensory processing, immediately postsynaptic to peripheral touch receptors. Specifically, we found that intersegmental neurons integrate excitatory touch signals from bristle neurons with inhibition from proprioceptive neurons in the femoral chordotonal organ. Studies of the femoral chordotonal organ in larger insects suggest that individual chordotonal neurons encode movements and static positions of the tibia (Field and Matheson, 1998). Thus, inhibitory input to ascending neurons may serve to suppress excitatory touch signals at specific phases of the walking cycle, or during grooming behavior. This reafferent signal may function in a manner analogous to corollary discharge, in which efferent motor commands are used to alter sensory signals that arise from self-generated movements (Poulet and Hedwig, 2007). Interestingly, a recent study in larval Drosophila found that nociceptive inputs and proprioceptive inputs can converge at the level of second-order neurons, and in this case the interaction is summation rather than suppression (Ohyama et al., 2015). Together, these results suggest that integration across submodalities is widespread and very early in this system, consistent with the evidence in vertebrates.
Comparison with other insect species
Many features of our data are similar to previous observations in larger insects such as the locust, cockroach, and stick insect (Burrows, 1996). For example, a single bristle on the locust leg can provide direct synaptic input to multiple classes of central neurons (Burrows, 1992), and the spatial gradients of tactile sensory input in some of these neurons resemble the receptive fields of the midline spiking neurons in our study. In addition, a study in the locust described second-order somatosensory neurons that integrate touch with signals from leg chordotonal neurons (Burrows, 1988). Another described a central neuron that integrated bristle signals from ipsilateral and contralateral legs (Nagayama, 1990). The morphologies of some of the neurons we identified resemble the morphologies of previously described locust neurons, including the ascending intersegmental neurons (Laurent and Burrows, 1988) and the midline local neurons (Burrows and Siegler, 1984).
By using genetic techniques to identify, target, and manipulate specific neuron populations, our study builds upon these previous results in several ways. First, population-level two-photon calcium imaging allowed us to estimate the total number and distribution of central neurons that process touch, and to situate our results within that map. Second, optogenetic tools allowed us to fully catalog the inputs to each central neuron class from different genetically-defined mechanoreceptor types, and to systematically investigate how these inputs are integrated. Third, by recording from the same genetically-identified neurons in multiple individuals, we were able to build up a cumulative picture of each cell class, and make explicit comparisons between classes. In the future, because all these neurons are genetically identifiable, it should be possible to trace their output connections, and to identify their functional role within the broader context of sensory and motor circuits in the VNC. By combining the classic advantages of insect neurophysiology with new genetic tools, Drosophila should prove a useful complement to other model organisms in dissecting the fundamental mechanisms of somatosensory processing.
EXPERIMENTAL PROCEDURES
Procedures are briefly summarized below. Details (including information on all genotypes and transgenes) are provided in the Supplemental Experimental Procedures.
Fly Stocks
Drosophila were raised on standard fly food and kept on a 12-h light, 12-h dark cycle at 25° C. All imaging and electrophysiology experiments were performed on female flies 1–3 days post-eclosion. Flies for all optogenetics experiments were raised on food supplemented with all-trans retinal.
CNS Electrophysiology and Imaging
A fly was fixed ventral side to the underside of a custom steel platform perforated with a precision-milled hole. The ventral head and ventral thorax were partly inserted through the hole so they were accessible from above. In all experiments except for those in Figure 7, the legs were glued down. The top side of the platform was perfused with oxygenated saline. A small hole was dissected in the cuticle of the ventral thorax to expose the prothoracic neuromeres. The perineural sheath under the hole was removed for electrophysiological recordings but left intact for calcium imaging. Whole-cell patch-clamp recordings were targeted to GFP-expressing neuronal cell bodies using an upright microscope with a 40× water-immersion objective. Calcium imaging was performed by expressing GCaMP6f in all neurons, and recording fluorescence signals from the VNC with a custom 2-photon microscope.
Mechanical and Optogenetic Stimulation
Bristles were mechanically stimulated with a glass pipette mounted on a piezoelectric actuator. Optogenetic stimulation of mechanoreceptors was achieved by illuminating the leg with green light (530 nm) through a fiber optic cannula. The optogenetically stimulated region encompassed 20–80 bristles, depending on its position on the leg. Although some LexA lines had expression in central neurons in the VNC (Figure S2), these cells were not directly stimulated by focal illumination of the leg (data not shown). We verified that axons of passage were not activated by the stimuli we used in these experiments (Figure S6B).
Supplementary Material
Acknowledgments
We are grateful to Barret Pfeiffer, David Anderson, and Brian Duistermars for sharing flies prior to publication. Daryl Gohl, Marion Silies, and Tom Clandinin created the InSITE GAL4 collection and provided advice on the LexA swap. Ofer Mazor and Pavel Gorelik provided technical assistance. We thank David Ginty, Michael Dickinson, Philip Holmes, Jan Ache, and members of the Wilson lab for feedback on the manuscript, and Robin Harris and Andy Seeds for helpful discussions. J.C.T. is the recipient of an NRSA fellowship from the NIH (F32-NS089259). This work was partly funded by NIH grant U01-NS090514 to R.I.W. R.I.W. is an HHMI Investigator.
Footnotes
AUTHOR CONTRIBUTIONS
J.C.T. performed the experiments and analyzed the data. J.C.T. and R.I.W. designed the experiments and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn AN, Meijer K, Full RJ. In situ muscle power differs without varying in vitro mechanical properties in two insect leg muscles innervated by the same motor neuron. J Exp Biol. 2006;209:3370–3382. doi: 10.1242/jeb.02392. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Possible principles underlying the transformation of sensory messages. In: Rosenblith WA, editor. Sensory Communication. Cambridge, MA: MIT Press; 1961. pp. 217–234. [Google Scholar]
- Brown AG, Franz DN. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Experimental brain research. 1969;7:231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Burrows M. Responses of spiking local interneurones in the locust to proprioceptive signals from the femoral chordotonal organ. J Comp Physiol A. 1988;164:207–217. doi: 10.1007/BF00603951. [DOI] [PubMed] [Google Scholar]
- Burrows M. Reliability and effectiveness of transmission from exteroceptive sensory neurons to spiking local interneurons in the locust. J Neurosci. 1992;12:1477–1489. doi: 10.1523/JNEUROSCI.12-04-01477.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M. Neurobiology of an Insect Brain. Oxford: Oxford University Press; 1996. [Google Scholar]
- Burrows M, Siegler MV. The morphological diversity and receptive fields of spiking local interneurons in the locust metathoracic ganglion. The Journal of comparative neurology. 1984;224:483–508. doi: 10.1002/cne.902240403. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Dudai Y. Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila. J Neurosci. 1989;9:56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Dudai Y. Adaptation and fatigue of a mechanosensory neuron in wild-type Drosophila and in memory mutants. J Neurosci. 1990;10:491–499. doi: 10.1523/JNEUROSCI.10-02-00491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field LH, Matheson T. Chordotonal Organs of Insects. In: Evans PD, editor. Advances in Insect Physiology. Academic Press; 1998. pp. 1–228. [Google Scholar]
- Gohl DM, Silies MA, Gao XJ, Bhalerao S, Luongo FJ, Lin CC, Potter CJ, Clandinin TR. A versatile in vivo system for directed dissection of gene expression patterns. Nature methods. 2011;8:231–237. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RM, Pfeiffer BD, Rubin GM, Truman JW. Neuron hemilineages provide the functional ground plan for the ventral nervous system. eLife. 2015;4 doi: 10.7554/eLife.04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckscher ES, Zarin AA, Faumont S, Clark MQ, Manning L, Fushiki A, Schneider-Mizell CM, Fetter RD, Truman JW, Zwart MF, et al. Even-Skipped(+) Interneurons Are Core Components of a Sensorimotor Circuit that Maintains Left-Right Symmetric Muscle Contraction Amplitude. Neuron. 2015;88:314–329. doi: 10.1016/j.neuron.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg R. The effect of pattern movement on the impulse activity of the cervical connective of Drosophila melanogaster. Zeitschrift fur Naturforschung Teil C: Biochemie, Biophysik, Biologie, Virologie. 1973;28:593–596. doi: 10.1515/znc-1973-9-1016. [DOI] [PubMed] [Google Scholar]
- Holtje M, Hustert R. Rapid mechano-sensory pathways code leg impact and elicit very rapid reflexes in insects. J Exp Biol. 2003;206:2715–2724. doi: 10.1242/jeb.00492. [DOI] [PubMed] [Google Scholar]
- Iwamura Y. Bilateral receptive field neurons and callosal connections in the somatosensory cortex. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2000;355:267–273. doi: 10.1098/rstb.2000.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindrich DL, Full RJ. Dynamic stabilization of rapid hexapedal locomotion. J Exp Biol. 2002;205:2803–2823. doi: 10.1242/jeb.205.18.2803. [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Current opinion in neurobiology. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Bengtsson F, Geborek P, Spanne A, Terekhov AV, Hayward V. Segregation of tactile input features in neurons of the cuneate nucleus. Neuron. 2014;83:1444–1452. doi: 10.1016/j.neuron.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Gomez-Ramirez M, Thakur PH, Hsiao SS. Multimodal Interactions between Proprioceptive and Cutaneous Signals in Primary Somatosensory Cortex. Neuron. 2015;86:555–566. doi: 10.1016/j.neuron.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. Independent optical excitation of distinct neural populations. Nature methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Burrows M. A population of ascending intersegmental interneurones in the locust with mechanosensory inputs from a hind leg. The Journal of comparative neurology. 1988;275:1–12. doi: 10.1002/cne.902750102. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Progress in brain research. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Koerber HR, Bannatyne BA. Light and electron microscopy of contacts between primary afferent fibres and neurones with axons ascending the dorsal columns of the feline spinal cord. Neuroscience. 1985;16:375–394. doi: 10.1016/0306-4522(85)90010-7. [DOI] [PubMed] [Google Scholar]
- Merritt DJ, Murphey RK. Projections of leg proprioceptors within the CNS of the fly Phormia in relation to the generalized insect ganglion. The Journal of comparative neurology. 1992;322:16–34. doi: 10.1002/cne.903220103. [DOI] [PubMed] [Google Scholar]
- Murphey RK, Possidente D, Pollack G, Merritt DJ. Modality-specific axonal projections in the CNS of the flies Phormia and Drosophila. The Journal of comparative neurology. 1989a;290:185–200. doi: 10.1002/cne.902900203. [DOI] [PubMed] [Google Scholar]
- Murphey RK, Possidente DR, Vandervorst P, Ghysen A. Compartments and the topography of leg afferent projections in Drosophila. J Neurosci. 1989b;9:3209–3217. doi: 10.1523/JNEUROSCI.09-09-03209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama T. The organization of receptive fields of an antero-medial group of spiking local interneurones in the locust with exteroceptive inputs from the legs. J Comp Physiol. 1990;166:471–476. [Google Scholar]
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- Pick S, Strauss R. Goal-driven behavioral adaptations in gap-climbing Drosophila. Current biology : CB. 2005;15:1473–1478. doi: 10.1016/j.cub.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Poulet JFA, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends in neurosciences. 2007;30:14–21. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Ramdya P, Lichocki P, Cruchet S, Frisch L, Tse W, Floreano D, Benton R. Mechanosensory interactions drive collective behaviour in Drosophila. Nature. 2015;519:233–236. doi: 10.1038/nature14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel AL, Frazier SF, Zill SN. Dynamic responses of tibial campaniform sensilla studied by substrate displacement in freely moving cockroaches. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 2001;187:405–420. doi: 10.1007/s003590100213. [DOI] [PubMed] [Google Scholar]
- Saal HP, Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends in neurosciences. 2014;37:689–697. doi: 10.1016/j.tins.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Akiyama M, Cai B, Scott A, Han BX, Takatoh J, Sigrist M, Arber S, Wang F. The Organization of Submodality-Specific Touch Afferent Inputs in the Vibrissa Column. Cell Rep. 2013;5:87–98. doi: 10.1016/j.celrep.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer PL, Kondagunta GV, Ritzmann RE. Motion analysis of escape movements evoked by tactile stimulation in the cockroach Periplaneta americana. J Exp Biol. 1994;190:287–294. doi: 10.1242/jeb.190.1.287. [DOI] [PubMed] [Google Scholar]
- Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM, Jr, Mensh BD, Simpson JH. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. eLife. 2014;3:e02951. doi: 10.7554/eLife.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Integration of bilateral whisker stimuli in rats: role of the whisker barrel cortices. Cerebral cortex (New York, NY : 1991) 2002;12:86–97. doi: 10.1093/cercor/12.1.86. [DOI] [PubMed] [Google Scholar]
- Smith SA, Shepherd D. Central afferent projections of proprioceptive sensory neurons in Drosophila revealed with the enhancer-trap technique. The Journal of comparative neurology. 1996;364:311–323. doi: 10.1002/(SICI)1096-9861(19960108)364:2<311::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Srinivasan MV, Laughlin SB, Dubs A. Predictive coding: a fresh view of inhibition in the retina. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character Royal Society (Great Britain) 1982;216:427–459. doi: 10.1098/rspb.1982.0085. [DOI] [PubMed] [Google Scholar]
- Vandervorst P, Ghysen A. Genetic control of sensory connections in Drosophila. Nature. 1980;286:65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- Wall PD. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967;188:403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill S, Schmitz J, Buschges A. Load sensing and control of posture and locomotion. Arthropod structure & development. 2004;33:273–286. doi: 10.1016/j.asd.2004.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.