Abstract
Brucellosis is a highly zoonotic disease that affects animals and human beings. Brucella suis is the etiological agent of porcine brucellosis and one of the major human brucellosis pathogens. Laboratory diagnosis of porcine brucellosis mainly relies on serological tests, and it has been widely demonstrated that serological assays based on the detection of anti O-polysaccharide antibodies are the most sensitive tests. Here, we validate a recombinant glycoprotein antigen, an N-formylperosamine O-polysaccharide–protein conjugate (OAg-AcrA), for diagnosis of porcine brucellosis. An indirect immunoassay based on the detection of anti-O-polysaccharide IgG antibodies was developed coupling OAg-AcrA to enzyme-linked immunosorbent assay plates (glyco-iELISA). To validate the assay, 563 serum samples obtained from experimentally infected and immunized pigs, as well as animals naturally infected with B. suis biovar 1 or 2, were tested. A receiver operating characteristic (ROC) analysis was performed, and based on this analysis, the optimum cutoff value was 0.56 (relative reactivity), which resulted in a diagnostic sensitivity and specificity of 100% and 99.7%, respectively. A cutoff value of 0.78 resulted in a test sensitivity of 98.4% and a test specificity of 100%. Overall, our results demonstrate that the glyco-iELISA is highly accurate for diagnosis of porcine brucellosis, improving the diagnostic performance of current serological tests. The recombinant glycoprotein OAg-AcrA can be produced in large homogeneous batches in a standardized way, making it an ideal candidate for further validation as a universal antigen for diagnosis of “smooth” brucellosis in animals and humans.
INTRODUCTION
Brucella spp. are Gram-negative facultative intracellular bacteria responsible for brucellosis in humans and animals. Animal brucellosis has a major economic impact because the infection causes abortions and stillbirths and reduces fertility in herds, while brucellosis in humans is a debilitating disease characterized by fever, sweating, and pain (1, 2). Brucella suis is the etiological agent of porcine brucellosis and one of the main human brucellosis pathogens, together with Brucella melitensis and Brucella abortus. There are five biovars of B. suis, with biovars 1, 2, and 3 being responsible for porcine brucellosis (3). B. suis biovars 1 and 3, endemic in Asia and America, are highly zoonotic and cause serious reproductive problems in pigs (infertility, abortion, and orchitis) and a serious disease in humans. Biovar 2 is restricted to Europe, where it represents an emerging problem with a high economic impact in pig farms and is less pathogenic for humans.
In the absence of an effective porcine brucellosis vaccine, control of the disease in pigs depends exclusively on detection and slaughter of infected animals. The gold standard method for confirmation of the infection is isolation of the pathogen; however, the slow growth of brucellae in primary cultures (up to 7 days), the risk involved in their handling, and the poor sensitivity of the method make diagnosis based solely on isolation of brucellae ineffective, not always feasible, and expensive. Therefore, laboratory diagnosis of porcine brucellosis mainly relies on serological tests using serum samples. Currently, all these tests are based on those that have been developed for the diagnosis of bovine brucellosis. The most commonly used serological tests are agglutination tests, such as the buffered plate agglutination test (BPAT) and Rose Bengal plate agglutination test (RBT), the complement fixation test (CFT), the fluorescence polarization assay (FPA), and competitive (cELISA) and indirect (iELISA) enzyme-linked immunosorbent assays (4, 5). With the exception of FPA and cELISA, which measure specific antibodies against the immunodominant O-polysaccharide section of lipopolysaccharide (LPS), all these tests use as antigens whole bacteria or bacterial extracts enriched in smooth or rough LPS, which are composed of a complex mixture of antigens (6, 7). Therefore, current serological tests suffer from false-positive reactions due to cross-reactivity with other antigens and/or common epitopes present in the lipid A and core sections of LPS. Additionally, a number of Gram-negative bacteria that possess similar O-polysaccharide structures (e.g., Escherichia coli O157 and Yersinia enterocolitica O:9) may induce antibodies that cross-react with Brucella antigens, causing false-positive reactions. Finally, a problem still unsolved in the serodiagnosis of brucellosis is the lack of a standardized reference antigen for diagnosis of the disease (8).
Since the identification and characterization of the N-glycosylation machinery of Campylobacter jejuni, the prototypical bacterial glycosylation system (9–12), and its functional transfer to E. coli (13), bacterial glycoengineering has emerged as a new discipline to produce recombinant glycoproteins that can be used as therapeutics, vaccines, or antigens for diagnosis (14–17). It has been largely demonstrated that the C. jejuni N-oligosaccharyltransferase (OTase) PglB (PglBCj), because of its low substrate specificity, can transfer a range of different LPS O-polysaccharides from its lipid donor to carrier proteins in a system that combines the N-glycosylation system of C. jejuni with the O-polysaccharide biosynthesis pathway of Gram-negative bacteria (11, 16, 18). In this in vivo bacterial glycosylation system, the O-polysaccharide linked to the lipid carrier undecaprenolphosphate is synthesized at the cytoplasmic face of the inner membrane, flipped to the periplasm, and polymerized. Subsequently, the O-polysaccharide is transferred by PglB from the lipid to a carrier protein, resulting in the synthesis of the O-polysaccharide–protein conjugate (18). Therefore, the glycoprotein of interest can be produced in a nonpathogenic Gram-negative bacterium coexpressing the enzymes required for the synthesis of the O-polysaccharide, PglB, and a carrier protein. Among the advantages of this novel technology in comparison with the traditional chemical methods used to produce glycoconjugates, we can highlight the versatility of the system, allowing the synthesis of a panel of glycoproteins with different sugar compositions, and the fact that the glycoproteins can be purified in one step from the periplasm of Gram-negative bacteria without the need for culturing pathogenic or slow-growing bacteria. Additionally, no chemical treatments are required for the isolation and cross-linking of the O-polysaccharide to the carrier protein, resulting in more homogeneous products that may facilitate the standardization of the antigens.
We previously produced and fully characterized the recombinant glycoprotein OAg-AcrA, consisting of the O-polysaccharide of B. abortus (OAg) covalently linked to the carrier protein AcrA (16). OAg-AcrA was used to develop new indirect immunoassays for the diagnosis of human and bovine brucellosis by coupling the antigen to magnetic beads or ELISA plates and using different detection systems (spectrophotometric, electrochemical, or fluorescent detection) and different types of samples (14, 15, 19). We have demonstrated that the OAg-AcrA-based assays have excellent performance for the diagnosis of human brucellosis caused by B. abortus, B. melitensis, and B. suis, the three main human brucellosis agents, despite the small structural differences between the O-polysaccharides of these species (14).
In this work, we validated the recombinant glycoprotein OAg-AcrA antigen for diagnosis of porcine brucellosis using serum samples obtained from experimentally infected and immunized pigs, as well as naturally infected animals. This new standardized antigen allowed the development of a highly accurate immunodiagnostic test for porcine brucellosis.
MATERIALS AND METHODS
Production and purification of the O-polysaccharide–protein conjugate (OAg-AcrA).
Production, purification, and characterization of the recombinant glycoprotein OAg-AcrA was performed as previously described (15, 16). Briefly, the Y. enterocolitica O:9 wild-type strain transformed with the plasmids pMAF10 (encoding the C. jejuni OTase PglB) and pMH5 (encoding the C. jejuni carrier protein AcrA fused to a histidine tag) was grown at 37°C in LB medium to an optical density at 600 nm (OD600) of ∼0.5. AcrA was constitutively expressed, and PglBCj expression was induced with arabinose 0.2% (wt/vol). Four hours after induction at 37°C, PglBCj was reinduced by a second addition of arabinose to maximize the glycosylation of AcrA. Cells were harvested by centrifugation after 20 h of induction, and periplasmic extracts were prepared by lysozyme treatment and subjected to Ni2+ affinity chromatography.
OAg-AcrA glyco-iELISA development and optimization.
Microtiter plates (Corning number 3590) were coated with OAg-AcrA at 2.5 μg/ml. The antigen was diluted in 0.05 M carbonate buffer, pH 9.6, and incubated for 18 h at 4°C. The plates were washed four times in 0.01 M phosphate-buffered saline, pH 7.2 (PBS), containing 0.2% Tween 20 (PBST) and blocked with 5% bovine skim milk in PBST (blocking buffer) for 1 h at 37°C. Serum samples were diluted in blocking buffer, added in duplicate, and incubated for 1 h at 37°C. Positive- and negative-control samples were included in each plate. Subsequently, horseradish peroxidase (HRP)-labeled goat anti-porcine IgG (Abcam) antibodies diluted in blocking buffer were added and incubated for 1 h at 37°C. Between reaction steps, the plates were washed three times with PBST to remove excess reagents. After incubation with the substrate (0.36% H2O2 and 0.01% 3,3′,5,5′-tetramethylbenzidine [TMB] in 0.1 M citric acid, pH 4.2) for 3 min at room temperature, the reaction was stopped with 0.16 M H2SO4 and the absorbance was determined at 450 nm using a plate reader (DTX 880 Multimode Detector; Beckman Coulter, Inc.). Optimization of the assay was performed as previously described (15). Based on these analyses, the optimal antigen concentration was 2.5 μg/ml (125 ng/well) and the optimal dilutions of the samples and conjugate were 1:200 and 1:5,000, respectively. These established parameters were used to test all the samples.
Other tests.
The BPAT and FPA were performed by the National Brucellosis Reference Laboratory (DILAB-SENASA) of Argentina as previously described (20, 21). For the BPAT, any degree of agglutination was considered positive, and for the FPA, a reactivity value of >85 milipolarization units (mP) was considered positive. The RBT and CFT were performed by the Unidade Estratégica de Investigação e Serviços em Produção e Saúde Animal, Instituto Nacional de Investigação Agrária e Veterinária (UEISPSA-INIAV) of Portugal as described previously (21).
Porcine samples.
To evaluate the performance of the assay, 563 serum samples obtained from the following animal groups were analyzed.
Ethics statement.
All the samples analyzed in this study were provided by the DILAB-SENASA and UEISPSA-INIAV, and all the studies with animals were done in accordance with institutional animal guidelines and approved by the local regulatory agencies (CICUAE-UNSAM).
(i) Experimentally infected animals.
Six animals (two males and four females) 4 months of age were intramuscularly inoculated with 5 × 106 CFU of B. suis 1330, and serum samples were obtained immediately before infection and 16 and 60 days postinfection. At 60 days postinfection, the animals were slaughtered, and samples from different organs (spleen, liver, lymph nodes, thymus, and tonsil) and tissues (whole blood) were obtained.
(ii) Experimentally immunized animals.
Twelve animals (six males and six females) 6 months of age were intramuscularly inoculated with 5 × 109 heat-killed bacteria (B. suis 1330 bacteria killed by heating for 1 h at 60°C), and serum samples were obtained immediately before and 30 days after inoculation.
(iii) Naturally infected animals.
Serum samples were obtained from animals coming from brucellosis-positive pig farms in which the circulation of B. suis was confirmed by isolation. Thirty-one samples were from an Argentinean farm, among which two animals were culture positive for B. suis biovar 1, and 10 samples were from a farm in Portugal in which all the animals were culture positive for B. suis biovar 2.
(iv) Serologically positive animals.
Serologically positive samples (positive by at least two different serological tests) were obtained from animals from herds with a history of brucellosis. Sera from 120 pigs from Argentina were positive by BPAT and FPA, and 26 serum samples from Portugal were positive by RBT and CFT.
(v) Serologically negative animals.
Serum samples were obtained from serologically negative animals from Argentina; this group included samples obtained from animals coming from herds with a history of brucellosis (NEG exposed) (n = 122) or from brucellosis-free herds (NEG unexposed) (n = 32) and from Canada, a country free of porcine brucellosis (n = 180).
Positive- and negative-control sera included in each assay run.
The positive-control serum was obtained from a culture-positive (B. suis biovar 1) and serologically positive (BPAT, positive; FPA, 96 mP) animal. The negative-control serum was obtained from a noninfected animal (BPAT, negative; FPA, 59 mP) coming from a brucellosis-free herd.
The serum samples from Argentina and Canada were provided by the DILAB-SENASA. The sera from Portugal were provided by the UEISPSA-INIAV.
Bacterial isolation and typing.
Organ samples were cut into pieces, disaggregated, and plated directly in petri dishes containing tryptic soy agar (TSA) supplemented with 20 mg/liter vancomycin (Sigma-Aldrich), 100 mg/liter cycloheximide (ICN Biomedicals), 10 U/ml bacitracin (Sigma-Aldrich), 5 mg/ml nalidixic acid (Sigma-Aldrich), 6,000 U/ml polymyxin B (Sigma-Aldrich), and 1 mg/ml amphotericin B (Sigma-Aldrich). The results were expressed as CFU per gram of tissue. Whole-blood samples (5 ml) were inoculated directly into biphasic Ruiz-Castañeda bottles containing TSA supplemented as described above. The bottles and plates were incubated at 37°C until the appearance of bacterial growth. Colonies suspected of being Brucella by macroscopic appearance were identified by Gram staining and typing by multiplex PCR (Bruce-ladder) (22). The results were expressed as positive or negative for isolation of Brucella.
Western blotting.
Nonglycosylated AcrA and the glycoprotein OAg-AcrA were subjected to SDS-10% PAGE and transferred to nitrocellulose membranes by semidry electroblotting. Immunoblotting was performed with the indicated serum samples at a 1:1,000 dilution in the blocking buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.5, 0.1% Tween 20, 5% caprine skim milk). Bound antibodies were visualized using HRP-labeled goat anti-porcine IgG (Abcam) secondary antibodies and enhanced chemiluminescence (SuperSignal West Pico chemiluminescent substrate detection reagents; Pierce Chemical Co.) according to the manufacturer's instructions.
Data analysis.
Reactivity values were relativized with respect to the absorbance at 450 nm (A450) of the positive-control serum included in each assay run. Relative reactivity (RR) was calculated as follows: RR = mean A450 of the test sample/mean A450 of the positive control. Dot plot, receiver-operating characteristic (ROC) (23, 24), two-graph ROC (TG-ROC) (a plot of the diagnostic sensitivity and specificity of the assay as a function of the cutoff values) (25), and Mann-Whitney test analyses were performed using GraphPad Prism software (version 5.01 for Windows; GraphPad, San Diego, CA, USA).
RESULTS
OAg-AcrA as an antigen for diagnosis of porcine brucellosis.
Since the O-polysaccharide of LPS is the immunodominant antigen in “smooth” brucella infections, and because serological assays based on the detection of antibodies against this antigen proved to be the most sensitive tests, we postulate that the recombinant glycoprotein OAg-AcrA would be an excellent tool for the diagnosis of porcine brucellosis. As a proof of concept and to demonstrate the usefulness of OAg-AcrA for the serodiagnosis of the disease, we developed an indirect ELISA coupling the antigen to microtiter plates (glyco-iELISA; see Materials and Methods) and analyzed serum samples obtained from experimentally infected and immunized pigs, as well as naturally infected animals.
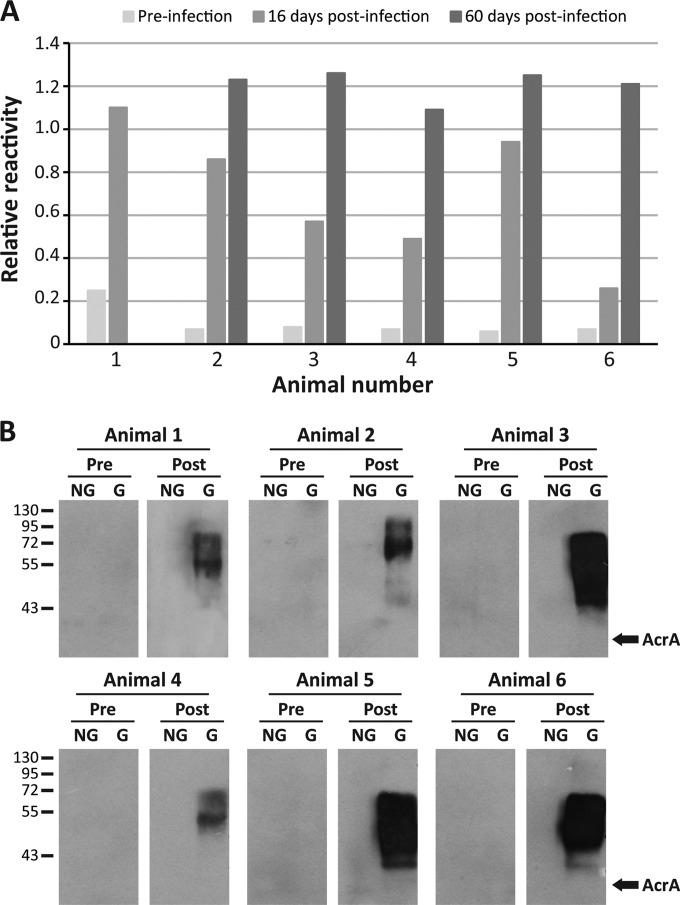
First, six animals were intramuscularly inoculated with 5 × 106 CFU of B. suis 1330, and serum samples were obtained immediately before infection and 16 and 60 days postinfection. In all cases, the infection was retrospectively confirmed by microbiological isolation of B. suis from different organs and tissues at the necropsies (Table 1). All preinfection samples were negative by the glyco-iELISA, and increasing reactivity values were observed at 16 and 60 days postinfection (Fig. 1A and Table 2). Animal 1 presented a higher basal reactivity value than the other animals. At 16 days postinfection, samples obtained from animals 1, 4, and 6 were negative by BPAT and FPA, and one sample (animal 3) was negative only by FPA. Two of these samples (animals 1 and 3) were positive by glyco-iELISA, using a cutoff value of 0.56 (see below). At 60 days postinfection, all the samples were positive by glyco-iELISA, while a serum sample from animal 4 was negative by BPAT (Table 2). These results indicate that by using the OAg-AcrA antigen it is possible to discriminate clearly between infected and noninfected animals. To evaluate the specificity of the reaction, preinfection and 60-day-postinfection sera were also analyzed by Western blotting against the nonglycosylated and glycosylated forms of AcrA (Fig. 1B). Reactivity against the glycoprotein was observed with the samples obtained at 60 days postinfection, but not with the preinfection samples obtained from the same animals. None of the samples was reactive against the nonglycosylated form of AcrA, indicating that the antibody response detected was specifically directed toward the O-polysaccharide moiety of the glycoprotein. Additionally, we analyzed serum samples obtained from experimentally immunized animals. Twelve animals were inoculated with 5 × 109 B. suis 1330 heat-killed bacteria, and sera were obtained immediately before and 30 days after the inoculation. As expected, preinoculation samples were negative by glyco-iELISA and BPAT (Table 3). At 30 days postinoculation, all the samples except one (animal 1) were positive by glyco-iELISA using a cutoff value of 0.56 (see below); at this time, all the samples were positive by BPAT, but 10 out of 12 were negative by FPA (Table 3). In the case of animal 1 (positive by BPAT but negative by glyco-iELISA), it is possible that at this early time of sampling (30 days postinoculation) the sample had a low titer of specific IgG antibodies. Because the glyco-iELISA detects specific IgG antibodies and the BPAT detects specific IgM and IgG antibodies, this could explain the differences observed.
TABLE 1.
Bacterial isolation from organs and tissues obtained from experimentally infected animals
| Animal no. | Bacterial isolation from organs and tissues (CFU/g)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spleen | Liver | GLN | ILN | SLN | PLN | Thymus | Tonsil | Blood | |
| 1 | 237 | 1 | 239 | NEG | 1 | NEG | ND | ND | NEG |
| 2 | 769 | 9 | 634 | 9 | UNC | 6 | 11 | ND | POS |
| 3 | 52 | 5 | 132 | 5 | 29 | 2 | ND | ND | POS |
| 4 | 1,440 | 17 | ND | 14 | 20 | ND | ND | ND | POS |
| 5 | 40 | 2 | 72 | 10 | 553 | ND | NEG | ND | POS |
| 6 | 145 | 40 | 916 | 25 | 2,200 | 18 | UNC | UNC | POS |
Bacterial isolation from organs or tissues obtained from experimentally infected animals at 60 days postinfection. GLN, gastrosplenic lymph nodes; ILN, inguinal lymph nodes; SLN, submandibular lymph nodes; PLN, prepectoral lymph nodes; NEG, negative isolation; POS, positive isolation; ND, not done (no sample available); UNC, uncountable CFU. For blood samples, 5 ml was inoculated directly into Ruiz-Castañeda bottles.
FIG 1.
OAg-AcrA recombinant glycoprotein as a novel antigen for the diagnosis of porcine brucellosis. Six animals were intramuscularly inoculated with 5 × 106 CFU of B. suis 1330, and serum samples were obtained before the infection and 16 and 60 days postinfection. (A) Glyco-iELISA analysis of the samples. The reactivity values are relative to the positive control included in each assay run. (B) Western blot analysis of the serum samples obtained before infection (Pre) and 60 days postinfection (Post). For animal 1, the analyzed sample corresponded to that obtained at 16 days postinfection. NG, nonglycosylated AcrA; G, glycosylated AcrA (OAg-AcrA). The positions of molecular mass standards (in kDa) are indicated on the left. The arrows on the right indicate the migration positions of nonglycosylated AcrA.
TABLE 2.
Serological test results for samples obtained from experimentally infected animalsa
| Animal no. | Test result |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preinfection |
Postinfection |
||||||||
| 16 days |
60 days |
||||||||
| BPATb | FPA (mP)c | Glyco-iELISA | BPAT | FPA (mP) | Glyco-iELISA | BPAT | FPA (mP) | Glyco-iELISA | |
| 1 | NEG | 70 | 0.25 | NEG | 80 | 1.1 | ND | ND | ND |
| 2 | NEG | 72 | 0.07 | POS | 100 | 0.86 | POS | 126 | 1.23 |
| 3 | NEG | 68 | 0.08 | POS | 75 | 0.57 | POS | 198 | 1.26 |
| 4 | NEG | 71 | 0.07 | NEG | 75 | 0.49 | NEG | 96 | 1.09 |
| 5 | NEG | 64 | 0.06 | POS | 95 | 0.94 | POS | 181 | 1.25 |
| 6 | NEG | 64 | 0.07 | NEG | 75 | 0.26 | POS | 121 | 1.21 |
Analysis of serum samples obtained from animals intramuscularly infected with 5 × 106 CFU of B. suis 1330.
BPAT, NEG, negative; POS, positive, ND, not done.
FPA cutoff value, >85 mP.
TABLE 3.
Serological test results for samples obtained from experimentally immunized animalsa
| Animal no. | Test result |
||||
|---|---|---|---|---|---|
| Preinoculation |
30 days postinoculation |
||||
| BPATb | Glyco-iELISA | BPAT | FPA (mP)c | Glyco-iELISA | |
| 1 | NEG | 0,07 | POS | 70 | 0.32 |
| 2 | NEG | 0,07 | POS | 67 | 1.40 |
| 3 | NEG | 0,07 | POS | 72 | 1.29 |
| 4 | NEG | 0,07 | POS | 83 | 1.81 |
| 5 | NEG | 0,07 | POS | 52 | 1.20 |
| 6 | NEG | 0,07 | POS | 71 | 0.68 |
| 7 | NEG | 0,08 | POS | 68 | 0.74 |
| 8 | NEG | 0,07 | POS | 74 | 1.17 |
| 9 | NEG | 0,08 | POS | 106 | 1.90 |
| 10 | NEG | 0,08 | POS | 73 | 0.65 |
| 11 | NEG | 0,08 | POS | 90 | 1.77 |
| 12 | NEG | 0,19 | POS | 75 | 1.60 |
Serological test results for serum samples obtained from animals intramuscularly inoculated with 5 × 109 B. suis 1330 heat-killed bacteria.
BPAT, NEG, negative; POS, positive.
FPA cutoff value, >85 mP.
To evaluate further the usefulness of the antigen for the diagnosis of porcine brucellosis, we analyzed serum samples obtained from animals coming from naturally infected herds. Thirty-one samples were obtained from a pig farm in Argentina (animals 1 to 31), among which 2 animals were culture positive for B. suis biovar 1, and 10 samples from a farm in Portugal (animals 32 to 41) where all the animals were culture positive for B. suis biovar 2 (Table 4). Samples 1 to 5 were obtained from animals with signs of infection, such as a high percentage of abortions, increased neonatal mortality, and infertility; in two of these pigs, B. suis biovar 1 was isolated from blood (Table 4). It is well known that B. suis can spread quickly in a pig farm with no history of brucellosis (3); thus, we decided to include in the analysis serum samples obtained from the remaining 26 pigs (animals 6 to 31) that were in close contact with the five infected animals. All the analyzed samples except two showed high reactivity values by glyco-iELISA, and these results were in complete agreement with those obtained by BPAT and FPA (Table 4). Finally, in order to determine the diagnostic value of the OAg-AcrA antigen for diagnosis of the disease in pigs infected with B. suis biovar 2, we analyzed the 10 samples obtained from animals with positive isolation of B. suis biovar 2. All the samples showed high reactivity by glyco-iELISA, in complete agreement with the results obtained by RBT and CFT (Table 4).
TABLE 4.
Serological test results for samples obtained from animals coming from naturally infected herdsa
| Animal no. | Result |
|||
|---|---|---|---|---|
| BPATb | FPA (mP)c | Glyco-iELISA | Isolation | |
| 1 | POS | 175 | 1.22 | B. suis biovar 1 |
| 2 | POS | 230 | 1.24 | B. suis biovar 1 |
| 3 | POS | 247 | 1.28 | NEG |
| 4 | POS | 258 | 1.24 | NEG |
| 5 | POS | 187 | 1.24 | NEG |
| 6 | POS | 250 | 1.25 | NDd |
| 7 | POS | 126 | 1.18 | ND |
| 8 | NEG | 72 | 0.41 | ND |
| 9 | POS | 254 | 1.25 | ND |
| 10 | POS | 126 | 1.12 | ND |
| 11 | POS | 148 | 1.22 | ND |
| 12 | POS | 235 | 1.25 | ND |
| 13 | POS | 273 | 1.22 | ND |
| 14 | POS | 250 | 1.22 | ND |
| 15 | NEG | 67 | 0.25 | ND |
| 16 | POS | 257 | 1.24 | ND |
| 17 | POS | 230 | 1.25 | ND |
| 18 | POS | 120 | 1.21 | ND |
| 19 | POS | 265 | 1.27 | ND |
| 20 | POS | 270 | 1.26 | ND |
| 21 | POS | 235 | 1.17 | ND |
| 22 | POS | 103 | 1.19 | ND |
| 23 | POS | 215 | 1.21 | ND |
| 24 | POS | 265 | 1.22 | ND |
| 25 | POS | 253 | 1.23 | ND |
| 26 | POS | 153 | 1.24 | ND |
| 27 | POS | 255 | 1.27 | ND |
| 28 | POS | 230 | 1.31 | ND |
| 29 | POS | 143 | 1.23 | ND |
| 30 | POS | 235 | 1.25 | ND |
| 31 | POS | 242 | 1.28 | ND |
| 32 | POS | ND | 1.17 | B. suis biovar 2 |
| 33 | POS | ND | 1.15 | B. suis biovar 2 |
| 34 | POS | ND | 1.14 | B. suis biovar 2 |
| 35 | POS | ND | 1.15 | B. suis biovar 2 |
| 36 | POS | ND | 1.11 | B. suis biovar 2 |
| 37 | POS | ND | 1.09 | B. suis biovar 2 |
| 38 | POS | ND | 1.13 | B. suis biovar 2 |
| 39 | POS | ND | 1.25 | B. suis biovar 2 |
| 40 | POS | ND | 1.22 | B. suis biovar 2 |
| 41 | POS | ND | 1.21 | B. suis biovar 2 |
Serological test results for serum samples obtained from animals coming from pig farms with at least one animal with positive isolation of B. suis.
BPAT, NEG, negative; POS, positive.
FPA cutoff value, >85 mP.
ND, not done. Brucella isolation from animals 6 to 31 was not attempted because no tissue and/or organs samples were available. None of these animals showed signs of illness up to the time of sampling.
Taken together, these results demonstrate the potential of the recombinant glycoprotein OAg-AcrA as a sensitive and specific diagnostic tool for porcine brucellosis caused by B. suis biovars 1 and 2.
OAg-AcrA antigen validation.
To further validate the antigen, a larger panel of positive and negative serum samples (n = 525) was analyzed by glyco-iELISA. The group of negative reference samples (NEG) included serologically negative samples obtained from animals coming from herds free of the disease (NEG unexposed; n = 32) and herds with a history of brucellosis (NEG exposed; n = 122) and from Canada, a country free of porcine brucellosis (n = 180). The group of positive reference samples (POS) included sera obtained from culture-positive animals (n = 6 experimentally infected animals; n = 12 animals naturally infected with B. suis biovar 1 or 2) and samples obtained from animals in which the infection was not confirmed by bacteriological culture but with positive results by at least two serological tests (n = 147 samples seropositive by BPAT and FPA from Argentina; n = 26 samples seropositive by RBT and CFT from Portugal). The glyco-iELISA reactivity values obtained with the POS and NEG groups of samples are outlined in the dot plot diagram shown in Fig. 2A. Almost no overlap between the POS and NEG groups of samples was observed; only one sample from the NEG-exposed group gave a reactivity value that overlapped those obtained with the POS group. These results indicate that, using the OAg-AcrA antigen, it is possible to discriminate clearly between brucellosis-positive animals and noninfected animals.
FIG 2.
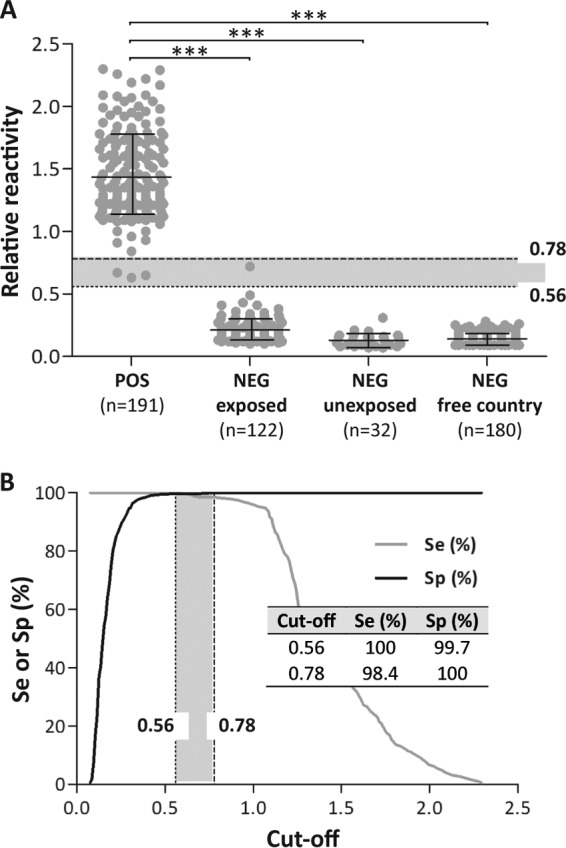
Dot plot and ROC analysis of glyco-iELISA results. (A) Dot plot analysis. Serum samples obtained from positive (POS) and negative (NEG) animals were tested by glyco-iELISA as indicated in Materials and Methods. The POS group included serum samples obtained from culture-positive and serologically positive (by at least two different serological tests) animals. The NEG group included samples obtained from animals coming from herds with a history of brucellosis (NEG exposed); herds without any history of the disease (NEG unexposed); and herds from Canada, a brucellosis-free country (NEG free country). The mean and standard deviation for each group are indicated: POS, 1.43 ± 0.33; NEG exposed, 0.21 ± 0.09; NEG unexposed, 0.13 ± 0.05; NEG free country, 0.14 ± 0.04. ***, P < 0.0001; Mann-Whitney test. (B) TG-ROC plot of the results. ROC analysis was carried out using as a positive reference samples the sera of the POS group and as a negative reference samples of the sera of the three NEG groups described for the dot plot in panel A. The dotted and dashed lines in the dot plot and TG-ROC plots indicate the cutoff values for which maximal diagnostic Se or Sp, respectively, were achieved. The two cutoff values represent the bounds of an intermediate range (IR) of reactivity values (shaded areas). (Inset) Se and Sp values obtained for the two cutoff values. The cutoff value that concurrently optimizes Se and Sp coincides with the cutoff value for which maximal Se was achieved (0.56; dotted line).
To evaluate the diagnostic performance of the glyco-iELISA, a ROC analysis was performed. ROC analysis was carried out using as positive and negative reference samples the POS and NEG groups described for the dot plot. Based on the ROC results, the area under the ROC curve (AUC) value was 0.9999 (95% confidence interval [CI], 0.9997 to 1.000), indicating that the test is highly accurate for the diagnosis of porcine brucellosis. With the objective of selecting the cutoff points that maximize the diagnostic sensitivity (Se) and/or specificity (Sp) of the test, a plot of the diagnostic Se and Sp as a function of the cutoff values (TG-ROC plot) was performed (Fig. 2B). This analysis allowed us to determine the cutoff value for which the diagnostic Se was 100% (cutoff = 0.56; Sp = 99.7%) and the cutoff value that gave a 100% Sp (cutoff = 0.78; Se = 98.4%). The cutoff value that concurrently optimized the Se and Sp coincided with the cutoff for which the maximum Se was reached (cutoff = 0.56) (Fig. 2B, dotted line).
Finally, we analyzed by glyco-iELISA serum samples obtained from animals coming from farms with a history of brucellosis but that did not meet the criteria established here for the selection of serologically positive samples (Table 5). These samples were positive in only one of the serological tests. Seven out of 12 samples that were BPAT positive/FPA negative were confirmed by the glyco-iELISA, whereas the three samples that were BPAT negative/FPA positive all had positive results by glyco-iELISA.
TABLE 5.
Analysis by glyco-iELISA of samples positive by BPAT or FPAa
| Animal no. | Test result |
||
|---|---|---|---|
| BPATb | FPA (mP)c | Glyco-iELISA | |
| 1 | POS | 55 | 0.81 |
| 2 | POS | 59 | 0.22 |
| 3 | POS | 69 | 0.95 |
| 4 | POS | 76 | 0.88 |
| 5 | POS | 79 | 0.54 |
| 6 | POS | 65 | 0.52 |
| 7 | POS | 57 | 1.56 |
| 8 | POS | 62 | 1.51 |
| 9 | POS | 74 | 0.78 |
| 10 | POS | 82 | 0.77 |
| 11 | POS | 56 | 0.39 |
| 12 | POS | 74 | 0.44 |
| 13 | NEG | 137 | 1.94 |
| 14 | NEG | 185 | 0.98 |
| 15 | NEG | 202 | 2.20 |
These samples did not meet the criteria established in this study for the selection of positive samples.
BPAT, NEG, negative; POS, positive.
FPA cutoff value, >85 mP.
Overall, our results demonstrate that the recombinant glycoprotein-based assay is highly accurate for diagnosis of porcine brucellosis, improving the diagnostic performance of current serological tests.
DISCUSSION
The hallmark of infection with smooth Brucella strains is the induction of high titers of antibodies against the immunodominant O-polysaccharide section of smooth LPS (sLPS). That is why the most sensitive and cost-effective way to diagnose the disease is by means of serological assays (26, 27). For these reasons, and due to the previously demonstrated excellent diagnostic performance of the recombinant glycoprotein OAg-AcrA for diagnosis of bovine and human brucellosis (14, 15), we decided to evaluate the antigen for the diagnosis of porcine brucellosis.
In this work, we validated the recombinant glycoprotein OAg-AcrA for diagnosis of porcine brucellosis by coupling the antigen to microtiter plates (glyco-iELISA) and using different sample panels. All glyco-iELISA parameters (antigen concentration and dilutions of sample and conjugate) were optimized as indicated in Materials and Methods. Initially, we analyzed serum samples obtained from experimentally infected and immunized pigs, as well as from naturally infected animals confirmed by isolation of B. suis biovar 1 or 2, allowing us to evaluate the performance of OAg-AcrA with a well-characterized panel of samples. The results obtained indicated that with this antigen, it is possible to discriminate unambiguously between infected and noninfected animals. By immunoblotting, we confirmed that the detected antibodies reacted specifically against the O-polysaccharide section of the glycoprotein, ruling out any potential interference from the carrier protein. Then, to further validate the antigen, a large panel of positive and negative samples (including culture-positive and serologically positive and negative samples) was evaluated and a ROC analysis was performed (23–25). The AUC (AUC = 0.9999; 95% CI, 0.9997 to 1.000) indicated that the glyco-iELISA is a highly accurate test to diagnose porcine brucellosis. Based on the ROC analysis, high values of diagnostic Se and Sp were obtained at the selected cutoffs, as well as a narrow intermediate range of reactivity values, which correlates with the low level of overlapping observed between the reactivity values of negative and positive samples. Finally, we analyzed serum samples obtained from animals coming from herds with a history of brucellosis that did not meet the criteria established here for the selection of serologically positive samples (i.e., positive by at least two serological tests). The results obtained with these samples, together with those obtained with the samples from experimentally infected and immunized animals, indicate that the glyco-iELISA may improve the diagnostic performance of the BPAT and FPA used for diagnosis of porcine brucellosis.
Historically all the serological tests for the diagnosis of porcine brucellosis have been based on those developed for bovine brucellosis, and therefore, they use as antigens whole bacteria (BPAT, RBT, and CFT), bacterial extracts enriched in sLPS (iELISA and cELISA), or a purified ∼22-kDa fragment of the O-polysaccharide of B. abortus sLPS (FPA). To obtain these antigens, level 3 biosafety facilities and multiple purification steps, in particular for sLPS and the 22-kDa O-polysaccharide fragment, are required. These procedures do not allow homogeneous and standardized batches of the antigens to be obtained, a problem still unresolved for all serological tests currently used for the diagnosis of human and animal brucellosis. Recently, a series of synthetic antigens based on the structure of the O-polysaccharide have been obtained and evaluated for diagnosis of bovine brucellosis, but with poor diagnostic performance in terms of sensitivity (28). Our glyco-iELISA proved to have high diagnostic performance, and additionally, production of the antigen can be easily scaled up from bacterial fermentations without the need for culturing pathogenic or slow-growing bacteria. Glycoengineering technology also facilitates the purification of the antigen, since it can be purified in a one-step process from periplasmic extracts by metal chelate affinity chromatography. Therefore, this technology reduces the upstream and downstream processing costs and makes the entire process more biosecure, since nonpathogenic bacteria are used. Additionally, the glycoengineering method overcomes many of the inherent disadvantages of the traditional chemical methods for the production of O-polysaccharide–protein conjugates (29). The production of OAg-AcrA does not require the purification of sLPS from the bacterium of interest. Furthermore, no chemical treatments are required either to release the O-polysaccharide from the lipid A core or to cross-link the carbohydrate to the carrier protein. In our system, all these steps are controlled in vivo by the bacteria, allowing the production of large homogeneous batches of antigen that in the future may facilitate the elaboration of a standardized reference antigen, with important implications for diagnosis of smooth brucellosis in humans and the animal species that constitute the reservoir of the disease. In this sense, we propose the recombinant glycoprotein OAg-AcrA as a universal antigen for diagnosis of smooth brucellosis. So far, we have validated the antigen for the diagnosis of brucellosis in humans (14), cattle (15), and pigs (this work). The versions of glyco-iELISA for goats and camels are under development. Further work will be required to assess the usefulness of the antigen for diagnosis of the disease in other animal species, as well as brucellosis caused by the new atypical strains reported recently.
In view of the excellent diagnostic performance in terms of sensitivity and specificity, we propose the glyco-iELISA as an improved test for diagnosis of porcine brucellosis. The excellent performance of the test in terms of diagnostic specificity could be explained by the fact that only the outer moiety of sLPS (i.e., the O-polysaccharide) is coupled to the carrier protein. Current tests have reduced serospecificity due to the presence of cross-reactive antibodies against common epitopes present in the core polysaccharide and lipid A of different bacteria. B. abortus and Y. enterocolitica O:9 have almost identical O-polysaccharide structures. B. abortus O-polysaccharide contains both A and M epitopes, with approximately 98% A epitope, while Y. enterocolitica O:9 O-polysaccharide is comprised solely of the A variant (30–32). False-positive serological reactions due to cross-reactivity linked to shared epitopes associated with the O-polysaccharide of Brucella and other bacteria could occur, but they constitute a relevant problem only in those regions with low prevalence of brucellosis or where the disease has been eradicated or controlled. In this scenario, a positive result should be confirmed by another method with high specificity (near 100%) but also with high sensitivity at the selected cutoff for which maximal specificity is achieved. In theory, the most specific methods are those based on non-O-polysaccharide antigens, but so far, all these methods suffer from very low sensitivity values at the cutoff that gives 100% specificity (26, 33, 34), and a cutoff yielding a specificity near 100% should always be applied when validating swine brucellosis tests in the context of low prevalence of the disease and/or the existence of false-positive serological reactions (26, 33, 34).
Here, we validated a recombinant glyco-iELISA and propose it as the test of choice for diagnosis of porcine brucellosis in regions with high prevalence of the disease. Although brucellosis has been eradicated from many industrialized countries, new foci of disease continually appear (35). The excellent diagnostic performance of the OAg-AcrA antigen for diagnosis of brucellosis in different species and the fact that the recombinant glycoprotein can be produced in large homogeneous batches in a standardized way make it an ideal candidate for further validation as a universal antigen for diagnosis of smooth brucellosis.
ACKNOWLEDGMENTS
This work was supported by grants PICT Start Up 2010/0144 (Préstamo BID 2437) and FONARSEC NANO 2010 Proyecto no. 005 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina. M.E.C. is a postdoctoral fellow of Fundación Bunge y Born. A.E.C., J.E.U., and D.J.C. are career investigators of Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET, Buenos Aires, Argentina.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
A patent has been filed regarding the diagnostic application of recombinant glycoproteins.
REFERENCES
- 1.Corbel MJ. 1997. Brucellosis: an overview. Emerg Infect Dis 3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young EJ. 1995. An overview of human brucellosis. Clin Infect Dis 21:283–289. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 3.Diaz Aparicio E. 2013. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev Sci Tech 32:43–51, 53–60. [PubMed] [Google Scholar]
- 4.Di Febo T, Luciani M, Portanti O, Bonfini B, Lelli R, Tittarelli M. 2012. Development and evaluation of diagnostic tests for the serological diagnosis of brucellosis in swine. Vet Ital 48:133–156. [PubMed] [Google Scholar]
- 5.Praud A, Gimenez O, Zanella G, Dufour B, Pozzi N, Antras V, Meyer L, Garin-Bastuji B. 2012. Estimation of sensitivity and specificity of five serological tests for the diagnosis of porcine brucellosis. Prev Vet Med 104:94–100. doi: 10.1016/j.prevetmed.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen K, Gall D, Jolley M, Leishman G, Balsevicius S, Smith P, Nicoletti P, Thomas F. 1996. A homogeneous fluorescence polarization assay for detection of antibody to Brucella abortus. J Immunol Methods 195:161–168. doi: 10.1016/0022-1759(96)00116-0. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen KH, Kelly L, Gall D, Nicoletti P, Kelly W. 1995. Improved competitive enzyme immunoassay for the diagnosis of bovine brucellosis. Vet Immunol Immunopathol 46:285–291. doi: 10.1016/0165-2427(94)05361-U. [DOI] [PubMed] [Google Scholar]
- 8.Al Dahouk S, Tomaso H, Nockler K, Neubauer H, Frangoulidis D. 2003. Laboratory-based diagnosis of brucellosis—a review of the literature. Part II: serological tests for brucellosis. Clin Lab 49:577–589. [PubMed] [Google Scholar]
- 9.Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol 32:1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 10.Kowarik M, Young NM, Numao S, Schulz BL, Hug I, Callewaert N, Mills DC, Watson DC, Hernandez M, Kelly JF, Wacker M, Aebi M. 2006. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J 25:1957–1966. doi: 10.1038/sj.emboj.7601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wacker M, Feldman MF, Callewaert N, Kowarik M, Clarke BR, Pohl NL, Hernandez M, Vines ED, Valvano MA, Whitfield C, Aebi M. 2006. Substrate specificity of bacterial oligosaccharyltransferase suggests a common transfer mechanism for the bacterial and eukaryotic systems. Proc Natl Acad Sci U S A 103:7088–7093. doi: 10.1073/pnas.0509207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nothaft H, Szymanski CM. 2010. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol 8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 13.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. 2002. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 14.Ciocchini AE, Rey Serantes DA, Melli LJ, Iwashkiw JA, Deodato B, Wallach J, Feldman MF, Ugalde JE, Comerci DJ. 2013. Development and validation of a novel diagnostic test for human brucellosis using a glyco-engineered antigen coupled to magnetic beads. PLoS Negl Trop Dis 7:e2048. doi: 10.1371/journal.pntd.0002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciocchini AE, Serantes DA, Melli LJ, Guidolin LS, Iwashkiw JA, Elena S, Franco C, Nicola AM, Feldman MF, Comerci DJ, Ugalde JE. 2014. A bacterial engineered glycoprotein as a novel antigen for diagnosis of bovine brucellosis. Vet Microbiol 172:455–465. doi: 10.1016/j.vetmic.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Iwashkiw JA, Fentabil MA, Faridmoayer A, Mills DC, Peppler M, Czibener C, Ciocchini AE, Comerci DJ, Ugalde JE, Feldman MF. 2012. Exploiting the Campylobacter jejuni protein glycosylation system for glycoengineering vaccines and diagnostic tools directed against brucellosis. Microb Cell Fact 11:13. doi: 10.1186/1475-2859-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melli LJ, Ciocchini AE, Caillava AJ, Vozza N, Chinen I, Rivas M, Feldman MF, Ugalde JE, Comerci DJ. 2015. Serogroup-specific bacterial engineered glycoproteins as novel antigenic targets for diagnosis of Shiga toxin-producing-Escherichia coli-associated hemolytic-uremic syndrome. J Clin Microbiol 53:528–538. doi: 10.1128/JCM.02262-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman MF, Wacker M, Hernandez M, Hitchen PG, Marolda CL, Kowarik M, Morris HR, Dell A, Valvano MA, Aebi M. 2005. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci U S A 102:3016–3021. doi: 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortina ME, Melli LJ, Roberti M, Mass M, Longinotti G, Tropea S, Lloret P, Serantes DA, Salomon F, Lloret M, Caillava AJ, Restuccia S, Altcheh J, Buscaglia CA, Malatto L, Ugalde JE, Fraigi L, Moina C, Ybarra G, Ciocchini AE, Comerci DJ. 2016. Electrochemical magnetic microbeads-based biosensor for point-of-care serodiagnosis of infectious diseases. Biosensors Bioelectronics 80:24–33. doi: 10.1016/j.bios.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 20.DILAB-SENASA. 2009. Manual de diagnóstico serológico de la brucellosis bovina versión 2. DILAB-SENASA, Buenos Aires, Argentina. [Google Scholar]
- 21.World Organization for Animal Health. 2012. Manual of diagnostic tests and vaccines for terrestrial animals, 7th ed World Organization for Animal Health, Paris, France. [Google Scholar]
- 22.Lopez-Goni I, Garcia-Yoldi D, Marin CM, de Miguel MJ, Munoz PM, Blasco JM, Jacques I, Grayon M, Cloeckaert A, Ferreira AC, Cardoso R, Correa de Sa MI, Walravens K, Albert D, Garin-Bastuji B. 2008. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol 46:3484–3487. doi: 10.1128/JCM.00837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greiner M, Gardner IA. 2000. Application of diagnostic tests in veterinary epidemiologic studies. Prev Vet Med 45:43–59. doi: 10.1016/S0167-5877(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 24.Swets JA. 1988. Measuring the accuracy of diagnostic systems. Science 240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 25.Greiner M, Sohr D, Gobel P. 1995. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods 185:123–132. doi: 10.1016/0022-1759(95)00121-P. [DOI] [PubMed] [Google Scholar]
- 26.Dieste-Perez L, Blasco JM, de Miguel MJ, Moriyon I, Munoz PM. 2015. Diagnostic performance of serological tests for swine brucellosis in the presence of false positive serological reactions. J Microbiol Methods 111:57–63. doi: 10.1016/j.mimet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz PM, Marín CM, Monreal D, González D, Garin-Bastuji B, Díaz R, Mainar-Jaime RC, Moriyón I, Blasco JM. 2005. Efficacy of several serological tests and antigens for diagnosis of bovine brucellosis in the presence of false-positive serological results due to Yersinia enterocolitica O:9. Clin Diagn Lab Immunol 12:141–151. doi: 10.1128/CDLI.12.1.141-151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGiven J, Howells L, Duncombe L, Stack J, Ganesh NV, Guiard J, Bundle DR. 2015. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping M epitope elements of Brucella O-polysaccharide. J Clin Microbiol 53:1204–1210. doi: 10.1128/JCM.03185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques I, Olivier-Bernardin V, Dubray G. 1991. Induction of antibody and protective responses in mice by Brucella O-polysaccharide-BSA conjugate. Vaccine 9:896–900. doi: 10.1016/0264-410X(91)90010-4. [DOI] [PubMed] [Google Scholar]
- 30.Bundle DR, Perry MB. 1985. Structure and serology of the Brucella abortus O-antigen. Biochem Soc Trans 13:980–982. doi: 10.1042/bst0130980. [DOI] [PubMed] [Google Scholar]
- 31.Caroff M, Bundle DR, Perry MB. 1984. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem 139:195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- 32.Meikle PJ, Perry MB, Cherwonogrodzky JW, Bundle DR. 1989. Fine structure of A and M antigens from Brucella biovars. Infect Immun 57:2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGiven JA, Nicola A, Commander NJ, Duncombe L, Taylor AV, Villari S, Dainty A, Thirlwall R, Bouzelmat N, Perrett LL, Brew SD, Stack JA. 2012. An evaluation of the capability of existing and novel serodiagnostic methods for porcine brucellosis to reduce false positive serological reactions. Vet Microbiol 160:378–386. doi: 10.1016/j.vetmic.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen K, Smith P, Yu W, Nicoletti P, Jungersen G, Stack J, Godfroid J. 2006. Serological discrimination by indirect enzyme immunoassay between the antibody response to Brucella sp. and Yersinia enterocolitica O:9 in cattle and pigs. Vet Immunol Immunopathol 109:69–78. doi: 10.1016/j.vetimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Chen JD, Ke CW, Deng X, Jiang S, Liang W, Ke BX, Li B, Tan H, Liu M. 2013. Brucellosis in Guangdong Province, People's Republic of China, 2005-2010. Emerg Infect Dis 19:817–818. doi: 10.3201/eid1905.120146. [DOI] [PMC free article] [PubMed] [Google Scholar]