Abstract
Rapid and sensitive detection of macrolide resistance in Mycoplasma genitalium is required for the guidance of adequate antimicrobial treatment. Previous studies have confirmed that single-base mutations at position 2058 or 2059 in domain V of the 23S rRNA gene of M. genitalium result in high-level macrolide resistance. Sequencing of PCR products remains the gold standard for the identification of mutations conferring resistance to macrolides but is laborious and time-consuming. The aim of the present study was to develop a 5′ nuclease genotyping assay to detect single nucleotide polymorphisms in the 23S rRNA gene of Mycoplasma genitalium that are associated with macrolide resistance by combining PCR with hydrolysis probes and subsequent endpoint genotyping analysis. The 5′ nuclease genotyping assay was used as a referral test to be used on M. genitalium-positive samples and was validated on 259 positive samples, of which 253 (97.7%) were successfully sequenced. With the newly developed assay, 237/259 (91.5%) investigated M. genitalium-positive samples were genotyped. The positive and the negative predictive values were 100% when evaluated on successfully genotyped samples. The newly developed assay discriminated macrolide-resistant M. genitalium in clinical specimens possessing A2058G, A2058C, A2058T, and A2059G mutations with a sensitivity of 94.4% (95% confidence interval [CI], 90.7% to 98.2%) and a specificity of 92.7% (95% CI, 87.8% to 97.6%) when evaluated on successfully sequenced samples. The assay can correctly guide antimicrobial treatment of M. genitalium infections.
INTRODUCTION
Mycoplasma genitalium is a sexually transmitted bacterium that causes nongonococcal urethritis in men and has been associated with cervicitis and pelvic inflammatory disease in women (1). The current recommended treatment of M. genitalium infection in Scandinavia is an extended course of oral macrolide. However, if empirical treatment of nongonococcal urethritis is initiated, usually only a single dose of azithromycin is given as treatment for suspected Chlamydia infection. Single-dose azithromycin treatment of M. genitalium infection is associated with a suboptimal treatment response and, in the case of treatment failure, with the development of M. genitalium macrolide resistance (2, 3). Macrolide-resistant M. genitalium strains are consequently prevalent, and resistance rates of 41% in a cohort of men with urethritis in London (4) and 38% in a recent population-based Danish survey (5) have been reported. In proven M. genitalium infection, macrolide susceptibility reporting is therefore necessary to guide therapy (6).
Macrolide resistance in M. genitalium is strongly associated with the mutations A2058G and A2059G in the gene encoding 23S rRNA (3, 7, 8). In a recent Dutch study, a high proportion of the A2058T mutation causing resistance was observed (9). Resistance-associated mutations can be identified by PCR amplification and sequencing. This method is best suited to process samples in bulk and may result in unacceptable reporting times. High-resolution melting (HRM) analysis is another powerful tool to detect mutations in a target sequence, and PCR assays using HRM analysis for detecting mutations associated with macrolide resistance have recently been reported (10, 11). An alternative method for identifying known mutations is genotyping assays using differentially labeled hydrolysis probes targeting wild-type and mutated alleles (12, 13). In the present study, we report such a 5′ nuclease genotyping assay for the detection of the macrolide resistance-associated mutations in M. genitalium. The assay was used to determine macrolide resistance in 259 samples that had previously tested positive for the presence of M. genitalium, and the results obtained using this assay were compared to sequencing of the region of interest in the 23S rRNA gene. The 5′ nuclease genotyping assay was highly accurate compared to sequencing.
MATERIALS AND METHODS
M. genitalium detection.
Between 8 October 2013 and 12 September 2014, the Department of Clinical Microbiology received 3,147 samples to test for the presence of M. genitalium. Testing was done using a laboratory-developed quantitative PCR (qPCR) using hydrolysis probes targeting the pdhD and mgpB genes of M. genitalium and an additional sample-processing control. The primers and probes of this laboratory-developed test have previously been published (14–16). In the multiplex qPCR, the final concentrations of M. genitalium-specific primers and of probes were 500 nM and 100 nM, respectively. Reactions were done in a total volume of 20 μl with LightCycler 480 probes master (Roche Applied Sciences, Penzberg, Germany) as the master mix. PCR was performed on the LightCycler 480 instrument (Roche Diagnostics, Basel, Switzerland) with the following PCR profile: 10 min of 95°C followed by 50 cycles of 95°C for 10 s and 60°C for 25 s. Fluorescence intensity was measured at the end of each cycle. Samples were identified as positive for the presence of M. genitalium if the two targets, pdhD and mgpB, were reactive. If only one target was detected, the sample was retested, and if reactive in either target upon retest, the sample was considered positive for M. genitalium.
Ethics statement.
This study was done using residual samples from routine clinical testing. The experimental protocol was reviewed by the Ethics Committee of the Capital Region of Denmark. As the study did not include patient data, the committee waived further evaluation of the protocol and advised that informed consent was not required (no. 14012610).
Nucleic acid purification.
Total nucleic acid (TNA) from samples was purified by MagNA Pure 96 (Roche Diagnostics) using the MagNA Pure 96 DNA and Viral NA small-volume kit (Roche Applied Sciences). The input included 190 μl of sample material mixed with 10 μl of sample processing control. TNA was eluted in 100 μl.
Sanger sequencing of 23S rRNA.
Previously published primers were used for amplification of the PCR product and sequencing of the domain V of the 23S rRNA gene encompassing the 2058, 2059, and 2062 positions (numbers referring to Escherichia coli sequence) (11). PCR was performed on an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) using the Qiagen OneStep reverse transcriptase PCR (RT-PCR) kit (Qiagen, Hilden, Germany) with the following cycling settings: 50°C for 30 min and 95°C for 15 min followed by 40 cycles of 94°C for 15 s, 55°C for 30 s, 72°C for 60 s, and finally 72°C for 10 min with cooling at 4°C. The expected 266-bp amplicon was exclusively generated for samples that tested positive for M. genitalium. Sanger sequencing of PCR products was done by Macrogen Europe (Macrogen, Amsterdam, the Netherlands). Sequence data obtained were analyzed using CLC Main Workbench software (CLC bio, Aarhus, Denmark), and BLAST analysis confirmed that the 23S rRNA gene of M. genitalium had been sequenced.
5′ nuclease genotyping assay for M. genitalium macrolide susceptibility testing.
For the detection of point mutations associated with macrolide resistance in M. genitalium, a PCR using hydrolysis probes and subsequent endpoint genotyping analysis was developed. The assay specifically targeted two mutations associated with macrolide resistance in the gene encoding 23S rRNA in M. genitalium, A2058G and A2059G (GenBank accession no. NR_077054.1). Samples were analyzed in the period between 26 August and 23 September 2014 as detailed below.
A region of the 23S rRNA gene (positions 1979 to 2125) was targeted using the following primers modified from Jensen et al. (7) and probes designed for this study: 200 nM forward primer 5′-CCATCTCTTGACTGTCTCGGC-3′, 300 nM reverse primer 5′-CCTACCTATTCTCTACATGGTGGTG-3′, and a mixture of three different probes (400 nM wild-type probe LC610-GGACGGAAAGACCCCGTGAAGCTTT-BBQ, 200 nM A2058G probe LC640-GACGGGAAGACCCCGTGAAGCTTT-BBQ, and 200 nM A2059G probe LC640-GACGGAGAGACCCCGTGAAGCTTT-BBQ (see Fig. 1). Reactions were performed using 8 μl of template in a total volume of 20 μl using LightCycler 480 probes master (Roche Applied Sciences) as the master mix. PCR was performed on the LightCycler 480 instrument (Roche Diagnostics) using the following PCR profile: 10 min at 95°C followed by 50 cycles of 95°C for 10 s and 60°C for 25 s. Fluorescence intensity was measured at the end of each cycle.
FIG 1.
Nucleotide sequences of the in-run controls used for the 5′ nuclease genotyping assay. The nucleotide sequences of wild-type Ultramer oligonucleotide (Wt) and Ultramer oligonucleotides containing the two most frequent macrolide resistance mutations, A2058G and A2059G, are shown. The location of the forward primer is indicated in blue and that of the reverse primer is indicated in green. Arrows indicate the 5′ to 3′ direction of the primer sequences. The nucleotide sequences of the three probes, wild-type probe, A2058G probe, and A2059G probe, are shown in the red boxes.
The data collected by the LightCycler 480 instrument were analyzed using the endpoint genotyping analysis module in the LightCycler 480 software. Based on the in-run controls and the endpoint fluorescence, the individual samples were categorized as either M. genitalium wild type or mutated by the software. As in-run controls, synthesized Ultramer oligonucleotides (Integrated DNA Technologies, Leuven, Belgium) comprising the entire PCR product were used. The sequences of the controls are shown in Fig. 1. A no-template control was included in each PCR run. Samples resulting in a negative call by the software (neither wild type nor mutated) or those that had fluorescence levels below 7,000 were repurified and tested a second time. No samples were processed more than twice.
RESULTS
Clinical samples.
During the period, 325 (10.3%) samples tested positive for the presence of M. genitalium, and for 259 of these, the residual sample was stored at −20°C for subsequent genotypic testing for macrolide resistance. Of the samples included, 113 (43.6%) were obtained from females (92 [81.4%] cervical swabs, 17 [15.0%] urethral swabs, and 4 [3.5%] urine samples), and 146 (56.4%) were obtained from males (94 [64.3%] urethral swabs and 52 [35.6%] urine samples). Of the samples not included, 30 (45.5%) originated from women (27 [90.0%] cervical swabs, 2 [6.7%] urethral swabs, and 1 [3.3%] urine sample), and 36 (54.5%) originated from men (19 [52.8%] urethral swabs and 17 [47.2%] urine samples). Samples included and not included did not differ with respect to patient gender (P = 0.89; Fisher's exact test). Furthermore, the crossing point values (Cp) that were obtained in diagnostic M. genitalium isolates did not vary between the included samples (Cp [pdhD], mean = 32.03) and those samples that were not included (Cp [pdhD], mean = 32.16) (P = 0.83; Student's t test; two-sided, independent samples, equal variance), which indicated that samples did not differ in bacterial load.
Sanger sequencing of 23S rRNA.
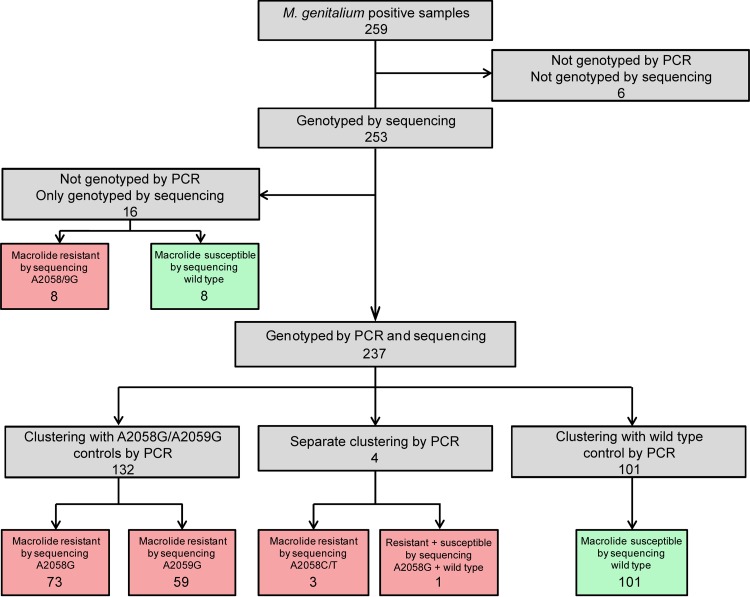
Using Sanger sequencing, we were able to amplify and sequence the 23S rRNA gene in 253/259 (97.7%; 95% confidence interval [CI], 95.9% to 99.5%) samples. Of the 253 successfully sequenced samples, 109 (43.1%) were wild type (i.e., macrolide susceptible), 75 (29.6%) contained the A2058G mutation (i.e., macrolide resistant), 65 (25.7%) contained the A2059G mutation (i.e., macrolide resistant), 2 (0.8%) contained the A2058T mutation (i.e., macrolide resistant), and 1 (0.4%) contained the A2058C mutation (i.e., macrolide resistant). The remaining sample displayed a mixed sequence chromatogram containing a wild-type and A2058G (macrolide resistant) result. These results are summarized in Fig. 2. In all, 144 of the 253 (56.9%; 95% CI, 50.8% to 63.0%) sequenced samples were categorized as containing mutations conferring resistance to macrolides (see Fig. 2, red boxes).
FIG 2.
Comparison of 5′ nuclease genotyping results with those of 23S rRNA sequencing. All 101 samples identified with the 5′ nuclease genotyping assay as wild type were verified by sequencing. All 132 samples, which by the 5′ nuclease genotyping assay were classified as containing M. genitalium with A2058G or A2059G mutations, were verified by sequencing. Four samples that clustered separately from the wild-type and A2058G/A2059G samples yielded either A2058C/T mutations or contained a mixture of wild-type and A2058G alleles when sequenced. Six of the 259 samples were not evaluated because the samples were not successfully genotyped by sequencing or by the 5′ nuclease genotyping assay. The figure samples that by sequencing contain mutations associated with macrolide resistance are indicated with red-shaded boxes. Samples without mutations, i.e., categorized by sequencing as macrolide susceptible, are indicated by green-shaded boxes. Of the 253 samples genotyped by sequencing, 109 (43.1%) were wild type, 75 (29.6%) contained the A2058G mutation, 65 (25.7%) contained the A2059G mutation, 2 (0.8%) contained the A2058T mutation, and 1 (0.4%) contained an A2058C mutation. The remaining sample displayed a mixed sequence chromatogram containing wild-type and A2058G results. In all, 144 of the 253 sequenced samples contained mutations conferring resistance to macrolides (56.9%).
5′ nuclease genotyping assay for M. genitalium macrolide susceptibility testing.
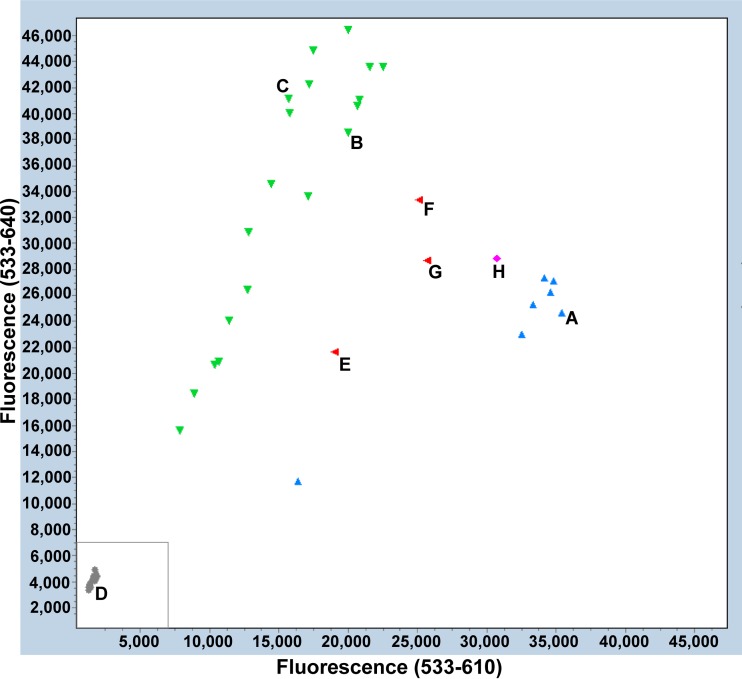
The 5′ nuclease genotyping assay successfully genotyped 223/259 (86.1%; 95% CI, 81.9% to 90.3%) samples. When the assay was repeated on the 36 samples that did not successfully genotype, 14 additional samples were successfully genotyped, yielding a total of 237 (91.5%; 95% CI, 88.5% to 94.9%) samples genotyped. Figure 3 shows an example of one of the plots. Of the 237 samples, 132 were genotyped as containing either the A2058G or the A2059G mutation (green in Fig. 3). The assay did not distinguish between the two mutations A2071G and A2072G, as these were grouped together in the endpoint fluorescence scatter plot. In all, 101 genotyped samples were classified as wild type (blue in Fig. 3).
FIG 3.
Endpoint fluorescence scatter plot containing 4 in-run controls and 40 clinical specimens. Control reactions using Ultramer oligonucleotides for wild type (Wt) (A), A2058G (B), and A2059G (C) are shown. (D) No template control. The gray box indicates the area where fluorescence levels were below 7,000 and samples were rerun. Clinical specimens were grouped by the software as either wild-type M. genitalium (blue), mutated M. genitalium (green), or inconclusive (gray). The plot shows four samples clustering between the wild-type and mutant groups in red (E, F, and G) and magenta (H). Calls of the software were in these cases “both alleles” (red) or “unknown” (magenta). These calls were generated by clinical specimens that contained A2058C/T mutations or a mixture of wild-type and A2058G results when sequenced.
Four samples were not genotyped as wild type or A2058G/A2059G but formed a separate cluster and were genotyped as “both alleles” or “unknown” by the software (red and magenta in Fig. 3) and were clustered in between the wild-type and A2058G/A2059G clusters. These results were reproducible when the samples were rerun. The four samples contained A2058C/T mutations (three samples) or a wild-type and A2058G result (one sample) by Sanger analysis.
Discrimination of the assay, i.e., the ability to remain nonreactive in the absence of M. genitalium in the sample, was evaluated using 141 clinical samples (92 samples from females [75 cervical swabs, 11 urethral swabs, and 6 urine samples] and 49 samples from males [21 urethral swabs and 28 urine samples]), 6 throat swabs that had tested positive for Mycoplasma pneumoniae (mean Cp, 25.9; range, 24.0 to 28.6), and a panel of Mycoplasma spp. (n = 14). The panel included Mycoplasma primatum, Mycoplasma orale, Mycoplasma lipophilum, M. pneumoniae, Mycoplasma alvi, Mycoplasma penetrans, Mycoplasma salivarium, Mycoplasma pirum, Mycoplasma arginini, Mycoplasma hominis, Mycoplasma buccale, Mycoplasma amphoriforme, and Mycoplasma gallisepticum (at concentrations between 105 and 108 genome equivalents/ml). All of the above samples were not reactive in the 5′ nuclease genotyping assay.
Comparison of 5′ nuclease genotyping results with those of 23S rRNA sequencing.
The results from the 5′ nuclease genotyping assay were compared to those of 23S rRNA sequencing. The results of the two methods are summarized in Fig. 2. The 5′ nuclease genotyping assay did not result in false-negative or false-positive results. All 101 samples that were identified with the 5′ nuclease genotyping assay as wild type were verified by sequencing. All 132 samples, which by the 5′ nuclease genotyping assay were classified as containing M. genitalium with an A2058G or A2059G mutation, were verified by sequencing. The four samples that resulted in ambiguous calls by the software yielded either A2058C/T mutations or contained a mixture of wild-type and A2058G alleles. The positive predictive value and the negative predictive value of the assay were each 100%.
The proportion of samples genotyped was higher for the sequencing assay than for the 5′ nuclease assay (253/259 [97.7%; 95% CI, 95.9% to 99.5%] versus 237/259 [91.5%; 95% CI, 88.1% to 94.9%]; P < 0.001 [McNemar's test]). If the sensitivity and specificity of the 5′ nuclease genotyping assay are calculated on the basis of all samples genotyped by sequencing, the sensitivity (i.e., the proportion correctly classified as macrolide resistant by the 5′ nuclease assay of the number of samples classified as macrolide resistant by sequencing) of the 5′ nuclease genotyping assay was (132 + 4)/(132 + 4 + 8) = 94.4% (95% CI, 90.7% to 98.2%) and the specificity (i.e., the proportion correctly classified as macrolide susceptible by the 5′ nuclease assay of the number of samples classified as macrolide susceptible by sequencing) was 101/(101 + 8) = 92.7% (95% CI, 87.8% to 97.6%). The accuracy of the 5′ nuclease genotyping assay was 93.7% (237/253 samples).
DISCUSSION
In this study, we report that 56.9% (95% CI, 50.8% to 63.0%) of samples positive for the presence M. genitalium contain mutations conferring resistance to first-line macrolide treatment, which indicates a further increase in resistance from the 38.1% resistance rate reported by Salado-Rasmussen and Jensen (5) based on Danish samples collected between January 2006 and December 2010. The high frequency of resistance and the limited treatment options necessitate rapid resistance assays to spare secondary treatment options (6). Indeed, emerging resistance to secondary treatment options using fluoroquinolones has already been reported in selected populations (3, 4, 8, 17).
Clinical M. genitalium infections are often associated with low bacterial loads in clinical samples (18), and these low loads may pose specific problems for genotyping assays used to determine macrolide susceptibility. Touati et al. recently reported on a fluorescence resonance energy transfer (FRET)-based HRM assay that is capable of identifying the most frequent resistance-causing mutations in 23S rRNA: A2058G, A2059G, and A2058C. In their report, they were capable of genotyping 155/202 (76.7%) specimens (11). Wold et al. described a 5′ nuclease-based assay using a common probe and 6 different forward primers specific to wild type, A2059G, A2059C, A2058G, A2058C, and A2058T results, respectively. They restricted their analysis to samples that were positive with a quantification cycle (Cq) value of <32 and obtained in this subset a genotype for 99/105 (94.3%) samples. However, the need for a preselection of samples with a relatively high bacterial load may suggest that sensitivity may limit the utility of this assay in clinical routine (11, 19).
The 5′ nuclease genotyping assay reported here was used to characterize samples containing M. genitalium. The assay successfully genotyped 86.1% of M. genitalium PCR-positive samples in a single attempt and 91.5% of samples when repeated. This finding may indicate that the proportion of samples genotyped is limited by sampling variation. Increasing the number of replicates used for genotyping may partially circumvent this sampling problem. Thus, we would expect a greater than 90% successful genotyping rate if the assay is done in duplicate and a further increase if more replicates are done. In addition, positive samples were stored at −20°C until genotyped. It has previously been reported that M. genitalium DNA may degrade when stored at this temperature for extended periods (18). For these reasons, a less than 100% successful genotyping rate was to be expected.
The assay does not discern between the most frequently detected resistance mutations A2058G and A2059G; however, the third most frequent resistance mutation A2058T consistently yielded a signal in between wild-type and the A2058G/A2059G variants. Although the A2058T mutation was only infrequently detected in the present study (2/136 [1.5%] resistant samples), isolates with this resistance mutation may be fit and transmissible, as samples containing this mutation were present in almost a third of the resistant samples in a recently published Dutch study (9).
In conclusion, we describe a 5′ nuclease genotyping assay that is easily interpretable and allows timely reporting of macrolide resistance in M. genitalium. The assay can genotype a large proportion of samples that test positive in a primary diagnostic assay for M. genitalium and displays a high concordance with sequencing.
ACKNOWLEDGMENTS
We thank Kaitlin A. Tagg at the Centre for Infectious Diseases and Microbiology, Institute of Clinical Pathology and Medical Research, Westmead Hospital, Australia for donating sequenced DNA from specimens positive for M. genitalium. We thank Jørgen Skov Jensen (State Serum Institute, Denmark) for kindly contributing the panel of Mycoplasma spp. We also thank the laboratorians at the Department of Clinical Microbiology, Hvidovre University Hospital, Denmark, especially Dilek Özdemir, for participating in the present investigation.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagrius C, Lore B, Jensen JS. 2013. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One 8(4):e61481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, Vodstrcil LA, Jensen JS, Hocking JS, Garland SM, Bradshaw CS. 2014. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 60:1228–1236. doi: 10.1093/cid/ciu1162. [DOI] [PubMed] [Google Scholar]
- 4.Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. 2014. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 58:631–637. doi: 10.1093/cid/cit752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salado-Rasmussen K, Jensen JS. 2014. Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis 59:24–30. doi: 10.1093/cid/ciu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manhart LE. 2014. Diagnostic and resistance testing for Mycoplasma genitalium: what will it take? Clin Infect Dis 59:31–33. doi: 10.1093/cid/ciu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. 2008. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 47:1546–1553. doi: 10.1086/593188. [DOI] [PubMed] [Google Scholar]
- 8.Tagg KA, Jeoffreys NJ, Couldwell DL, Donald JA, Gilbert GL. 2013. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol 51:2245–2249. doi: 10.1128/JCM.00495-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nijhuis RH, Severs TT, Van der Vegt DS, Van Zwet AA, Kusters JG. 2015. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother 70:2515–2518. doi: 10.1093/jac/dkv136. [DOI] [PubMed] [Google Scholar]
- 10.Twin J, Jensen JS, Bradshaw CS, Garland SM, Fairley CK, Min LY, Tabrizi SN. 2012. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One 7(4):e35593. doi: 10.1371/journal.pone.0035593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touati A, Peuchant O, Jensen JS, Bebear C, Pereyre S. 2014. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol 52:1549–1555. doi: 10.1128/JCM.03318-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles J, Hardick J, Yuenger J, Dan M, Reich K, Zenilman J. 2004. Use of applied biosystems 7900HT sequence detection system and TaqMan assay for detection of quinolone-resistant Neisseria gonorrhoeae. J Clin Microbiol 42:3281–3283. doi: 10.1128/JCM.42.7.3281-3283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ. 1999. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14:143–149. doi: 10.1016/S1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JS, Bjornelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 42:683–692. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller EE, Venter JM, Magooa MP, Morrison C, Lewis DA, Mavedzenge SN. 2012. Development of a rotor-gene real-time PCR assay for the detection and quantification of Mycoplasma genitalium. J Microbiol Methods 88:311–315. doi: 10.1016/j.mimet.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Hoegh AM, Nielsen JB, Lester A, Friis-Moller A, Schonning K. 2012. A multiplex, internally controlled real-time PCR assay for detection of toxigenic Clostridium difficile and identification of hypervirulent strain 027/ST-1. Eur J Clin Microbiol Infect Dis 31:1073–1079. doi: 10.1007/s10096-011-1409-5. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi M, Ito S, Yasuda M, Tsuchiya T, Hatazaki K, Takanashi M, Ezaki T, Deguchi T. 2014. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother 69:2376–2382. doi: 10.1093/jac/dku164. [DOI] [PubMed] [Google Scholar]
- 18.Carlsen KH, Jensen JS. 2010. Mycoplasma genitalium PCR: does freezing of specimens affect sensitivity? J Clin Microbiol 48:3624–3627. doi: 10.1128/JCM.00232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wold C, Sorthe J, Hartgill U, Olsen AO, Moghaddam A, Reinton N. 2015. Identification of macrolide-resistant Mycoplasma genitalium using real-time PCR. J Eur Acad Dermatol Venereol 29:1616–1620. doi: 10.1111/jdv.12963. [DOI] [PubMed] [Google Scholar]