Abstract
Sputum acid-fast bacilli (AFB) smear microscopy has suboptimal sensitivity but remains the most commonly used laboratory test to diagnose pulmonary tuberculosis (TB). We prospectively evaluated the small membrane filtration (SMF) method that concentrates AFB in a smaller area to facilitate detection to improve the diagnostic performance of microscopy. We enrolled adults with suspicion of pulmonary TB from health facilities in southwestern Uganda. Clinical history, physical examination, and 3 sputum samples were obtained for direct fluorescent AFB smear, SMF, Xpert MTB/RIF, and MGIT culture media. Sensitivity and specificity were estimated for SMF, AFB smear, and Xpert MTB/RIF, using MGIT as the reference standard. The analysis was stratified according to HIV status. From September 2012 to April 2014, 737 participants were included in the HIV-infected stratum (146 [20.5%] were culture positive) and 313 were in the HIV-uninfected stratum (85 [28%] were culture positive). In HIV-infected patients, the sensitivity of a single SMF was 67.4% (95% confidence interval [CI], 59.9% to 74.1%); for AFB, 68.0% (95% CI, 60.6% to 74.6%); and for Xpert MTB/RIF, 91.0% (95% CI, 85.0% to 94.8%). In HIV-uninfected patients, the corresponding sensitivities were 72.5% (95% CI, 62.1% to 80.9%), 80.3% (95% CI, 70.8% to 87.2%), and 93.5% (95% CI, 85.7% to 97.2%). The specificity for all 3 tests in both HIV groups was ≥96%. In this setting, the SMF method did not improve the diagnostic accuracy of sputum AFB. The Xpert MTB/RIF assay performed well in both HIV-infected and -uninfected groups.
INTRODUCTION
Direct acid-fast bacilli (AFB) smear microscopy of unconcentrated sputum has been the mainstay of tuberculosis (TB) diagnosis for more than a century, especially in resource-constrained settings, where more than 90% of the cases and TB-related deaths occur (1). Direct AFB smear microscopy is inexpensive, is specific in high-TB-prevalence settings, and can rapidly identify the most infectious patients (i.e., sputum AFB positive) (2, 3). However, sputum AFB has suboptimal sensitivity, particularly in populations with a low Mycobacterium tuberculosis bacillary load in sputum (i.e., paucibacillary TB), such as children, those with extrapulmonary TB, and persons with HIV infection (4). Overall, the diagnostic sensitivity of the direct sputum AFB smear ranges between 30% and 75%, often leading to delayed diagnosis, continued transmission, and poor outcomes (5, 6).
Several methods have been evaluated to increase the sensitivity of smear microscopy. These include the use of chemicals, such as sodium hydroxide (NaOH), N-acetyl-l-cysteine (NALC), or sodium hypochlorite (bleach), to liquefy and concentrate sputum (7–9), use of light-emitting diodes to improve visualization (10, 11), and physical measures, such as centrifugation or gravity sedimentation (12, 13). A systematic review showed that sputum centrifugation plus a chemical (NALC or NaOH) can improve the yield of smear microscopy up to 39% (14). However, centrifuges are expensive, require electricity, and are biosafety level 3, as they carry a potential risk of aerosolization, and some bacilli may be lost in the supernatant. In general, given the widespread use and operational benefits of smear microscopy, any technology that increases its yield would be of great value.
The small membrane filtration (SMF) is a novel method that concentrates bacilli from clinical samples by filtrating the sputum digests through a 25-mm-diameter polycarbonate membrane filter (15). After filtration, the membrane is fixed to a glass slide that can be stained and examined for AFB with standard methods. In the initial proof-of-concept studies that used individual filters connected to a rudimentary, plastic-made vacuum manifold, SMF showed an absolute incremental yield of up to 40% compared to direct smear, but the filtration failure rate (5% to 20%) was unacceptably high (15, 16). Based on these promising results, the method was modified to include an aluminum-made, multitest vacuum manifold designed for semiautomation and a new sample dilution protocol to prevent filtration failure. In a prospective evaluation in mostly hospitalized TB suspects with advanced HIV disease in Kampala, Uganda (17), the semiautomated SMF method had a lower (48.5%) sensitivity than direct smear (60.9%), but questions were raised regarding the laboratory protocol used and the generalizability of the study population (18). This study reports on a prospective diagnostic study stratified by HIV status evaluating the SMF method in comparison to the Xpert MTB/RIF and using culture as the reference in a large cohort of predominantly outpatient TB suspects in a high-HIV- and -TB-burden setting.
MATERIALS AND METHODS
Participants.
We prospectively enrolled adults with suspicion of pulmonary TB at the Mbarara Regional Referral Hospital (MRRH) and the Mbarara Municipality Centre in Mbarara, Uganda. MRRH is a tertiary-care hospital with over 250 inpatient beds and serves as the main referral health facility for the southwestern region of Uganda. With an estimated TB incidence of 166 cases per 100,000 inhabitants, Uganda is on the WHO list of high-burden TB countries; the prevalence of HIV infection among TB patients is 48% (19). Health personnel caring for patients referred potential participants to the study team. We included pulmonary TB suspects 18 years or older with cough for ≥2 weeks and at least one additional TB symptom (fever, weight loss, or night sweats). We excluded patients who had received >2 days of antituberculosis therapy within the last 60 days.
Clinical data.
We collected clinical and demographic data using a structured data capture form and performed HIV testing in TB suspects with unknown status after obtaining consent. A CD4 cell count was obtained in those with a positive HIV test. A posteroanterior chest radiograph was performed in nonpregnant participants and interpreted by a radiologist experienced in TB.
Laboratory methods.
All laboratory testing was done at the biosafety level 3 laboratory of Epicentre/Médecins sans Frontières Mbarara Research Centre in Mbarara, Uganda. The research laboratory has strict quality control and quality assurance methods in place and extensive experience in laboratory-based research. External quality assessment is provided on a yearly basis by the Institute of Tropical Medicine in Antwerp, Belgium, and the National Health Laboratory Services in South Africa. We obtained three sputum samples (≥2 ml each), one from an early morning (EM) specimen and two from spot sampling performed 1 day apart.
Study design.
All three samples were tested for direct fluorescent smear and mycobacterial growth indicator tube (MGIT) culture. EM samples were dedicated to SMF testing, while spot samples were randomized in a 1:1 ratio using a block size of 4, so that one sample was tested by SMF (S1) and one, by Xpert MTB/RIF (S2). Therefore, all three samples were tested on direct smear and culture, two by SMF, and one by Xpert MTB/RIF. Given the randomization, the primary comparison of samples was as follows: SMF versus direct smear used EM and S1 samples, SMF versus Xpert MTB/RIF used S1 and S2 samples, and direct smear versus Xpert MTB/RIF used S2 samples.
Sample handling and processing.
Samples were collected at the Epicentre laboratory or at the participating health centers. After informed consent was obtained, study participants were instructed by a study nurse on how to provide an adequate sputum specimen, which included rinsing the mouth with water before expectorating the sample. The instructions were printed and handed to participants in the sputum collection room. Once obtained, the volume and appearance of the specimen was visually assessed by the nurse and then placed in an iced-filled container before transportation to the laboratory within 3 h. Upon arrival, samples were labeled as EM, spot 1, or spot 2. Samples for SMF analysis (EM and randomized spot) were then treated with an equal volume (50 mg/ml of sterile sodium citrate buffer) of N-acetyl-l-cysteine (NALC). In order to prevent filtration failure due to debris clogging the SMF membrane, the digest was then filtered through sterile gauze into a second tube, vortexed, and split in half by volume. One half was processed for standard direct fluorescent AFB smear microscopy and culture, and the other half was used for SMF testing. On the other spot specimen, a direct smear was performed before decontamination of the sample using NALC/sodium hydroxide (NaOH) solution and then processed for culture and molecular testing (Xpert MTB/RIF).
Standard laboratory methods.
Specimens were suspended in 1.5 ml of buffer. Direct smear was done using the auramine technique, examined using an Olympus microscope equipped with the fluorescence system (LED system; BergmanLabora, Danderyd, Sweden), analyzed for AFB, and graded according to WHO criteria (20). After standard processing, 0.5 ml of the specimen was incubated using the manual mycobacterial growth indicator tube (MGIT; Becton Dickinson, NJ, USA) system for up to 6 weeks. Contamination in MGIT media was ruled out using Ziehl-Neelsen (ZN) microscopy and culture on blood agar. For all positive MGIT cultures, we differentiated between Mycoplasma tuberculosis and nontuberculous mycobacteria (NTM) using the SD TB Ag MPT64 rapid system (SD Bioline, Kyongi-do, South Korea), following the manufacturer's instructions. The GenoType mycobacterium CM/AS identification kit (Hain Lifescience, Nehren, Germany) was used for identification of NTM. The Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) was performed according to the manufacturer's instructions.
SMF method.
Prior to study initiation, two developers of the SMF test (K. P. Fennelly and S. Vinhas) trained study laboratory personnel on the SMF methods according to a standard operating procedure. Briefly, the SMF sample was mixed with 3% sodium hypochlorite (JIK brand; Reckitt Benckiser, Kampala, Uganda) in a 3:1 ratio and vortexed every 5 min for 15 min; 1 volume of Triton X-100-plus-ethanol (final concentration, 95% ethanol to 1% Triton X-100) (Triton X-100, Sigma-Aldrich, USA) was added. The digest was then prefiltered through a 30-μm pore size filter for easy flow and collected into a conical tube that was then attached to a 6-place stainless steel vacuum manifold (Merck Millipore Corporation, Darmstadt, Germany) controlled at a pressure of 25 mm of mercury. The sample (median sample volume, 4 ml; range, 1 to 22 ml) was then filtered through a 0.8-μm Isopore membrane filter (Merck Millipore Corporation, Darmstadt, Germany). Once the sample had been completely filtered, the containing trapped material was attached to a standard glass microscopy slide (Fisher Scientific), heat fixed at 80°C for 10 min, and stained and graded using the standard WHO microscopy grading (20). All laboratory methods were quality controlled, and the personnel interpreting the SMF smears were blinded to the results of Xpert MTB/RIF and direct microscopy using separate registers concealed by coded numbers.
Statistical analysis.
We report the results according to the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines (21, 22). The index test under evaluation for this study was SMF, which was compared to direct smear and to Xpert MTB/RIF using M. tuberculosis culture, using a composite of three culture results as a reference standard. Participants with any M. tuberculosis culture-positive result were used to estimate sensitivity, and those with all M. tuberculosis culture-negative results were used to estimate specificity. Comparisons were made using the generalized estimating equation (GEE) approach with an independent working correlation matrix to fit a logistic regression model using a generalized score test statistic (23). A sandwich variance was used to account for correlation of test results within a single individual. Secondary comparisons of SMF versus Xpert MTB/RIF used McNemar's test, using data with complete definitive results for the two tests.
For estimation when a single outcome per study participant was used to estimate sensitivity or specificity, Wilson's score method was used to calculate 95% confidence intervals (CIs). When more than one outcome per study participant was used to estimate sensitivity and specificity, estimates were obtained from the logistic regression using the GEE method, and confidence intervals were calculated on the log scale and then exponentiated. Secondary comparisons using a combination of results based on (i) two SMF and two direct smears used the EM and S1 samples, (ii) two SMF used the EM and S1 samples, and (iii) three direct smear was performed using EM, S1, and S2 samples. When generating outcomes based on combination of results, any positive test resulted in a positive combination outcome. In the case of missing data, all available results were used, e.g., if only one SMF result was available, the worst of two SMF was based on that result. All testing and estimation were two sided at the 5% significance level and were not adjusted for multiple comparisons. The GENMOD and FREQ procedures using SAS 9.3 (Cary, NC) were used for all testing and estimation. A sample size estimate is provided in the supplemental material online.
Ethical approvals.
The study was approved by the institutional review boards of Mbarara University of Science and Technology, the Uganda National Council for Science and Technology, the Comité de Protection des Personnes de St Germain en Layes, France, and, Boston University Medical Center, with oversight of statistical analysis by the Rutgers University Newark Institutional Review Board. We obtained written informed consent from participants in accordance with ethical guidelines from participating institutions.
RESULTS
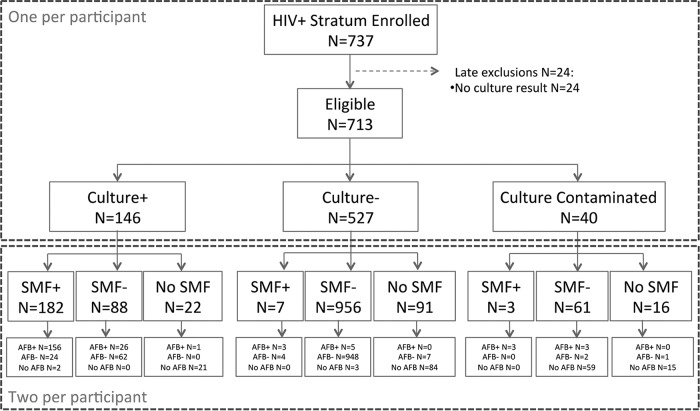
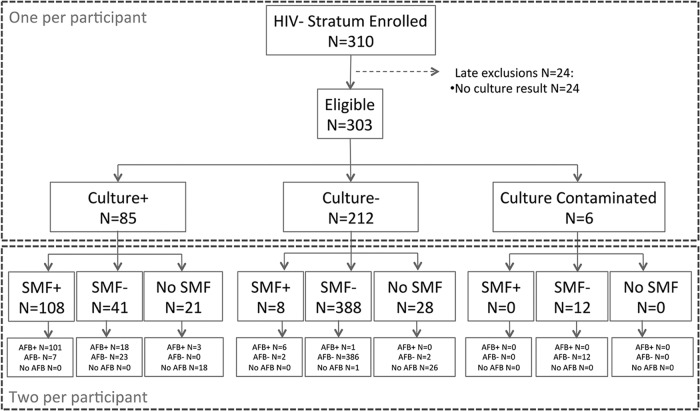
From September 2012 to April 2014, we screened 2,019 potentially eligible individuals but excluded 972 of them, because they did not meet study criteria for suspected pulmonary TB (Fig. 1). We enrolled 1,047 participants into the study, 737 into the HIV-infected cohort and 310 into the HIV-uninfected cohort. Of these, we excluded 24 (3.3%) HIV-infected and 7 (2.3%) HIV-uninfected subjects, because culture results were unavailable or because the HIV stratum was mismatched (1). Therefore, this analysis includes 713 (97% of originally enrolled participants) HIV-infected and 303 (98% of originally enrolled participants) HIV-uninfected patients with available culture results (Fig. 2 and 3).
FIG 1.
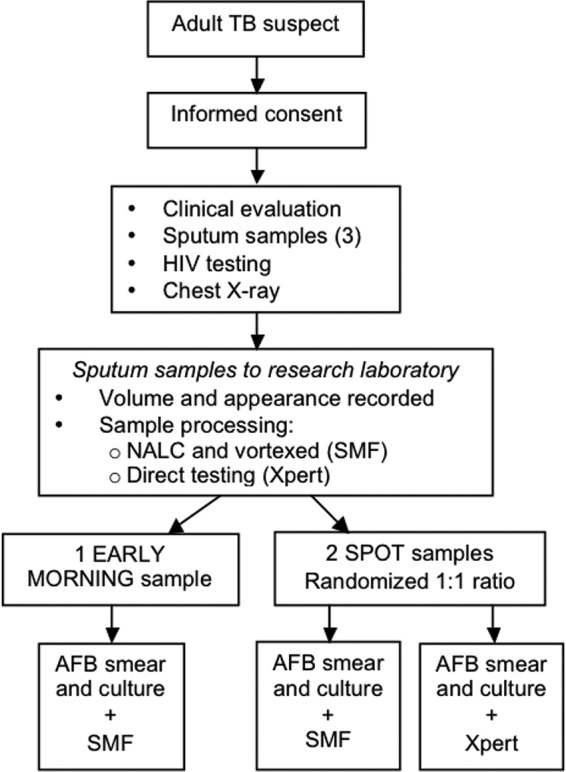
Study design.
FIG 2.
Comparison of a single AFB versus SMF using early morning and randomized spot samples in HIV-infected patients.
FIG 3.
Comparison of a single AFB versus SMF using early morning and randomized spot samples in HIV-uninfected patients.
Study population.
The demographic and clinical characteristics of study participants by HIV status are shown in Table 1. HIV-infected patients were more likely to be younger (P < 0.001), female (P = 0.02), unmarried (P < 0.001), and unemployed (P < 0.001) than those HIV uninfected. There were no differences in smoking, alcohol intake, and history of TB between the two HIV groups. In the 354 HIV-infected patients with CD4 results, the median (interquartile range [IQR]) was 308 cells/ml (111 to 484 cells/ml). In a per-patient analysis that included all available samples, 146 (20.5%) in the HIV-infected cohort were culture positive, 527 (73.9%) were culture negative, and 40 (5.6%) had contaminated cultures; the median (IQR) sputum volume was 4 ml (2 to 5 ml). In the HIV-uninfected cohort, 85 (28%) were culture positive, 212 (70%) were culture negative, and 6 (2%) had contaminated cultures; the median (IQR) sputum volume was 4 ml (3 to 5 ml).
TABLE 1.
Characteristics of 1,016 pulmonary tuberculosis suspects in Mbarara, Uganda, by HIV infection statusa
| Variable | HIV infected (n = 713) | HIV uninfected (n = 303) | P |
|---|---|---|---|
| Median (IQRb) age (yr) | 35 (29–43) | 46 (30–60) | <0.001 |
| No. (%) female gender | 374 (52) | 134 (44) | 0.02 |
| No. (%) any smoking | 69 (10) | 38 (13) | 0.12 |
| No. (%) previous TB history | 88 (12) | 32 (11) | 0.42 |
| No. (%) BCGc scar present | 439 (64) | 170 (57) | 0.15 |
| Median (IQR) BMId (kg/m2) | 20.3 (18–23) | 20 (17–22) | 0.06 |
| No. (%) symptoms | |||
| Fever | 520 (74) | 198 (66) | 0.02 |
| Hemoptysis | 111 (16) | 75 (25) | <0.001 |
| Night sweats | 466 (66) | 213 (71) | 0.11 |
| Extent of disease by chest radiograph | 0.06 | ||
| Normal | 63 (27) | 31 (33) | |
| Minimal | 53 (23) | 22 (23) | |
| Moderately advanced | 86 (37) | 21 (22) | |
| Far advanced | 32 (14) | 20 (21) | |
| No. (%) cavities | 37 (16) | 19 (20) | 0.32 |
| No. (%) miliary disease | 33 (14) | 15 (16) | 0.65 |
| No. (%) direct AFB smeare | |||
| Negative | 587 (82) | 223 (74) | |
| Positive | 125 (18) | 80 (26) | |
| Scanty | 20 (16) | 3 (4) | |
| 1+ | 25 (20) | 10 (12) | |
| 2+ | 22 (18) | 15 (19) | |
| 3+ | 58 (46) | 52 (65) | |
| CD4 cell count (cells/ml) | |||
| Median (IQR) | 308 (111–484) | ||
| Range | 0–1,482 |
Missing data: HIV-infected group: sputum AFB (n = 1), chest radiograph (n = 476), CD4 cell count (n = 359); HIV-uninfected group: chest radiograph (n = 209).
IQR, interquartile range.
BCG, bacille Calmette-Guérin vaccine.
BMI, body mass index.
AFB; acid-fast bacilli. Highest grade of all available results per patient.
Direct AFB results and effect of increasing number of samples.
For the HIV-infected cohort, a single direct AFB (here referred to as AFB) had a sensitivity of 68.3% (95% CI, 61.4% to 74.5%); two AFB had a sensitivity of 75.4% (95% CI, 67.7% to 81.7%); and three had a sensitivity of 79.3% (95% CI, 72.0% to 85.1%). All pairwise comparisons of sensitivity were statistically significant (comparison of 1 versus 2, P < 0.001; comparison of 2 versus 3, P = 0.026; and comparison of 1 versus 3, P < 0.001). The estimated specificity decreased with increasing number of samples from 99.2% (95% CI, 98.3% to 99.7%) to 99.0% (95% CI, 97.7% to 99.6%) and 98.7% (95% CI, 97.3% to 99.4%) for 1, 2, and 3 samples, respectively. While each increment did not result in a significant difference in specificity, increasing from 1 to 3 did result in a significant decrease (comparison of 1 versus 2, P = 0.21; comparison of 2 versus 3, P = 0.22; and comparison of 1 versus 3, P = 0.001) (Table 2).
TABLE 2.
Results of direct fluorescent AFBa smear microscopy, SMF, and Xpert MTB/RIF by HIV status in 1,016 pulmonary tuberculosis suspects in Mbarara, Uganda
| Method by HIV status | Number of test results with valid results by reference standard |
Number positive for test: % sensitivity (95% CIb) | Pc | Number negative for test: % specificity (95% CIb) | Pc | ||
|---|---|---|---|---|---|---|---|
| M. tuberculosis detected | M. tuberculosis not detected | Contaminated | |||||
| HIV-infected stratum | |||||||
| Reference standard (MGIT)d | 146 | 527 | 40 | ||||
| Comparison of direct AFB to SMFe | |||||||
| Direct AFB EM + spotf for SMF comparison | 135 + 134 | 479 + 488 | 30 + 35 | 183: 68.0 (60.6 to 74.6) | 0.81 | 959: 99.2 (97.9 to 99.7) | 0.79 |
| SMF EM + spotf | 136 + 134 | 479 + 481 | 29 + 35 | 18: 67.4 (59.9 to 74.1) | 956: 99.3 (98.2 to 99.7) | ||
| Comparison of direct AFB or SMF to Xpert MTB/RIFe | |||||||
| Xpert MTB/RIF | 134 | 479g | 34 | 122: 91.0 (85.0 to 94.8) | 464: 96.9 (94.9 to 98.1) | ||
| Direct AFB spot for Xpert comparison | 135 | 491 | 35 | 93: 68.9 (60.6 to 76.1) | <0.001 | 488: 99.4 (98.2 to 99.8) | 0.003 |
| SMF for Xpert comparison | 134 | 482 | 29 + 35 | 90: 67.2 (58.8 to 74.5) | <0.001 | 478: 99.2 (97.9 to 99.7) | 0.011 |
| HIV-uninfected stratum | |||||||
| Reference standard (MGIT)d | 85 | 212 | 6 | ||||
| Comparison of direct AFB to SMFe | |||||||
| Direct AFB EM + spotf for SMF comparison | 76 + 76 | 197 + 200 | 6 + 6 | 122: 80.3 (70.8 to 87.2) | 0.067 | 390: 98.2 (95.6 to 99.3) | 0.56 |
| SMF EM + spotf | 74 + 75 | 196 + 200 | 6 + 6 | 108: 72.5 (62.1 to 80.9) | 388: 98.0 (95.4 to 99.1) | ||
| Comparison of direct AFB or SMF to Xpert MTB/RIFe | |||||||
| Xpert MTB/RIF | 77 | 197 † | 6 | 72: 93.5 (85.7 to 97.2) | 190: 96.4 (92.8 to 98.3) | ||
| Direct AFB spot for Xpert comparison | 79 | 199 | 6 | 64: 81.0 (71.0 to 88.1) | 0.001 | 196: 98.5 (95.7 to 99.5) | 0.044 |
| SMF for Xpert comparison | 75 | 200 | 6 | 54: 72.0 (61.0 to 80.9) | <0.001 | 196: 98.0 (95.0 to 99.2) | 0.24 |
AFB, acid-fast bacilli.
CI, confidence interval.
Since all comparisons used multiple results per person, P values were calculated using the generalized estimating equations score test.
Reference standard was based on manual MGIT liquid culture (three samples), with the final MGIT culture result determined as a composite of three values; any positive culture determined a positive composite result.
Comparisons of direct AFB versus SMF use an early morning (EM) sample plus a spot sample randomized for SMF testing. Comparisons of direct AFB versus Xpert MTB/RIF use one sample randomized for Xpert MTB/RIF testing. Comparisons of SMF versus Xpert MTB/RIF use one sample randomized to Xpert MTB/RIF.
EM, early morning. When two samples from a participant are used for analysis, the number of EM and spot samples was delineated (EM + spot).
One Xpert MTB/RIF result was contaminated and excluded.
For the HIV-uninfected cohort, a single AFB test had a sensitivity of 80.5% (95% CI, 71.7% to 87.1%), two tests combined had a sensitivity of 85.2% (95% CI, 75.9% to 91.3%), and three had a sensitivity of 88.2% (95% CI, 79.7% to 93.5%). Each additional AFB improved the sensitivity estimate; increases from 1 to 2 AFB tests (P = 0.018) and from 1 to 3 (P < 0.001) were statistically significant, whereas a comparison of 2 versus 3 AFB had borderline significance (P = 0.075). AFB specificity was 98.3% (95% CI, 95.7% to 99.4%), 97.6% (95% CI, 94.5% to 99.0%), and 97.6% (95% CI, 94.6% to 99.0%) for 1, 2, and 3 AFB tests, respectively, and pairwise comparisons were as follows: 1 versus 2 (P = 0.068), 2 versus 3 (P = 0.025), and 1 versus 3 (P = 0.088).
SMF test performance.
For the HIV-infected cohort, a single SMF test had a sensitivity of 67.4% (95% CI, 59.9% to 74.1%), and two SMF combined had a sensitivity of 73.4% (95% CI, 65.6% to 80.0%), with the improvement being statistically significant (P = 0.006). The estimated specificity decreased slightly from 99.3% (95% CI, 98.2% to 99.7%) for 1 sample to 99.0% (95% CI, 97.7% to 99.6%) for 2 samples (P = 0.11). For the HIV-uninfected cohort, a single SMF test had sensitivity of 72.5% (95% CI, 62.1% to 80.9%), and two SMF combined had a sensitivity of 78.8% (95% CI, 68.6% to 86.3%), with the improvement being statistically significant (P < 0.001). The specificity of SMF decreased from 98.0% (95% CI, 95.4% to 99.1%) for 1 sample to 97.1% (95% CI, 93.8% to 98.7%) for 2 samples (P = 0.057). Processing time was similar for SMF and direct microscopy (approximately 10 min each).
Xpert MTB/RIF performance.
For the HIV-infected cohort, a single Xpert MTB/RIF test had estimated sensitivity of 91.0% (95% CI, 85.0% to 94.8%) and specificity of 96.9% (95% CI, 94.9% to 98.1%). For the HIV-uninfected cohort, a single Xpert MTB/RIF test had a sensitivity of 93.5% (95% CI, 85.7% to 97.2%) and a specificity of 96.4% (95% CI, 92.8% to 98.3%).
Comparison of SMF versus Xpert MTB/RIF.
One spot sample tested by Xpert MTB/RIF performed better than SMF for sensitivity (P < 0.001), but SMF had significantly higher specificity (P = 0.011) for the HIV-infected cohort. Likewise, for the HIV-uninfected cohort, one Xpert MTB/RIF performed better than SMF in terms of sensitivity (P < 0.001) but not specificity (P = 0.24). Combining SMF results from the EM sample to a spot did not improve sensitivity of SMF to the level of Xpert MTB/RIF for HIV-infected or HIV-uninfected individuals.
Comparison of combination of two SMF versus combination of two AFB.
Two SMF tests using an EM and one spot sample did not perform better than two direct AFB smears in terms of sensitivity (P = 0.48 and P = 0.09 for HIV-infected and -uninfected groups, respectively) or specificity (P = 0.99 and P = 0.56 for HIV-infected and -uninfected groups, respectively).
DISCUSSION
In this large cross-sectional diagnostic study, the SMF method did not improve the sensitivity of smear microscopy and, as expected, was inferior to Xpert MTB/RIF to diagnose culture-positive pulmonary TB in both HIV-infected and -uninfected patients. The results were similar when a single sample or two samples (one each of EM and spot) for SMF were compared to a single or two direct smears, respectively.
The SMF method is based on the principle that concentrating AFB over a smaller area can facilitate microscopic detection. In an initial study from Brazil of 313 HIV-uninfected individuals using Ogawa-Kudoh culture as the reference standard, the sensitivity of the SMF method on the first sputum specimen was 89%, compared to 60% for centrifuged and 56.1% for direct smears (15). Among smear-negative TB patients, SMF had a sensitivity of 73%, similar to the 67% achieved by Xpert MTB/RIF in a recent meta-analysis (25). The volumes of sputum used in that study were considerably larger (median, 6.8 ml) than those in this current study (4 ml). In a second Brazilian study that included 432 patients, the sensitivity of the SMF method in both HIV-infected and -uninfected participants (61.9% and 81.8%, respectively) was significantly higher than those of centrifuged sputum smears (47.6% and 63.6%, respectively) (16).
Our findings from this study are consistent with the only other prospective evaluation that was performed in Kampala, Uganda (17). In a study involving 212 mostly HIV-infected TB suspects, the sensitivity of the SMF method (48.5%) was significantly lower than that of direct (60.9%) or concentrated (63.3%) smear, using liquid and solid cultures as the standards of reference. SMF was particularly sensitive to sputum quality, with lower sensitivities noted in viscous and low-volume samples. However, the methods used in that study differed from the original two studies from Brazil, not only in using lower sputa volumes but also in using an unvalidated manifold apparatus (18). In this study, we found a 67.4% sensitivity of a single SMF in the HIV-infected cohort, which, although better than the 48.5% reported on the first prospective evaluation (17), still remains significantly lower than the 82% to 89% sensitivity found in the initial proof-of-concept studies (15, 16). Quality and volume of sputum specimens are known to predict smear positivity, as shown by a recent study from Korea where sputum volumes >4 ml were more likely to yield positive results (26). We hypothesized that the dilution protocol used in the Kampala study might have affected the SMF performance when distilled water was added to the samples to achieve a 5-ml volume. The median volume of sputum in the Kampala study was 3 ml (later divided into 3 aliquots), compared with 6.8-ml samples (divided in 2 aliquots) used in the initial study (15). Further, the initial studies excluded patients who were unable to produce sputum and used solid Ogawa media as the standard of reference; taken together, these factors may explain the lower sensitivity against the reference standard of liquid culture. However, the much higher performance of direct AFB smears in this setting was unexpected.
An integral component of the SMF test is the vacuum manifold, which is connected to the membrane filters to facilitate sample concentration. The initial studies used a simple vacuum manifold that did not require electricity, whereas the Kampala study used a stainless aluminum manifold that was designed for semiautomation and to decrease filtration failures. Indeed, the second-generation manifold might have led to decreased flow and loss of sputum digest, which could explain the suboptimal performance reported. A potential pitfall in the SMF method is loss of activity of chlorine during storage in hot conditions or if unprotected from light (16). It is possible that the sputum characteristics in the two Ugandan cohorts differed from those in the other studies in Brazil.
Importantly, direct AFB and Xpert MTB/RIF performed particularly well in our study, which is likely a reflection of using an experienced research laboratory with internal and external quality control methods and of sampling a study population with relatively advanced TB disease, as suggested by the AFB smear grade breakdown of study participants. In a systematic review evaluating 14 studies, direct smear had a sensitivity of up to 80% to detect culture proven pulmonary TB (14). However, the performance of AFB smear microscopy is likely to decline in less-experienced laboratories. The diagnostic yield of Xpert MTB/RIF in this study is in agreement with several other studies that evaluated the performance and cost-effectiveness of Xpert MTB/RIF to diagnose pulmonary TB in high-burden settings (24, 27, 28). Given its heightened sensitivity, diagnostic accuracy, and cost-saving and affordability potentials (29), Xpert MTB/RIF could replace sputum AFB smear microscopy as the standard TB diagnostic method in certain settings, but its benefit may be limited without a concomitant improvement in linkage to care (30). However, we found a measurably lower Xpert MTB/RIF specificity (96.4% to 96.9%) compared to a recent meta-analysis (99%) (25). Recent studies have reported false-positive results of Xpert MTB/RIF in previously treated TB patients as a possible indication of dead M. tuberculosis DNA after antituberculous treatment (31–33); in this study, 12% of HIV-infected and 11% of HIV-uninfected patients had a history of TB treatment.
Our study has limitations. We enrolled TB suspects from a large hospital outpatient clinic and a considerable number of hospitalized patients; thus, our results may not be applicable to smaller, more distant health care centers. The sputum samples for SMF testing underwent a homogenization step before AFB smear microscopy compared to the sample randomized for Xpert MTB/RIF testing; however, the homogenization process did not affect the sensitivity of direct AFB smear across samples (data not shown), and the comparisons of AFB versus SMF and AFB versus Xpert MTB/RIF always used comparable samples. Also, we did not collect data on sputum characteristics to address whether the presence of liquid, bloody, or viscous specimens had any impact on the yield of SMF. Due to logistical constraints, a significant proportion of study participants did not have CD4 and chest radiograph results. Finally, we did not have data on quantitative cultures to evaluate the performance of SMF in paucibacillary specimens.
In conclusion, the SMF test did not improve the yield of sputum AFB smear in this setting. As expected, the Xpert MTB/RIF was highly sensitive and specific and may be considered an initial standard TB diagnostic tool in Uganda.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Arthur Mugisha and Racheal Taganya (study clinicians) and Stella Kyasimire and Mastulah Nakanwagi (study nurses) for their contributions and Stephanie Moine (research coordinator at Boston Medical Center), Francis Varaine (Epicentre), and Andrew Ramsay (World Health Organization) for their input as part of the scientific committee overseeing this study and for critically reviewing the manuscript.
The study was funded by Médecins Sans Frontières. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Y.B., S.K., M.B., K.P.F., and E.C.J.-L. contributed to conception and design. Y.B., P.O., D.N., and J.M.-A. contributed to acquisition of data. Y.B., S.V., C.A.-V., K.P.F., and E.C.J.-L. contributed to analysis and interpretation. S.V. and K.P.F. provided on-site training of the SMF method and quality control throughout the study. All authors contributed to either drafting or revising the manuscript and gave final approval.
Funding Statement
The study was funded by Médecins sans Frontiéres.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00017-16.
REFERENCES
- 1.Trébucq A. 2004. Revisiting sputum smear microscopy. Int J Tuberc Lung Dis 8:805. [PubMed] [Google Scholar]
- 2.Sepkowitz KA. 1996. How contagious is tuberculosis? Clin Infect Dis 23:954–962. doi: 10.1093/clinids/23.5.954. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JB, Wynn-Williams N. 1954. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc 69:724–732. [DOI] [PubMed] [Google Scholar]
- 4.Getahun H, Harrington M, O'Brien R, Nunn P. 2007. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 5.Davies PD, Pai M. 2008. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis 12:1226–1234. [PubMed] [Google Scholar]
- 6.Dorman SE. 2010. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis 50 (Suppl 3):S173–S177. [DOI] [PubMed] [Google Scholar]
- 7.Angeby KA, Hoffner SE, Diwan VK. 2004. Should the bleach microscopy method be recommended for improved case detection of tuberculosis? Literature review and key person analysis. Int J Tuberc Lung Dis 8:806–815. [PubMed] [Google Scholar]
- 8.Chew R, Calderon C, Schumacher SG, Sherman JM, Caviedes L, Fuentes P, Coronel J, Valencia T, Hererra B, Zimic M, Huaroto L, Sabogal I, Escombe AR, Gilman RH, Evans CA. 2011. Evaluation of bleach-sedimentation for sterilizing and concentrating Mycobacterium tuberculosis in sputum specimens. BMC Infect Dis 11:269. doi: 10.1186/1471-2334-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet M, Ramsay A, Githui W, Gagnidze L, Varaine F, Guerin PJ. 2008. Bleach sedimentation: an opportunity to optimize smear microscopy for tuberculosis diagnosis in settings of high prevalence of HIV. Clin Infect Dis 46:1710–1716. doi: 10.1086/587891. [DOI] [PubMed] [Google Scholar]
- 10.Minion J, Sohn H, Pai M. 2009. Light-emitting diode technologies for TB diagnosis: what is on the market? Expert Rev Med Devices 6:341–345. doi: 10.1586/erd.09.26. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet M, Gagnidze L, Guerin PJ, Bonte L, Ramsay A, Githui W, Varaine F. 2011. Evaluation of combined LED-fluorescence microscopy and bleach sedimentation for diagnosis of tuberculosis at peripheral health service level. PLoS One 6:e20175. doi: 10.1371/journal.pone.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeby KA, Alvarado-Galvez C, Pineda-Garcia L, Hoffner SE. 2000. Improved sputum microscopy for a more sensitive diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis 4:684–687. [PubMed] [Google Scholar]
- 13.Apers L, Mutsvangwa J, Magwenzi J, Chigara N, Butterworth A, Mason P, Van der Stuyft P. 2003. A comparison of direct microscopy, the concentration method, and the mycobacterial growth indicator tube for the examination of sputum for acid-fast bacilli. Int J Tuberc Lung Dis 7:376–381. [PubMed] [Google Scholar]
- 14.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. 2006. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 6:664–674. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 15.Fennelly KP, Morais CG, Hadad DJ, Vinhas S, Dietze R, Palaci M. 2012. The small membrane filter method of microscopy to diagnose pulmonary tuberculosis. J Clin Microbiol 50:2096–2099. doi: 10.1128/JCM.00572-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quincó P, Buhrer-Sekula S, Brandao W, Monte R, Souza SL, Saraceni V, Palaci M, Dietze R, Cordeiro-Santos M. 2013. Increased sensitivity in diagnosis of tuberculosis in HIV-positive patients through the small membrane filter method of microscopy. J Clin Microbiol 51:2921–2925. doi: 10.1128/JCM.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones-López E, Manabe YC, Palaci M, Kayiza C, Armstrong D, Nakiyingi L, Ssengooba W, Gaeddert M, Kubiak R, Almeida Junior P, Alland D, Dietze R, Joloba M, Ellner JJ, Dorman SE. 2014. Prospective cross-sectional evaluation of the small membrane filtration method for diagnosis of pulmonary tuberculosis. J Clin Microbiol 52:2513–2520. doi: 10.1128/JCM.00642-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fennelly KP. 2014. Evaluation of a modified small membrane filtration method. J Clin Microbiol 52:4447. doi: 10.1128/JCM.02567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 20.WHO. 1998. Laboratory services in tuberculosis control: part II—microscopy. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 21.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. 2003. Toward complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Intern Med 138:40–44. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 22.Fontela PS, Pant Pai N, Schiller I, Dendukuri N, Ramsay A, Pai M. 2009. Quality and reporting of diagnostic accuracy studies in TB, HIV, and malaria: evaluation using QUADAS and STARD standards. PLoS One 4:e7753. doi: 10.1371/journal.pone.0007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY, Albert PS. 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44:1049–1060. doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
- 24.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicenter implementation study. Lancet 377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. 2014. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 1:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon SH, Lee NK, Yim JJ. 2012. Impact of sputum gross appearance and volume on smear positivity of pulmonary tuberculosis: a prospective cohort study. BMC Infect Dis 12:172. doi: 10.1186/1471-2334-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, Davis JL, Whitelaw A, Nicol MP, Gler MT, Khaliqov A, Zamudio C, Perkins MD, Boehme CC, Cobelens F. 2011. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high-burden countries: a cost-effectiveness analysis. PLoS Med 8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantoja A, Fitzpatrick C, Vassall A, Weyer K, Floyd K. 2013. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: a cost and affordability analysis. Eur Respir J 42:708–720. doi: 10.1183/09031936.00147912. [DOI] [PubMed] [Google Scholar]
- 30.Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, Erasmus LK, Ndjeka NO, Mvusi L, Vassall A, Sinanovic E, Cox HS, Dye C, Grant AD, Fielding KL. 2015. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomized trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 3:e450–457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 31.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. 2014. False-positive Xpert MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis 18:876–878. doi: 10.5588/ijtld.13.0853. [DOI] [PubMed] [Google Scholar]
- 32.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. 2015. False-positive Xpert MTB/RIF assays and previous treatment. Int J Tuberc Lung Dis 19:495–496. doi: 10.5588/ijtld.14.0800-2. [DOI] [PubMed] [Google Scholar]
- 33.Theron G, Venter R, Calligaro G, Smith L, Limberis J, Meldau R, Chanda D, Esmail A, Peter J, Dheda K. 2016. Xpert MTB/RIF results in patients with previous tuberculosis: can we distinguish true from false-positive results? Clin Infect Dis 62:995–1011. doi: 10.1093/cid/civ1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.