Abstract
Detecting resistance to fluoroquinolones (FQ) and second-line injectable drugs (amikacin [AMK], kanamycin [KAN], and capreomycin [CAP]) is crucial given the worldwide increase in the incidence of extensively drug-resistant tuberculosis (XDR-TB). A new version of the GenoType MTBDRsl test (v2.0) has been developed to improve the detection of resistance to FQ (involving gyrA and gyrB mutations) and to second-line injectable drugs (involving rrs and eis promoter mutations) in Mycobacterium tuberculosis. A collection of 127 multidrug-resistant (MDR) M. tuberculosis complex strains was tested using the first (v1) and second (v2.0) versions of the MTBDRsl test, as well as DNA sequencing. The specificities in resistance detection of v1 and v2.0 were similar throughout, whereas the levels of sensitivity of v2.0 were superior for FQ (94.8% versus 89.6%) and KAN (90.5% versus 59.5%) but similar for AMK (91.3%) and CAP (83.0%). The sensitivity and specificity of v2.0 were superior to those of v1 for the detection of pre-XDR strains (83.3% versus 75.0% and 88.6% versus 67.1%, respectively), whereas the sensitivity of v2.0 was superior to that of v1 only for the detection of XDR strains (83.0% versus 49.1%). In conclusion, MTBDRsl v2.0 is superior to MTBDRsl v1 and efficiently detects the most common mutations involved in resistance to FQ and aminoglycosides/CAP. However, due to mutations not recognized by v2.0 or to the presence of resistance mechanisms not yet characterized (particularly mechanisms related to monoresistance to aminoglycosides or CAP), the results for wild-type strains obtained with MTBDRsl v2.0 should be confirmed by further DNA sequencing and phenotypic drug susceptibility testing.
INTRODUCTION
In 2014, six million new cases of tuberculosis (TB) were reported to the WHO, an increasing part of them being caused by multidrug-resistant (MDR) strains, defined as strains resistant to both isoniazid and rifampin, the two main anti-TB drugs (1). For the past 20 years, treatment of MDR TB was based on the association of fluoroquinolones (FQ) and aminoglycosides/capreomycin (CAP), i.e., the aminoglycosides kanamycin (KAN) and amikacin (AMK) and the cyclic peptide CAP (2). Unfortunately, due to inadequate use of second-line treatments, extensively drug-resistant (XDR) TB, defined as MDR TB caused by Mycobacterium tuberculosis strains with resistance to FQ and at least one of three injectable second-line drugs (AMK, KAN, and CAP), has emerged (1). The misuse of antituberculous drugs is partly due to the inability of institutions in several countries to perform drug susceptibility testing (DST). Worldwide, only 12% of patients with new bacteriologically confirmed TB cases and 58% of previously treated TB patients were tested for drug-resistant strains in 2014 (1). XDR strains currently represent a worrisome threat to global health, since treatment failure can be as high as 50%; therefore, the prognosis may be similar to that of untreated TB (3).
Since conventional phenotypic methods are cumbersome and require weeks to months to obtain a drug resistance profile, molecular assays allowing more-rapid drug resistance detection have been developed and implemented, even in areas where DST capacities are very limited or not available. The molecular tests for detecting resistance to antituberculous drugs are based on the detection of mutations affecting the function and/or expression of chromosome-encoded targets, since they are the sole mechanisms of drug resistance described in M. tuberculosis. Concerning FQ, the main mechanism of acquired resistance is an alteration of the DNA gyrase (consisting of two subunits, GyrA and GyrB, encoded by the gyrA and gyrB genes) (4), which is associated with decreased drug activity levels. Most mutations conferring FQ resistance (FQ-R) occur in a short segment termed the quinolone resistance-determining region (QRDR) in gyrA (substitutions mostly affecting residues A90 and D94 and, more rarely, G88 and S91) and, less frequently, in gyrB (substitutions mostly affecting D500, N538, T539, and E540) (4, 5). Resistance to second-line injectable drugs (CAP, AMK, and KAN) is caused by mutations at positions 1401, 1402, and 1484 in the rrs gene with the following expression patterns: rrs substitution A1401G entails low-level resistance to CAP and high-level resistance to AMK and KAN (6), whereas the rrs C1402T substitution entails low-level resistance to KAN and high-level resistance to CAP but no resistance to AMK. The rrs G1484T substitution entails high-level resistance to all three drugs (6). Several studies showed that mutations in the promoter region of eis (a gene encoding an aminoglycoside acetyltransferase), mainly at the −10 to −14 region and at −37, are responsible for low-level resistance to KAN in 30% to 80% of the strains resistant to KAN that have no mutation in rrs (7, 8, 9).
The most commonly used commercial kit for genotypic DST for second-line drugs is MTBDRsl. However, due to the low sensitivity of this test, the WHO decided not to endorse it in 2013 (10). Additional targeting of mutations in the eis promoter (region −10 to −14) and in gyrB (codons 536 to 541) was included, while targeting of the likely ethambutol resistance gene embB was abandoned because of its low sensitivity for resistance detection (11).
The goal of the present study was to compare the ability of the first (v1) and second (v2.0) versions of the GenoType MTBDRsl DNA strip assay to properly identify susceptibility or resistance to FQ and aminoglycosides/CAP of MDR clinical isolates displaying a wide variety of molecular mechanisms of resistance and representing a broad range of additional resistance to FQ and second-line injectable drugs (KAN, AMK, and CAP). The performance of the tests was evaluated in comparison with that of phenotypic DST and DNA sequencing.
MATERIALS AND METHODS
Strains.
MDR M. tuberculosis complex clinical isolates (n = 127) (identified by GenoType Mycobacterium CM) isolated in France and received at the French Reference Center for Mycobacteria during the study period (2005 to 2015) were included based on their MDR (n = 26), pre-XDR (defined as MDR strains with resistance to FQ [n = 24] or to at least one of the three injectable second-line drugs AMK, KAN, and CAP [n = 24]), or XDR (n = 53) status. In vitro DST for ofloxacin (OFX), AMK, KAN, and CAP was performed on Löwenstein-Jensen medium following the proportion method (12), using the following concentrations: 2 mg/liter for OFX, 20 mg/liter for AMK, 30 mg/liter for KAN, and 40 mg/liter for CAP (13).
DNA sequencing of drug resistance-associated genes.
Genomic DNA was isolated from bacteria grown on Löwenstein-Jensen medium. A loop of culture was suspended in water (500 μl) and heated at 95°C for 15 min. The DNA used for amplification by PCR was obtained by heat shock extraction (1 min at 95°C and 1 min on ice, repeated five times). A volume of 5 μl was used in PCR with the oligonucleotide primers described below. To detect FQ resistance, the QRDRs of gyrA and gyrB were amplified and sequenced using primers PRI8 (5′-YGGTGGRTCRTTRCCYGGCGA-3′) and PRI9 (5′-CGCCGCGTGCTSTATGCRATG-3′) for gyrA and primers gyrBa (5′-GAGTTGGTGCGGCGTAAGAGC-3′) and gyrBe (5′-CGGCCATCAAGCACGATCTTG-3′) for gyrB. For detection of aminoglycoside/CAP resistance, the rrs gene (positions 1401 to 1484) and eis promoter were amplified and sequenced using, respectively, primers RRSA (5′-GGCGTTCCCTTGTGGCCTGTG-3′) and RRS1539 (5′-GGGGCGTTTTGCTGGTGCTCC-3′) and primers eis-F (5′-ATTCAGGGCCGATGAAATC-3′) and eis-R (5′-GATGATCGACCGGGTTTG-3′). After amplification, unincorporated nucleotides and primers were removed by filtration with Microcon 100 microconcentrators (Amicon Inc., Beverly, MA) and the amplicons were sequenced using a BigDye Terminator cycle sequencing ready kit (Applied Biosystems) following the manufacturer's instructions.
MTBDRsl tests.
Amplification and hybridization of DNA extracted from isolates were performed according to the manufacturer's instructions (14). The GenoType MTBDRsl v1 test uses a strip coated with 22 probes (15). Briefly, detection of FQ resistance is based on the use of three wild-type (WT) probes covering GyrA codons 85 to 97. The presence of the most frequently observed mutations is confirmed by positive hybridization with six probes for the detection of substitutions in GyrA (A90V, S91P, D94A, D94N/Y, D94G, and D94H). For detection of aminoglycoside/CAP resistance, two WT probes cover nucleotides 1401 and 1402 and nucleotide 1484, and two mutant probes specifically detect the A1401G and G1484T exchanges. The GenoType MTBDRsl v2.0 DNA strip is coated with 27 probes (see Fig. 1), and detection of FQ resistance is based on the use of three WT probes covering GyrA codons 85 to 97 and one WT probe covering GyrB codons 536 to 541. The presence of the most frequently observed mutations is confirmed by positive hybridization with six probes for the detection of substitutions in GyrA (A90V, S91P, D94A, D94N/Y, D94G, and D94H; see Fig. 1) and two probes for the detection of substitutions in GyrB (N538D and E540V). For detection of aminoglycoside/CAP resistance, two WT probes cover nucleotides 1401 plus 1402 and nucleotide 1484 in rrs and three probes cover nucleotides G-37, C-14 plus C-12 plus G-10, and C-2 in eis, while three mutant probes specifically detect the A1401G, G1484T, and C-14T exchanges.
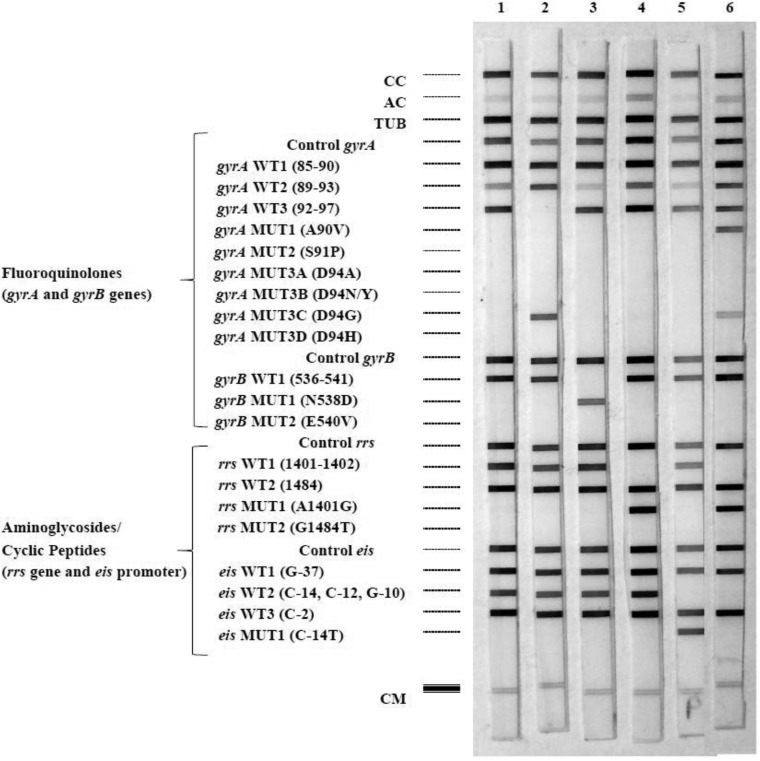
FIG 1.
Hybridization patterns obtained with the GenoType MTBDRsl v2.0 assay. The controls, targeted genes, and mutations are given to the left of the figure. CC, conjugate control; AC, amplification control (23S rRNA); TUB, M. tuberculosis complex-specific control (23S rRNA); Control gyrA, control for gyrA amplification; gyrA WT1 to WT3, gyrA wild-type (WT) probes located in regions of codons 85 to 97; gyrA MUT1 to MUT3D, gyrA mutant probes testing for mutations entailing A90V, S91P, D94A, D94N/Y, D94G, and D94H substitutions; Control gyrB, control for gyrB amplification; gyrB WT, gyrB WT probe testing for mutations in codons 536 to 541; gyrB MUTA and MUT2, gyrB mutant probes testing for mutations entailing N538D and E540V substitutions; Control rrs, control for rrs amplification; rrs WT1 and WT2, rrs WT probes covering nucleotides 1401 and 1402 and nucleotide 1484; rrs MUT1 and MUT2, rrs mutant probes testing for A1401G and G1484T mutations; Control eis, amplification control for eis; eis WT1, WT2, and WT3, eis WT probes located in regions for nucleotide G-37, nucleotides C-14, C-12, and G-10, and nucleotide C-2, respectively; eis MUT1, mutant probe testing for mutation C-14T; CM, colored marker. Typical hybridization patterns were obtained and are shown in the figure as follows: lane 1, H37Rv (WT); lane 2, gyrA D94G; lane 3, gyrB E540V; lane 4, rrs C1402T; lane 5, eis C-14T; lane 6, gyrA D94G-A90V-WT (gyrA D94G and A90V and WT), rrs A1401G, and eis G-10A.
Statistical analysis.
Performance of MTBDRsl v2.0 was evaluated using conventional phenotypic DST and Sanger sequencing as reference standards. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio, and diagnostic accuracy values were calculated according to the Wilson score. All statistical analyses were carried out using Open Source Epidemiologic Statistics for Public Health version 3.03 (16).
The nucleotide sequences determined for the mutant genes included in the present report were deposited in the GenBank database (see below). Particular attention must be given to the numbering system of GyrA and GyrB (4). The amino acids at positions 88, 90, 91, and 94 in the M. tuberculosis numbering system correspond to the amino acids at positions 81, 83, 84, and 87 in the Escherichia coli numbering system, respectively. For GyrB, codons D500, N538, and E540 are according to the 1998 numbering system and correspond, respectively, to codons 426, 464, and 466 according to the numbering system in E. coli and codons 461, 499, and 501 according to the numbering system proposed in 2002 by Camus et al. (17).
Nucleotide sequence accession numbers.
The nucleotide sequences determined for the mutant genes included in the present report were deposited in the GenBank database under the following accession numbers: GU323381, GU323382, KU160143, KU160144, KU160145, and KU291456 for GyrB mutants N538D, D500A, E540V, E540D, D500V, and D500N, respectively; GU323384, GU323385, GU323386, GU323387, GU323388, GU323389, GU323391, GU323393, GU323394, KU160146, KU160147, and KU160148 for GyrA mutants D94A, D94G, D94N, D94H, G88A, G88C, T80A-A90G, D94G-A90V, A90V, D94Y, T80A, and A90V-S91P, respectively; GU323404, GU323405, and KU160149 for rrs mutants A1401G, G1484T, and C1402T, respectively; and KU160151, KU160152, KU160153, and KU160154 for eis promoters G-10A, C-12T, C-14T, and G-37T, respectively.
RESULTS
Description of strains.
Among the 127 strains, 77 were FQ resistant (FQ-R) and 50 were FQ susceptible (FQ-S) (Table 1). Among the 77 FQ-R strains, 69/77 (89.61%) were found by DNA sequencing to display various substitutions in the GyrA QRDR, corresponding either to substitutions frequently reported in the literature (mutations in codon 90 [22/77] and codon 94 [44/77]) or to more rarely encountered substitutions (codon 88 [3/77] and codon 91 [3/77]). Seven were found by DNA sequencing to display various substitutions in the GyrB QRDR (E540V plus WT, E540V, E540D, N538D, D500V, D500N, and D500A, respectively), whereas one isolate harbored no substitution in GyrA or GyrB QRDRs. Among the 50 FQ-S strains, 46 were WT, three harbored a T80A substitution in GyrA, and one harbored a T80A substitution and an A90G substitution in GyrA.
TABLE 1.
GenoType MTBDRsl first version and MTBDRsl v2.0 test results for the detection of fluoroquinolone resistance in 127 M. tuberculosis strains
| Total no. of strains (no. of XDR strains), fluoroquinolone resistance phenotype | Total no. of strains (no. of XDR strains) with indicated result(s) | Amino acid change(s) |
Result(s) by MTBDRsl first version for GyrA | Result(s) by MTBDRsl v2.0 |
||
|---|---|---|---|---|---|---|
| GyrA | GyrB | GyrA | GyrB | |||
| 77 (53), R | 1 | G88C | WT | ΔWT1 | ΔWT1 | WT |
| 2 | G88A | WT | ΔWT1 | ΔWT1 | WT | |
| 14 (12) | A90V | WT | ΔWT2, MUTA90V | ΔWT2, MUTA90V | WT | |
| 5 (3) | A90V + WT | WT | MUTA90V (+ ΔWT2 for 1) | MUTA90V | WT | |
| 2 (1) | S91P | WT | ΔWT2, MUTS91P | ΔWT2, MUTS91P | WT | |
| 6 (5) | D94A | WT | ΔWT3, MUTD94A | ΔWT3, MUTD94A | WT | |
| 22 (15) | D94G | WT | ΔWT3, MUTD94G | ΔWT3, MUTD94G | WT | |
| 4 (4) | D94G + WT | WT | MUTD94G | MUTD94G | WT | |
| 6 (4) | D94N | WT | ΔWT3, MUTD94N/Y | ΔWT3, MUTD94N/Y | WT | |
| 3 (1) | D94Y | WT | ΔWT3 | ΔWT3 | WT | |
| 1 (1) | D94H | WT | ΔWT3, MUTD94H | ΔWT3, MUTD94H | WT | |
| 1 (1) | A90V + S91P + WT | WT | MUTA90V, MUTS91P | MUTA90V, MUTS91P | WT | |
| 2 (2) | A90V + D94G + WT | WT | MUTA90V, MUTD94G | MUTA90V, MUTD94G | WT | |
| 1 | WT | E540V + WT | WT | WT | MUTE540V | |
| 1 (1) | WT | E540V | WT | WT | ΔWT, MUTE540V | |
| 1 (1) | WT | E540D | WT | WT | ΔWT | |
| 1 (1) | WT | N538D | WT | WT | ΔWT, MUTN538D | |
| 1 | WT | D500V | WT | WT | WT | |
| 1 | WT | D500N | WT | WT | WT | |
| 1 | WT | D500A | WT | WT | WT | |
| 1 (1) | WT | WT | WT | WT | WT | |
| 50, S | 3 | T80A | WT | WT | WT | WT |
| 1 | T80A + A90G | WT | ΔWT2 | ΔWT2 | WT | |
| 46 | WT | WT | WT | WT | WT | |
Among the 127 strains, 77 were resistant to at least KAN, AMK, or CAP (45 KAN-AMK-CAP-R, 1 KAN-AMK-R, 5 KAN-CAP-R, 3 mono-CAP-R, and 23 mono-KAN-R) and 50 were susceptible to KAN, AMK, and CAP (Table 2). Among the 45 strains resistant to KAN, AMK, and CAP, 43 had a mutation in the rrs gene between nucleotides 1400 and 1500; substitution A1401G was found in 39 strains (with an additional mutation in the eis promoter in 4 strains: G-10A and C-12T in 2 strains each), C1402T in 1 strain, and G1484T in 3 strains.
TABLE 2.
GenoType MTBDRsl first version and MTBDRsl v2.0 test results for the detection of resistance to aminoglycosides and cyclic peptide in 127 M. tuberculosis strains
| Total no. of strains (no. of XDR strains) | Drug resistance phenotype |
Total no. of strains (no. of XDR strains) | Amino acid change |
Result(s) by MTBDRsl first version (rrs 1400 region) | Result by MTBDRsl v2.0 |
||||
|---|---|---|---|---|---|---|---|---|---|
| KAN | AMK | CAP | rrs 1400 region | eis | rrs 1400 region | eis | |||
| 45 (28) | R | R | R | 34 (19) | A1401G | WT | ΔWT1, MUTA1401G | ΔWT1, MUTA1401G | WT |
| R | R | R | 2 (2) | A1401G | G-10A | ΔWT1, MUTA1401G | ΔWT1, MUTA1401G | ΔWT2 | |
| R | R | R | 2 (2) | A1401G | C-12T | ΔWT1, MUTA1401G | ΔWT1, MUTA1401G | ΔWT1 | |
| R | R | R | 1 | A1401G + WT | WT | MUT1 | MUT1 | WT | |
| R | R | R | 1 (1) | C1402T | WT | ΔWT1 | ΔWT1 | WT | |
| R | R | R | 3 (3) | G1484T | WT | ΔWT2, MUTG1484T | ΔWT2, MUTG1484T | WT | |
| R | R | R | 2 (1) | WT | C-14T | WT | WT | ΔWT2, MUTC-14T | |
| 1 (1) | R | R | S | 1 (1) | WT | C-14T | WT | WT | ΔWT2, MUTC-14T |
| 5 (5) | R | S | R | 1 (1) | C1402T | WT | ΔWT1 | ΔWT1 | WT |
| R | S | R | 1 (1) | WT | G-10A | WT | WT | ΔWT2 | |
| R | S | R | 1 (1) | WT | G-37T | WT | WT | ΔWT1 | |
| R | S | R | 2 (2) | WT | WT | WT | WT | WT | |
| 3 (2) | S | S | R | 3 (2) | WT | WT | WT | WT | WT |
| 23 (17) | R | S | S | 7 (4) | WT | G-10A | WT | WT | ΔWT2 |
| R | S | S | 2 (2) | WT | C-12T | WT | WT | ΔWT1 | |
| R | S | S | 4 (4) | WT | C-14T | WT | WT | ΔWT2, MUTC-14T | |
| R | S | S | 5 (3) | WT | G-37T | WT | WT | ΔWT1 | |
| R | S | S | 5 (4) | WT | WT | WT | WT | WT | |
| 50 | S | S | S | 1 | WT | G-10A | WT | WT | ΔWT2 |
| S | S | S | 1 | WT | C-14T | WT | WT | ΔWT2, MUTC-14T | |
| S | S | S | 1 | WT | G-37T | WT | WT | ΔWT1 | |
| S | S | S | 47 | WT | WT | WT | WT | WT | |
GenoType MTBDRsl test results obtained for the detection of resistance to fluoroquinolones (Table 1).
All 69 strains displaying mutations in gyrA among the 77 FQ-R strains were detected by MTBDRsl v1 and v2.0. Sixty-three of the 69 mutations (91.3%) were detected directly by hybridization with gyrA probes MUTA90V, MUTS91P, MUTD94A, and/or MUTD94H. These mutations corresponded to the following substitutions: D94G in 34.9% (22/63), A90V in 22.2% (14/63), D94N in 9.5% (6/63), D94A in 9.5% (6/63), S91P in 3.2% (2/63), and D94H in 1.6% (1/63) (Table 1). Among the six mutant strains detected indirectly, three were detected because of lack of hybridization with the WT1 gyrA probe (corresponding to G88A and G88C substitutions which are not included as specific probes in MTBDRsl v2.0), and three strains were detected because of lack of hybridization with the WT3 gyrA probe. For the three latter strains, hybridization with the mutant probe gyrA MUTD94N/Y should also have been detected since they harbored a D94Y substitution. For all 69 strains, the same results were obtained with versions v1 and v2.0 of MTBDRsl (Table 1).
Four of the seven FQ-R strains with the GyrB substitutions N538D, E540V, and E540D were detected by GenoType MTBDRsl v2.0, either directly (substitutions N538D and E540V) or indirectly (ΔWT; substitution E540D). The three FQ-R strains not detected by MTBDRsl v2.0 harbored substitutions D500V/N/A, which were not recognized by the test. None of the GyrB substitutions was detected by v1 (Table 1).
The final FQ-R strain without a mutation in GyrA or GyrB was not detected by MTBDRsl v1 or v2.0 (Table 1).
All except 1 of the 50 FQ-S strains were accurately detected by both MTBDRsl versions. The strain misidentified as resistant by both tests harbored a double substitution, T80A plus A90G, in GyrA, leading to lack of hybridization with WT probe WT2.
MTBDRsl test results for the detection of resistance to aminoglycosides/CAP (Table 2).
All 45 strains resistant to the three injectable drugs KAN, AMK, and CAP were detected by the MTBDRsl v2.0 and 43 by v1. Forty-four (97.8%) strains were detected either directly by hybridization with one of the rrs probes (MUTA1401G or MUTG1484T) in the case of 42 strains (detected by MTBDRsl v1) or with one of the eis probes (MUTC-14T) in the case of 2 strains (not detected by MTBDRsl v1) or else indirectly because of lack of hybridization in 1 strain (2.2%) bearing a mutation (C1402T) which is not covered by a specific probe in the MTBDRsl tests.
The only KAN-AMK-R strain harboring a C-14T mutation in the eis promoter was detected by MTBDRsl v2.0 but not by v1.
Among the five KAN-CAP-R strains, three were accurately detected by the MTBDRsl v2.0 test: one showed an rrs mutation (C1402T) and two an eis promoter mutation (G-10A or G-37T). The same result was observed for the three mono-CAP-R strains. MTBDRsl v1 detected only one of those strains, i.e., that harboring a C1402T substitution (Table 2).
Among the 23 mono-KAN-R strains, 18 strains harboring a mutation in the eis promoter (7 harboring G-10A, 2 C-12T, 4 C-14T, and 5 G-37T) were accurately diagnosed by MTBDRsl v2.0 either directly (eis probe MUTC-14T with deletion of the WT2 eis probe) or indirectly (deletion of the WT1 eis probe for G-37T and deletion of the WT2 eis probe for C-12T and G-10A). The remaining five strains which harbored no mutation according to DNA sequencing results were also not detected by the MTBDRsl v2.0 test. None of the 23 mono-KAN-R strains were detected by the MTBDRsl v1 test (Table 2).
Among the 50 strains susceptible to KAN, AMK, and CAP, three were misidentified as resistant to KAN by MTBDRsl v2.0 and also by DNA sequencing due to eis promoter mutations (G-10A, C-14T, and G-37T), whereas v1 accurately classified these strains as susceptible (Table 2).
MTBDRsl test results for the detection of pre-XDR and XDR strains.
Among the 48 pre-XDR strains (24 pre-XDR FQ-R strains and 24 pre-XDR [KAN-AMK-CAP-R] strains) and the 53 XDR strains, MTBDRsl v2.0 correctly identified, respectively, 43 (21 pre-XDR FQ-R and 22 pre-XDR [KAN-AMK-CAP-R] strains) and 44 strains. Among the 24 pre-XDR FQ-R strains, 3 strains harboring a D500 GyrB substitution were misclassified by MTBDRsl v1 and v2.0, and one additional strain harboring an E540 GyrB substitution was misclassified only by v1 (Table 1). Among the 24 pre-XDR strains with KAN-AMK-CAP resistance, 2 (including 1 mono-CAP-R strain and 1 mono-KAN-R strain) harboring no mutation in rrs or the eis promoter were misclassified by the two versions of MTBDRsl, and 6 additional strains (1 KAN-AMK-CAP-R strain and 5 mono-KAN-R strain) harboring eis promoter mutations were misclassified only by v1 (Table 2).
Among the 53 XDR strains, 1 (with no mutation in GyrA or GyrB) was misclassified as FQ-S and 8 (with no mutation in the rrs 1400 region or the eis promoter) were misclassified as KAN-AMK-CAP-susceptible by MTBDRsl v2.0, whereas 3 strains (harboring no mutation in GyrA or GyrB or harboring an E540 or N538 GyrB substitution) were misclassified as FQ-S and 17 strains (harboring only an eis promoter mutation) were misclassified as KAN-AMK-CAP-susceptible strains by v1.
Sensitivity and specificity.
The sensitivity and specificity of MTBDRsl v1 and v2.0 were calculated by comparing the results of the DNA strip assay to those of the in vitro phenotypic tests taken as the reference test. The values for sensitivity and specificity of both MTBDRsl test versions in the detection of resistance to the drugs studied are shown in Table 3.
TABLE 3.
Performance of Genotype MTBDRsl v2.0 and the first version of the MTBDRsl assay compared to phenotypic DST on 127 clinical isolatesa
| Parameter | Performance of MTBDRsl v2.0 [and MTBDRsl first version] compared to phenotypic DST |
pre-XDR |
XDR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluoroquinolone |
Kanamycin |
Amikacin |
Capreomycin |
|||||||||
| Value | (95% CI) | Value | (95% CI) | Value | (95% CI) | Value | (95% CI) | Value | (95% CI) | Value | (95% CI) | |
| % sensitivity | 94.8[89.6 | (87.4, 98.0)(80.8, 94.6)] | 90.5[59.5 | (81.7, 95.3)(48.1, 69.9)] | 91.3[91.3 | (79.7, 96.6)(79.7, 96.6)] | 83.0[83.0 | (70.8, 90.8)(70.8, 90.8)] | 83.3[75.0 | (70.4, 91.3)(61.2, 85.1)] | 83.0[49.1 | (70.8, 90.8)(36.1, 62.1)] |
| % specificity | 98[98 | (89.5, 99.7)(89.5, 99.7)] | 94.3[100 | (84.6, 98.1)(93.2, 100)] | 100[100 | (95.5, 100)(95.5, 100)] | 100[100 | (95.1, 100)(95.1, 100)] | 88.6[67.1 | (70.7, 93.9)(56.1, 76.5)] | 100[100 | (95.1, 100)(95.1, 100)] |
| PPV (%) | 98.7[98.6 | (92.7, 99.8)(92.3, 99.8)] | 95.7[100 | (88.1, 98.5)(92.0, 100)] | 100[100 | (91.6, 100)(91.6, 100)] | 100[100 | (92.0, 100)(92.0, 100)] | 81.6[58.1 | (68.6, 90.0)(45.7, 69.5)] | 100[100 | (92.0, 100)(87.1, 100)] |
| NPV (%) | 92.4[86.0 | (82.1, 97.0)(74.7, 92.7)] | 87.7[63.9 | (76.75, 93.9)(53.1, 73.4)] | 95.3[95.3 | (88.5, 98.2)(88.5, 98.2)] | 89.2[89.2 | (80.7, 94.2)(80.7, 94.2)] | 89.7[81.5 | (81.0, 94.7)(70.4, 89.1)] | 89.2[73.3 | (80.7, 94.2)(63.9, 80.9)] |
| Positive likelihood ratio | 47.4[44.8 | (6.7, 337)(6.3, 319.1)] | 16.0[UD | (8.3, 30.8)UD] | UD[UD | UDUD] | UD[UD | UDUD] | 7.3[2.3 | (5.8 9.2)(2.1, 2.5)] | UD[UD | UDUD] |
| Negative likelihood ratio | 0.05[0.11 | (0.03, 0.09)(0.08, 0.14)] | 0.10[0.41 | (0.08, 0.13)(0.38, 0.43)] | 0.09[0.09 | (0.05, 0.14)(0.05, 0.14)] | 0.17[0.17 | (0.14, 0.21)(0.14, 0.21)] | 0.19[0.37 | (0.14, 0.24)(0.31, 0.45)] | 0.17[0.51 | (0.14, 0.21)(0.47, 0.55)] |
| Diagnostic accuracy (%) | 96.1[92.9 | (91.1, 98.3)(87.1, 96.2)] | 92.1[76.4 | (86.1, 95.7)(68.2, 82.9)] | 96.8[96.8 | (92.2, 98.8)(92.2, 98.8)] | 92.9[92.9 | (87.1, 96.2)(87.1, 96,2)] | 86.6[70.1 | (79.6, 91.5)(61.6, 77.4)] | 92.9[78.7 | (87.1, 96.2)(70.8, 85.0)] |
CI, confidence interval; DST, drug susceptibility testing; PPV, positive predictive value; NPV, negative predictive value; UD, undefined.
DISCUSSION
When MDR-TB is detected, the main therapeutic issue that must be addressed is that of determining the susceptibility of the strain to the remaining first-line drugs, to second-line drugs, particularly KAN, AMK, and CAP, and to fluoroquinolones. In the present study, we assessed the capacity of GenoType MTBDRsl v2.0 and v1 comparatively to detect mutations linked to resistance to FQ, AMK, KAN, and CAP in MDR (including XDR) clinical strains.
The target region for detection of ethambutol (a first-line antituberculosis drug) resistance (embB codon 306), present in MTBDRsl v1, has been removed from v2.0. Therefore, ethambutol resistance was not considered in the present study. However, we showed recently that adding the search for mutations at codon 406 or 497 in embB and for mutations in the embC-embA intergenic region to the search for mutations at codon 306 in embB could dramatically increase the sensitivity of the test (18).
The performance of MTBDRsl v1 (Table 3) was concordant with results of recent meta-analyses (11, 19) as well as with our previous evaluation (15). These results confirm that v1 has high sensitivity and specificity for the detection of resistance to FQ, AMK, and CAP but that it performs poorly in the detection of KAN resistance and therefore of pre-XDR and XDR status.
The overall performance of MTBDRsl v2.0 determined in our study was similar to that reported in the only other published study evaluating this test. Tagliani et al. found sensitivity and specificity values for direct testing of 83.6% and 100% for FQ, 95.5% and 91.4% for KAN, and, globally, 86.4% and 90.1% for second-line injectable drugs (AMK, KAN, and CAP) (20). However, the ability to detect resistance to FQ was superior in our study, mainly due to the difference between the proportions of strains resistant to FQ without a mutation in DNA gyrase in the two studies (1/77 in our study [1.3%] versus 10/73 [13.7%] in that of Tagliani et al. [20]). The performance of MTBDRsl v2.0 was superior to that of v1 (Tables 1, 2, and 3), mainly due to the addition of probes corresponding to the eis promoter and the gyrB gene, which allow the detection of 5.2% more FQ-R strains and 31% more KAN-R strains.
Although the global performance of the test was good, some discrepancies and errors occurred. Given their major impact on treatment, we analyze these discrepancies here in detail. Regarding fluoroquinolones, there were five discrepancies or errors (5/127 = 3.9%). The analysis of the latter results indicated that four strains were wrongly classified as FQ-S (yielding false-negative results) and that one was wrongly classified as FQ-R by MTBDRsl v2.0 (yielding a false-positive result). Concerning the four misclassified FQ-S strains, three harbored a substitution in GyrB at amino acid 500 which is not covered by MTBDRsl v2.0 and one had no mutation in GyrA or GyrB. Three of the four false-negative results could have been avoided if the mutation at position 500 in GyrB had been detected, a mutation that makes up 9% of the GyrB mutations responsible for FQ resistance (21) and that is implicated in low-level resistance (22).
The strain misclassified by MTBDRsl v2.0 as FQ-R harbored a double substitution in GyrA (T80A-A90G) and was classified as resistant because of the absence of hybridization with WT gyrA probe WT2. Previous studies have demonstrated that these substitutions, commonly identified in African countries such as Republic of the Congo, do not confer resistance and are thus responsible for misclassification of FQ resistance by line probe assay (23, 24). This problem, already encountered with MTBDRsl v1, could be avoided by inclusion of a comment in the recommendations for interpretation of MTBDRsl v2.0 results in case of the absence of the WT2 probe.
Surprisingly, three FQ-R strains with the mutation GyrA D94Y, which should theoretically be detected directly by hybridization with a mutant probe, gyrA MUTD94N/Y, were detected only indirectly by the absence of hybridization with WT gyrA probe WT3. This absence of hybridization does not result in a false diagnosis of susceptibility, as was also shown in the study of Tagliani et al. for two strains with GyrA D94Y and one strain with GyrA D94G (20).
Regarding the injectable second-line drugs, there were 13 (10.2%) discrepancies (10/127 [7.9%] for KAN, 2/127 [1.6%] for AMK, and 9/127 [7.1%] for CAP). Ten strains were wrongly classified as susceptible to second-line injectable drugs (7/127 [5.5%] for KAN, 4/127 [3.1%] for AMK, and 9/127 [7.1%] for CAP), and 3 were wrongly classified as resistant (KAN, 3/127 [2.3%]). The 10 strains wrongly classified as susceptible to second-line injectable drugs (5 mono-KAN-R, 3 mono-CAP-R, and 2 KAN-CAP-R strains) did not have any mutation in rrs or the eis promoter. The three strains wrongly classified as resistant to second-line injectable drugs (three KAN-AMK-CAP-S strains) had an eis promoter mutation (G-10A, C-14T, or G-37T). The implication of these mutations in KAN resistance is being debated, since they are responsible for low-level KAN resistance at most (7, 9, 25–27). Moreover, one study has shown that the absolute concentration method using Löwenstein-Jensen medium for susceptibility testing does not adequately detect low-level KAN resistance (7). This could explain why we missed the detection of KAN resistance in the three strains harboring an eis promoter mutation.
Regarding the possible improvements of MTBDRsl v2.0 for the detection of aminoglycoside/CAP resistance, the available data from the literature do not support the need for modification since the mutations of the genes involved in resistance which have been described are rare and their implication in resistance has not been firmly established (e.g., tylA for CAP resistance [28] and the whiB7 5′ untranslated region [UTR] for KAN resistance [29]) (7, 25, 26, 30, 31).
Our study has some limitations. First, as with many other publications evaluating commercial kits, our study used a representative collection of strains received at only one center, i.e., the French Reference Center for Mycobacteria. This may introduce a country-dependent bias of the results. For example, our collection contained more strains harboring GyrB substitutions than that of Tagliani et al. (7/127 compared to 1/228 isolates). Second, we evaluated the performance of the new version of the Genotype MTBDRsl assay, v2.0, only on M. tuberculosis complex strains, and not on clinical specimens, as Tagliani et al. did (20).
The performance of molecular tests in the detection of resistance has markedly improved in recent years, and substantial further improvements are unlikely to be made in the future. These tests, with their inherent complexity as a drawback, are now facing the “glass ceiling” of the ability of existing DST methods, the generally accepted gold standards, to properly classify strains as susceptible or resistant. Indeed, some mutations implicated in low levels of resistance that have an impact on patient outcome cannot be detected adequately with phenotypic methods (32–34).
ACKNOWLEDGMENTS
This work was supported by grants from the Ministère de la Recherche (grant UPRES EA 1541) and from the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Université Pierre et Marie Curie (UPMC).
We thank Ekkehard Collatz for English editing.
Members of the French National Reference Center for Mycobacteria are as follows: Christine Bernard, Emmanuelle Cambau, Vincent Jarlier, Faiza Mougari, Laurent Raskine, and Jérôme Robert.
Funding Statement
This work was supported by grants from the Ministère de la Recherche (grant UPRES EA 1541), the Institut National de la Santé et de la Recherche Médicale (INSERM), and the Université Pierre et Marie Curie (UPMC).
REFERENCES
- 1.World Health Organization. 2015. Global tuberculosis report 2015. WHO/HTM/TB/2015.22. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2008. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. WHO HTM/TB/2008.402. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Kliiman K, Altraja A. 2009. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. Eur Respir J 33:1085–1094. doi: 10.1183/09031936.00155708. [DOI] [PubMed] [Google Scholar]
- 4.Maruri F, Sterling TR, Kaiga AW, Blackman A, van der Heijden YF, Mayer C, Cambau E, Aubry A. 2012. A systematic review of gyrase mutations associated with fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed gyrase numbering system. J Antimicrob Chemother 67:819–831. doi: 10.1093/jac/dkr566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantel A, Petrella S, Veziris N, Brossier F, Bastian S, Jarlier V, Mayer C, Aubry A. 2012. Extending the definition of the GyrB quinolone resistance-determining region in Mycobacterium tuberculosis DNA gyrase for assessing fluoroquinolone resistance in M. tuberculosis. Antimicrob Agents Chemother 56:1990–1996. doi: 10.1128/AAC.06272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maus CE, Plikaytis BB, Shinnick TM. 2005. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother 49:3192–3197. doi: 10.1128/AAC.49.8.3192-3197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravorty S, Lee JS, Cho EJ, Roh SS, Smith LE, Lee J, Kim CT, Via LE, Cho SN, Barry CE III, Alland D. 2015. Genotypic susceptibility testing of Mycobacterium tuberculosis isolates for amikacin and kanamycin resistance by use of a rapid sloppy molecular beacon-based assay identifies more cases of low level drug resistance than phenotypic Lowenstein-Jensen testing. J Clin Microbiol 53:43–51. doi: 10.1128/JCM.02059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gikalo MB, Nosova EY, Krylova LY, Moroz AM. 2012. The role of eis mutations in the development of kanamycin resistance in Mycobacterium tuberculosis isolates from the Moscow region. J Antimicrob Chemother 67:2107–2109. doi: 10.1093/jac/dks178. [DOI] [PubMed] [Google Scholar]
- 9.Zaunbrecher MA, Sikes RD Jr, Metchock B, Shinnick TM, Posey JE. 2009. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. 2013. Expert group meeting report: the use of molecular line probe assay for the detection of resistance to second-line anti-tuberculosis drugs. WHO/HTM/TB/2013.01. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 11.Feng Y, Liu S, Wang Q, Wang L, Tang S, Wang J, Lu W. 2013. Rapid diagnosis of drug resistance to fluoroquinolones, amikacin, capreomycin, kanamycin and ethambutol using genotype MTBDRsl assay: a meta-analysis. PLoS One 8:e55292. doi: 10.1371/journal.pone.0055292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canetti G, Rist N, Grosset J. 1963. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation. Rev Tuberc Pneumol (Paris) 27:217–272. (In French.) [PubMed] [Google Scholar]
- 13.World Health Organization. 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO/HTM/TB/2008.392. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 14.Hain Lifescience GmbH. 2015. GenoType MTBDRsl Ver2.0: instruction manual. Hain Lifescience GmbH, Nehren, Germany. [Google Scholar]
- 15.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. 2010. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-Resistant mycobacterium tuberculosis complex isolates. J Clin Microbiol 48:1683–1689. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean AG, Sullivan KM, Soe MM. 2014. OpenEpi: open source epidemiologic statistics for public health, version 3.03. www.OpenEpi.com Updated 22 September 2014.
- 17.Camus JC, Pryor MJ, Medigue C, Cole ST. 2002. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 148:2967–2973. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- 18.Brossier F, Sougakoff W, Bernard C, Petrou M, Adeyema K, Pham A, Amy de la Breteque D, Vallet M, Jarlier V, Sola C, Veziris N. 2015. Molecular analysis of the embCAB locus and embR gene involved in ethambutol resistance in clinical isolates of Mycobacterium tuberculosis in France. Antimicrob Agents Chemother 59:4800–4808. doi: 10.1128/AAC.00150-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theron G, Peter J, Richardson M, Barnard M, Donegan S, Warren R, Steingart KR, Dheda K. 2014. The diagnostic accuracy of the GenoType(®) MTBDRsl assay for the detection of resistance to second-line anti-tuberculosis drugs. Cochrane Database Syst Rev 10:CD010705. doi: 10.1002/14651858.CD010705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagliani E, Cabibbe AM, Miotto P, Borroni E, Toro JC, Mansjö M, Hoffner S, Hillemann D, Zalutskaya A, Skrahina A, Cirillo DM. 2015. Diagnostic performance of the new version of GenoType MTBDRsl (v2.0) assay for detection of resistance to fluoroquinolones and second-line injectable drugs: a multicenter study. J Clin Microbiol 53:2961–2969. doi: 10.1128/JCM.01257-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard C, Veziris N, Brossier F, Sougakoff W, Jarlier V, Robert J, Aubry A. 2015. Molecular diagnosis of fluoroquinolone resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:1519–1524. doi: 10.1128/AAC.04058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik S, Willby M, Sikes D, Tsodikov OV, Posey JE. 2012. New insights into fluoroquinolone resistance in Mycobacterium tuberculosis: functional genetic analysis of gyrA and gyrB mutations. PLoS One 7:e39754. doi: 10.1371/journal.pone.0039754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aubry A, Sougakoff W, Bodzongo P, Delcroix G, Armand S, Millot G, Jarlier V, Courcol R, Lemaître N. 2014. First evaluation of drug-resistant Mycobacterium tuberculosis clinical isolates from Congo revealed misdetection of fluoroquinolone resistance by line probe assay due to a double substitution T80A-A90G in GyrA. PLoS One 9:e95083. doi: 10.1371/journal.pone.0095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Groll A, Martin A, Jureen P, Hoffner S, Vandamme P, Portaels F, Palomino JC, da Silva PA. 2009. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob Agents Chemother 53:4498–4500. doi: 10.1128/AAC.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, Posey JE. 2011. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother 55:2032–2041. doi: 10.1128/AAC.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engström A, Perskvist N, Werngren J, Hoffner SE, Juréen P. 2011. Comparison of clinical isolates and in vitro selected mutants reveals that tlyA is not a sensitive genetic marker for capreomycin resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 66:1247–1254. doi: 10.1093/jac/dkr109. [DOI] [PubMed] [Google Scholar]
- 27.Hoshide M, Qian L, Rodrigues C, Warren R, Victor T, Evasco HB II, Tupasi T, Crudu V, Douglas JT. 2014. Geographical differences associated with single-nucleotide polymorphisms (SNPs) in nine gene targets among resistant clinical isolates of Mycobacterium tuberculosis. J Clin Microbiol 52:1322–1329. doi: 10.1128/JCM.00857-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maus CE, Plikaytis BB, Shinnick TM. 2005. Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 49:571–577. doi: 10.1128/AAC.49.2.571-577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves AZ, Campbell PJ, Sultana R, Malik S, Murray M, Plikaytis BB, Shinnick TM, Posey JE. 2013. Aminoglycoside cross-resistance in Mycobacterium tuberculosis due to mutations in the 5′ untranslated region of whiB7. Antimicrob Agents Chemother 57:1857–1865. doi: 10.1128/AAC.02191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Q, Dai G, Long Q, Yu X, Dong L, Huang H, Xie J. 2013. Mycobacterium tuberculosis rrs A1401G mutation correlates with high-level resistance to kanamycin, amikacin, and capreomycin in clinical isolates from mainland China. Diagn Microbiol Infect Dis 77:138–142. doi: 10.1016/j.diagmicrobio.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Georghiou SB, Magana M, Garfein RS, Catanzaro DG, Catanzaro A, Rodwell TC. 2012. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One 7:e33275. doi: 10.1371/journal.pone.0033275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fillion A, Aubry A, Brossier F, Chauffour A, Jarlier V, Veziris N. 2013. Impact of fluoroquinolone resistance on bactericidal and sterilizing activity of a moxifloxacin-containing regimen in murine tuberculosis. Antimicrob Agents Chemother 57:4496–4500. doi: 10.1128/AAC.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poissy J, Aubry A, Fernandez C, Lott MC, Chauffour A, Jarlier V, Farinotti R, Veziris N. 2010. Should moxifloxacin be used for the treatment of extensively drug-resistant tuberculosis? An answer from a murine model. Antimicrob Agents Chemother 54:4765–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Deun A, Aung KJM, Bola V, Lebeke R, Hossain MA, de Rijk WB, Rigouts L, Gumusboga A, Torrea G, de Jong BC. 2013. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 51:2633–2640. doi: 10.1128/JCM.00553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]