Abstract
Chagas disease has spread to areas that are nonendemic for the disease with human migration. Since no single reference standard test is available, serological diagnosis of chronic Chagas disease requires at least two tests. New-generation techniques have significantly improved the accuracy of Chagas disease diagnosis by the use of a large mixture of recombinant antigens with different detection systems, such as chemiluminescence. The aim of the present study was to assess the overall accuracy of a new-generation kit, the Architect Chagas (cutoff, ≥1 sample relative light units/cutoff value [S/CO]), as a single technique for the diagnosis of chronic Chagas disease. The Architect Chagas showed a sensitivity of 100% (95% confidence interval [CI], 99.5 to 100%) and a specificity of 97.6% (95% CI, 95.2 to 99.9%). Five out of six false-positive serum samples were a consequence of cross-reactivity with Leishmania spp., and all of them achieved results of <5 S/CO. We propose the Architect Chagas as a single technique for screening in blood banks and for routine diagnosis in clinical laboratories. Only gray-zone and positive sera with a result of ≤6 S/CO would need to be confirmed by a second serological assay, thus avoiding false-positive sera and the problem of cross-reactivity with Leishmania species. The application of this proposal would result in important savings in the cost of Chagas disease diagnosis and therefore in the management and control of the disease.
INTRODUCTION
Chagas disease, or American trypanosomiasis, is a parasitic infection traditionally linked to rural areas of Latin America (1). Based on 2010 data, an estimated 5,742,167 people are infected in 21 Latin American countries (2). The epidemiology of Chagas disease has changed because of migratory trends, and it is now an emerging public health problem in the United States and Europe (3, 4), notably in Spain, the European country with the largest number of immigrants from Latin America (3, 5).
The flagellated protozoan Trypanosoma cruzi is mainly transmitted in areas endemic for the disease through contact with the dejections of blood-feeding triatomine bugs (6, 7) and more rarely by oral transmission through contaminated food (8, 9). The infection may also occur in both areas that are endemic and nonendemic through blood transfusion (10), organ transplant (11), congenital transmission (12), and laboratory accidents (13), allowing the disease to spread to urbanized areas (14).
Chagas disease occurs in two stages: the acute phase, which is without symptoms or with nonspecific manifestations in the majority of cases; and the chronic phase, characterized by cardiac and/or gastrointestinal disorders. In the chronic indeterminate phase of the disease, most patients remain asymptomatic all of their lives (15, 16).
Due to low and intermittent parasitemia, diagnosis during the chronic phase of Chagas disease is made by serological methods (10, 15, 16). There are two types of serological techniques for the detection of anti-T. cruzi antibodies: conventional tests using a whole-parasite antigen, and nonconventional tests based on recombinant antigens (17, 18). Cross-reactivity, especially in conventional assays, is a particular problem for the serological diagnosis of Chagas disease in regions where leishmaniasis also occurs (15, 19). Although numerous assays are available for diagnosing Chagas disease, no single test is considered the reference standard (19–21).
To date, an individual is diagnosed as infected with T. cruzi in the chronic phase of the disease when the results of two serological tests are positive (17). When inconclusive or discordant results appear, a third technique (17) or additional samples are required (22), thereby increasing the cost of diagnosis. The plethora of serological tests used to identify T. cruzi infections often demonstrate discrepant results, which makes serum interpretation difficult (22, 23). Moreover, T. cruzi has great genetic diversity and is currently divided into six genotypes known as discrete typing units (DTUs) (TcI to TcVI) (24). Discordant results between assays are often attributed to antigenic differences among recombinant proteins or T. cruzi DTUs (23, 25).
New-generation tests with potentially improved accuracy have been developed recently. The use of a large mixture of recombinant antigens and the incorporation of different detection systems, such as chemiluminescence, increase the sensitivity and specificity of the techniques. Other advantages of new-generation tests are automation, rapidity, and high performance. Among them, the Architect Chagas (Abbott Laboratories, Wiesbaden, Germany), a chemiluminescent microparticle immunoassay (CMIA), uses four recombinant proteins as the antigen (26–28).
The aim of the present study was to assess the overall accuracy of a new-generation kit that combines a mixture of recombinant proteins with chemiluminescence (Architect Chagas). The application of this single technique in the diagnosis of chronic Chagas disease modifies the aforementioned diagnostic recommendations. Accordingly, it might lead to a reduction in the cost and time of diagnosis and be the first step toward reaching a consensus on a standard protocol.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the Clinical Research Ethics Committee (CEIC) of the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain (project code IIBSP-CHA-2013-33; CEIC no. 53/2013). All samples were anonymized before being evaluated and included in the study.
Study population and serum samples.
A total of 315 serum samples from adults admitted to the Hospital de la Santa Creu i Sant Pau of Barcelona (Spain) were used in this work. Clinical data were recorded by a retrospective review of patient files through the computer system Systems, Applications, and Products for Data Processing (SAP). Serum samples (conserved at −40°C) were collected during the period from January 2009 to December 2012 and divided into four panels (I to IV).
Panel I (n = 107) contained samples from chronic Chagas-seropositive patients from countries endemic for Chagas disease in Latin America who were diagnosed in Spain (96% from Bolivia, 2% from Argentina, and 2% from Paraguay).
Panel II (n = 125) contained samples from nonchagasic individuals from countries that were both endemic (n = 64) and nonendemic (n = 61) for Chagas disease.
For panels I and II, samples had concordant results for two enzyme-linked immunosorbent assays (ELISAs) using whole-parasite antigen (ELISAc) (29) and recombinant antigens (ELISAr) (BioELISA Chagas; Biokit, Lliçà d'Amunt, Spain). Clinical and epidemiological data were considered for the selection.
Panel III (n = 12) contained samples from individuals from countries endemic for Chagas disease with discrepant serological results diagnosed in Spain. These samples had discordant results for ELISAc and ELISAr and were also tested by Western blotting (WB) (19) in order to get the final interpretation (11 considered negative and 1 positive). Clinical and epidemiological data were also considered for the selection.
Panel IV (n = 71) contained samples from patients with other infectious diseases to evaluate cross-reactions (8 individuals with leishmaniasis, 7 with toxoplasmosis, 6 with amebic hepatic abscess, 3 with malaria, 6 with strongyloidiasis, 1 with visceral larva migrans [VLM], 3 with cytomegalovirus, 7 with human immunodeficiency virus [HIV], 4 with parvovirus B19, 5 with Epstein-Barr virus [EBV], 5 with hepatitis B virus [HBV], 2 with hepatitis C virus [HCV], 9 with syphilis, and 5 with Lyme borreliosis). All samples had serological and/or parasitological or molecular evidence of the presence of the infectious diseases studied.
Serological assays and interpretation of results.
Since there is no single widely accepted reference standard test for the diagnosis of T. cruzi infections, 244 serum samples were precharacterized using two serological tests, according to WHO recommendations (17). The remaining 71 samples were taken from patients with other diagnoses (panel IV). For the serum precharacterization, the techniques used were two ELISAs, one of them in house and using sonicated epimastigotes of T. cruzi (ELISAc) (cutoff, ≥20 units) (29), and the second one with recombinant antigens (ELISAr) (results [sample ratio absorbance/cutoff value] of <0.9 were considered negative, ≥1 was considered positive, and the gray zone was from ≥0.9 to <1). Samples with positive results for both assays were included in panel I, and sera with negative results were included in panel II. Samples with discordant results by these techniques were included in panel III, and they were tested by an in-house WB based on lysate T. cruzi epimastigotes, as described elsewhere (19). The final interpretation of panel III samples was based on results coinciding in two out of the three techniques performed; thus, 11 were considered negative and one was considered positive. In order to rule out Chagas disease, samples from patients with other infectious diseases (panel IV) were also analyzed through WB.
All sera were tested for the presence of T. cruzi antibodies by the CMIA Architect Chagas assay. This fully automated assay is based on recombinant proteins FP3, FP6, FP10, and TcF. In aggregate, these four hybrid recombinant proteins represent 14 distinct antigenic regions (30, 31). Testing was performed according to the manufacturer's instructions. The chemiluminescent reaction is measured in relative light units (RLUs). The results are expressed as sample RLUs/cutoff value (S/CO). Ratios of <0.8 are considered negative, ratios of ≥1 are considered positive, and the gray zone was from ≥0.8 to <1.
Data analysis.
The following measures of diagnostic accuracy were calculated (TP, true positive; TN, true negative; FP, false positive; FN, false negative): sensitivity (calculated as TP/[TP + FN]); specificity (calculated as TN/[TN + FP]); validity index, defined as the percentage of patients correctly classified (32) (calculated as [TP + TN]/[TP + TN + FP + FN]), positive and negative predictive values (PPV and NPV, respectively), which are the proportion of correctly diagnosed individuals with positive (PPV) or negative (NPV) results (33) (calculated as TP/[TP + FP] and TN/[TN + FN], respectively), positive and negative likelihood ratios (LR+, the highest value being the best result; and LR−, with the lowest value being the best result), which express how many times more or less frequently, respectively, the test result is obtained among individuals with the disease compared with those without the disease (34) (calculated as sensitivity/[1 − specificity] and [1 − sensitivity]/specificity, respectively), the Youden index, which is a measure of the overall discriminative power of a diagnostic procedure (35) (calculated as [sensitivity + specificity] − 1), and Cohen's kappa coefficient, which describes the level of concordance among tests relating the observed agreement (Ao) and the agreement expected by chance (Ae) (36) (calculated as [Ao − Ae]/[1 − Ae]) (values, >0.8 indicate a high level of agreement) (37). Calculations were performed with the software EPIDAT 3.1, which is available online at http://www.sergas.es/Saude-publica.
Economic evaluation.
An economic assessment of the annual cost of Chagas disease serology at the Hospital de la Santa Creu i Sant Pau in Barcelona was done. During the period from March 2014 to February 2015, a total of 718 serum samples were analyzed for the presence of T. cruzi antibodies in our hospital. Several calculations were done: (i) the annual cost of performing two assays (Architect Chagas and ELISAr) for all the 718 serum samples, according to the WHO recommendations; (ii) the annual cost of performing the Architect Chagas for all sera and confirming by the ELISAr gray zone (2 serum samples) and all positive samples (98 serum samples); and (iii) the annual cost of having to confirm by the second test only samples with gray-zone (2 serum samples) and positive results of ≤6 S/CO (19 serum samples), the strategy proposed in this study.
RESULTS
Sera were divided into four panels: panel I (samples from chronic chagasic patients), panel II (samples from nonchagasic patients), panel III (samples with discrepant serological results), and panel IV (samples from patients with other infectious diseases).
A coincident result of the Architect Chagas with the precharacterization was considered true positive (TP) or true negative (TN), and a discordant result with the precharacterization was considered false positive (FP) or false negative (FN) (Table 1). In this study, no FN results for Architect Chagas were observed.
TABLE 1.
Overview of the results obtained with the Architect Chagas assay for the four panels of serum samples studied
| Test result | No. with precharacterized sera |
No. in panel IV (n = 71) | Total (n = 315) | ||
|---|---|---|---|---|---|
| Panel I (n = 107) | Panel II (n = 125) | Panel III (n = 12) | |||
| CMIA positive | 107 | 1 | 1 | 5 | 114 |
| CMIA negative | 0 | 124 | 10 | 66 | 200 |
| Gray zone | 0 | 0 | 1 | 0 | 1 |
| Total | 107 | 125 | 12 | 71 | 315 |
Among the 244 serum samples precharacterized as positive or negative for Chagas disease, 242 samples were concordant with the Architect Chagas results. Only one serum sample from panel II tested positive and was considered FP, and one serum sample from panel III gave a result in the gray zone. Therefore, the concordance level between precharacterized sera and the results obtained with the Architect Chagas was 99.2%.
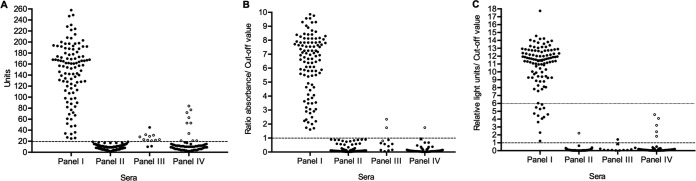
The overall serum value distribution of ELISAc, ELISAr, and the Architect Chagas is shown in Fig. 1.
FIG 1.
Overall serum value distribution of ELISAc (A), ELISAr (B), and Architect Chagas (C). Serum samples from panel I (samples from chronic chagasic seropositive patients, n = 107), panel II (samples from nonchagasic patients, n = 125), panel III (samples with discrepant serological results, n = 12), and panel IV (samples from patients with other infections, n = 71) are represented. Filled circles (●) indicate true-positive and negative results, open circles (○) indicate false-positive and negative results, and Xs (×) represent results in the gray zone. Dashed lines represent the cutoff value established for each test: 20 units for ELISAc (A), 1 absorbance/cutoff value for ELISAr (B), and 1 relative light unit/cutoff value for Architect Chagas (C). The dotted line in panel C indicates the point of the 6 relative light units/cutoff value on the y axis.
In reference to TP serum values (n = 108), 94 samples (87.04%) achieved results of >6 S/CO. The remaining 14 serum samples (12.96%) obtained values of ≤6 S/CO; 9 samples (8.33%) obtained S/CO values from 1 to 4.9, and 5 samples (4.63%) obtained S/CO values from 5 to 6.
When sera from patients with other infectious diseases were analyzed, 5 out of 71 samples were reactive by the Architect Chagas. All of them came from Leishmania-infected patients with Chagas disease ruled out by a WB method (19). These FP sera for the Architect Chagas also showed positive results for ELISAc (values between 53 and 84 units) and negative results for ELISAr, except in one case, in which the sample obtained a value in the gray zone.
The serum sample from panel III with a gray-zone result for the Architect Chagas was positive for ELISAc (FP), negative for ELISAr, and negative for WB. The serum sample from panel IV (Leishmania infection) with a gray-zone result for ELISAr was positive for both ELISAc and the Architect Chagas (FP) and negative for WB. These samples were not included in the calculations, resulting in a final panel of 313 serum samples.
The measures of diagnostic accuracy of the Architect Chagas assay are shown in Table 2. Sensitivity, calculated using panels I and III, was 100%. Specificity, calculated using panels II, III and IV, was 97.6%. FP sera obtained S/CO results between 1.8 and 4.6, and 5 out of 6 samples came from Leishmania-infected patients (Table 3). A high proportion of patients were correctly classified (validity index, 98.4%), and the test showed a high level of agreement with the two techniques used in the precharacterization; a kappa index of 0.91 (95% confidence interval [CI], 0.86 to 0.95) with ELISAc and a value of 0.94 (95% CI, 0.90 to 0.98) with ELISAr.
TABLE 2.
Measures of diagnostic accuracy of the Architect Chagas assay results
| Measurea | Result (no./total no.) | 95% CIb |
|---|---|---|
| Sensitivity (%) | 100 (108/108) | 99.54–100 |
| Specificity (%) | 97.56 (200/205) | 95.21–99.92 |
| Validity index (%) | 98.40 (308/313) | 96.85–99.95 |
| PPV (%) | 95.58 (108/113) | 91.34–99.81 |
| NPV (%) | 100 (200/200) | 99.75–100 |
| LR+ | 41.00 | 17.25–97.45 |
| LR− | ||
| Youden index | 0.98 | 0.95–1 |
PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio.
95% CI, 95% confidence interval.
TABLE 3.
FP serum results of the Architect Chagas assay (n = 6)
| FP serum sample | Architect Chagas (S/CO)a | Other infection(s) |
|---|---|---|
| 1 | 2.22 | Unknown |
| 2 | 1.83 | Leishmaniasis |
| 3 | 4.57 | Leishmaniasis |
| 4 | 4.09 | Leishmaniasis |
| 5 | 3.21 | Leishmaniasis |
| 6 | 2.40 | Leishmaniasis |
S/CO, sample relative light units/cutoff value.
ELISAc scored 17 FP results, with 8 in panel III and 9 in panel IV (7 serum samples with Leishmania infection and 2 with EBV). Therefore, the test showed 100% sensitivity (95% CI, 99.5 to 100%), 91.7% specificity (95% CI, 87.7 to 95.7%), and the validity index was 94.6% (95% CI, 91.9 to 97.2%). ELISAr achieved 3 FP results and 1 FN result, with 2 FP results and the FN result in panel III, and 1 FP result in panel IV (serum with EBV). Consequently, the sensitivity and specificity of the technique were 99.1% (95% CI, 96.8 to 100%) and 98.5% (95% CI, 96.7 to 100%), respectively, and the validity index was 98.7% (95% CI, 97.3 to 100%).
The annual cost of performing two assays for Chagas disease diagnosis in our hospital in Barcelona is €6,864.08 or $7,413.21. From the 718 samples analyzed from March 2014 to February 2015, 618 (86.1%) samples tested negative using the Architect Chagas. Taking into account the 100% sensitivity of the test found in this study, it was possible to classify the sera as negative using only a single technique. The remaining 100 serum samples (13.9%) were analyzed by two tests (Architect Chagas and ELISAr), since the Architect Chagas gave gray-zone (2 serum samples [0.3%]) or positive results (98 serum samples; [13.6%]). Positive samples with results of >6 S/CO (79 serum samples [11%]) were also analyzed with a second test (ELISAr), confirming that all of them were TP. This represents an annual cost of €3,156.08 or $3,408.57. We propose that gray-zone (2 serum samples [0.3%]) and samples with positive results of ≤6 S/CO (19 serum samples [2.6%]) require further confirmation (TP, 57.9%). If inconclusive results appear, a third technique or additional samples are required. Confirmation by a second test was necessary in only 21 serum samples instead of the 100 positive and inconclusive samples. As a result, the annual cost by not having to confirm all positive samples would be €2,682.08 or $2,896.65 in the hospital population, which represents savings of €4,182 or $4,516.56 per year.
DISCUSSION
Despite the absence of the vector, Chagas disease is now an emerging public health problem in Europe and the United States due to immigration from areas endemic for the disease (3, 4). Chronic forms of the disease have appeared in countries that are nonendemic (4, 38, 39), as well as acute forms, principally due to vertical transmission (40–42). In Europe, chronic forms are more abundant than congenital cases.
Chronic forms of Chagas disease are diagnosed serologically, requiring two tests for confirmation (17). According to the World Health Organization (17), an ideal serological test should be easy to perform in a single step, be fast, cheap, require no special equipment or refrigeration of reagents, and have 100% sensitivity and specificity; unfortunately, no such test exists for Chagas disease. The lack of a reference standard serological assay for the diagnosis of T. cruzi infection has prompted the development of new tests, which require further evaluation. Among them, the Architect Chagas, a fully automated assay using four recombinant proteins as the antigen, has been scarcely studied to date (26–28).
Serum precharacterization was performed by ELISAc, a conventional method using parasite lysate as the antigen (29), and ELISAr, based on T. cruzi TcF antigen, a recombinant fusion protein that comprises four serologically active peptides (PEP-II, TcD, TcE, and TcLo1.2) (43, 44). The assay evaluated here, the Architect Chagas, incorporates three recombinant proteins (FP3, FP6, and FP10) in addition to the TcF of ELISAr (30, 31, 45, 46). These four proteins in aggregate represent 14 different antigenic regions present throughout the life cycle of T. cruzi (30, 45). Moreover, T. cruzi is currently divided into six DTUs with distinct genetic profiles (24). The Architect Chagas is capable of detecting the genetic diversity of T. cruzi by the incorporation of highly conserved antigenic proteins with tandemly repeated amino acid domains (26, 45).
A well-known problem in the serological diagnosis of Chagas disease is cross-reaction with antibodies produced by other pathogens, especially Leishmania species (15, 19, 47). All FP sera for the Architect Chagas except one (5 out of 6) came from patients with leishmaniasis (panel IV) (see Table 3). Although all patients were from Spain, these samples were analyzed by WB using T. cruzi lysate epimastigotes as an antigen (19) in order to check for possible Leishmania-T. cruzi coinfections. Chagas disease was ruled out in all five cases because of negative results. The remaining FP serum sample belonged to a precharacterized negative patient (panel II) from an area endemic for the disease, in which leishmaniasis was ruled out. No data of other possible pathologies of the patient were known.
In this report, the Architect Chagas recombinant test showed 100% sensitivity, while its specificity was 97.6% due to cross-reactions in the leishmaniasis patients. The specificity achieved by the Architect Chagas assay excluding cross-reactions with Leishmania spp. would be 99.5%. The Architect Chagas results were highly concordant with tests using crude antigens, such as ELISAc (kappa index, 0.91), but with higher specificity (ELISAc sensitivity, 100%; specificity, 91.7%). While the Architect Chagas gave positive results in 5 out of 8 serum samples from Leishmania-infected patients, indicating cross-reactions, ELISAc scored positive results in all 8 serum samples with Leishmania species. The technique evaluated here also showed a high level of agreement with the ELISAr results (kappa index, 0.94). Although the specificity shown by ELISAr and even the validity index were higher than those with the Architect Chagas, this technique did not detect all positive sera (ELISAr sensitivity, 99.1%; specificity, 98.5%; validity index, 98.7%). Indeed, the Architect Chagas is better able than the ELISAc and ELISAr to discriminate between positive and negative sera (see Fig. 1). The higher sensitivity of the Architect Chagas is probably due to the greater diversity of proteins used as antigens, representing the three morphological forms (trypomastigotes, epimastigotes, and amastigotes) and the genetic diversity of T. cruzi (26, 45). Among current tests in which the number of recombinant proteins is known, the Architect Chagas uses the most. This higher number of recombinant antigens might also explain the high level of cross-reactions with Leishmania species infection. Consequently, this fact should be considered when studying the diagnosis of Chagas disease in areas endemic for visceral leishmaniasis. Other authors previously reported that mixtures of recombinant proteins are very useful as antigens for the immunodiagnosis of Chagas disease (48, 49).
New-generation techniques, such as the Architect Chagas or Bio-Flash Chagas (Biokit, Lliçà d'Amunt, Spain) (50), have improved the diagnosis of Chagas disease with innovative new tools (large mixture of recombinant antigens and chemiluminescence as detection system). Previous studies have also proposed a chemiluminescent ELISA (CL-ELISA) with purified trypomastigote glycoproteins for the detection of lytic protective antibodies against T. cruzi in human serum (33, 51, 52). CL-ELISA achieved high diagnostic accuracy in both areas that are endemic (51, 52) and nonendemic (33) for the disease. Detection systems, such as chemiluminescence, increase light amplification and signal duration in comparison with traditional ELISAs.
Both characteristics, the larger number of recombinant antigens and signal amplification, lead to higher accuracy in the diagnosis of Chagas disease compared to that with the conventional and recombinant techniques used in this study.
Other authors have evaluated the Architect Chagas using different populations or sample conditions (26–28). Their overall results (26–28) suggest that the Architect Chagas is a highly suitable assay for the diagnosis of chronic T. cruzi infection, and its use as a single technique for routine testing in high-prevalence areas has already been recommended (26). In contrast to what is proposed here, a reduction from 1 to 0.88 in the CO value has been recommended, but only when blood samples on filter paper are used (28).
According to the results in the present study, and preserving the manufacturer's criteria for the interpretation of results, we propose the Architect Chagas or other similar new-generation tests, as a single technique for the diagnosis of chronic Chagas disease in blood banks and clinical laboratories in both areas that are endemic and nonendemic for the disease. Taking into account the positive and cross-reactivity results obtained and the overall distribution of serum values (see Fig. 1C), we suggest that only gray-zone and positive sera with results of ≤6 S/CO would need to be confirmed by a second serological assay, in agreement with WHO recommendations. Sera with these results represented <18% of the positive samples and 6.3% of the total sera analyzed in this study. Further studies with other new-generation techniques with similar characteristics (recombinant antigens and chemiluminescence) are necessary.
Several control measures exist for Chagas disease, according to the different transmission scenarios (7, 14, 53), some of which have been applied by health organizations or administrative governments (54–58). Previous studies on the cost-effectiveness of Chagas disease management have been undertaken (59–62), but the costs of different diagnostic methods have not been compared.
The adoption of a single high-performance technique, like the one studied here, would entail a significant savings. Indeed, the savings would be €4,182 or $4,516.56 per year in our hospital, if the comparison is with the cost of performing two assays for all sera, the strategy recommended by the WHO that is used to date. Our proposal would allow the optimization of screening procedures and cost according to the document of the 63rd World Health Assembly (63).
According to Sicuri et al. (59), 1.7 million migrants from Latin American countries endemic for Chagas disease live in Spain, where 42,173 adult immigrants are estimated to be infected with T. cruzi (64). By 2009, an estimated 68,000 to 122,000 Latin American immigrants in Europe were thought to be infected by T. cruzi, but only 4,290 of them were diagnosed (65). Although Chagas disease has become a real problem for countries hosting Latin American migrants, not all European countries screen for the infection (57, 66), a problem that may have been exacerbated by the recent economic crisis (57). Therefore, the management of Chagas disease in countries nonendemic for the disease is crucial to controlling the infection. For an individual with chronic Chagas disease, the estimated average lifetime cost of health care is $27,684, with considerable variations between countries (60). Other authors have reported that in the long term, it is cheaper to diagnose and treat individuals with Chagas disease than not (61). Accordingly, the high rate of underdiagnosis in countries that are nonendemic might contribute to increases in the final cost of Chagas disease patients. The use of a single technique would reduce diagnosis costs and therefore allow the application of screening and control programs in countries where such systems have not yet been implemented.
In conclusion, the Architect Chagas is a highly effective assay for the diagnosis of Chagas disease, with 100% sensitivity, and it allows the correct diagnosis of the majority of samples when applied as a single technique. The Architect Chagas can be used as a single assay in blood banks and clinical laboratories for routine diagnosis. Only gray-zone and positive serum samples with a result of ≤6 S/CO would need to be confirmed by a second serological assay to avoid both FP sera and cross-reactions with Leishmania species. The application of this proposal would result in important savings in the cost of Chagas disease diagnosis and therefore in the management and control of the disease.
ACKNOWLEDGMENTS
We thank Montserrat Portús, Joaquim Gascón, and Pere Coll for their support and helpful scientific discussions.
We declare no conflicts of interest.
This work was partially supported by Departament d'Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya, Spain (grant 2014SGR026). A.A., M.G., and S.T. belong to RICET, a Tropical Disease Cooperative Research Network in Spain (grant RD12/0018/0010). C.M. belongs to the Spanish Network for Research in Infectious Diseases (grant REIPIRD12/0015), Instituto de Salud Carlos III, Madrid, Spain.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Rassi A Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–44. [PubMed] [Google Scholar]
- 3.Schmunis GA. 2007. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz 102(Suppl 1):75–85. [DOI] [PubMed] [Google Scholar]
- 4.Gascon J, Bern C, Pinazo MJ. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Roca C, Pinazo MJ, López-Chejade P, Bayó J, Posada E, López-Solana J, Gállego M, Portús M, Gascón J, Chagas-Clot Research Group. 2011. Chagas disease among the Latin American adult population attending in a primary care center in Barcelona, Spain. PLoS Negl Trop Dis 5:e1135. doi: 10.1371/journal.pntd.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prata A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 7.Sosa-Estani S, Segura EL. 2015. Integrated control of Chagas disease for its elimination as public health problem–a review. Mem Inst Oswaldo Cruz 110:289–298. doi: 10.1590/0074-02760140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benchimol-Barbosa PR. 2010. Trends on acute Chagas' disease transmitted by oral route in Brazil: steady increase in new cases and a concealed residual fluctuation. Int J Cardiol 145:494–496. doi: 10.1016/j.ijcard.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Alarcón de Noya B, Noya González O. 2015. An ecological overview on the factors that drives to Trypanosoma cruzi oral transmission. Acta Trop 151:94–102. doi: 10.1016/j.actatropica.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Angheben A, Boix L, Buonfrate D, Gobbi F, Bisoffi Z, Pupella S, Gandini G, Aprili G. 2015. Chagas disease and transfusion medicine: a perspective from non-endemic countries. Blood Transfus 13:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kransdorf EP, Zakowski PC, Kobashigawa JA. 2014. Chagas disease in solid organ and heart transplantation. Curr Opin Infect Dis 27:418–424. doi: 10.1097/QCO.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 12.Carlier Y, Sosa-Estani S, Luquetti AO, Buekens P. 2015. Congenital Chagas disease: an update. Mem Inst Oswaldo Cruz 110:363–368. doi: 10.1590/0074-02760140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herwaldt BL. 2001. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev 14:659–688. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinazo MJ, Gascon J. 2015. The importance of the multidisciplinary approach to deal with the new epidemiological scenario of Chagas disease (global health). Acta Trop 151:16–20. doi: 10.1016/j.actatropica.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Flores-Chávez M, de Fuentes I, Gárate T, Cañavate C. 2007. Diagnóstico de laboratorio de la enfermedad de Chagas importada. Enferm Infecc Microbiol Clin 25(Suppl 3):29–37. [DOI] [PubMed] [Google Scholar]
- 16.Bern C. 2015. Chagas' disease. N Engl J Med 373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 2002. Control of Chagas disease: second report of the WHO expert committee. World Health Organ Tech Rep Ser 905:1–109. [PubMed] [Google Scholar]
- 18.Longhi SA, Brandariz SB, Lafon SO, Niborski LL, Luquetti AO, Schijman AG, Levin MJ, Gómez KA. 2012. Evaluation of in-house ELISA using Trypanosoma cruzi lysate and recombinant antigens for diagnosis of Chagas disease and discrimination of its clinical forms. Am J Trop Med Hyg 87:267–271. doi: 10.4269/ajtmh.2012.11-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riera C, Verges M, Iniesta L, Fisa R, Gállego M, Tebar S, Portús M. 2012. Short report: identification of a Western blot pattern for the specific diagnosis of Trypanosoma cruzi infection in human sera. Am J Trop Med Hyg 86:412–416. doi: 10.4269/ajtmh.2012.11-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores-Chávez M, Cruz I, Rodríguez M, Nieto J, Franco E, Gárate T, Cañavate C. 2010. Comparación de técnicas serológicas convencionales y no convencionales para el diagnóstico de la enfermedad de Chagas importada en España. Enferm Infecc Microbiol Clin 28:284–293. doi: 10.1016/j.eimc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, Albajar-Viñas P. 2011. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis 5:e1250. doi: 10.1371/journal.pntd.0001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapa JS, Saraiva RM, Hasslocher-Moreno AM, Georg I, Souza AS, Xavier SS, do Brasil PE. 2012. Dealing with initial inconclusive serological results for chronic Chagas disease in clinical practice. Eur J Clin Microbiol Infect Dis 31:965–974. doi: 10.1007/s10096-011-1393-9. [DOI] [PubMed] [Google Scholar]
- 23.Guzmán-Gómez D, López-Monteon A, de la Soledad Lagunes-Castro MS, Álvarez-Martínez C, Hernández-Lutzon MJ, Dumonteil E, Ramos-Ligonio A. 2015. Highly discordant serology against Trypanosoma cruzi in central Veracruz, Mexico: role of the antigen used for diagnostic; Parasit Vectors: 8:466. doi: 10.1186/s13071-015-1072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. 2012. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol 12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Reis-Cunha JL, Mendes TA, de Almeida Lourdes R, Ribeiro DR, Machado-de-Avila RA, de Oliveira Tavares M, Lemos DS, Câmara AC, Olórtegui CC, de Lana M, da Cunha Galvão LM, Fujiwara RT, Bartholomeu DC. 2014. Genome-wide screening and identification of new Trypanosoma cruzi antigens with potential application for chronic Chagas disease diagnosis. PLoS One 9:e106304. doi: 10.1371/journal.pone.0106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Praast G, Herzogenrath J, Bernhardt S, Christ H, Sickinger E. 2011. Evaluation of the Abbott ARCHITECT Chagas prototype assay. Diagn Microbiol Infect Dis 69:74–81. doi: 10.1016/j.diagmicrobio.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Iborra-Bendicho MA, Albert-Hernández M, Márquez-Contreras C, Segovia-Hernández M. 2012. ARCHITECT Chagas: una nueva herramienta diagnóstica en la enfermedad de Chagas. Enferm Infecc Microbiol Clin 30:463–465. doi: 10.1016/j.eimc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Holguín A, Norman F, Martín L, Mateos ML, Chacón J, López-Vélez R, Pérez-Molina JA. 2013. Dried blood as an alternative to plasma or serum for Trypanosoma cruzi IgG detection in screening programs. Clin Vaccine Immunol 20:1197–1202. doi: 10.1128/CVI.00221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riera C, Vergés M, López-Chejade P, Piron M, Gascón J, Gállego M, Portús M. 2009. Desarrollo y evaluación de una técnica ELISA con antígeno crudo de Trypanosoma cruzi para el diagnóstico de la enfermedad de Chagas. Enferm Emerg 11:22–29. [Google Scholar]
- 30.Chang CD, Cheng KY, Jiang LX, Salbilla VA, Haller AS, Yem AW, Bryant JD, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. 2006. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion 46:1737–1744. doi: 10.1111/j.1537-2995.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheng KY, Chang CD, Salbilla VA, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. 2007. Immunoblot assay using recombinant antigens as a supplemental test to confirm the presence of antibodies to Trypanosoma cruzi. Clin Vaccine Immunol 14:355–361. doi: 10.1128/CVI.00401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TDR Diagnostics Evaluation Expert Panel, Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. 2010. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 8(Suppl 12):S17–S29. [PubMed] [Google Scholar]
- 33.Izquierdo L, Marques AF, Gállego M, Sanz S, Tebar S, Riera C, Quintó L, Aldasoro E, Almeida IC, Gascon J. 2013. Evaluation of a chemiluminescent enzyme-linked immunosorbent assay for the diagnosis of Trypanosoma cruzi infection in a nonendemic setting. Mem Inst Oswaldo Cruz 108:928–931. doi: 10.1590/0074-0276130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Cortés A, Ojeda A, Francino O, López-Fuertes L, Timón M, Alberola J. 2010. Leishmania infection: laboratory diagnosing in the absence of a “gold standard.” Am J Trop Med Hyg 82:251–256. doi: 10.4269/ajtmh.2010.09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youden WJ. 1950. Index for rating diagnostic tests. Cancer 3:32–35. doi:. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. 1960. A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 37.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 38.Bern C, Montgomery SP. 2009. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 39.Herrador Z, Rivas E, Gherasim A, Gomez-Barroso D, García J, Benito A, Aparicio P. 2015. Using hospital discharge database to characterize Chagas disease evolution in Spain: there is a need for a systematic approach towards disease detection and control. PLoS Negl Trop Dis 9:e0003710. doi: 10.1371/journal.pntd.0003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muñoz J, Gómez i Prat J, Gállego M, Gimeno F, Treviño B, López-Chejade P, Ribera O, Molina L, Sanz S, Pinazo MJ, Riera C, Posada EJ, Sanz G, Portús M, Gascon J. 2009. Clinical profile of Trypanosoma cruzi infection in a non-endemic setting: immigration and Chagas disease in Barcelona (Spain). Acta Trop 111:51–55. doi: 10.1016/j.actatropica.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz J, Coll O, Juncosa T, Vergés M, del Pino M, Fumado V, Bosch J, Posada EJ, Hernandez S, Fisa R, Boguña JM, Gállego M, Sanz S, Portús M, Gascón J. 2009. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clin Infect Dis 48:1736–1740. doi: 10.1086/599223. [DOI] [PubMed] [Google Scholar]
- 42.Riera C, Guarro A, El Kassab H, Jorba JM, Castro M, Angrill R, Gállego M, Fisa R, Martin C, Lobato A, Portús M. 2006. Congenital transmission of Trypanosoma cruzi in Europe (Spain): a case report. Am J Trop Med Hyg 75:1078–1081. [PubMed] [Google Scholar]
- 43.Ferreira AW, Belem ZR, Lemos EA, Reed SG, Campos-Neto A. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J Clin Microbiol 39:4390–4395. doi: 10.1128/JCM.39.12.4390-4395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houghton RL, Benson DR, Reynolds LD, McNeill PD, Sleath PR, Lodes MJ, Skeiky YAW, Leiby DA, Badaro R, Reed SG. 1999. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J Infect Dis 179:1226–1234. doi: 10.1086/314723. [DOI] [PubMed] [Google Scholar]
- 45.da Silveira JF, Umezawa ES, Luquetti AO. 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol 17:286–291. doi: 10.1016/S1471-4922(01)01897-9. [DOI] [PubMed] [Google Scholar]
- 46.Frasch ACC, Cazzulo JJ, Åslund L, Pettersson U. 1991. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol Today 7:148–151. doi: 10.1016/0169-4758(91)90284-U. [DOI] [PubMed] [Google Scholar]
- 47.Berrizbeitia M, Ndao M, Bubis J, Gottschalk M, Aché A, Lacouture S, Medina M, Ward BJ. 2006. Purified excreted-secreted antigens from Trypanosoma cruzi trypomastigotes as tools for diagnosis of Chagas' disease. J Clin Microbiol 44:291–296. doi: 10.1128/JCM.44.2.291-296.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umezawa ES, Bastos SF, Coura JR, Levin MJ, Gonzalez A, Rangel-Aldao R, Zingales B, Luquetti AO, da Silveira JF. 2003. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 43:91–97. doi: 10.1046/j.1537-2995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 49.Umezawa ES, Luquetti AO, Levitus G, Ponce C, Ponce E, Henriquez D, Revollo S, Espinoza B, Sousa O, Khan B, da Silveira JF. 2004. Serodiagnosis of chronic and acute Chagas' disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J Clin Microbiol 42:449–452. doi: 10.1128/JCM.42.1.449-452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faraudo S, López N, Canela B, Guimarães A, Sáez-Alquezar A. 2015. Evaluation in Brazil of the new Bio-Flash Chagas assay on Biokit's Bio-Flash analyzer. Rep Esp Salud Pública 2015:65–66. [Google Scholar]
- 51.Almeida IC, Covas DT, Soussumi LM, Travassos LR. 1997. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 37:850–857. doi: 10.1046/j.1537-2995.1997.37897424410.x. [DOI] [PubMed] [Google Scholar]
- 52.De Marchi CR, Di Noia JM, Frasch ACC, Amato Neto V, Almeida IC, Buscaglia CA. 2011. Evaluation of a recombinant Trypanosoma cruzi mucin-like antigen for serodiagnosis of Chagas' disease. Clin Vaccine Immunol 18:1850–1855. doi: 10.1128/CVI.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nouvellet P, Cucunubá ZM, Gourbière S. 2015. Ecology, evolution and control of Chagas disease: a century of neglected modelling and a promising future. Adv Parasitol 87:135–191. doi: 10.1016/bs.apar.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Generalitat de Catalunya. 2010. Protocolo de cribado y diagnóstico de la enfermedad de Chagas en mujeres embarazadas latinoamericanas y sus bebés. Departament de Salud, Generalitat de Catalunya, Barcelona, Spain: http://canalsalut.gencat.cat/web/.content/home_canal_salut/professionals/temes_de_salut/chagas/documents/arxius/chagas_espanyol.pdf. [Google Scholar]
- 55.Basile L, Oliveira I, Ciruela P, Plasencia A, Working Group For Developing The Catalonian Screening Programme For Congenital Transmission Of Chagas Disease. 2011. The current screening programme for congenital transmission of Chagas disease in Catalonia, Spain. Euro Surveill 16:pii=19972 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19972. [DOI] [PubMed] [Google Scholar]
- 56.Albajar-Viñas P, Jannin J. 2011. The hidden Chagas disease burden in Europe. Euro Surveill 16:pii=19975 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19975. [DOI] [PubMed] [Google Scholar]
- 57.Requena-Méndez A, Albajar-Viñas P, Angheben A, Chiodini P, Gascón J, Muñoz J, Chagas Disease COHEMI Working Group. 2014. Health policies to control Chagas disease transmission in European countries. PLoS Negl Trop Dis 8:e3245. doi: 10.1371/journal.pntd.0003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonney KM. 2014. Chagas disease in the 21st century: a public health success or an emerging threat? Parasite 21:11. doi: 10.1051/parasite/2014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sicuri E, Muñoz J, Pinazo MJ, Posada E, Sanchez J, Alonso PL, Gascon J. 2011. Economic evaluation of Chagas disease screening of pregnant Latin American women and of their infants in a non endemic area. Acta Trop 118:110–117. doi: 10.1016/j.actatropica.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. 2013. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis 13:342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramsey JM, Elizondo-Cano M, Sanchez-González G, Peña-Nieves A, Figueroa-Lara A. 2014. Opportunity cost for early treatment of Chagas disease in Mexico. PLoS Negl Trop Dis 8:e2776. doi: 10.1371/journal.pntd.0002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imaz-Iglesia I, Miguel LG-S, Ayala-Morillas LE, García-Pérez L, González-Enríquez J, Blasco-Hernández T, Martín-Águeda MB, Sarría-Santamera A. 2015. Economic evaluation of Chagas disease screening in Spain. Acta Trop 148:77–88. doi: 10.1016/j.actatropica.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 63.63rd World Health Assembly. 2010. WHA63.20. Chagas disease: control and elimination. World Health Organization, Geneva, Switzerland: http://www.who.int/neglected_diseases/mediacentre/WHA_63.20_Eng.pdf. [Google Scholar]
- 64.Navarro M, Navaza B, Guionnet A, López-Vélez R. 2012. Chagas disease in Spain: need for further public health measures. PLoS Negl Trop Dis 6:e1962. doi: 10.1371/journal.pntd.0001962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van Gool T, Canavate C, Flores-Chavez M, Jackson Y, Chiodini PL, Albajar-Viñas P, Working Group on Chagas Disease. 2011. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill 16:pii=19968 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19968. [PubMed] [Google Scholar]
- 66.Pérez-Molina JA, Perez AM, Norman FF, Monge-Maillo B, López-Vélez R. 2015. Old and new challenges in Chagas disease. Lancet Infect Dis 15:1347–1356. doi: 10.1016/S1473-3099(15)00243-1. [DOI] [PubMed] [Google Scholar]