Abstract
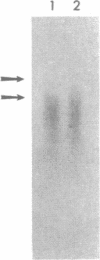
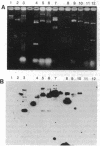
We present here a method for broadly characterizing single cells at the molecular level beyond the more common morphological and transmitter/receptor classifications. The RNA from defined single cells is amplified by microinjecting primer, nucleotides, and enzyme into acutely dissociated cells from a defined region of rat brain. Further processing yields amplified antisense RNA. A second round of amplification results in greater than 10(6)-fold amplification of the original starting material, which is adequate for analysis--e.g., use as a probe, making of cDNA libraries, etc. We demonstrate this method by constructing expression profiles of single live cells from rat hippocampus. This profiling suggests that cells that appear to be morphologically similar may show marked differences in patterns of expression. In addition, we characterize several mRNAs from a single cell, some of which were previously undescribed, perhaps due to "rarity" when averaged over many cell types. Electrophysiological analysis coupled with molecular biology within the same cell will facilitate a better understanding of how changes at the molecular level are manifested in functional properties. This approach should be applicable to a wide variety of studies, including development, mutant models, aging, and neurodegenerative disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belyavsky A., Vinogradova T., Rajewsky K. PCR-based cDNA library construction: general cDNA libraries at the level of a few cells. Nucleic Acids Res. 1989 Apr 25;17(8):2919–2932. doi: 10.1093/nar/17.8.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Near J. C., Kmetz P. J., Sathe G. M., Southan C., Strickler J. E., Gross M., Young J. F., Wells T. N., Groot P. H. Rat ATP citrate-lyase. Molecular cloning and sequence analysis of a full-length cDNA and mRNA abundance as a function of diet, organ, and age. J Biol Chem. 1990 Jan 25;265(3):1430–1435. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamprecht B., Glaser T., Reiser G., Bayer E., Propst F. Culture and characteristics of hormone-responsive neuroblastoma X glioma hybrid cells. Methods Enzymol. 1985;109:316–341. doi: 10.1016/0076-6879(85)09096-6. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986 Jul 25;14(14):5936–5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Tam A. W., Smith M. M., Fry K. E., Larrick J. W. Construction of cDNA libraries from small numbers of cells using sequence independent primers. Nucleic Acids Res. 1989 Feb 11;17(3):1269–1269. doi: 10.1093/nar/17.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder R. N., von Zastrow M. E., Yool A., Dement W. C., Barchas J. D., Eberwine J. H. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. H., Lee M. B., Cheun J. E. Properties of GABA-activated whole-cell currents in bipolar cells of the rat retina. Vis Neurosci. 1990 Apr;4(4):349–357. doi: 10.1017/s0952523800004557. [DOI] [PubMed] [Google Scholar]