Abstract
Identification of bloodstream infections is among the most critical tasks performed by the clinical microbiology laboratory. While the criteria for achieving an adequate blood culture specimen in adults have been well described, there is much more ambiguity in pediatric populations. This minireview focuses on the available pediatric literature pertaining to the collection of an optimal blood culture specimen, including timing, volume, and bottle selection, as well as rapid diagnostic approaches and their role in the management of pediatric bloodstream infections.
INTRODUCTION
Blood cultures remain the mainstay of laboratory diagnosis of bloodstream infections (BSIs) in infants and children. Recovery of a pathogen is advantageous, as it confirms the diagnosis of bacteremia and allows for identification and susceptibility testing on the organism to optimize antimicrobial therapy and duration. A negative blood culture is just as important, as it rules out cases of bacteremia and prompts continued investigation of other infectious or noninfectious etiologies or cessation of unnecessary empirical antimicrobial therapy.
The spectrum of pathogens causing pediatric BSI varies widely by age, presenting symptoms, and immune status. In 1979, an evaluation of pediatric blood culture found Haemophilus influenzae to be the most prevalent organism followed by Streptococcus pneumoniae and Staphylococcus aureus (1). Today, H. influenzae and S. pneumoniae are rare bloodstream pathogens due to widespread vaccination. A 2012 study of infants of <3 months of age found the leading causes of bacteremia to be Escherichia coli, group B Streptococcus (Streptococcus agalactiae), and S. aureus (2). The rate of BSI in otherwise healthy children drops precipitously after the first few months of life, but if occurring, the most common pathogens are S. aureus, S. pneumoniae due to community-acquired pneumonia, and Neisseria meningitidis in adolescents. Immunocompromised children are susceptible to a broad range of bloodstream pathogens, including all of those previously mentioned as well as Pseudomonas aeruginosa and Candida spp. (3).
The majority of studies related to the laboratory diagnosis of BSI focus on the adult population. Thus, this minireview will be devoted to children and the multifactorial aspects involved in obtaining an optimal pediatric blood culture specimen, including timing, volume, and bottle selection. Lastly, a discussion on the rapid diagnostic approaches currently available and their impact on pediatric management and outcomes will be reviewed.
BLOOD CULTURE COLLECTION
Factors that may influence the recovery of pathogens from the blood include the timing of blood collection, number of sets collected, and blood volume. It is well accepted that the volume of blood collected is the single most important factor. Evidence from both adult and pediatric studies show that the probability of recovering a pathogen from blood culture increases with the volume of blood obtained. In addition, the time to detection inversely correlates with the volume of blood cultured (4). Optimal collection of blood volume is particularly relevant in the pediatric population, as collecting a sufficient volume can be difficult due to the diminutive size of the patients and the risk of requiring blood transfusion to compensate for repeated phlebotomy (5).
Importance of blood volume.
The inherent difficulties in obtaining blood from children have contributed to the common misconception that children have higher levels of bacteremia compared to adults; thus, a blood volume of 0.5 to 1.0 ml is presumed sufficient to detect bloodstream pathogens (6, 7). This was supported by reports of high CFU per milliliter (CFU/ml) of blood in children with H. influenzae (6,293 CFU/ml) and S. pneumoniae (51 CFU/ml) (8). Multiple recent studies have confirmed that low-level bacteremia is more common than previously thought, occurring in 38% to 68% of all pediatric patients with a positive blood culture (9, 10), and <1 ml of blood volume is inadequate for the detection of pathogens present at a density of <4 CFU/ml (5). A study of infants of ≤2 months of age demonstrated that over two-thirds of culture-positive patients had colony counts of ≤10 CFU/ml, and between 2 and 6 ml of blood was required for pathogen detection (11). The same group confirmed these findings in a subsequent study in children of ≤15 years of age where 60.3% had <10 CFU/ml, and 23.1% of pathogens were present at ≤1 CFU/ml (10). The two studies confirmed low-level bacteremia in 71% to 75% of patients that died from sepsis (10, 11).
Despite the presence of low-level bacteremia in children, procurement of insufficient blood volume remains a significant concern. A study performed at a tertiary children's hospital in Australia evaluated the blood volume of 1,358 blood culture bottles, 1,067 of which were collected prior to educational intervention. Using a criteria of ≥0.5 ml for patients who are <1 month old, ≥1.0 ml for patients who are 1 to 36 months old, and ≥4.0 ml for patients who are 36 months old and older, only 46.0% of blood culture bottles collected in the preintervention period contained an adequate blood volume. Patient age appears to impact the blood volume obtained, as the proportion of blood culture bottles with <0.5 ml of blood increased from 12.4% in all patients to 30.0% in patients who are <1 month of age (12). Similarly, when evaluating blood culture submissions, inadequate volume (defined by patient weight and not age) was obtained in 40.0% of cases in a single study of 843 patients who were ≤18 years of age with at least 1 positive blood culture.
The two above studies reported correlation between blood cultures that were positive with noncontaminant bacteria and adequate blood volume (incidence rates of 60% to 71%). In contrast, the studies found that procurement of low blood volume inversely correlated with blood culture contamination rates (12, 13). Sixty-five percent of positive blood cultures deemed to be contaminants had inadequate blood volume (13), and recovery of contaminant was twice as likely when inadequate blood volume was obtained (5.1% versus 2.8%) (12). These findings further support the necessity of obtaining sufficient blood culture volume, as the recovery of contaminants has been reported in 25% to 69% of all positive blood cultures in pediatric patients (12–14) and is associated with unnecessary antimicrobial therapy, prolonged hospitalization, and incurred cost. It is not particularly clear why low volume blood cultures are more prone to yield contaminants; one theory is that the acquisition of contaminants is independent of blood volume, and rather the collection of larger blood volume dilutes the concentration of the contaminant present in the blood culture bottle, reducing the chance of detection during the incubation period (13, 15).
Several studies in the adult population have reported an increased detection yield of 0.6% to 4.7% for each additional milliliter of blood procured for culture, concluding that increases in blood culture volume directly correlate with an increase in the detection rate (16, 17). Similar findings have been reported in the pediatric population (4, 13, 18); specifically, a large study performed in Kenya with blood culture data on 19,339 children who were <13 years of age found that the proportion of positive blood cultures significantly increased with each milliliter of blood (5.6% at 1 ml, 6.8% at 2 ml, and 7.9% at 3 ml) (18).
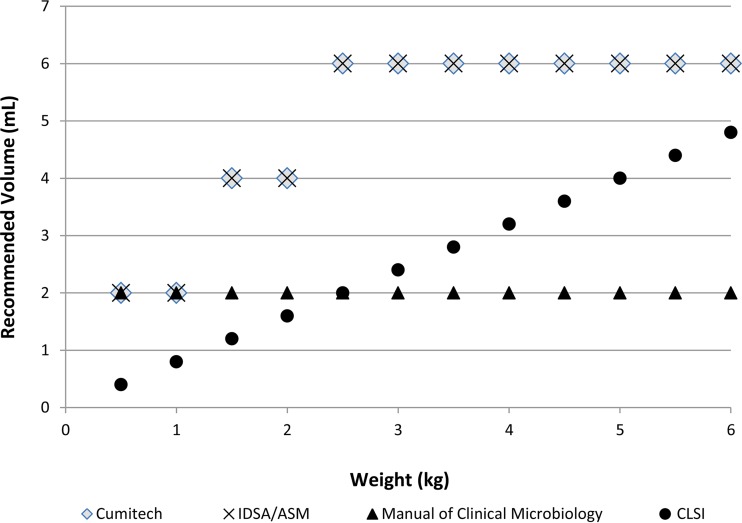
In conclusion, there are limited data to establish the optimal collection volume in children, and the majority of information is extrapolated from studies in adult populations. A safe and appropriate approach is that the collection of blood should be proportionate to the patient's total blood volume and, more specifically, the patient's weight. As illustrated in Fig. 1, there is a lack of consensus among the current recommendations for pediatric blood collection (19–22). The most current guidelines from the Infectious Diseases Society of America (IDSA) and the American Society of Microbiology (ASM) recommend the collection of 3% to 4% of total patient blood volume in patients weighing <12.7 kg and 1.8% to 2.7% in patients weighing >12.8 kg (22). This is supported by a past study that showed that collection of up to 4.5% of a patient's total blood volume will increase the yield of pathogen detection without jeopardizing patient safety (10). Weight-based guidelines are simple on paper but are complicated to carry out in practice. In critically ill patients, collection of an adequate amount of blood, even when based on the patient weight, is often not feasible. Additionally, there is a limit on the total blood volume that can be removed from a patient in a 24-h period, and blood culture is just one of the many laboratory tests that may be competing for the limited amount of blood available. Future studies are needed to determine how effective the weight-based guideline is at optimizing the detection of bloodstream pathogens.
FIG 1.
There is no consensus on blood volume collection recommendations by weight. Modified with permission from Lancaster et al. (34). Summary of recommended blood volume by Cumitech 1C blood cultures IV, the Infectious Diseases Society of America (IDSA) and the American Society of Microbiology (ASM), the Manual of Clinical Microbiology, 10th ed, and the Clinical and Laboratory Standards Institute (CLSI) guideline M47-A. An estimated total blood volume of 80 kg/ml was used to illustrate CLSI's recommendation of not exceeding 1% of total blood volume.
Other factors impacting blood culture positivity.
Traditionally, it was perceived that drawing blood around the time of a temperature spike would increase the likelihood of recovery of an organism. However, the presence of fever lacks the ability to independently predict BSI; rather, other findings must be taken into account, including hypotension and elevated white blood cells (23). To our knowledge, there are currently no pediatric studies available on this subject. A retrospective, multicenter study of 1,436 adult patients found no significant association between enhanced recovery of bacteria and collection of blood cultures at the time of fever spike. The authors concluded that, at least in the adult population, the timing of blood culture collection is not important and should be based on convenience (24). This coincides with IDSA/ASM guidelines that collection should be dictated based on the patient's acuity (22).
The number of blood culture sets and what constitutes a “set” may also influence the laboratory detection of BSIs. The IDSA/ASM document offers concrete guidance for adult patients, recommending 2 to 4 blood culture sets (1 aerobic and 1 anaerobic bottle per set) for each septic episode. In the pediatric population, collection of blood culture sets, which can include 1 or 2 bottles, is solely based on patient weight. Only one set is recommended for children weighing <1 kg, and an additional set is recommended in patients weighing >1 kg (22). Although collection of multiple sets may also assist clinicians in ruling out probable contaminants when only 1 bottle is positive, in practice it is uncommon to collect multiple blood culture bottles in pediatric patients at any weight (12). The question is whether additional blood draws would improve the recovery rate in pediatric patients. There are a number of studies that concluded that ensuring sufficient blood volume within 1 bottle is more beneficial than obtaining multiple blood bottles. A study of 300 patients ranging from 2 months to 18 years of age presenting to the emergency department found that collection of a single 6-ml blood culture had a higher recovery yield compared to 2 separate 2-ml bottles (4). These findings were further supported by a prospective study of 216 neonates with 2 sets of blood cultures collected from 2 separate sites within 15 to 30 min of each other. Twenty of the 216 neonates (9.2%) had culture-proven sepsis, and all cases were positive from the two sites, demonstrating that a single blood culture set with at least 1 ml of blood is sufficient for detection of bacteremia in neonates (25), which allows for further avoidance of blood transfusion and unnecessary pain. In contrast, a recent study on febrile neutropenic patients who were <21 years of age with hematological malignancies identified 9.2% additional cases of BSI upon repeat culture, highlighting the necessity of repeat blood cultures in this patient population (26). This study did not report the blood volume that was inoculated into each bottle, which raises the question of adequate volume and whether that may have increased the recovery yield in the first blood draw.
The administration of antibiotics prior to obtaining blood cultures is detrimental to organism recovery. To combat this issue, antibiotic-neutralizing substances, such as charcoal or antibiotic-binding resin beads, are often added to commercial blood culture bottles. A study to determine the effectiveness of antibiotic-neutralizing agents spiked BacT/Alert Pediatric FAN (bioMérieux, Durham, NC) and Bactec Peds Plus (BD Diagnostics, Sparks, MD) blood culture bottles with 8 common pediatric blood culture pathogens with and without an organism-appropriate antibiotic. Organisms grew in all but one bottle without antibiotics, but in the presence of antibiotic, organisms were only recovered in 19% of the BacT/Alert Pediatric FAN and 50% of the Bactec Peds Plus blood culture bottles (27). These data demonstrate that pediatric patients that are pretreated with antibiotics have drastically reduced recovery of bacteria from blood culture and that the protective effect of antibiotic-neutralizing substances varies by manufacturer.
Time to positivity for detection of catheter-associated bacteremia.
Many bacteria are adept at adhering to plastic surfaces and forming biofilms. Often in immunocompromised patients and neonates with positive blood cultures, it is difficult to determine if the source of a patient's bacteremia is an indwelling catheter or another source. To further complicate the picture, catheters are often colonized with skin flora such as coagulase-negative staphylococci, which make a true infection and contaminating organism difficult to distinguish with only a single positive blood culture. In the past, quantitative blood cultures were used to help elucidate the true source of infection. There are many methods of interpretation, but in general, a blood culture drawn from a central line that grows >100 CFU/ml bacteria or has a colony count that is 3- to 5-fold greater than a paired peripheral blood draw was indicative of a catheter-associated bloodstream infection (28–30).
Quantitative cultures have fallen out of favor in recent years because of the low blood volume that is cultured, because cultures are only checked for growth once or twice per day compared to every 10 min for automated blood culture systems, and because of questions about the sensitivity and specificity of the procedure for detection of catheter-associated bacteremia. Automated blood cultures do not provide quantitative colony counts, but the same objective can be achieved by measuring the difference in time to positivity (TTP) of blood cultures when an equal volume is drawn from a catheter and a peripheral source within minutes of each other. A study of spiked pediatric blood culture bottles with a known quantity of bacteria showed that, for coagulase-negative staphylococci, a 10-fold increase in bacterial density correlated to a 2.3- to 2.6-h reduction in TTP, while a 5-fold increase in bacterial density correlated to a 1.6- to 1.8-h reduction (31). Another study from St. Jude's Children's Hospital found that the sensitivity of TTP to diagnose catheter-associated bloodstream infection using a differential of 180 min was only 61% with a specificity of 94% (32).
In conclusion, for diagnosis of pediatric catheter-associated infections, few microbiology laboratories perform quantitative cultures. A >2-h differential in time to positivity between catheter and peripheral blood cultures is indicative of the catheter being the nidus of infection, but this testing method must be used with caution due to its low sensitivity.
BLOOD CULTURE BOTTLES
The utility of pediatric blood culture bottles.
Pediatric blood culture bottles were developed to support the specific needs of pediatric patients, namely, the culture of low blood volumes and the detection of fastidious organisms. The reduced amount of broth optimizes the blood to broth ratio and improves the time to detection for culture of small blood volumes. Of commonly used aerobic bottles, BD Bactec Peds Plus/F Medium (Becton Dickinson, Franklin Lakes, NJ, USA) and BacT/Alert PF (bioMérieux, Marcy I'Etoile, France) recommend a minimum of 0.5 ml of blood, while the VersaTREK Redox (Oakwood Village, OH, USA) claims it can detect bacteremia with as little as 0.1 ml of blood. Pediatric bottles also contain a different broth formulation to support the growth of fastidious pediatric pathogens, such as S. pneumoniae, H. influenzae, and Neisseria meningitidis. These organisms are rarely detected from blood cultures these days due to widespread vaccination, which leads one to question if pediatric-specific blood culture bottles are still necessary, especially for hospitals that serve both adult and pediatric populations. Unfortunately, there are very few data to support either position. On the subject of the optimal blood-to-broth ratio, a study from 1984 demonstrated that common pediatric bloodstream pathogens, including fastidious organisms, were routinely recovered at a blood/broth ratio of 1:100 and there was no difference in time-to-positivity compared to a ratio of 1:10 (33). Schreckenberger et al. compared the time to positivity of BacT/Alert pediatric bottles to standard aerobic bottles for the same system by seeding bottles with 17 bacterial pathogens and one yeast pathogen at two concentrations. They found that the pediatric bottles did not reduce the time to positivity, and in fact, the standard bottles signaled positive more quickly than the pediatric bottles in almost all cases (P. Schreckenberger et al., presented at the 109th American Society for Microbiology General Meeting, Philadelphia, PA, 17 to 21 May 2009). The only published data directly comparing pediatric and adult blood culture bottles for their ability to recover organisms is a recent manuscript in which a total of 600 Bactec Peds Plus/F and Bactec Plus Aerobic/F blood culture bottles were seeded with 0.5 to 3 ml of 10 common bacteria and yeasts at concentrations of 1 to 10 CFU/ml and <1 CFU/ml (34). At a concentration of 1 to 10 CFU/ml, the two bottle types had similar detection rates. At concentrations of <1 CFU/ml, the seeded organisms were more frequently detected in the pediatric bottles for 6 organisms, they were more frequently detected in the adult bottles for 3 organisms, and for one organism they were detected in the same number of bottles. These data suggest that pediatric blood culture bottles may offer a slight advantage in terms of detecting bacteremia at very low concentrations submitted in very low volumes. Further clinical studies are necessary to definitively answer this question.
Anaerobic blood culture for pediatric patients.
Anaerobic organisms contribute to 10% to 20% of all bacteremia in adults (35). Recent studies have demonstrated that the level of anaerobic bacteremia in children is much lower than that in adults. A study of nearly 10,000 paired aerobic and anaerobic blood culture bottles submitted for culture revealed that 7.7% were positive for clinically significant microorganisms, but of this group, only 15 blood cultures (0.16%) were positive for obligate anaerobes (36). Chart review determined that the 15 cultures yielding anaerobes belonged to 5 unique patients who were all determined to be at increased risk for anaerobic bloodstream infections. The low prevalence of pediatric anaerobic bacteremia has also been reported in other groups (1, 37–39). These studies support the practice of pediatric anaerobic blood cultures only for those at increased risk, such as immunocompromised patients and those with head, neck, and intraabdominal infections.
Children are at greater risk for bacteremia caused by obligate aerobic organisms, such as Pseudomonas spp. and Candida spp. Two studies have shown that culturing the entire blood volume under aerobic conditions increased detection of clinically significant pathogens compared to culture with aerobic and anaerobic bottles (39, 40). In these studies, with such a small volume of blood being cultured from many pediatric patients, the benefit of detecting clinically significant obligate aerobic organisms outweighed the very low rate of anaerobic pathogen detection. There is a notion that anaerobic blood culture bottles are superior for detection of facultative anaerobes, as occasionally the anaerobic bottle of a blood culture set will detect an organism that is not present in the aerobic bottle. Unfortunately there is no data in the literature to support this position. It is more likely that these situations are due to a random sampling event, which is especially common when very small amounts of blood are cultured from patients with low-level bacteremia (36).
Due to the very low prevalence of clinically significant anaerobic bacteremia in pediatric patients, there is a push by some institutions to eliminate routine anaerobic blood culture as a cost-saving measure (38, 41). One hospital demonstrated that various interventions put in place to reduce nonindicated anaerobic blood culture resulted in an 80% decrease in anaerobic blood culture, while the number of aerobic blood cultures submitted for culture was unaffected. The reduction in anaerobic blood cultures was still present 1 year after the intervention, resulting in a substantial cost savings for the hospital (41).
In conclusion, pediatric patients are at low risk for development of anaerobic sepsis and are at higher risk for sepsis with obligate aerobic organisms, such as Candida and Pseudomonas. With limited blood volume, the data show that it is prudent to culture the entire volume under aerobic conditions unless the patient is at increased risk for anaerobic bacteremia. This practice also has the beneficial side effect of reducing hospital costs associated with anaerobic blood cultures.
SPEEDING UP THE LABORATORY DIAGNOSIS OF BLOODSTREAM INFECTIONS
In the past few years, the development of a number of rapid diagnostic methodologies has revolutionized the approach to diagnosing BSIs in all patient populations. There are many commercially available assays that are approved by the United States Food and Drug Administration (FDA) for in vitro diagnostic use on positive blood cultures, including peptide nucleic acid fluorescent in situ hybridization (PNA FISH; AdvanDx, Woburn, MA), FilmArray blood culture identification panel (BCID; BioFire, Salt Lake City, UT), and Verigene Gram-positive blood culture (BC-GP) and Gram-negative blood culture (BC-GN) panels (Nanosphere, Northbrook, IL). Utilization of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) directly from positive blood culture has been extensively explored by multiple institutions using either the CE-marked Sepsityper kit (Bruker Daltonics, Brenham, Germany) or laboratory-developed methods (42, 43) and is deemed to be a reliable and less expensive alternative to molecular panels. A recent review by Kothari et al. provides a thorough summary of the most recent technologies for diagnosis of BSI (44). A novel approach to rapid diagnostics from positive blood cultures is the Accelerate ID/AST system (Accelerate Diagnostics, Tucson, AZ), which offers identification for 19 bacteria and yeast targets from positive blood cultures. What is unique about this system is the delivery of phenotypic susceptibility results rather than mere detection of resistant genes. The test is currently not approved by the FDA, but preliminary data appear promising (C. Price et al., presented at the 25th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 25 to 28 April 2015).
The movement toward organism identification directly from whole blood further expedites the detection process and addresses the multifactorial complexity of obtaining appropriate blood cultures. T2Candida (T2 Biosystems, Inc., Lexington, MA) was approved by the FDA in 2014 and allows for the detection of Candida spp. directly from whole blood. There are currently no studies available in children; a multicenter study in 1,801 adult patients demonstrated overall sensitivity and specificity of 91.1% and 99.4%, respectively, with a limit of detection of 1 to 3 CFU/ml (45). Lastly, PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) technology can detect a broad range of bacteria and yeast pathogens directly from whole blood. The IRIDICA platform using the IRIDICA BAC BSI assay (Ibis Biosciences, Abbott Molecular, Des Plaines, IL) is CE marked but not FDA cleared. The system detects >750 bacteria and Candida targets and resistance markers from 5 ml of whole blood with reported positive and negative agreements of 74.8% and 78.6%, respectively, compared to blood cultures (46). There are a number of limitations to these rapid diagnostic methods. The tests complement conventional identification and susceptibility testing rather than replace them. Moreover, aside from the T2Candida test and IRIDICA BAC BSI, all assays described above are dependent on a positive blood culture. A pitfall of the multiplex molecular assays is that they consist of predefined targets, whereas the MALDI-TOF MS database is significantly broader. In addition, the Verigene BC-GP and MALDI-TOF MS can misidentify viridans group streptococci as S. pneumoniae, resulting in an increased number of false positives that must be confirmed by conventional biochemical methods (42, 47). Lastly, the feasibility of the T2Candida and IRIDICA BAC BSI in young children and infants may be limited due to the 3 to 5 ml of blood required.
In contrast to the adult population, there are extremely few data on the impact of rapid laboratory diagnostics on antimicrobial optimization, patient management, and costs to the health care system in the pediatric population. A single-center study evaluated the impact of implementing the Verigene BC-GP assay in a pediatric tertiary care setting. In this study, 440 BSI episodes from 383 patients who were ≤18 years of age were enrolled, 221 preimplementation and 219 postimplementation. Although the study did evaluate all targets included in the panel, the most significant findings were noted for S. aureus BSIs, particularly in the general pediatric population, where the mean time to identification and antimicrobial resistance detection was shortened by 45.9 h and antimicrobial optimization was shortened by 11.9 h. Furthermore, median length of stay (LOS) from onset of BSI in this group was decreased by 5.8 days with a median cost savings of $13,341 per patient. Of note, no significant impact on LOS and hospital cost were observed when all patient data were analyzed and is likely due to the significant comorbidities associated with approximately 60% of the patients (48).
SUMMARY
While performing adult blood culture is a fairly standardized process with definitive guidelines supported by copious data, pediatric blood culture practices vary by institution because published data and guidelines are lacking. Based on the currently available data, best practices for pediatric blood culture include obtaining a sample of adequate blood volume prior to the administration of antibiotics from any child experiencing clinical signs of sepsis. Anaerobic blood culture is unnecessary for the majority of patients, and there is debate on the utility of pediatric-specific blood culture bottles. The scant literature on rapid molecular identification of blood culture pathogens for pediatric patients has shown that these technologies may directly impact antimicrobial optimization and reduce length of hospital stay and hospital cost. It goes without saying that more research into many of the topics touched upon in this review are needed to further elucidate best practices for blood culture collection from pediatric patients.
Biography

Jennifer Dien Bard, Ph.D., D(ABMM), is the director of clinical microbiology and virology laboratories at Children's Hospital Los Angeles (CHLA) and holds a faculty appointment as Assistant Professor of Clinical Pathology in the Department of Pathology and Laboratory Medicine at the University of Southern California Keck School of Medicine. She completed the CPEP training program in Medical and Public Health Microbiology at the University of California, Los Angeles and recently established a CPEP training program at CHLA for which she serves as program director. Dr. Dien Bard is a Diplomate of the American Board of Medical Microbiology and a Fellow of the Canadian College of Microbiologists. She is currently a member of the editorial board of the Journal of Clinical Microbiology and the American Society for Microbiology Professional Development Committee. Her current research interests include the development and utilization of rapid laboratory diagnostics and their subsequent impact on patient management.
REFERENCES
- 1.Szymczak EG, Barr JT, Durbin WA, Goldmann DA. 1979. Evaluation of blood culture procedures in a pediatric hospital. J Clin Microbiol 9:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhow TL, Hung YY, Herz AM. 2012. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics 129:e590–e596. doi: 10.1542/peds.2011-1546. [DOI] [PubMed] [Google Scholar]
- 3.Bateman SL, Seed PC. 2010. Procession to pediatric bacteremia and sepsis: covert operations and failures in diplomacy. Pediatrics 126:137–150. doi: 10.1542/peds.2009-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacman DJ, Karasic RB, Reynolds EA, Kost SI. 1996. Effect of number of blood cultures and volume of blood on detection of bacteremia in children. J Pediatr 128:190–195. doi: 10.1016/S0022-3476(96)70388-8. [DOI] [PubMed] [Google Scholar]
- 5.Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. 1996. Volume of blood required to detect common neonatal pathogens. J Pediatr 129:275–278. doi: 10.1016/S0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 6.O'Grady NP, Barie PS, Bartlett JG, Bleck T, Garvey G, Jacobi J, Linden P, Maki DG, Nam M, Pasculle W, Pasquale MD, Tribett DL, Masur H. 1998. Practice guidelines for evaluating new fever in critically ill adult patients. Task Force of the Society of Critical Care Medicine and the Infectious Diseases Society of America. Clin Infect Dis 26:1042–1059. doi: 10.1086/520308. [DOI] [PubMed] [Google Scholar]
- 7.Yagupsky P, Nolte FS. 1990. Quantitative aspects of septicemia. Clin Microbiol Rev 3:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durbin WA, Szymczak EG, Goldmann DA. 1978. Quantitative blood cultures in childhood bacteremia. J Pediatr 92:778–780. doi: 10.1016/S0022-3476(78)80151-6. [DOI] [PubMed] [Google Scholar]
- 9.Kellogg JA, Bankert DA, Manzella JP, Parsey KS, Scott SL, Cavanaugh SH. 1994. Clinical comparison of isolator and thiol broth with ESP aerobic and anaerobic bottles for recovery of pathogens from blood. J Clin Microbiol 32:2050–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellogg JA, Manzella JP, Bankert DA. 2000. Frequency of low-level bacteremia in children from birth to fifteen years of age. J Clin Microbiol 38:2181–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA. 1997. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J 16:381–385. doi: 10.1097/00006454-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. 2007. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children's hospital. Pediatrics 119:891–896. [DOI] [PubMed] [Google Scholar]
- 13.Gonsalves WI, Cornish N, Moore M, Chen A, Varman M. 2009. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol 47:3482–3485. doi: 10.1128/JCM.02107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biondi EA, Mischler M, Jerardi KE, Statile AM, French J, Evans R, Lee V, Chen C, Asche C, Ren J, Shah SS. 2014. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr 168:844–849. doi: 10.1001/jamapediatrics.2014.895. [DOI] [PubMed] [Google Scholar]
- 15.Bekeris LG, Tworek JA, Walsh MK, Valenstein PN. 2005. Trends in blood culture contamination: a College of American Pathologists Q-Tracks study of 356 institutions. Arch Pathol Lab Med 129:1222–1225. [DOI] [PubMed] [Google Scholar]
- 16.Bouza E, Sousa D, Rodriguez-Creixems M, Lechuz JG, Munoz P. 2007. Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections? J Clin Microbiol 45:2765–2769. doi: 10.1128/JCM.00140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mermel LA, Maki DG. 1993. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 119:270–272. doi: 10.7326/0003-4819-119-4-199308150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 19.Clinical Laboratory and Standard Institute. 2007. Principles and procedures for blood cultures; approved guideline—1st ed CLSI document M47-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Baron EJ, Thomson RB Jr. Specimen collection, transport, and processing: bacteriology, p 228–271. In Versalovic J, Carroll KC, Jorgensen JH, Funke G, Landry ML, Warnock DW (ed), Manual of clinical microbiology, 10th ed, vol 2.z ASM Press, Washington, DC. [Google Scholar]
- 21.Baron EJ, Weinstein MP, Dunne WM, Yagupsky P, Welch DF, Wilson DM. 2005. Cumitech 1C, blood cultures IV. ASM Press, Washington, DC. [Google Scholar]
- 22.Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, Bourbeau P, Carroll KC, Kehl SC, Dunne WM, Robinson-Dunn B, Schwartzman JD, Chapin KC, Snyder JW, Forbes BA, Patel R, Rosenblatt JE, Pritt BS. 2013. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis 57:e22–e121. doi: 10.1093/cid/cit278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaimes F, Arango C, Ruiz G, Cuervo J, Botero J, Velez G, Upegui N, Machado F. 2004. Predicting bacteremia at the bedside. Clin Infect Dis 38:357–362. doi: 10.1086/380967. [DOI] [PubMed] [Google Scholar]
- 24.Riedel S, Bourbeau P, Swartz B, Brecher S, Carroll KC, Stamper PD, Dunne WM, McCardle T, Walk N, Fiebelkorn K, Sewell D, Richter SS, Beekmann S, Doern GV. 2008. Timing of specimen collection for blood cultures from febrile patients with bacteremia. J Clin Microbiol 46:1381–1385. doi: 10.1128/JCM.02033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar S, Bhagat I, DeCristofaro JD, Wiswell TE, Spitzer AR. 2006. A study of the role of multiple site blood cultures in the evaluation of neonatal sepsis. J Perinatol 26:18–22. doi: 10.1038/sj.jp.7211410. [DOI] [PubMed] [Google Scholar]
- 26.Wattier RL, Dvorak CC, Auerbach AD, Weintrub PS. 2015. Repeat blood cultures in children with persistent fever and neutropenia: diagnostic and clinical implications. Pediatr Blood Cancer 62:1421–1426. doi: 10.1002/pbc.25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan KV, Turner NN, Lancaster DP, Shah AR, Chandler LJ, Friedman DF, Blecker-Shelly DL. 2013. Superior sensitivity and decreased time to detection with the Bactec Peds Plus/F system compared to the BacT/Alert Pediatric FAN blood culture system. J Clin Microbiol 51:4083–4086. doi: 10.1128/JCM.02205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raad I, Hanna H, Maki D. 2007. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis 7:645–657. doi: 10.1016/S1473-3099(07)70235-9. [DOI] [PubMed] [Google Scholar]
- 29.Douard MC, Arlet G, Leverger G, Paulien R, Waintrop C, Clementi E, Eurin B, Schaison G. 1991. Quantitative blood cultures for diagnosis and management of catheter-related sepsis in pediatric hematology and oncology patients. Intensive Care Med 17:30–35. doi: 10.1007/BF01708406. [DOI] [PubMed] [Google Scholar]
- 30.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haimi-Cohen Y, Vellozzi EM, Rubin LG. 2002. Initial concentration of Staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J Clin Microbiol 40:898–901. doi: 10.1128/JCM.40.3.898-901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaur AH, Flynn PM, Heine DJ, Giannini MA, Shenep JL, Hayden RT. 2005. Diagnosis of catheter-related bloodstream infections among pediatric oncology patients lacking a peripheral culture, using differential time to detection. Pediatr Infect Dis J 24:445–449. doi: 10.1097/01.inf.0000160950.83583.7f. [DOI] [PubMed] [Google Scholar]
- 33.Kennaugh JK, Gregory WW, Powell KR, Hendley JO. 1984. The effect of dilution during culture on detection of low concentrations of bacteria in blood. Pediatr Infect Dis 3:317–318. doi: 10.1097/00006454-198407000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster DP, Friedman DF, Chiotos K, Sullivan KV. 2015. Blood volume required for detection of low levels and ultralow levels of organisms responsible for neonatal bacteremia by use of Bactec Peds Plus/F, Plus Aerobic/F Medium, and the BD Bactec FX system: an in vitro study. J Clin Microbiol 53:3609–3613. doi: 10.1128/JCM.01706-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein EJ. 1996. Anaerobic bacteremia. Clin Infect Dis 23(Suppl):S97–S101. doi: 10.1093/clinids/23.Supplement_1.S97. [DOI] [PubMed] [Google Scholar]
- 36.Zaidi AK, Knaut AL, Mirrett S, Reller LB. 1995. Value of routine anaerobic blood cultures for pediatric patients. J Pediatr 127:263–268. doi: 10.1016/S0022-3476(95)70305-5. [DOI] [PubMed] [Google Scholar]
- 37.Echeverria P, Smith AL. 1978. Anaerobic bacteremia as observed in a children's hospital. Clinically this may signify true anaerobic sepsis. Clin Pediatr (Phila) 17:688–690. [DOI] [PubMed] [Google Scholar]
- 38.Iwata K, Takahashi M. 2008. Is anaerobic blood culture necessary? If so, who needs it? Am J Med Sci 336:58–63. [DOI] [PubMed] [Google Scholar]
- 39.Dunne WM Jr, Tillman J, Havens PL. 1994. Assessing the need for anaerobic medium for the recovery of clinically significant blood culture isolates in children. Pediatr Infect Dis J 13:203–206. doi: 10.1097/00006454-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Morris AJ, Wilson ML, Mirrett S, Reller LB. 1993. Rationale for selective use of anaerobic blood cultures. J Clin Microbiol 31:2110–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong I, Jayagobi PA, Raut P, Chong CY. 2015. Judicious utilization of healthcare resources: reducing unindicated pediatric anaerobic blood cultures in a pediatric hospital. J Healthc Qual 37:199–204, quiz 204–205. [DOI] [PubMed] [Google Scholar]
- 42.Kok J, Thomas LC, Olma T, Chen SC, Iredell JR. 2011. Identification of bacteria in blood culture broths using matrix-assisted laser desorption-ionization Sepsityper and time of flight mass spectrometry. PLoS One 6(8):e23285. doi: 10.1371/journal.pone.0023285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mestas J, Felsenstein S, Bard JD. 2014. Direct identification of bacteria from positive BacT/ALERT blood culture bottles using matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Diagn Microbiol Infect Dis 80:193–196. doi: 10.1016/j.diagmicrobio.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Kothari A, Morgan M, Haake DA. 2014. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis 59:272–278. doi: 10.1093/cid/ciu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, Garey KW, Alangaden GJ, Vazquez JA, Groeger JS, Judson MA, Vinagre YM, Heard SO, Zervou FN, Zacharioudakis IM, Kontoyiannis DP, Pappas PG. 2015. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 60:892–899. doi: 10.1093/cid/ciu959. [DOI] [PubMed] [Google Scholar]
- 46.Jordana-Lluch E, Gimenez M, Quesada MD, Rivaya B, Marco C, Dominguez MJ, Armestar F, Martro E, Ausina V. 2015. Evaluation of the broad-range PCR/ESI-MS technology in blood specimens for the molecular diagnosis of bloodstream infections. PLoS One 10:e0140865. doi: 10.1371/journal.pone.0140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mestas J, Polanco CM, Felsenstein S, Dien Bard J. 2014. Performance of the Verigene Gram-positive blood culture assay for direct detection of Gram-positive organisms and resistance markers in a pediatric hospital. J Clin Microbiol 52:283–287. doi: 10.1128/JCM.02322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felsenstein S, Bender JM, Sposto R, Gentry M, Takemoto C, Dien Bard J. 2016. Impact of a rapid blood culture assay for Gram-positive identification and detection of resistance markers in a pediatric hospital. Arch Pathol Lab Med 140:267–275. doi: 10.5858/arpa.2015-0119-OA. [DOI] [PubMed] [Google Scholar]