Abstract
Surotomycin (formerly called CB-183,315) is a novel, orally administered cyclic lipopeptide antibacterial in development for the treatment of Clostridium difficile infection (CDI) that has potent activity against vancomycin-resistant enterococci (VRE) but limited activity against Gram-negative bacilli, including Bacteroides spp. We used a mouse model to investigate the impact of surotomycin exposure on the microbiome, and to test the consequences of the disruption on colonization by vancomycin-resistant enterococci (VRE) and extended-spectrum β-lactamase-producing Klebsiella pneumoniae (ESBL-KP), in comparison with the effects of oral vancomycin and metronidazole. Mice (8 per group) received saline, vancomycin, metronidazole, or surotomycin through an orogastric tube daily for 5 days and were challenged with 105 CFU of VRE or ESBL-KP administered through an orogastric tube on day 2 of treatment. The concentrations of the pathogens in stool were determined during and after treatment by plating on selective media. A second experiment was conducted to determine if the antibiotics would inhibit established VRE colonization. In comparison to controls, oral vancomycin promoted VRE and ESBL-KP overgrowth in stool (8 log10 to 10 log10 CFU/g; P < 0.001), whereas metronidazole did not (<4 log10 CFU/g; P > 0.5). Surotomycin promoted ESBL-KP overgrowth (>8 log10 CFU/g; P, <0.001 for comparison with saline controls) but not VRE overgrowth. Surotomycin suppressed preexisting VRE colonization, whereas metronidazole and vancomycin did not. These results suggest that treatment of CDI with surotomycin could reduce levels of VRE acquisition and overgrowth from those with agents such as vancomycin and metronidazole. However, surotomycin and vancomycin may promote colonization by antibiotic-resistant Gram-negative bacilli.
INTRODUCTION
Oral vancomycin and oral metronidazole are the most commonly used antibiotics for the treatment of Clostridium difficile infection (CDI). One limitation of these agents is that they are nonselective (i.e., they inhibit normal anaerobic intestinal microbiota in addition to C. difficile) (1–3). For example, oral vancomycin treatment may result in the suppression of Bacteroides/Prevotella, Clostridium coccoides, and Clostridium leptum group organisms in stool (2, 3). Inhibition of the anaerobic microbiota by vancomycin and metronidazole may contribute to recurrences of CDI and to colonization by health care-associated pathogens, such as vancomycin-resistant enterococci (VRE) (4, 5). In comparison to vancomycin, fidaxomicin causes minimal disruption of the anaerobic microbiota and is associated with fewer recurrences and less acquisition of VRE and Candida spp. during CDI treatment (1, 6). However, selection of preexisting subpopulations of VRE with elevated fidaxomicin MICs was common during fidaxomicin therapy (6).
Surotomycin (formerly called CB-183,315) is a novel, nonabsorbed lipopeptide antibiotic that is being studied in phase 3 trials for the treatment of CDI (7–10). Surotomycin has excellent activity against Gram-positive organisms, including VRE, and no significant activity against Gram-negative bacilli, including Bacteroides spp. (8, 9). Surotomycin reduced recoverable C. difficile levels and was sparing of gut microbes, primarily Bacteroides and Prevotella spp., in a subset of patients from a recently conducted phase 2 clinical trial (11). Therefore, we hypothesized that surotomycin would inhibit preexisting VRE colonization and that the minimal microbiome disruption upon surotomycin exposure would preserve Bacteroides spp. and other anaerobes, preventing colonization by health care-associated pathogens, such as extended-spectrum β-lactamase-producing Klebsiella pneumoniae (ESBL-KP) and VRE. Here we used a mouse model to compare the effects of surotomycin with those of vancomycin and metronidazole on the establishment and persistence of intestinal colonization by VRE and ESBL-KP.
MATERIALS AND METHODS
Pathogens studied.
Enterococcus faecium C68 is a previously described VanB-type clinical VRE isolate (12). K. pneumoniae P62 is a clinical isolate that produces an SHV-type extended-spectrum β-lactamase (ESBL). Both organisms have been used in previous mouse model studies (12, 13).
Susceptibility testing.
Broth dilution MICs of the test antibiotics for the test organisms were determined using standard methods for testing the susceptibility of aerobic bacteria (14, 15). For surotomycin, the calcium in the medium was adjusted to a final concentration of 50 mg/liter, the optimal concentration for antibacterial activity (16).
Bioassay for antibiotic concentrations.
The concentrations of the test antibiotics in stool were determined by an agar diffusion assay with Clostridium perfringens as the indicator strain (17).
Quantification of stool pathogens.
Fresh stool specimens were processed as described elsewhere (12, 13). In order to quantify VRE and ESBL-KP, diluted samples were plated onto Enterococcosel agar (Becton Dickinson, Cockeysville, MD) containing 20 μg/ml vancomycin and MacConkey agar (Becton Dickinson) containing 10 μg/ml ceftazidime, respectively. The plates were incubated in room air at 37°C for 48 h, and the number of CFU of each pathogen per gram of sample was calculated. For a subset of 20 ceftazidime-resistant Gram-negative isolates, identification and susceptibility testing were performed using standard methods to confirm that the isolates were K. pneumoniae strains with susceptibility patterns identical to that of the initial strain inoculated (14, 15).
Antibiotic dose selection.
Dose-ranging experiments were run to determine the amounts of vancomycin and surotomycin that had to be dosed in order to result in concentrations in the stools of mice similar to those measured in humans (i.e., 1,000 to 2,000 μg/g of stool) (11, 18; unpublished data, Merck and Co.). Mice (5 per group) received a single oral administration of vancomycin or surotomycin at 2.25 to 7.5 mg/day or 75 to 250 mg/kg of body weight. Fecal pellets were collected within 3 intervals of 0 to 4, 4 to 8, and 8 to 24 h after dosing. Levels of the drugs in feces were measured by liquid chromatography (LC)–mass spectrometry (MS) and were confirmed using satellite animals dosed at 1.125 mg/day or 37.5 mg/kg. This dosing regimen resulted in peak levels of 1,492 μg of surotomycin/g of feces and 1,370 μg of vancomycin/g of feces by 4 to 8 h postdosing.
For metronidazole, we administered 3.75 mg/day, which is 5 times the usual human dose administered over a 24-h period (in milligrams of antibiotic per gram of body weight). This dose was chosen because it has been shown in previous mouse model studies to promote persistent colonization with VRE when administered subcutaneously and to reduce colonization resistance when administered by oral gavage (19, 20). In contrast, a dose of metronidazole equivalent to the human dose on a milligram-per-kilogram basis did not promote VRE colonization in mice (19).
Effects of the antibiotics on intestinal microbiota.
The Animal Care Committee of the Cleveland Veterans Affairs Medical Center approved the experimental protocol. Initial experiments were conducted to assess the effects of treatment with the test antibiotics or saline on the intestinal microbiota of mice. Female CF-1 mice (6 per group) weighing ∼30 g (Harlan Sprague-Dawley, Indianapolis, IN) were housed in individual cages. (CF-1 mice are outbred white mice.) Mice received daily oroesophageal instillation of the test antibiotics (total volume, 0.2 ml) for 5 days using a stainless steel feeding tube (Perfektum; Popper & Sons, New Hyde Park, NY). Mice were dosed with surotomycin (1.125 mg/day), vancomycin (1.125 mg/day), or metronidazole (3.75 mg/day). For all experiments, cages were changed daily during and after treatment in order to reduce reexposure to contaminated bedding and cage material and to minimize the potential for reingestion through coprophagy.
Stool samples were collected at baseline, on days 2 and 5 of treatment, and 3, 5, and 10 days after treatment for evaluation of the effects of the antibiotics on the microbiota. Quantitative cultures for facultative and aerobic Gram-negative bacilli and enterococci were performed by plating serially diluted specimens onto MacConkey agar (Difco Laboratories, Detroit, MI) and Enterococcosel agar (Becton Dickinson), respectively.
Deep-sequencing analysis of stool microbiota.
Fecal bacterial DNA was extracted from ∼500 mg of feces by use of the QIAamp DNA Stool minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Sequencing and analysis were carried out by Second Genome (San Bruno, CA). To enrich the samples for the V4 region of the bacterial 16S rRNA gene, DNA was PCR amplified using fusion primers designed against surrounding conserved regions and tailed with sequences to incorporate Illumina (San Diego, CA) adapter and indexing bar codes. After Illumina library construction, amplicons were sequenced using a MiSeq benchtop sequencer instrument (Illumina). Using QIIME and custom scripts, sequences were quality filtered and demultiplexed using exact matches to the DNA bar codes supplied. The resulting sequences were used to search the Greengenes reference database of 16S rRNA sequences, clustered at 97% by uclust (closed-reference operational taxonomic unit [OTU] picking). The longest sequence from each OTU thus formed was used as the representative OTU sequence and was assigned a taxonomic classification via MOTHUR's Bayesian classifier, trained against the Greengenes database clustered at 98%. Principal coordinate analysis (PCoA) was carried out to visualize complex relationships between samples. The Adonis test was used to assess whole-microbiome differences among groups. Bar plot representations were generated to show the top 8 microbial groups at the phylum level.
Analysis of Bacteroides spp. and Clostridium leptum by real-time PCR.
To determine the effects of antibiotic treatment on the concentrations of Bacteroides spp. and C. leptum, a representative Firmicutes organism, quantitative PCR (qPCR) was performed using the methods and primers of Louie et al. (2). Fecal bacterial DNA was extracted from 100 mg of feces by use of the QIAamp DNA Stool minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Purified template DNA from Bacteroides fragilis and C. leptum was used for melting curve analysis and for the generation of standard curves for each primer set using 10-fold serial dilutions of DNA ranging from 10 to 10−6 ng. qPCR was performed using the CFX96 detection system (Bio-Rad, Hercules, CA). Amplification and detection were conducted in 96-well plates with SYBR green 2× qPCR master mix (Bio-Rad). Each sample was run in triplicate in a final volume of 20 μl containing a final concentration of 0.3 μM each primer and 5 μl of 2-ng/μl template DNA using the following parameters: 1 cycle at 94°C for 5 min, followed by 49 cycles at 94°C for 20 s, 56°C to 58°C for 20 s, and 72°C for 20 s.
Effects of the antibiotics on the establishment of colonization by VRE and ESBL-KP.
To assess the effects of antibiotic exposure on the initial establishment of colonization, mice (8 per group) received oroesophageal instillation of 10,000 CFU of VRE or ESBL-KP on day 2 of 5 of daily treatment with the antibiotics or saline. The concentrations of VRE and ESBL-KP in stool were measured on day 5 of antibiotic treatment and 3, 5, and 10 days after the completion of antibiotic treatment.
Effects of the antibiotics on the persistence of VRE colonization.
To assess the effects of the antibiotics on the persistence of VRE colonization, mice (8 per group) received clindamycin (1.2 mg) subcutaneously and 10,000 CFU of VRE by oroesophageal instillation to establish colonization. Subsequently, the test antibiotics were administered daily for 5 days by oroesophageal instillation, VRE concentrations were measured on day 5 of antibiotic treatment and on days 3, 5, and 10 after treatment.
Statistical analysis.
One-way analysis of variance (ANOVA) was performed to compare the concentrations of organisms among the treatment groups. P values were adjusted for multiple comparisons using the Scheffe correction. Computations were performed by the use of Stata software (version 5.0; Stata, College Station, TX).
RESULTS
Susceptibility testing.
Vancomycin, metronidazole, and surotomycin MICs for ESBL-KP were >256 μg/ml. Vancomycin, metronidazole, and surotomycin MICs for VRE were 256, >256, and 1 μg/ml, respectively.
Effects of the antibiotics on indigenous enterococci and facultative Gram-negative bacilli by culture.
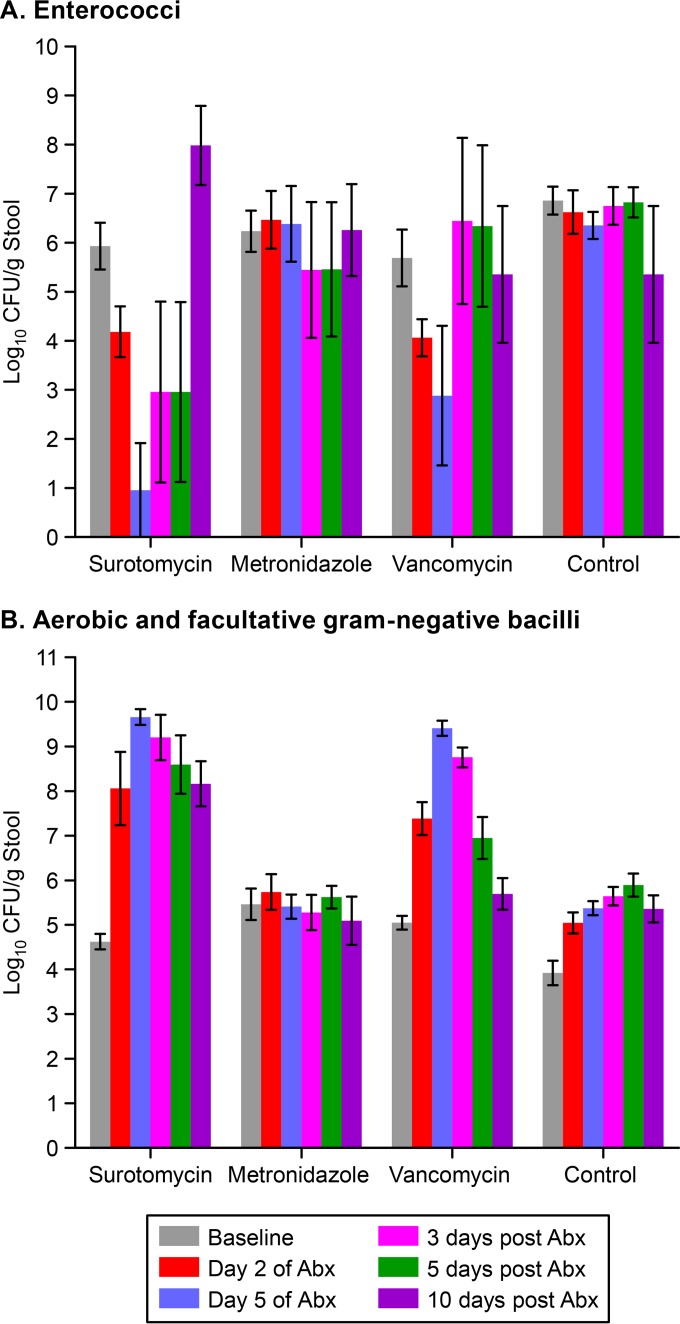
Figure 1 shows the effects of antibiotic treatment on the concentrations of enterococci (A) and aerobic and facultative Gram-negative bacilli (B) by culture. Surotomycin and vancomycin suppressed levels of enterococci during treatment, whereas metronidazole did not; levels of enterococci returned to baseline concentrations by 3 days after the discontinuation of vancomycin and by 10 days after the discontinuation of surotomycin. In comparison to controls, surotomycin and vancomycin permitted the overgrowth of Gram-negative bacilli, whereas metronidazole did not; by 10 days after the discontinuation of antibiotics, levels of Gram-negative bacilli remained significantly elevated over the baseline in surotomycin-treated mice but not in vancomycin-treated mice.
FIG 1.
Effects of antibiotic treatment on the concentrations of enterococci (A) and aerobic and facultative Gram-negative bacilli (B) in stool by culture. Mice received daily subcutaneous antibiotic treatment for 5 days. Error bars represent standard errors. Abx, antibiotics.
Effects of the antibiotics on indigenous microbiota by deep sequencing and qPCR.
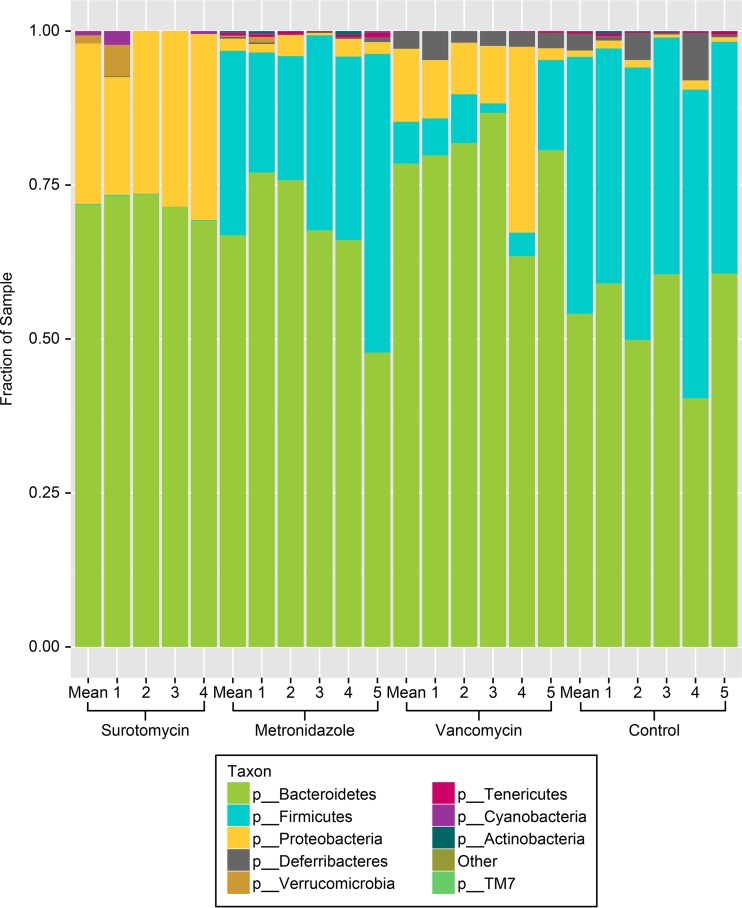
Figure 2 shows the relative proportions of different bacterial phyla on day 5 of antibiotic treatment in comparison to those for the saline control group, including the summed total for each treatment group and data for individual mice. In control mice, Bacteroidetes and Firmicutes were predominant, with Proteobacteria making up only a minor proportion of the indigenous microbiota. Metronidazole treatment was associated with minor reductions in the proportion of Firmicutes, with no increase in the proportion of Proteobacteria. In contrast, surotomycin treatment and vancomycin treatment were associated with marked suppression of Firmicutes and expansion of Proteobacteria. On day 5 of treatment, the alpha diversity estimates for each of the antibiotic treatment groups were significantly reduced from those for the saline control group, but the greatest reductions were found in the vancomycin and surotomycin groups (alpha diversity estimates, 4.56 ± 0.14 for the control group, 1.42 ± 0.29 [P < 0.001] for the vancomycin group, 2.15 ± 0.40 [P < 0.001] for the surotomycin group, and 4.16 ± 0.17 [P = 0.004] for the metronidazole group).
FIG 2.
Comparison of the stool microbiota of mice by 16S rRNA deep-sequencing analysis after 5 days of antibiotic treatment. The relative abundances of the major bacterial phyla are shown. Mean results for the total number of mice in each group (4 mice for the surotomycin group and 5 each for the metronidazole, vancomycin, and control groups) are shown. Numbers indicate data for individual mice in each group.
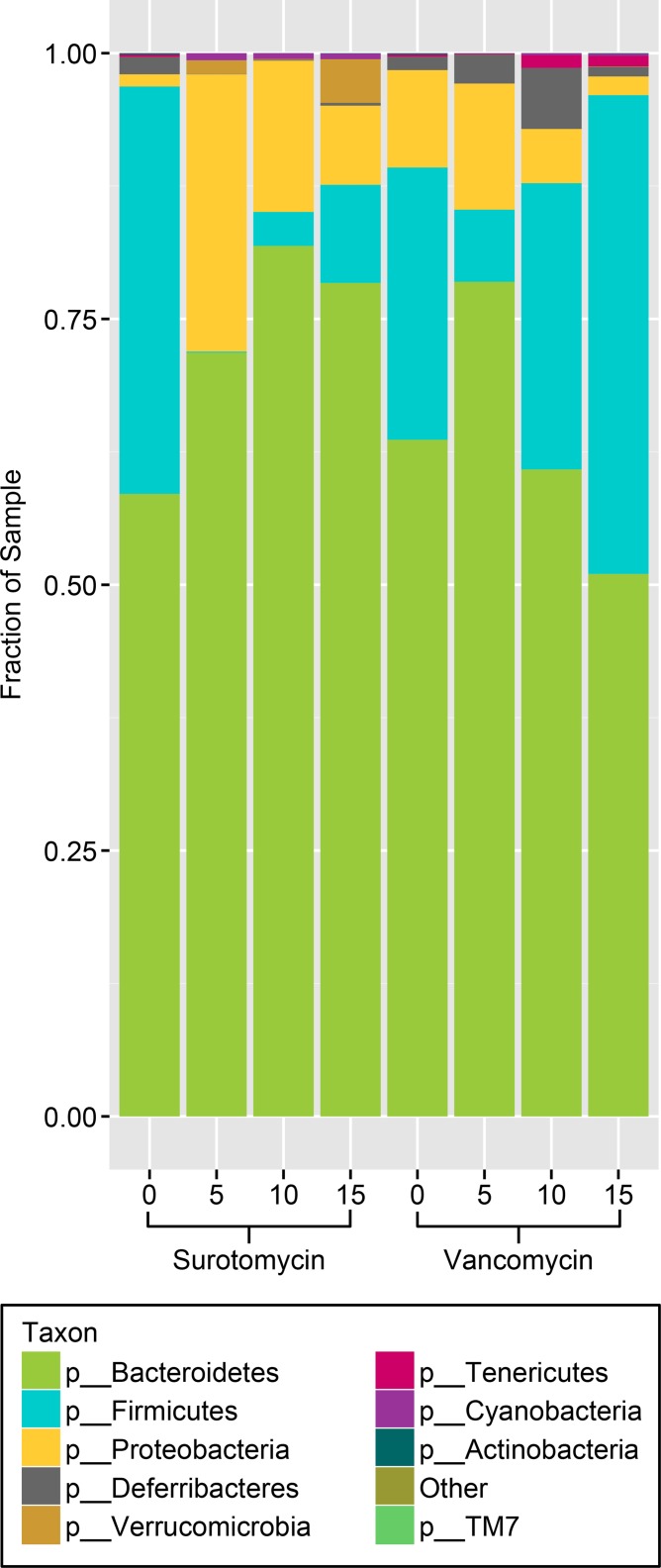
Figure 3 shows the relative proportions of the different taxa in the surotomycin and vancomycin groups before, during, and after treatment. For both antibiotic treatment groups, the proportion of Firmicutes increased from the end of treatment (day 5) to 10 days posttreatment (day 15), while the proportion of Proteobacteria decreased. With surotomycin, the changes were more pronounced, with less evidence of recovery by day 15.
FIG 3.
Comparison of the stool microbiota of mice by 16S rRNA deep-sequencing analysis before, during, and after treatment with oral surotomycin or vancomycin. Mice received daily oral antibiotic treatment for 5 days (day 0 to day 5). Numbers indicate the day of sample collection: day 0, prior to treatment; day 5, after 5 days of antibiotic treatment; day 10, 5 days after the last antibiotic dose; day 15, 10 days after the last antibiotic dose. The relative abundances of the major bacterial phyla are shown as a composite for 5 total mice in each group at each time point.
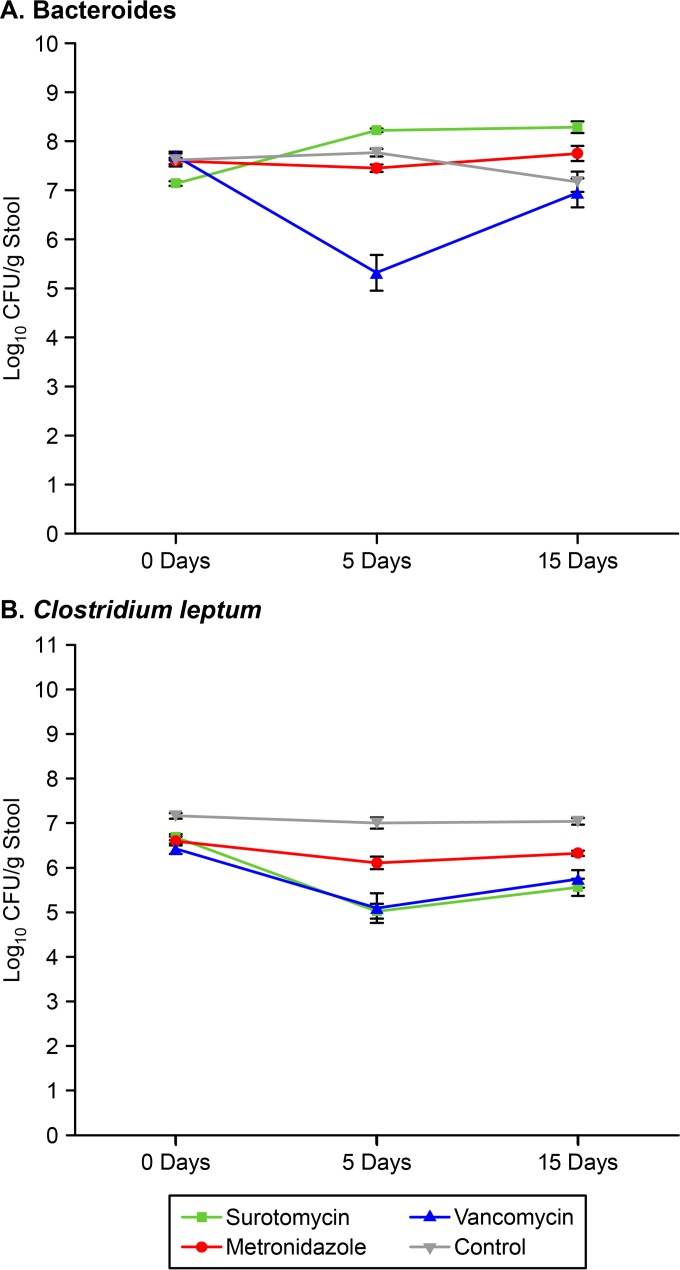
As shown in Fig. 4, vancomycin significantly reduced the concentration of Bacteroides spp. on day 5 of treatment (P < 0.001), whereas surotomycin and metronidazole did not (P > 0.05). Vancomycin and surotomycin both significantly reduced the concentration of C. leptum on day 5 of treatment (P < 0.01), whereas metronidazole did not (P > 0.05).
FIG 4.
Effects of antibiotic treatment on the concentrations of Bacteroides spp. (A) and Clostridium leptum (B) in the stool specimens of mice as determined by real-time PCR. Mice (4 in the surotomycin group and 5 each in the metronidazole, vancomycin, and control groups) received daily oral antibiotic treatment for 5 days (day 0 to day 5). Error bars represent standard errors.
Effects of the antibiotics on the establishment of colonization by VRE and ESBL-KP.
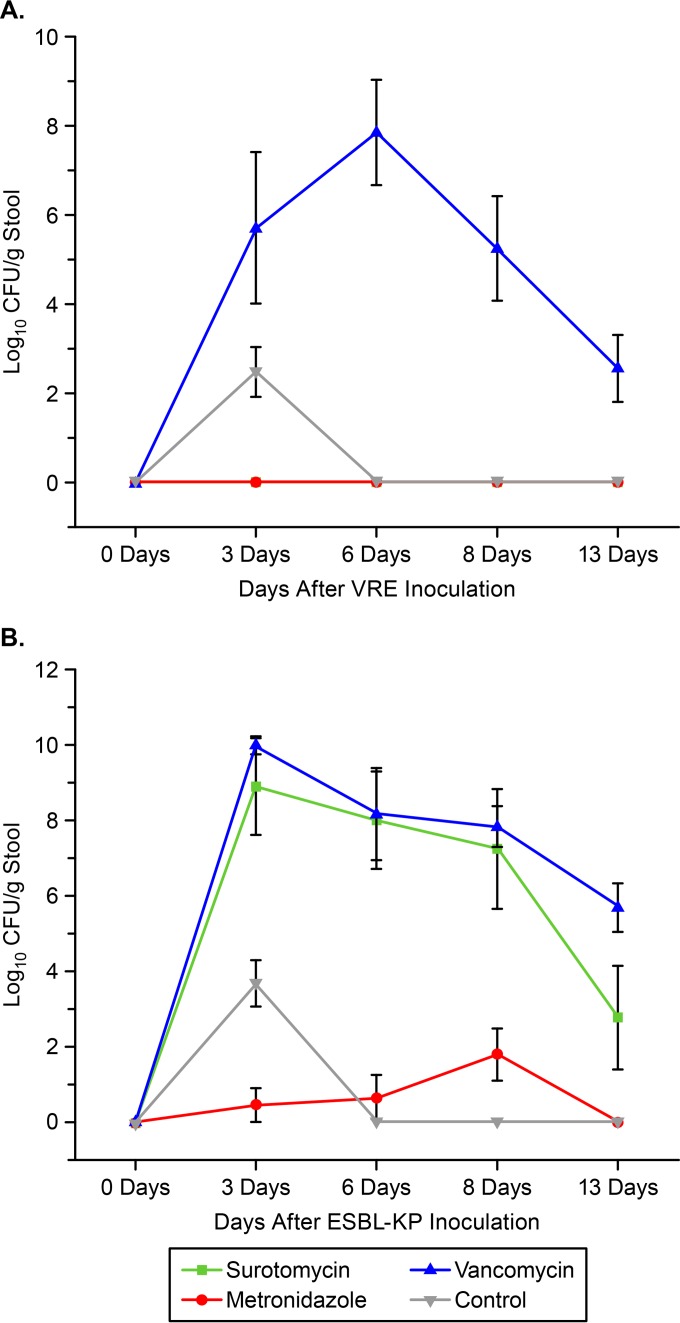
Figure 5 shows the effects of antibiotic treatment on the establishment of colonization by VRE (Fig. 5A) and ESBL-KP (Fig. 5B). In comparison to controls, oral vancomycin promoted the overgrowth of both pathogens (P < 0.001), and surotomycin promoted the overgrowth of ESBL-KP (P < 0.001), whereas metronidazole did not promote the overgrowth of either pathogen. None of the surotomycin-treated mice had detectable VRE at any time point.
FIG 5.
Effects of antibiotic treatment on the establishment of colonization by vancomycin-resistant enterococci (VRE) (A) and extended-spectrum β-lactamase-producing Klebsiella pneumoniae (ESBL-KP) (B) in mice. Mice (8 per group) received daily oral antibiotic treatment for 5 days (day 0 to day 5). Error bars represent standard errors.
Effects of the antibiotics on the persistence of VRE colonization.
In mice with preexisting high-density VRE colonization, surotomycin suppressed VRE to below the limit of detection during treatment (∼1 log10 CFU/g of stool), whereas VRE concentrations did not differ from those for saline controls in the metronidazole and vancomycin groups. However, high levels of VRE (>5 log10 CFU/g of stool), were again detectable in the stool specimens of all surotomycin-treated mice at 5 and 10 days after the discontinuation of treatment.
DISCUSSION
We found that oral vancomycin promoted the acquisition of VRE colonization in mice, whereas oral surotomycin and metronidazole did not. Surotomycin also suppressed indigenous enterococci and preexisting high-density VRE colonization to undetectable levels during treatment. However, surotomycin disrupted the anaerobic intestinal microbiota, allowing the overgrowth of indigenous facultative Gram-negative bacilli and exogenously administered ESBL-KP during treatment and the overgrowth of indigenous enterococci and previously suppressed VRE after treatment.
Our results for vancomycin are consistent with those of previous studies demonstrating that oral vancomycin promotes the acquisition and persistent overgrowth of VRE in mice and humans (4, 12, 19, 20). However, our results for metronidazole differ from those of previous studies with mice demonstrating that the dose administered may promote persistent colonization with VRE and transient loss of resistance to colonization with C. difficile, VRE, and Gram-negative bacilli (19, 20). The fact that metronidazole did not alter the anaerobic microbiota or colonization resistance in the present study could potentially be related to the use of a mouse strain different from that used by Lewis et al. (20) and a route of administration different from that in our previous study (19). In addition, it should be noted that in the absence of CDI, oral metronidazole treatment does not result in detectable drug levels in the stool specimens of humans, due to low levels of excretion into the intestinal tract and/or inactivation in intestinal contents (21). In contrast, in CDI patients treated with metronidazole, low levels of drug are detectable in stool, presumably due to the presence of diarrhea or inflammation of the colon, resulting in the promotion of VRE overgrowth in patients with concurrent VRE colonization (4, 22).
Our findings have implications for the selection of CDI therapy. In settings where VRE colonization and infection are a significant concern (e.g., organ transplant or stem cell transplant patients), the use of surotomycin rather than vancomycin or metronidazole for CDI treatment may result in less-frequent acquisition and overgrowth of VRE. However, the benefits of this approach may be limited, because our results suggest that recurrence of high-density VRE colonization may be common after surotomycin treatment. Surotomycin may also promote the overgrowth of Gram-negative bacilli; however, our results demonstrate that similar overgrowth may occur during oral vancomycin treatment. Although metronidazole did not promote colonization by VRE and ESBL-KP in healthy mice in the current study, the potential benefits of using this agent are questionable given the fact that previous studies on patients with CDI have demonstrated detectable levels of metronidazole in stool specimens and the promotion of colonization by VRE (4, 22).
It is notable that surotomycin markedly suppressed Firmicutes but did not reduce concentrations of Bacteroides spp. as determined by qPCR, suggesting that Firmicutes rather than Bacteroidetes may be essential in providing resistance against colonization with Gram-negative bacilli and enterococci. Our findings are consistent with other recent evidence suggesting that bacteria from the phylum Firmicutes may play an important role in colonization resistance. For mice, we previously demonstrated that the recovery of bacteria from the families Lachnospiraceae and Ruminococcaceae (phylum Firmicutes, order Clostridiales) corresponded directly with the timing of the recovery of in vivo resistance to colonization with VRE and C. difficile (23). For hospitalized patients, a reduction in the abundance of members of the order Clostridiales was independently associated with an increased risk of nosocomial CDI (24).
Our study has some limitations. The study was conducted using a mouse model with healthy mice. Additional studies will be required to confirm that the findings are applicable to patients with CDI. We studied only one strain each of VRE and K. pneumoniae. However, we have shown previously that multiple VRE and K. pneumoniae strains gave similar results in the mouse model (12, 13). Finally, we studied only one species of antimicrobial-resistant Gram-negative bacilli. Further studies that include other species, such as Acinetobacter spp., are needed.
ACKNOWLEDGMENTS
Curtis J. Donskey is a member of advisory boards for 3M and Seres and has received research funding from Clorox, Ecolab, Cepheid, GOJO, STERIS, 3M, Merck, and Synthetic Biologics. Abhishek Deshpande has received research funding from Clorox, STERIS, and 3M. Laurent Chesnel and Lihong Gao are employees of Merck & Co., Inc. Luisa Chan is an employee of Second Genome Inc. The remaining authors have no relevant disclosures.
Funding Statement
This work, including the efforts of Laurent Chesnel, was funded by Cubist and Merck and Co., Inc., and supported by the Department of Veterans Affairs (VA).
REFERENCES
- 1.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 2.Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, Krulicki W. 2012. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis 55(Suppl 2):S132–S142. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. 2010. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 156:3354–3359. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 4.Al-Nassir W, Sethi AK, Riggs MM, Li Y, Pultz MJ, Donskey CJ. 2008. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother 52:2403–2406. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi AK, Al-Nassir W, Nerandzic MM, Donskey CJ. 2009. Skin and environmental contamination with vancomycin-resistant enterococci in patients being treated with oral metronidazole versus oral vancomycin for Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 30:13–17. doi: 10.1086/592710. [DOI] [PubMed] [Google Scholar]
- 6.Nerandzic MM, Mullane K, Miller MA, Babakhani F, Donskey CJ. 2012. Reduced acquisition and overgrowth of vancomycin-resistant enterococci in patients treated with fidaxomicin versus vancomycin for C. difficile infection. Clin Infect Dis 55(Suppl 2):S121–S126. doi: 10.1093/cid/cis440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citron DM, Goldstein EJ. 2011. Reproducibility of broth microdilution and comparison to agar dilution for testing CB-183,315 against clinical isolates of Clostridium difficile. Diagn Microbiol Infect Dis 70:554–556. doi: 10.1016/j.diagmicrobio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Snydman DR, Jacobus NV, McDermott LA. 2012. Activity of a novel cyclic lipopeptide, CB-183,315, against resistant Clostridium difficile and other Gram-positive aerobic and anaerobic intestinal pathogens. Antimicrob Agents Chemother 56:3448–3452. doi: 10.1128/AAC.06257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citron DM, Tyrrell KL, Merriam CV, Goldstein EJ. 2012. In vitro activities of CB-183,315, vancomycin, and metronidazole against 556 strains of Clostridium difficile, 445 other intestinal anaerobes, and 56 Enterobacteriaceae species. Antimicrob Agents Chemother 56:1613–1615. doi: 10.1128/AAC.05655-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascio CT, Mortin LI, Howland KT, Van Praagh AD, Zhang S, Arya A, Chuong CL, Kang C, Li T, Silverman JA. 2012. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob Agents Chemother 56:5023–5030. doi: 10.1128/AAC.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon K, Byrne B, Happe J, Louie T. 2012. Abstr 22nd Eur Cong Clin Microbiol Infect Dis, abstr P-2250. Enteric microbiome profiles during a phase 2 clinical trial of CB-183, 315 or vancomycin for treatment of Clostridium difficile infection. [DOI] [PubMed] [Google Scholar]
- 12.Donskey CJ, Hanrahan JA, Hutton RA, Rice LB. 2000. Effect of parenteral antibiotic administration on the establishment of colonization with vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis 181:1830–1833. doi: 10.1086/315428. [DOI] [PubMed] [Google Scholar]
- 13.Hoyen CK, Pultz NJ, Paterson DL, Aron DC, Donskey CJ. 2003. Effect of parenteral antibiotic administration on establishment of intestinal colonization in mice by Klebsiella pneumoniae strains producing extended-spectrum β-lactamases. Antimicrob Agents Chemother 47:3610–3612. doi: 10.1128/AAC.47.11.3610-3612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2005. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Traczewski MM, Deane J, Sahm D, Brown SD, Chesnel L. 16 December 2015. Impact of variations in test method parameters on in vitro activity of surotomycin against Clostridium difficile and surotomycin quality control limits for broth microdilution and agar dilution susceptibility testing. J Clin Microbiol doi: 10.1128/JCM.02881-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolfe RD, Finegold SM. 1983. Intestinal β-lactamase activity in ampicillin-induced, Clostridium difficile-associated ileocecitis. J Infect Dis 147:227–235. doi: 10.1093/infdis/147.2.227. [DOI] [PubMed] [Google Scholar]
- 18.Gonzales M, Pepin J, Frost EH, Carrier JC, Sirard S, Fortier LC, Valiquette L. 2010. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis 10:363. doi: 10.1186/1471-2334-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donskey CJ, Hanrahan JA, Hutton RA, Rice LB. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis 180:384–390. doi: 10.1086/314874. [DOI] [PubMed] [Google Scholar]
- 20.Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, Gobourne A, Ling L, Pamer EG. 2015. Loss of microbiota-mediated colonization resistance to Clostridium difficile infection with oral vancomycin compared with metronidazole. J Infect Dis 212:1656–1665. doi: 10.1093/infdis/jiv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, Gerding DN. 1992. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med 117:297–302. [DOI] [PubMed] [Google Scholar]
- 22.Bolton RP, Culshaw MA. 1986. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27:1169–1172. doi: 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jump RL, Polinkovsky A, Hurless K, Sitzlar B, Eckart K, Tomas M, Deshpande A, Nerandzic MM, Donskey CJ. 2014. Metabolomics analysis identifies intestinal microbiota-derived biomarkers of colonization resistance in clindamycin-treated mice. PLoS One 9:e101267. doi: 10.1371/journal.pone.0101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent C, Stephens DA, Loo VG, Edens TJ, Behr MA, Dewar K, Manges AR. 2013. Reductions in intestinal Clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome 1:18. doi: 10.1186/2049-2618-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]