Abstract
Despite their common use as an empirical combination therapy for the better part of a decade, there has been a recent association between combination therapy with vancomycin and piperacillin-tazobactam and high rates of acute kidney injury (AKI). The reasons for this increased association are unclear, and this analysis was designed to investigate the association. Retrospective cohort and case-control studies were performed. The primary objective was to assess if there is an association between extended-infusion piperacillin-tazobactam in combination with vancomycin and development of AKI. The secondary objectives were to identify risk factors for AKI in patients on the combination, regardless of infusion strategy, and to evaluate the impact of AKI on clinical outcomes. AKI occurred in 105/320 (33%) patients from the cohort receiving combination therapy with vancomycin and piperacillin-tazobactam, with similar rates seen in those receiving intermittent (53/160 [33.1%]) and extended infusions (52/160 [32.5%]) of piperacillin-tazobactam. Independent risk factors for AKI in the cohort included having a documented Gram-positive infection, the presence of sepsis, receipt of a vancomycin loading dose (odds ratio [OR], 2.22; 95% confidence interval [CI], 1.05 to 4.71), and receipt of any concomitant nephrotoxin (OR, 2.44; 95% CI, 1.41 to 4.22). For at-risk patients remaining on combination therapy, the highest rates of AKI occurred on days 4 (10.7%) and 5 (19.3%). The incidence of AKI in patients on combination therapy with vancomycin and piperacillin-tazobactam is high, occurring in 33% of patients. Receipt of piperacillin-tazobactam as an extended infusion did not increase this risk. Modifiable risk factors for AKI include receipt of a vancomycin loading dose, concomitant nephrotoxins, and longer durations of therapy.
INTRODUCTION
The combination of vancomycin and piperacillin-tazobactam is a commonly prescribed empirical therapy for patients with health care-associated infections, as it provides coverage against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (1). There have been recent reports of relatively high rates of acute kidney injury (AKI), in the range of 15 to 35%, for patients receiving a combination of vancomycin and piperacillin-tazobactam (2–5), which are higher than those reported for patients receiving vancomycin alone (2, 5) and for patients receiving vancomycin and cefepime concomitantly (3). Although vancomycin has long been recognized as a nephrotoxic agent, and piperacillin-tazobactam has been associated with interstitial nephritis, the combination of these agents has been routinely used for many years; however, reports of increased AKI risk have only recently emerged (2–5).
The reason for the high rates of AKI in patients receiving vancomycin and piperacillin-tazobactam is unclear, as is the reason for the recent increase in the frequency of reports regarding an association between patients receiving this combination and AKI. Some have hypothesized that the recently reported increased risk is related to more-aggressive vancomycin dosing based on the recently published vancomycin consensus guidelines (6).
In addition to the changes in vancomycin dosing, there has also been increased interest in implementing extended-duration infusions of piperacillin-tazobactam in an attempt to optimize the time that free concentrations of the drug are above the MIC of target pathogens, as this is the pharmacokinetic/pharmacodynamic predictor of efficacy. Evidence has been mounting that extended infusion of piperacillin-tazobactam can improve outcomes in patients with invasive infections (7).
In 2013, there was an anecdotal increase in cases of AKI among patients receiving vancomycin at the Detroit Medical Center (DMC), Detroit, MI. This perceived increase coincided temporally with a switch of the preferred antipseudomonal agent from cefepime to piperacillin-tazobactam, as well as the implementation of extended-infusion (3-h) administration of all antipseudomonal β-lactams (previously administered via standard intermittent 30-min infusions). Prior to 2013, extended infusions were considered on a case-by-case basis for the treatment of organisms with elevated MICs; however, this practice was infrequent. Furthermore, at the time of switch from standard to extended infusions, a transition to a more consistent and higher dose of piperacillin-tazobactam was also made. Prior to the intervention, most patients received a base dose of piperacillin-tazobactam of 3.375 g every 6 h, whereas after the intervention, all patients received a higher dose of 4.5 g every 6 h as an extended infusion, with appropriate renal dose adjustments.
Given the perceived increase in vancomycin-associated nephrotoxicity at the DMC that coincided with these changes in the utilization of piperacillin-tazobactam, as well as the increasing reports in the literature of associations between administration of vancomycin in combination with piperacillin-tazobactam, this retrospective cohort analysis of patients who received combination therapy with vancomycin and piperacillin-tazobactam was designed. The first objective of this study was to evaluate whether there was an association between administration of piperacillin-tazobactam via an extended infusion in combination with vancomycin and development of AKI. The second objective was to identify risk factors for AKI among patients who received the combination, regardless of the infusion strategy used.
MATERIALS AND METHODS
Study design and setting.
This study consisted of retrospective cohort and nested case-control analyses of patients who received combination therapy with vancomycin and piperacillin-tazobactam at the DMC. The DMC is a tertiary-care health system in metropolitan Detroit, MI, and consists of 5 acute-care hospitals with >2,000 inpatient beds. This study was approved by the institutional review boards of the Detroit Medical Center and Wayne State University.
Study population.
All patients who were admitted to the DMC from 1 December 2011 to 31 December 2013 and who received both vancomycin and piperacillin-tazobactam were screened for inclusion by a review of medical records. Eligible subjects were those who were ≥18 years of age at the time of admission, were admitted to one of the study hospitals, and received combination therapy with vancomycin and piperacillin-tazobactam. Combination therapy was defined as the initiation of both vancomycin and piperacillin-tazobactam within 24 h of one another and coadministration of these agents for ≥48 h. Patients were excluded if they had a baseline serum creatinine level of ≥1.2 mg/dl or if they were receiving renal replacement therapy at the time of the initiation of therapy.
Definition of acute kidney injury.
AKI was assessed by three different definitions in this study: the risk, injury, failure, loss, and end-stage kidney disease (RIFLE) criteria (8), the Acute Kidney Injury Network (AKIN) criteria (9), and the vancomycin consensus guideline definition (6). AKI according to the RIFLE criteria was categorized using the following criteria: risk, defined as a 1.5-fold increase in the serum creatinine level or glomerular filtration rate (GFR) decrease by 25%; injury, defined as a 2-fold increase in the serum creatinine level or GFR decrease by 50%; and failure, defined as a 3-fold increase in the serum creatinine level or GFR decrease by 75%. AKI according to the AKIN criteria was categorized using the following criteria: stage 1 was defined as an increase in the serum creatinine level of 0.3 mg/dl or >50%, stage 2 was defined as an increase in the serum creatinine level of >100%, and stage 3 was defined as an increase in the serum creatinine level of >200%. The definition of AKI according to the vancomycin consensus guidelines was defined as an increase in the serum creatinine level of ≥0.5 mg/dl or >50% increase from baseline, whichever was greater in at least 2 consecutive measurements during the period from the initiation of vancomycin therapy to 72 h after the completion of therapy (6). For the second objective of identifying independent predictors for acute kidney injury, all analyses were completed using the RIFLE criteria as the definition of AKI, with cases meeting at least the risk category and controls not developing AKI.
Variables collected.
Covariates obtained through a review of medical records included admission source; demographics; body mass index (BMI); the presence of comorbidities, including the Charlson comorbidity index (10); severity of sepsis (11); intensive care unit (ICU) stay; mechanical ventilation; relevant laboratory parameters; physician diagnosis of infection; microbiology data; concomitant nephrotoxic agents; administration of a vancomycin loading dose (defined as an initial vancomycin dosage that was greater than the subsequent maintenance dose); and vancomycin trough concentrations. The dose and duration of vancomycin and piperacillin-tazobactam treatments were also captured. Severity of sepsis, ICU stay, and mechanical ventilation were assessed during the period of 2 days before to 2 days after the initiation of combination therapy. Concomitant nephrotoxic agents evaluated included vasopressors, aminoglycosides, colistin, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, and intravenous contrast.
Statistics.
All statistics were calculated using SAS version 9.3 (Cary, NC). Bivariate analyses were conducted using Fisher's exact test and/or chi-square tests for unmatched dichotomous variables, and the Student t test and Wilcoxon rank sum test were used for analysis of continuous variables. Matched analyses of bivariate variables were conducted using conditional logistic regression modeling.
For the first objective, eligible patients were classified into two cohorts based on the administration time of piperacillin-tazobactam. The first was the standard infusion group, in which subjects were administered piperacillin-tazobactam via a 30-min infusion (SI), and the second was the extended-infusion group, in which subjects were administered piperacillin-tazobactam via a 3-h infusion (EI). Patients who received vancomycin in combination with piperacillin-tazobactam via EI were matched at a 1:1 ratio to patients who received vancomycin in combination with piperacillin-tazobactam via SI on the following risk factors for AKI: severity of sepsis at the time of initiation of combination therapy (dichotomized as the presence or absence of either severe sepsis or septic shock [11]), ICU stay at the onset of combination therapy, duration of combination therapy, daily vancomycin dosage, and number of concomitant nephrotoxic agents received. For this objective, patients receiving EI and SI were compared using matched analyses. Variables associated with EI in bivariate analyses (P < 0.10) were included in a multivariable logistic regression model to predict the independent effect of EI on AKI.
For the second objective (to identify risk factors for AKI in the entire patient population, including patients who received piperacillin-tazobactam as either EI or SI), a nested case-control study was performed. For this analysis, cases were defined as those who developed AKI, and controls were defined as those who did not develop AKI. Case and control groups were compared to identify the risk factors associated with acute kidney injury. In order to identify independent overall predictors of acute kidney injury, cases and controls were compared using unmatched bivariate analyses of patient covariates and AKI. Variables with a P value of <0.1 in bivariate analysis were considered for inclusion in the multivariate models. Two types of multivariate models were evaluated for dichotomous outcomes, with the first using logistic regression, and the second using Cox proportional hazards methodology. For all multivariate models, variables with a P value of <0.05 in the final multivariable model were considered significant. The final model was evaluated for confounding by determining if the removal of any covariates with a P value of >0.05 changed the β-coefficient of variables left in the model by >10%. If confounding variables were identified, they were placed in the final model. In order to assess the impact of AKI development on the length of stay, linear regression was also used to identify predictors of length of stay, following log transformation of this variable. The inverse natural log of the regression coefficient for independent predictors of log length of stay was calculated and reported as the fold increase in the length of stay. Attributable length of stay associated with AKI was calculated as follows: fold increase in length of stay with AKI × median length of stay for controls − median length of stay for controls.
A power calculation was performed prior to study initiation. Assuming an AKI rate of 10% in the SI group, a 2-fold increase in AKI rates associated with EI, and an α value of 0.05, it was determined that 157 patients in each group would be required to obtain a statistical power of 80%.
RESULTS
A total of 320 patients who received combination therapy with vancomycin and piperacillin-tazobactam were included in the study. One-hundred sixty patients who received piperacillin-tazobactam via SI were matched to 160 patients who received piperacillin-tazobactam as a 3-h EI. One hundred seventy (53%) patients in the cohort were male, and 116 (32%) patients were white. The mean ± standard deviation age of the cohort was 54.8 ± 16.9 years. In all cases, vancomycin and piperacillin-tazobactam were started as empirical therapy. Thirty-three percent (n = 106) of patients had a physician diagnosis of infection in combination with positive bacterial culture results. Among patients with a diagnosis of infection, the most common diagnosis was skin or soft tissue infection in 40 cases (38%), followed by bone or joint infection in 22 cases (21%) and pneumonia in 15 cases (14%).
Impact of infusion strategy on toxicity.
The baseline characteristics of the SI and EI groups were similar (Table 1). Subjects who received SI were more likely to have been admitted from home (P < 0.01), to have peripheral vascular disease (P < 0.01) and diabetes mellitus (P = 0.01), and have a Gram-positive pathogen isolated (P = 0.04). Rates of AKI, according to the vancomycin consensus guideline, RIFLE criteria, and AKIN, were similar between groups: 28.1% versus 26.9% (P = 0.90), 33.1% versus 32.5% (P = 1.0), and 35.6% versus 35.0% (P = 1.0) for SI and EI, respectively. When controlling for the residual differences between the two groups (presence of diabetes, peripheral vascular disease, admission from home, and a documented Gram-positive infection) in multivariable analysis, there was no association between receipt of piperacillin-tazobactam via extended infusion and RIFLE-defined AKI (odds ratio, 1.00; 95% confidence interval, 0.80 to 1.26).
TABLE 1.
Baseline characteristics of the cohort
| Characteristica | Standard infusion (n = 160) | Extended infusion (n = 160) | P value |
|---|---|---|---|
| Age (mean ± SD) (yr) | 53.8 ± 17.4 | 55.8 ± 16.5 | 0.30 |
| Women | 73 (46) | 77 (48) | 0.66 |
| African-American | 97 (61) | 107 (67) | 0.11 |
| Admission source | <0.01 | ||
| Home | 149 (93) | 130 (81) | |
| Nursing home | 7 (4) | 27 (17) | |
| Other hospital | 4 (3) | 3 (2) | |
| Ht (mean ± SD) (cm) | 170 ± 11 | 171 ± 11 | 0.38 |
| Wt (median [IQR]) (kg) | 79.3 (63.6–97.6) | 78.4 (69.0–95.0) | 0.87 |
| BMI (median [IQR]) (kg/m2) | 26.7 (22.3–33.0) | 26.8 (22.8–31.6) | 0.88 |
| Comorbid conditions | |||
| Peripheral vascular disease | 24 (15) | 8 (5) | <0.01 |
| Chronic pulmonary disease | 45 (28) | 52 (33) | 0.40 |
| Diabetes mellitus | 50 (31) | 30 (19) | 0.01 |
| Charlson comorbidity index (median [IQR]) | 1 (0–3) | 1 (0–2.5) | 0.59 |
| Hospital and infection-related variables | |||
| No sepsis | 29 (18) | 27 (17) | 0.95 |
| Sepsis | 88 (55) | 90 (56) | |
| Severe sepsis | 31 (19) | 33 (21) | |
| Septic shock | 12 (8) | 10 (6) | |
| ICU stay | 34 (21) | 33 (21) | 0.89 |
| Mechanical ventilation | 24 (15) | 25 (16) | 0.88 |
| WBC count at start day of combination therapy (median [IQR]) | 10.6 (7.2–14.4) | 11.6 (8.0–15.5) | 0.14 |
| Baseline creatinine (mean ± SD) (mg/dl) | 0.88 ± 0.21 | 0.84 ± 0.23 | |
| Length of stay before combination therapy (median [IQR]) (days) | 1.0 (1.0–3.5) | 1.0 (1.0–3.0) | 0.14 |
| Infection type and diagnosis | |||
| Physician diagnosis with positive culture | 57 (36) | 49 (31) | 0.34 |
| Pneumonia | 4 (3) | 11 (7) | 0.07 |
| Endocarditis | 4 (3) | 1 (1) | 0.21 |
| Intra-abdominal infection | 5 (3) | 4 (3) | 0.73 |
| Skin/soft tissue infection | 25 (16) | 15 (9) | 0.10 |
| Bone/joint infection | 15 (9) | 7 (4) | 0.09 |
| Urinary tract infection | 1 (1) | 7 (4) | 0.06 |
| Catheter-associated infection | 1 (1) | 2 (1) | 0.57 |
| Other/unknown | 2 (1) | 2 (1) | 1.00 |
| Bacteremia | 18 (11) | 12 (8) | 0.25 |
| Polymicrobial infection | 27 (17) | 19 (12) | 0.27 |
| Pathogens | |||
| Gram-positive bacteria | 45 (28) | 30 (19) | 0.04 |
| Gram-negative bacteria | 21 (13) | 26 (16) | 0.43 |
| MRSA | 8 (5) | 12 (8) | 0.35 |
| MSSA | 14 (9) | 4 (3) | 0.02 |
| Nephrotoxic agent | |||
| No. of nephrotoxins (mean ± SD) | 0.96 ± 0.96 | 0.94 ± 0.90 | 0.81 |
| No. (%) of nephrotoxins | |||
| 0 | 64 (40) | 61 (38) | 0.91 |
| 1 | 49 (31) | 56 (35) | |
| 2 | 38 (24) | 36 (23) | |
| 3 | 7 (4) | 6 (4) | |
| 4 | 2 (1) | 1 (1) | |
| Vasopressors | 10 (6) | 10 (6) | 1.00 |
| Aminoglycoside | 12 (8) | 6 (4) | 0.15 |
| Colistin | 3 (2) | 1 (1) | 0.33 |
| ACEI/ARB | 42 (26) | 40 (25) | 0.79 |
| Diuretics | 42 (26) | 48 (30) | 0.45 |
| Contrast | 45 (28) | 45 (28) | 1.00 |
| Any nephrotoxins | 96 (60) | 99 (62) | 0.73 |
| Vancomycin administration | |||
| Loading doses given (n [%]) | 135 (84) | 134 (84) | 0.87 |
| Loading dose (mg/day) (mean ± SD) | 1,968 ± 498 | 1,896 ± 431 | 0.20 |
| First maintenance dose (mg/day)(mean ± SD) | 3,092 ± 1,409 | 2,945 ± 1,267 | 0.25 |
| Median trough concn (mg/liter [IQR]) (n) | 18.1 (14.1–22.9) (138) | 17.5 (13.1–21.9) (135) | 0.36 |
| Duration of combination therapy (median [IQR]) (days) | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 0.89 |
IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; WBC, white blood cell; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker. Unless otherwise indicated, data are presented as no. (%).
Predictors of AKI in patients receiving concomitant vancomycin and piperacillin-tazobactam.
In the entire cohort, AKI occurred in 105 subjects (32.8%) (Table 2). In bivariate analysis, patients in the AKI group, compared to those without AKI, were younger (P = 0.02) and more likely to have a physician diagnosis of infection in combination with positive culture (P = 0.04), have an invasive infection (i.e., pneumonia, endocarditis, or bone/joint infection) (P = 0.01), have a documented Gram-positive pathogen (P = 0.01), and have received any concomitant nephrotoxic agent (P = 0.007). No association between median vancomycin trough concentration or daily dose of vancomycin and AKI was identified. In a multivariate logistic regression model (Table 3), when controlling for age, race, median vancomycin trough concentration, and the presence of comorbidities, independent predictors for AKI were receipt of a vancomycin loading dose (odds ratio [OR], 2.30; 95% confidence interval [CI], 1.08 to 4.90), receipt of any concomitant nephrotoxin (OR, 2.33; 95% CI, 1.34 to 4.04), and treatment of a documented Gram-positive infection (OR, 2.13; 95% CI, 1.19 to 3.81). When multivariate Cox proportional hazards modeling was completed, the results were similar (data not shown).
TABLE 2.
Bivariate analysis of predictors for acute kidney injury in the total population who received vancomycin and piperacillin-tazobactam combination therapya
| Variableb | Non-AKI (n = 215) | AKI (n = 105) | P value |
|---|---|---|---|
| Age (mean ± SD) (yr) | 56.4 ± 16.6 | 51.6 ± 17.4 | 0.02 |
| Women | 97 (45.1) | 53 (50.5) | 0.40 |
| Race | |||
| White | 76 (35.4) | 27 (25.7) | 0.08 |
| Black | 133 (61.9) | 71 (67.6) | |
| Other | 6 (2.8) | 7 (6.7) | |
| Admission source | |||
| Home | 185 (86.1) | 94 (89.5) | 0.68 |
| Nursing home | 25 (11.6) | 9 (8.6) | |
| Other hospital | 5 (2.3) | 2 (1.9) | |
| Ht (mean ± SD) (cm) | 171.3 ± 11.2 | 170.1 ± 11.5 | 0.36 |
| Wt (median [IQR]) (kg) | 79.4 (66.0–95.0) | 79.0 (66.0–97.0) | 0.62 |
| BMI (median [IQR]) (kg/m2) | 26.9 (22.4–32.4) | 26.8 (23.0–32.9) | 0.42 |
| Comorbid conditions | |||
| AIDS | 3 (1.4) | 0 (0) | 0.55 |
| Myocardial infarction | 13 (6.1) | 2 (1.9) | 0.16 |
| Congestive heart failure | 28 (13.0) | 9 (8.6) | 0.27 |
| Peripheral vascular disease | 24 (11.2) | 8 (7.6) | 0.43 |
| Dementia | 18 (8.4) | 7 (6.7) | 0.66 |
| Chronic pulmonary disease | 66 (30.7) | 31 (29.5) | 0.90 |
| Connective tissue disease | 8 (3.7) | 4 (3.8) | 1.00 |
| Peptic ulcer disease | 3 (1.4) | 0 (0) | 0.55 |
| Chronic kidney disease | 5 (2.3) | 1 (1.0) | 0.67 |
| Leukemia | 2 (0.9) | 1 (1.0) | 1.00 |
| Lymphoma | 1 (0.5) | 0 (0) | 1.00 |
| Malignant solid tumor | 29 (13.5) | 14 (13.3) | 1.00 |
| Cerebrovascular disease | 20 (9.3) | 11 (10.5) | 0.84 |
| Liver disease | 11 (5.1) | 6 (5.7) | 0.80 |
| Diabetes mellitus | 57 (26.5) | 23 (21.9) | 0.41 |
| Hypertension | 125 (58.1) | 44 (41.9) | 0.01 |
| Charlson comorbidity index (median [IQR]) | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 0.09 |
| Score >0 | 155 (72.1) | 64 (61.0) | 0.055 |
| Hospital and infection-related variables | |||
| No sepsis | 43 (20.0) | 13 (12.4) | 0.15 |
| Sepsis | 117 (54.4) | 61 (58.1) | |
| Severe sepsis | 38 (17.7) | 26 (24.8) | |
| Septic shock | 17 (7.9) | 5 (4.8) | |
| Any sepsis | 172 (80.0) | 92 (87.6) | 0.12 |
| Severe sepsis/septic shock | 55 (25.6) | 31 (29.5) | 0.50 |
| ICU stay | 47 (21.9) | 20 (19.1) | 0.66 |
| Mechanical ventilation | 34 (15.8) | 15 (14.3) | 0.87 |
| Length of stay before combination therapy (median [IQR]) (days) | 1.0 (1.0–3.0) | 1.0 (1.0–4.0) | 0.09 |
| Infection type and diagnosis | |||
| Physician diagnosis in combination with positive culture | 63 (29.3) | 43 (41.0) | 0.04 |
| Pneumonia | 8 (3.7) | 7 (6.7) | 0.27 |
| Endocarditis | 2 (0.9) | 3 (2.9) | 0.34 |
| Intra-abdominal infection | 6 (2.8) | 3 (2.9) | 1.00 |
| Skin/soft tissue infection | 27 (12.6) | 13 (12.4) | 1.00 |
| Bone/joint infection | 11 (5.1) | 11 (10.5) | 0.10 |
| Urinary tract infection | 6 (2.8) | 2 (1.9) | 1.00 |
| Catheter-associated infection | 2 (0.9) | 1 (1.0) | 1.00 |
| Other/unknown | 1 (0.5) | 3 (2.9) | 0.11 |
| Invasive infectionc | 21 (9.8) | 21 (20.0) | 0.01 |
| Bacteremia | 17 (7.9) | 13 (12.4) | 0.22 |
| Polymicrobial infection | 27 (12.6) | 19 (18.1) | 0.23 |
| Gram-positive bacteria | 41 (19.1) | 34 (32.4) | 0.01 |
| MRSA | 10 (4.7) | 10 (9.5) | 0.14 |
| MSSA | 10 (4.7) | 8 (7.6) | 0.31 |
| Gram-negative bacteria | 31 (14.4) | 16 (15.2) | 0.87 |
| Pseudomonas aeruginosa | 11 (5.1) | 3 (2.9) | 0.56 |
| Enterobacteriaceae | 18 (8.4) | 8 (7.6) | 1.00 |
| Any nephrotoxins | 120 (55.8) | 75 (71.4) | 0.007 |
| Vasopressors | 17 (7.9) | 3 (2.9) | 0.09 |
| Aminoglycoside | 10 (4.7) | 8 (7.6) | 0.31 |
| Colistin | 2 (0.9) | 2 (1.9) | 0.60 |
| ACEI/ARB | 57 (26.5) | 25 (23.8) | 0.68 |
| Diuretics | 54 (25.1) | 36 (34.3) | 0.11 |
| Contrast | 54 (25.1) | 36 (34.3) | 0.11 |
| Vancomycin administration | |||
| Loading doses given (n [%]) | 176 (81.9) | 93 (88.6) | 0.14 |
| Loading dose (mg/day) (mean ± SD) | 1,932.2 ± 477.1 | 1,933.5 ± 449.3 | 0.98 |
| Mean first maintenance dose (mg/day) (mean ± SD) | 2,992.6 ± 1,285.0 | 3,071.4 ± 1,451.0 | 0.64 |
| Median trough concnd (mg/liter [IQR]) | 17.1 (12.4–21.3) | 17.9 (13.7–23.2) | 0.07 |
| No. (%) of patients with median trough concn (mg/liter) of: | |||
| 0–10 | 24 (11.2) | 5 (4.8) | |
| 10–15 | 37 (17.2) | 23 (21.9) | 0.05 |
| 15–20 | 55 (25.6) | 17 (16.2) | 0.49 |
| >20 | 58 (27.0) | 16 (15.2) | 0.62 |
All cases received vancomycin and piperacillin-tazobactam as empirical therapy. The criteria for AKI were those for RIFLE.
IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; ACEI, angiotensin-converting enzyme inhibitor, ARB, angiotensin II receptor blocker. Unless otherwise indicated, data are presented as no. (%).
Combination of pneumonia, bone/joint infection, and/or endocarditis.
Vancomycin troughs were only considered if they were obtained prior to AKI development. Patients without vancomycin troughs obtained at all during treatment (n = 47 [15%], 41 [19%] patients in the non-AKI group, and 6 [6%] patients in the AKI group) and those who had no troughs obtained prior to AKI (n = 38, all in AKI group [36% of patients in AKI group]) were excluded from trough analyses.
TABLE 3.
Multivariate model for independent predictors of AKI in population who received vancomycin and piperacillin-tazobactam combination therapya
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| Receipt of any concomitant nephrotoxin | 2.33 | 1.19–3.81 |
| Receipt of a vancomycin loading dose | 2.30 | 1.08–4.90 |
| Presence of ≥2 SIRS criteriab | 1.84 | 0.86–3.92 |
| Documented Gram-positive infection | 2.13 | 1.19–3.81 |
Controlled for age, race, median vancomycin trough, and Charlson comorbidity index.
SIRS, systemic inflammatory response syndrome.
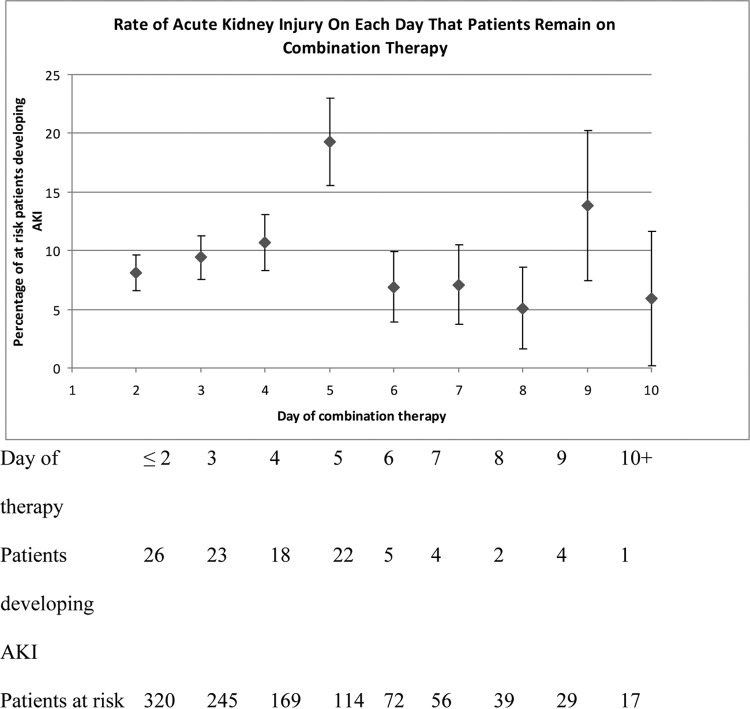
Figure 1 displays the impact of duration of combination therapy on the risk of developing AKI. An increase in AKI rate was seen on days 4 and 5 of combination therapy, with 10.7% and 19.3% of at-risk patients developing AKI on days 4 and 5 of combination therapy, respectively.
FIG 1.
Incidence of AKI as a function of duration of combination therapy. “Patients at risk” consisted of patients receiving combination therapy who had not yet developed AKI.
Impact of AKI on outcomes.
There was an association between the development of AKI and increased length of stay from the onset of combination therapy (median, 7 days; interquartile range, 4 to 11 days in patients who did not develop AKI, versus median, 12 days; interquartile range, 8 to 16 days, in those who did develop AKI) (P < 0.001). In adjusted analysis, after controlling for other differences between patients who developed AKI and those who did not (age, presence of an invasive infection, Charlson score, and concomitant nephrotoxins), the development of AKI was associated with a 1.58-fold increase in length of stay (95% confidence interval, 1.36-fold to 1.83-fold). However, there was no association between the development of AKI and 30-day mortality (4.2% and 7.6%, in patients without and with AKI, respectively; P = 0.56).
DISCUSSION
To the best of our knowledge, this study represents the largest analysis assessing the association between vancomycin and piperacillin-tazobactam combination therapy and development of AKI and is the first that identifies treatment-related factors associated with AKI in this population. The first important finding in this study is that the administration of piperacillin-tazobactam via EI does not increase the AKI risk compared to that with administration via SI. This is an encouraging finding, as EI administration of piperacillin-tazobactam is an increasingly popular stewardship strategy designed to optimize the pharmacodynamic exposures of this agent to improve patient outcomes. This finding suggests that the recently noted increase in AKI incidence among patients receiving combination therapy with vancomycin and piperacillin-tazobactam is not explained by the more frequent use of EI piperacillin-tazobactam. Given the large sample size in this study and the stringent matching criteria between patients receiving EI and SI based on known risk factors for AKI, the findings in this study are particularly robust, and clinicians should feel confident that EI does not increase AKI risk.
The second important finding was the identification of potentially modifiable risk factors for AKI in patients receiving the combination, which provides important opportunities for antimicrobial stewardship programs. In addition to the presence of sepsis and isolation of a Gram-positive pathogen, the two strongest predictors of toxicity in patients on the combination were receipt of a vancomycin loading dose and the receipt of any concomitant nephrotoxin. Both of these variables are potentially modifiable, and careful attention should be given to whether or not concomitant nephrotoxin exposures can be avoided and whether or not a vancomycin loading dose is needed. In our cohort, ∼20% of patients presented with no signs of sepsis, and therefore, the need for a loading dose in these patients is questionable. The vancomycin consensus guidelines state that loading doses should be considered only when patients are “seriously ill” (6).
The third and particularly interesting finding in this study was the association between the duration of combination therapy and the development of AKI. Although 49 out of the total 105 AKI events (47%) in this cohort occurred within the first 72 h, the daily rates of AKI in at-risk patients were <10% on both days 2 and 3 of therapy due to the large number of patients who received combination therapy for a minimum of 2 to 3 days. The daily rates of AKI in at-risk patients increased to 10.7% on day 4 and then sharply increased to 19.3% on day 5. Almost 40% of AKI cases occurred on days 4 and 5 of therapy. This finding presents a striking opportunity for stewardship programs to limit the occurrence of AKI. As all patients in this cohort were started empirically on the combination, appropriately obtained cultures and deescalation of antimicrobial therapy by 72 h (i.e., stopping vancomycin and/or piperacillin-tazobactam) could have potentially prevented a large percentage of AKI events. Thus, in addition to limiting exposures that might drive antimicrobial resistance or the development of superinfections, such as Clostridium difficile, appropriate deescalation in the first 72 h could significantly decrease the incidence of AKI. This is critically important, as in adjusted analyses, the development of AKI was associated with a 58% increase in length of stay, which equates to an ∼4-day increase in the median length of stay.
Despite the robust methodology, there are limitations to this study. The population in this study was not particularly ill. Therefore, the generalizability of these data to a more critically ill population is limited. Additionally, the decision was made a priori to limit the study to patients with normal baseline renal function, and thus, our study excludes patients with baseline renal insufficiency. This decision was made purposefully, as the increased incidence in AKI at our institution was seen in patients who did not have baseline renal insufficiency. Furthermore, it was felt that if infusion strategy was a risk for toxicity, any association would have been less pronounced in those with baseline renal insufficiency, as the increased half-life of piperacillin-tazobactam in these patients would make the concentration-time profiles more similar between the SI and EI groups.
Another important consideration is that when the DMC changed from standard to extended infusions of piperacillin-tazobactam, this also led to a change in the daily dose given. The most common dose utilized in the SI group was 3.375 g every 6 h (received by 79% of patients), whereas in the EI group, the most common dose was higher, at 4.5 g every 6 h (received by 80% of patients). This higher dosing strategy for EI administration led to a disparity in the daily dose of piperacillin-tazobactam between the groups, with the EI group receiving a larger daily dose. However, the lack of association between EI and AKI suggests that this did not impact results. It is also important to note that independent predictors of AKI were evaluated using logistic regression. The reason that this was done was so the impact of the duration of therapy on AKI risk could be specifically analyzed (Fig. 1). In addition, Cox proportion hazards modeling was also performed, and the predictors of AKI were similar.
Despite the lack of association between EI and AKI, it is important to note that in this analysis, the overall rate of AKI in patients on the combination was higher than would have been expected in patients receiving vancomycin alone (2). AKI occurred in 105/320 (33%) patients who received combination therapy with vancomycin and piperacillin-tazobactam, a rate that is similar to that in two studies recently reported in the literature (3, 4). The high rate of AKI reported in these analyses is concerning, particularly considering the frequency with which this combination is administered and the fact that in our analysis, use occurred entirely in the empirical setting. However, it is important to appreciate that it was not the objective of this study to investigate whether combination therapy with vancomycin and piperacillin-tazobactam was associated with higher rates of toxicity than those with vancomycin monotherapy or combination therapy with alternative Gram-negative coverage. Further investigation comparing the effects of the vancomycin and piperacillin-tazobactam combination with either vancomycin monotherapy or vancomycin and cefepime combination therapy is warranted.
The findings in this study present unique opportunities upon which antimicrobial stewardship programs can act. Among patients receiving concomitant vancomycin and piperacillin-tazobactam, modifiable risk factors, including receipt of a vancomycin loading dose and concomitant nephrotoxins, and duration of empirical therapy for >72 h should be targeted to prevent AKI and improve patient outcomes.
ACKNOWLEDGMENTS
K.S.K. is supported by the National Institute of Allergy and Infectious Diseases (Division of Microbiology and Infectious Diseases protocol 10-0065 and grant RO1 1R01AI119446-01). M.J.R. is supported in part by NIAID grant R21 AI109266-01. E.T.M. is supported in part by NIAID grant K01AI099006.
We have no financial conflicts with commercial entities.
REFERENCES
- 1.Magill SS, Edwards JR, Beldavs ZG, Dumyati G, Janelle SJ, Kainer MA, Lynfield R, Nadle J, Neuhauser MM, Ray SM, Richards K, Rodriguez R, Thompson DL, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . 2014. Prevalence of antimicrobial use in U.S. acute care hospitals, May–September 2011. JAMA 312:1438–1446. doi: 10.1001/jama.2014.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgess LD, Drew RH. 2014. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 34:670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 3.Gomes DM, Smotherman C, Birch A, Dupree L, Della Vecchia BJ, Kraemer DF, Jankowski CA. 2014. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy 34:662–669. [DOI] [PubMed] [Google Scholar]
- 4.Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. 2014. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect 20:O384–O389. doi: 10.1111/1469-0691.12410. [DOI] [PubMed] [Google Scholar]
- 5.Meaney CJ, Hynicka LM, Tsoukleris MG. 2014. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy 34:653–661. [DOI] [PubMed] [Google Scholar]
- 6.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP Jr, Lomaestro B, Drusano GL. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 44:357–363. doi: 10.1086/510590. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup . 2004. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network . 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup . 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. [DOI] [PubMed] [Google Scholar]