Abstract
The increasing global burden of multidrug-resistant tuberculosis (MDR-TB) requires reliable drug susceptibility testing that accurately characterizes susceptibility and resistance of pathogenic bacteria to effectively treat patients with this deadly disease. Delamanid is an anti-TB agent first approved in the European Union in 2014 for the treatment of pulmonary MDR-TB in adults. Using the agar proportion method, delamanid MIC was determined for 460 isolates: 316 from patients enrolled in a phase 2 global clinical trial, 76 from two phase 2 early bactericidal activity trials conducted in South Africa, and 68 isolates obtained outside clinical trials (45 from Japanese patients and 23 from South African patients). With the exception of two isolates, MICs ranged from 0.001 to 0.05 μg/ml, resulting in an MIC50 of 0.004 μg/ml and an MIC90 of 0.012 μg/ml. Various degrees of resistance to other anti-TB drugs did not affect the distribution of MICs, nor did origin of isolates from regions/countries other than South Africa. A critical concentration/breakpoint of 0.2 μg/ml can be used to define susceptible and resistant isolates based on the distribution of MICs and available pharmacokinetic data. Thus, clinical isolates from delamanid-naive patients with tuberculosis have a very low MIC for delamanid and baseline resistance is rare, demonstrating the potential potency of delamanid and supporting its use in an optimized background treatment regimen for MDR-TB.
INTRODUCTION
Tuberculosis (TB) continues to be a major source of morbidity and mortality worldwide, with an estimated 9.6 million new cases and 1.5 million deaths reported in 2014 (1). Multidrug-resistant TB (MDR-TB), bacteria resistant to the two key drugs isoniazid and rifampin, complicates efforts in TB control, making up 3.3% of new cases and 20% of previously treated cases. MDR-TB requires longer and more toxic treatment regimens in comparison to treating drug-susceptible TB, and has a lower success rate (50%) as reported by the World Health Organization (WHO) (1). Even more deadly is extensively drug-resistant TB (XDR-TB), which comprises an estimated 9.7% of MDR-TB cases. This form of MDR-TB has additional resistance to at least one of the fluoroquinolones and at least one of the three second-line injectable drugs, amikacin, kanamycin, or capreomycin (1), dramatically limiting treatment options for infected patients.
Delamanid (OPC-67683; Deltyba) is from the nitro-dihydro-imidazooxazole class of compounds that inhibits the synthesis of key mycolic acids. It showed potent in vitro and in vivo activity against both drug-susceptible and drug-resistant strains of Mycobacterium tuberculosis in preclinical development (2) and in an early bactericidal activity trial (3). Trial 204 (ClinicalTrials.gov NCT00685360), a large, global, phase 2 clinical trial comparing delamanid to placebo in combination with an optimized background regimen (OBR) for pulmonary MDR-TB patients, demonstrated significant improvement of sputum culture conversion with 2 months of delamanid treatment (4). Analysis of follow-up data from these patients in an extension trial (ClinicalTrials.gov NCT02573350) revealed that 6 months or more of treatment with delamanid plus OBR was associated with higher favorable treatment outcomes compared with ≤2 months of treatment with delamanid (including initial placebo group) plus OBR (74.5% versus 55.0%, P < 0.001) (5), and significantly lower mortality (2.9% versus 12.0%, P = 0.001) (6), even among patients with XDR-TB (7).
In April 2014, marketing authorization for delamanid was granted by the European Commission for treatment of pulmonary MDR-TB in adult patients in the European Union. The Japanese and Korean regulatory authorities granted approval in July 2014 and October 2014, respectively.
A key characteristic of any antimicrobial agent is the distribution of MICs of that agent for its target organisms. The MIC is a fundamental property of the agent, representing its potency, and distribution of MICs can contribute to an interpretation of whether target organisms are susceptible or resistant to that agent. The Clinical and Laboratory Standards Institute (CLSI) provides definitions of “susceptible” as “a category that implies that isolates are inhibited by an antimicrobial agent at concentrations that are usually attained (i.e., the concentration-time profile of the antimicrobial agent is favorable) when the recommended dosage is used” and “resistant” as “a category for isolates that are not inhibited by the usually achievable concentrations of the agent with normal dosage regimens” (8). Therefore, the combination of the distribution of the MICs and the pharmacokinetic (PK) properties of the agent is ideal for defining the critical concentration that separates susceptibility and resistance.
The objectives of this paper are to (i) define delamanid-susceptible and -resistant populations of M. tuberculosis using recently generated MIC data from 460 clinical isolates and (ii) propose a critical concentration suggested by the distribution of MICs and the plasma concentrations achieved by the marketed dose of 100 mg twice daily (BID).
MATERIALS AND METHODS
Clinical isolates and testing laboratories.
Three laboratories contributed data for this study. (i) The Otsuka Microbiological Research Institute (MRI) in Tokushima, Japan, tested a total of 48 isolates from Japanese MDR- and XDR-TB patients collected from 2007 to 2012: these included 25 isolates provided by the Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association (Tokyo, Japan), a WHO supranational reference laboratory and 23 isolates provided by Miroku Medical Laboratory Co., Ltd. (Nagano, Japan), a laboratory certified by the Research Institute of Tuberculosis to perform susceptibility testing. (ii) The Department of Medical Biochemistry at Stellenbosch University in Tygerberg, South Africa, determined MICs for isolates from 76 patients enrolled in two phase 2 early bactericidal activity (EBA) studies of delamanid. The first study, trial 102, was conducted in 2005 with an early formulation of delamanid. The second trial in 2006, trial 101 (ClinicalTrials.gov NCT00401271), used the current marketed formulation of delamanid, and the methods and results for this trial have been described (3). Under contract with Otsuka, the Stellenbosch University laboratory also tested 43 isolates from South African TB patients from their strain library, with various susceptibility patterns (Otsuka, unpublished data). (iii) The Public Health Research Institute Tuberculosis Center at Rutgers University, New Jersey Medical School, Newark, NJ (PHRI), tested baseline isolates from 337 patients enrolled between 2008 and 2010 in the Otsuka phase 2 clinical trial 204 (4). Isolates were tested in batches at each laboratory, thawed from frozen stock, subcultured on either liquid or solid media, and checked for purity prior to use.
All clinical trial protocols were approved by independent ethics committees and institutional review boards for all sites. All patients provided written informed consent before enrollment occurred. The trials were performed in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonization and adhered to the ethical principles of the Declaration of Helsinki.
Subspecies identification using standard methods based on PCR amplification for regions of difference and other polymorphisms (9, 10) was performed for trial 102, trial 101, and trial 204 isolates. Japanese isolates were identified as M. tuberculosis by the suppliers. South African isolates were determined to be M. tuberculosis by Stellenbosch University.
Determination of MIC.
Delamanid powder was provided by Otsuka Pharmaceutical Co., Ltd. (Tokushima, Japan), and was dissolved in dimethyl sulfoxide (DMSO) to prepare a range of drug concentrations. MICs were determined by the proportion method. Middlebrook 7H10 or 7H11 agar media, containing 0.5% glycerol and 10% oleic acid-albumin-dextrose-catalase (OADC) enrichment, was prepared according to the manufacturer's instructions, to which the appropriate delamanid drug solution (or DMSO for control plates) was added before dispensing into divided plates. The plates were dried for at least 1 h inside a biosafety cabinet and used on the same day of preparation.
A range of 2-fold dilutions of delamanid were tested in each study. Since MICs were expected to be very low for isolates never exposed to delamanid, a low range of delamanid concentrations were examined first. The higher range of concentrations was tested only in the event an MIC value could not be obtained using the low range. The ranges tested for each collection of isolates were as follows: Japanese collection and trial 204, 0.001 to 8 μg/ml; South African collection, 0.00625 to 12.8 μg/ml; trial 102, 0.003 to 25 μg/ml; and trial 101, 0.006 to 25 μg/ml.
Inocula were prepared from isolates grown on 7H11 agar media, on Lowenstein-Jensen (LJ) slants, or in 7H9 broth. The bacterial suspensions were then diluted to an estimated 107 CFU/ml, from which serial dilutions (100-, 1,000-, and 10,000-fold) were prepared, plated on drug-free medium and delamanid-containing medium (100 μl), sealed to prevent dehydration, and incubated at 37°C for up to 6 weeks.
The MIC value was reported as the lowest plate concentration for which the growth on the drug-containing plate was ≤1% that of the drug-free plate. (The South African clinical isolates study, trial 102, and trial 101 reported MIC values for which resistance was <1%.)
Quality control.
Each batch of agar media was tested for sterility in parallel with test isolates, and tested for performance using the quality control (QC) organism M. tuberculosis H37Rv (American Type Culture Collection, catalog no. 25618). The organism was prepared in parallel with and according to the exact procedures as the test isolates in each batch, and an acceptable MIC range (determined from nonclinical studies) was defined as 0.002 to 0.016 μg/ml.
Pharmacokinetic evaluation.
Blood samples were collected from all trial 204 patients for determination of delamanid plasma concentrations, and the area under the curve during 24 h (AUC0–24), minimum concentration (Cmin), and maximum concentration (Cmax) were determined as described previously (4). Delamanid concentrations in plasma were determined using ultrahigh performance liquid chromatographic-tandem mass spectrometry (11). (The full protocol of trial 204 can be found at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1112433/suppl_file/nejmoa1112433_protocol.pdf.)
RESULTS
The source, disposition, and resistance phenotypes of all isolates are shown in Fig. 1.
FIG 1.
Source, disposition, and resistance phenotypes of isolates collected for delamanid MIC. *, isolates either did not grow on subculture (n = 32), the MIC test failed (n = 5), the isolates were not tested due to clerical errors (n = 4), the isolates were not determined to be MTB (n = 1), or the MIC was indeterminate (n = 1). DS-TB, fully drug-susceptible TB; MDR-TB, isolates with resistance to isoniazid and rifampin; XDR-TB, MDR-TB plus resistance to a fluoroquinolone and a second-line injectable drug; Other TB, includes monoresistant TB (resistant to only one drug) and polyresistant TB (resistant to multiple drugs but does not meet the definition of MDR-TB).
Delamanid MIC determination and distribution.
The QC organism H37Rv was tested once with each batch of patient isolates and generated very reproducible results in this study, with MIC values between 0.002 and 0.012 μg/ml (n = 44; mean, median, and mode = 0.004 μg/ml).
A total of 460 of the 503 (91%) available isolates were subcultured and successfully tested for delamanid MIC. Clinical isolates from 48 Japanese patients were tested at the MRI laboratory, 45 (94%) of which gave delamanid MIC values. All 76 enrolled patients from the EBA trials in South Africa that provided sputum grew isolates that were confirmed as M. tuberculosis and produced delamanid MIC values. Isolates from 43 South African patients were subcultured at Stellenbosch University laboratory, of which 23 (53%) grew and were tested for delamanid MIC. A total of 337 isolates from patients participating in trial 204 were tested at PHRI. These isolates were received from Egypt, Estonia, Japan, Latvia, Peru, the Philippines, South Korea (Seoul), and the United States; isolates from patients enrolled in China and Masan, South Korea, were not shipped to PHRI, owing to regulatory restrictions. Of these isolates, 316 (94%) were confirmed as M. tuberculosis and generated delamanid MIC values. All isolates from the clinical trials are from baseline sputum samples taken prior to patients receiving delamanid or the comparator treatment in each trial.
The overall MIC distribution for delamanid for the combined 460 isolates is shown by isolate source in Fig. 2. With the exception of two isolates, MICs ranged from 0.001 to 0.05 μg/ml, with a median and mode at 0.004 μg/ml, an MIC95 of 0.012 μg/ml, and an MIC99 of 0.031 μg/ml. Two isolates had higher MIC values, one at 1 μg/ml (from Egypt) and another with an MIC value of >8 μg/ml (from Korea). Thus, two distinct populations were seen. The population with lower MICs (n = 458; range, 0.001 to 0.05 μg/ml) are all at least two dilutions below 0.2 μg/ml, whereas the two isolates with MICs in the higher group (range 1 to >8 μg/ml) are at least two dilutions higher than 0.2 μg/ml, suggesting that 0.2 μg/ml is an appropriate concentration to separate these two groups.
FIG 2.
Delamanid MIC distribution (μg/ml) from 460 clinical isolates. *, MICs of 0.006 and ≤0.006 μg/ml were combined in this figure. See Table S1 in the supplemental material for source data.
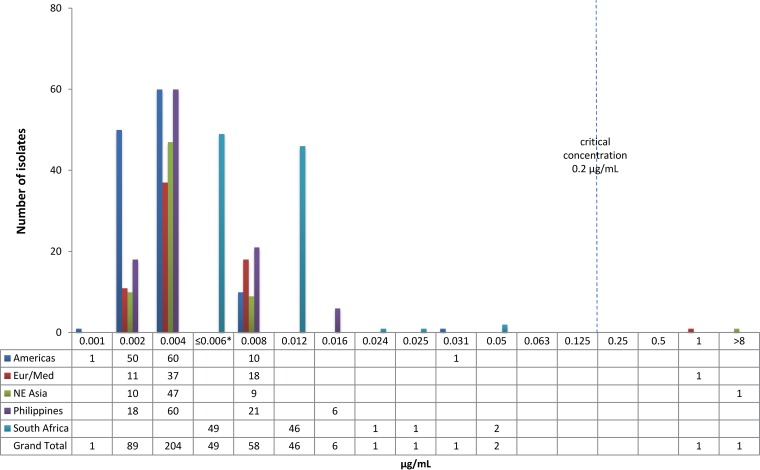
The distribution of MICs from isolates in the different geographic regions/countries—the Americas (Peru, United States), Europe/Mediterranean (Estonia, Latvia, Egypt), Northeast Asia (Japan, South Korea), Philippines, and South Africa—is shown in Fig. 3. MIC distributions by individual country can be found in Table S1 in the supplemental material. Figure 4 shows the distribution of MICs from isolates characterized by the degree of resistance to other anti-TB drugs: drug-sensitive TB (DS-TB), MDR-TB, XDR-TB, and “other” (resistant to some anti-TB drugs but not MDR-TB). The majority of isolates from South Africa are DS-TB isolates from the two EBA trials, and all but one of the DS-TB isolates in the collection are from South Africa. Excluding the two isolates with elevated MICs, the median MIC for all regions, except South Africa, and all drug resistance categories, except DS-TB, is 0.004 μg/ml. The median MIC for the South African and the DS-TB isolates (largely the same isolates) is 0.012 μg/ml, raising the possibility of a higher MIC for South African or DS-TB isolates.
FIG 3.
Delamanid MIC distribution (μg/ml) by geographic region/country. *, MICs of 0.006 and ≤0.006 μg/ml were combined in this figure. See Table S1 in the supplemental material for source data. The “Europe/Mediterranean” (Eur/Med) region consists of Estonia, Latvia, and Egypt. The “Northeast Asia” (NE Asia) region consists of Japan and South Korea. The “Americas” region consists of Peru and the United States.
FIG 4.
Delamanid MIC distribution (μg/ml) by resistance phenotype. *, MICs of 0.006 and ≤0.006 μg/ml were combined in this figure. See Table S1 in the supplemental material for source data. DS-TB, fully drug-susceptible TB; MDR-TB, isolates with resistance to isoniazid and rifampin; XDR-TB, MDR-TB plus resistance to a fluoroquinolone and a second-line injectable drug; Other TB, includes monoresistant TB (resistant to only one drug) and polyresistant TB (resistant to multiple drugs but does not meet the definition of MDR-TB).
Pharmacokinetic results from trial 204.
The results of pharmacokinetic testing in trial 204 have been described previously (4). Data from patients receiving the now-approved dose of 100 mg BID (twice daily) are presented here. At day 56 of the trial (after steady state had been achieved), following a 100-mg dose, the mean Cmax was 0.4 μg/ml, the mean Cmin was 0.3 μg/ml, and the mean AUC0–24 was 7.9 h·μg/ml (4). Relative to the provisional critical concentration of 0.2 μg/ml, additional analysis of the PK data demonstrated that, at steady state and following a 100 mg BID dose, 95.8% of patients receiving delamanid at 100 mg BID had a Cmax ≥ 0.2 μg/ml and 79.8% had a Cmin ≥ 0.2 μg/ml.
DISCUSSION
The MIC parameter represents the potency of a particular antimicrobial agent, and the generation of MIC data against relevant clinical isolates is necessary to establish the critical concentration for susceptibility of new anti-TB drugs. This study determined delamanid MIC values for 460 MTB clinical isolates from geographically diverse populations, mostly from MDR-TB patients. Excluding two isolates with elevated MICs of 1 μg/ml and >8 μg/ml, MIC values ranged between 0.001 and 0.05 μg/ml, with a median and mode at 0.004 μg/ml, an MIC50 of 0.004 μg/ml, and an MIC90 of 0.012 μg/ml. There was no difference noted in the distribution in most regions/countries or for patients with drug-resistant isolates. A difference could not be excluded for isolates from patients with drug susceptible tuberculosis, all but one of whom were from South Africa. This difference could represent a biological difference in drug-susceptible isolates, a geographic difference in isolates from South Africa, or could reflect a difference in laboratory procedures. The ongoing delamanid phase 3 Trial 213 (ClinicalTrials.gov NCT01424670) has recruited MDR-TB patients from South Africa, and determining the MICs for isolates obtained in this trial could provide more insight into potential geographic variations.
The distribution of MICs from clinical isolates collected from delamanid-naive patients fall into two distinct populations, one with 458 MICs ranging from 0.001 to 0.05 μg/ml and another with two MICs ranging from 1 to >8 μg/ml. Looking at the delamanid MIC data alone, one may suggest an epidemiological cutoff value (ECOFF; the MIC value identifying the upper limit of the wild-type population) (12) of 0.05 μg/ml. However, both the CLSI and the U.S. Food and Drug Administration recommend the analysis of PK data to provide a more complete clinical picture for defining the critical concentration of an agent (8, 13).
An MIC of 0.2 μg/ml meets the definition of a critical concentration in that it (i) clearly divides the observed MICs for M. tuberculosis into two separate and distinct populations, and (ii) it has clinical relevance based on the PK data from trial 204, since >95% of patients receiving delamanid (100 mg BID) had peak delamanid concentrations of >0.2 μg/ml, and ca. 80% had a minimum concentration of >0.2 μg/ml at steady state.
Delamanid requires activation by the target organism through the F420 coenzyme-dependent bioreduction pathway (2), and mutations in one of the five genes that are part of this activation mechanism—ddn (Rv3547), fgd1 (Rv0407), fbiA (Rv3261), fbiB (Rv3262), and fbiC (Rv1173)—are known to cause resistance to nitroimidazoles (14, 15). Two baseline isolates from trial 204 had higher MIC values (see Fig. 2). Gene sequencing revealed nonsynonymous mutations in the ddn genes of both isolates (M. Fujiwara, unpublished data). One other report of a naturally delamanid-resistant isolate has been described by Doi and Disratthakit (16). This otherwise drug-susceptible isolate had a delamanid MIC of >100 μg/ml and was also resistant to PA-824, another nitroimidazole currently in tuberculosis clinical trials. Gene sequencing was not performed on this isolate, and it is unknown whether the patient received prior nitroimidazole therapy.
There are several limitations to this analysis. First, data have been pooled from multiple studies, and testing was performed in different laboratories using slightly different protocols. Nevertheless, the range of MICs is consistently low, with 458/460 (99.6%) of the isolates having a delamanid MIC of ≤0.05 μg/ml, and QC was performed with each assay. Second, pharmacodynamic (PD) data to complement the MIC and PK data were not available and may have provided a more robust assessment of the appropriate critical concentration. PD data are also being collected in trial 213, which randomizes patients to receive either 6 months of delamanid or placebo, plus OBR, for the treatment of MDR-TB, with patient follow-up for 30 months. Further analysis of this additional PK/PD data may enrich the analysis of the critical concentration.
Another limitation is that, while the isolates for this analysis come from a wide range of geographic areas and likely represent a wide range of lineages of M. tuberculosis, genotypic analysis to identify those lineages was beyond the scope of this project. Similarly, whole-genome sequencing of the isolates was outside the scope of this analysis and may have identified gene sequences that explain small variations in MICs.
In conclusion, delamanid has a very low MIC in clinical isolates obtained from geographically diverse populations and against DS-TB, drug-resistant TB, MDR-TB, and XDR-TB strains. Naturally occurring resistance to delamanid would appear to be rare (<1%), but adherence to the recent WHO recommendation that delamanid may be added to a well-constructed MDR-TB treatment regimen will be necessary to prevent the emergence of additional resistance (17, 18). Further studies of MIC values from additional clinical trial isolates, and genetic characterization of isolates with high MIC values, along with PK/PD analysis, will provide additional insight into the proposed use of a critical concentration of 0.2 μg/ml and the genetic basis for resistance.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the patients, principal investigators, and laboratory managers and staff that participated in trials 204, 102, and 101. We also acknowledge Stellenbosch University, Miroku Medical Laboratory Co., Ltd., and the Research Institute of Tuberculosis for providing clinical isolates for this research.
Funding Statement
All of the authors were employees of or provided contract services to Otsuka Pharmaceuticals, Inc., and all of the trials were funded by Otsuka. There was no additional grant funding from any other agency.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03014-15.
REFERENCES
- 1.World Health Organization. 2015. Global tuberculosis report. Document WHO/HTM/TB/2015.22. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, Geiter LJ, Wells CD, Paccaly AJ, Donald PR. 2011. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis 15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 4.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, Suh GY, Danilovits M, Ogata H, Kurve A, Chang J, Suzuki K, Tupasi T, Koh WJ, Seaworth B, Geiter LJ, Wells CD. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 5.Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, Cirule A, Leimane V, Kurve A, Levina K, Geiter LJ, Manissero D, Wells CD. 2013. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 41:1393–1400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells CD, Gupta R, Hittel N, Geiter LJ. 2015. Long-term mortality assessment of multidrug-resistant tuberculosis patients treated with delamanid. Eur Respir J 45:1498–1501. doi: 10.1183/09031936.00176314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta R, Geiter LJ, Wells CD, Gao M, Cirule A, Xiao H. 2015. Delamanid for extensively drug-resistant tuberculosis. N Engl J Med 373:291–292. doi: 10.1056/NEJMc1415332. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2008. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 3rd ed Document M23-A3. CLSI, Wayne, PA. [Google Scholar]
- 9.Parsons LM, Brosch R, Cole ST, Somoskovi A, Loder A, Bretzel G, Van Soolingen D, Hale YM, Salfinger M. 2002. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J Clin Microbiol 40:2339–2345. doi: 10.1128/JCM.40.7.2339-2345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren RM, Gey van Pittius NC, Barnard M, Hesseling A, Engelke E, de Kock M, Gutierrez MC, Chege GK, Victor TC, Hoal EG, van Helden PD. 2006. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int J Tuberc Lung Dis 10:818–822. [PubMed] [Google Scholar]
- 11.Meng M, Smith B, Johnston B, Carter S, Brisson J, Roth SE. 2015. Simultaneous quantitation of delamanid (OPC-67683) and its eight metabolites in human plasma using UHPLC–MS/MS. J Chromatogr B 1002:78–91. doi: 10.1016/j.jchromb.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 12.Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Odenholt I, Rodloff A, Soussy CJ, Steinbakk M, Soriano F, Stetsiouk O. 2006. European Committee on Antimicrobial Susceptibility Testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect 12:501–503. doi: 10.1111/j.1469-0691.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services/Food and Drug Administration Center for Drug Evaluation and Research. 2009. Guidance for industry: microbiological data for systemic antibacterial drug products: development, analysis, and presentation. HHS/FDA, Rockville, MD. [Google Scholar]
- 14.European Medicines Agency. 2013. Assessment report: Deltyba. European Medicines Agency, London, England. [Google Scholar]
- 15.Haver HL, Chua A, Ghode P, Lakshminarayana SB, Singhal A, Mathema B, Wintjens R, Bifani P. 2015. Mutations in genes for the F420 biosynthetic pathway and a nitroreductase enzyme are the primary resistance determinants in spontaneous in vitro-selected PA-824-resistant mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:5316–5323. doi: 10.1128/AAC.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi N, Disratthakit A. 2006. Characteristic anti-mycobacterial spectra of the novel anti-TB drug candidates OPC-67683 and PA-824, poster F1-1377a. Int Chemother Antimicrob Agents Chemother (ICAAC). American Society for Microbiology, Washington, DC. [Google Scholar]
- 17.World Health Organization. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Document WHO/HTM/TB/2014.11. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf. [PubMed] [Google Scholar]
- 18.World Health Organization. 2014. The use of delamanid in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Document WHO/HTM/TB2014.23. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.