Abstract
The echinocandins and liposomal amphotericin B are active against biofilm produced by echinocandin-susceptible Candida strains. However, few data have been reported on the production of biofilm by echinocandin-resistant isolates and their antifungal susceptibility. We studied the production of biofilm by fks mutant Candida strains and intrinsically echinocandin-resistant non-Candida isolates and the susceptibility of both entities to liposomal amphotericin B and echinocandins. We analyzed the production of biofilm by isolates from patients with fungemia (fks mutant Candida, n = 5; intrinsically echinocandin-resistant non-Candida, n = 12; and Candida wild type, n = 10). Biofilm formation was measured to classify strains according to biomass (crystal violet assay) and metabolic activity (XTT reduction assay). Preformed biofilms were tested against liposomal amphotericin B, caspofungin, micafungin, and anidulafungin. The sessile MIC was defined as the antifungal concentration yielding a 50% or 80% reduction in the metabolic activity of the biofilm compared to that of the growth control (SMIC50 and SMIC80, respectively). fks mutant Candida isolates formed biofilms in a fashion similar to that of Candida wild-type strains. The echinocandins had the highest activity against biofilms formed by wild-type Candida isolates, followed by fks mutant Candida isolates and non-Candida isolates. Liposomal amphotericin B had the highest activity against fks mutant Candida biofilms. The formation of biofilm by echinocandin-resistant strains was similar to that of wild-type strains, although resistance to echinocandins remained high.
INTRODUCTION
Biofilm production allows Candida spp. to attach to catheters and other bioprosthetic devices, leading to catheter-related candidemia and complicating the management of patients with invasive candidiasis (1). Candida biofilms are difficult to eradicate because they are resistant to many antifungal agents and host immune mechanisms. In addition, associated infections increase morbidity and mortality rates (1–4). Echinocandins and liposomal amphotericin B are active against biofilm (5, 6), and patients with fungemia receiving these antifungal agents may not need early catheter removal (7).
Echinocandin resistance is increasingly frequent and complicates the management of patients with candidemia caused by C. glabrata (8). Resistance to echinocandins in Candida spp. is a consequence of mutations in the fks gene family (9–11). Other non-Candida species, such as Trichosporon, Rhodotorula, and Saccharomyces, rarely cause fungemia, although they are intrinsically resistant to echinocandins. Compared with wild-type Candida isolates, fks mutant Candida isolates and intrinsically echinocandin-resistant non-Candida isolates are thought to form biofilms that are highly resistant to echinocandins. In contrast, biofilms may be susceptible to liposomal amphotericin B regardless of their susceptibility to echinocandins. Only two studies have proved that fks mutant Candida isolates are able to form biofilms; however, very few isolates were tested (12) or only fks mutant C. albicans isolates were characterized (13). Little is known about the antifungal susceptibility of the biofilms formed by isolates belonging to non-albicans Candida species or intrinsically echinocandin-resistant non-Candida species to echinocandins or liposomal amphotericin B.
We characterized biofilm production, measured in terms of biomass production and metabolic activity, in clinical wild-type Candida, fks mutant Candida, and intrinsically echinocandin-resistant non-Candida strains. We also studied the antifungal susceptibility of these strains to echinocandins and liposomal amphotericin B.
(Results from this study were presented at the 7th Congress on Trends in Medical Mycology in Lisbon, Portugal, 2015 [14].)
MATERIALS AND METHODS
Organisms and identification.
We studied 5 previously reported fks mutant Candida isolates (Candida albicans, n = 1; Candida glabrata, n = 1; Candida tropicalis, n = 3) (15, 16) (Table 1) and 10 wild-type Candida species isolates, 2 for each fks mutant isolate, matching the biofilm profile and species of the mutant isolates (C. albicans, n = 2; C. glabrata, n = 2; C. tropicalis, n = 6). In addition, we analyzed 12 intrinsically echinocandin-resistant non-Candida species (Rhodotorula mucilaginosa, n = 7; Trichosporon asahii, n = 2; Trichosporon japonicum, n = 1; Trichosporon dermatis, n = 1; Arxula adeninivorans, n = 1). All isolates were obtained from positive blood cultures of patients with fungemia and were identified using the ID 32C system (bioMérieux, Marcy l'Etoile, France) and confirmed by amplification and sequencing of the ITS1-5.8S-ITS2 region (15, 16).
TABLE 1.
Formation of biofilm by fks mutant Candida isolates (n = 5) and intrinsically echinocandin-resistant non-Candida isolates (n = 12)a
| fks mutation or resistance status | Species | Biofilm biomass (classification) | Biofilm metabolic activity (classification) |
|---|---|---|---|
| F641S fks1 | C. albicans | 1.16 (MBF) | 0.19 (MMA) |
| Δ649 fks2 | C. glabrata | 0.03 (LBF) | 0.20 (HMA) |
| S645F fks1 | C. tropicalis | 1.84 (HBF) | 0.17 (MMA) |
| F641L fks1 | C. tropicalis | 0.77 (MBF) | 0.15 (MMA) |
| R647G fks1 | C. tropicalis | 2.33 (HBF) | 0.14 (MMA) |
| Wild type | C. albicans | 0.47 (MBF) | 0.18 (MMA) |
| Wild type | C. albicans | 0.86 (MBF) | 0.16 (MMA) |
| Wild type | C. glabrata | 0.16 (LBF) | 0.24 (HMA) |
| Wild type | C. glabrata | 0.25 (LBF) | 0.21 (HMA) |
| Wild type | C. tropicalis | 0.94 (MBF) | 0.13 (MMA) |
| Wild type | C. tropicalis | 1.04 (MBF) | 0.10 (MMA) |
| Wild type | C. tropicalis | 1.98 (HBF) | 0.17 (MMA) |
| Wild type | C. tropicalis | 1.75 (HBF) | 0.16 (MMA) |
| Wild type | C. tropicalis | 1.92 (HBF) | 0.14 (MMA) |
| Wild type | C. tropicalis | 2.32 (HBF) | 0.18 (MMA) |
| Intrinsically resistant | A. adeninivorans | 0.05 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.05 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.13 (LBF) | 0.03 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.13 (LBF) | 0.03 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.06 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.09 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.09 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | R. mucilaginosa | 0.06 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | T. asahii | 0.11 (LBF) | 0.01 (LMA) |
| Intrinsically resistant | T. asahii | 0.08 (LBF) | 0.03 (LMA) |
| Intrinsically resistant | T. dermatis | 0.29 (LBF) | 0.02 (LMA) |
| Intrinsically resistant | T. japonicum | 0.54 (MBF) | 0.13 (MMA) |
The formation of biofilm by fks mutant Candida isolates and intrinsically echinocandin-resistant non-Candida isolates was studied using biomass production (crystal violet) and biofilm metabolic activity (XTT reduction). The biofilms are classified according to our previously reported scores (17).
Biofilm formation.
Biofilm was formed according to our previously reported methodology (17). Briefly, a loopful of cultured yeasts was inoculated in 20 ml of yeast-peptone-dextrose broth (Difco, Becton Dickinson, Madrid, Spain) and incubated overnight at 30°C. After centrifugation, cells were resuspended in 20 ml of phosphate-buffered saline (PBS) and centrifuged at 3,000 × g for 5 min for washing. This procedure was performed twice, and washed cells were resuspended in 10 ml of RPMI 1640 broth medium. The suspension was adjusted to 1 × 106 cells/ml, 100 μl of the suspension was inoculated in 96-well trays and incubated for 24 h at 37°C, and biofilm was quantified by following two procedures: (i) crystal violet staining to assess biofilm production (18) and (ii) the XTT reduction assay to assess the metabolic activity of the biofilm (19). Strains were tested in triplicate and classified based on our previously reported score (17) (i.e., according to the biomass production obtained by crystal violet assay) as low biofilm forming (LBF; <0.44), moderate biofilm forming (MBF; 0.44 to 1.17), and high biofilm forming (HBF; >1.17). Strains were also classified based on their metabolic activity using the XTT reduction assay as low metabolic activity (LMA; <0.097), moderate metabolic activity (MMA; 0.097 to 0.2), and high metabolic activity (HMA; >0.2).
Antifungal susceptibility.
The antifungal activity of anidulafungin (Pfizer Pharmaceutical Group, New York, NY, USA), caspofungin (Merck & Co., Inc., Rahway, NJ, USA), micafungin (Astellas Pharma, Inc., Tokyo, Japan), and liposomal amphotericin B (AmBisome Gilead Sciences) was assessed against planktonic yeasts and biofilms using the EUCAST EDEf 7.2 procedure and the XTT reduction assay, respectively (20, 21). Echinocandins were obtained from standard powders and the concentrations prepared according to the potency of the drug. Liposomal amphotericin B was prepared according to the manufacturer's instructions. Briefly, 12 ml of distilled water was added to a vial containing 50 mg of liposomal amphotericin B to obtain a solution with an amphotericin B concentration equal to 4,000 μg/ml. The filtered solution was subsequently diluted in distilled water to a final stock solution of 1,280 μg/ml.
Preformed biofilms were treated with increasing 2-fold concentrations of the four drugs, ranging from 0.015 μg/ml to 16 μg/ml, and plates were incubated at 37°C for 24 h. After the antifungal was removed, a solution comprising 0.5 mg/ml XTT and 0.1 mM menadione (Sigma-Aldrich, Madrid, Spain) was added, and the plates were incubated in darkness for 2 h. The XTT solution was read spectrophotometrically at 490 nm (Multiskan FC microplate photometer; Thermo Scientific, Madrid, Spain). The sessile MIC50 (SMIC50) and MIC80 (SMIC80) were defined as a 50% and 80% reduction, respectively, in the metabolic activity of the biofilm treated with the antifungal compared to activity in the control well.
SEM.
For scanning electron microscopy (SEM), we studied the biofilm structure of the fks mutant Candida isolates (n = 5), intrinsically echinocandin-resistant non-Candida isolates (n = 3), and Candida wild-type strains (n = 4). The wild-type strains were chosen according to the biofilm production profile (biomass production and metabolic activity) that matched the fks mutant Candida isolates. We also studied the impact of micafungin and liposomal amphotericin B on the biofilm structure.
Biofilms were formed on 50-mm polystyrene discs and prepared for SEM as previously reported (17). In order to study the antifungal effect, preformed biofilms of each isolate were incubated for 24 h at 37°C with 100 μl of RPMI solution containing a drug concentration equal to the SMIC80. The structure of the treated and nontreated biofilms was visualized using a scanning electron microscope (JEOL-JSM 6400; Jeol, Tokyo, Japan).
Data analysis.
The in vitro activity of caspofungin, anidulafungin, micafungin, and liposomal amphotericin B against planktonic and sessile forms is shown as MIC50s and MIC90s, geometric means, and MIC ranges. Differences between the four drugs in antifungal activity against the biofilms were assessed and compared using the Friedman test. Differences in the susceptibility of the biofilms formed by all three types of isolates were studied using the Kruskal-Wallis test.
This study was approved by the local ethics committee (Comité Ético de Investigación Clínica del Hospital Gregorio Marañón [CEIC-A1], study number 316/15).
RESULTS AND DISCUSSION
We found that the biofilm structure of fks mutant Candida isolates did not differ considerably from that of wild-type Candida strains (Fig. 1) (Table 1). Given the importance of glucan synthase in cell wall biosynthesis, fks mutant Candida isolates may not be able to form biofilms. However, Vavala and colleagues (12) found that one C. albicans fks mutant isolate was more able to form biofilm than the susceptible wild-type strain. Our study confirms these observations and shows that biofilm formation may be more related to the species of Candida than to the presence of fks mutations. We previously reported that the pattern of biofilm production is a species-specific feature (17); the degree of biofilm formation of the fks mutant Candida isolates reported here is consistent with the species-specific pattern, with a high proportion of C. tropicalis isolates being classified as HBF and MBF and a high proportion of C. glabrata isolates classified as HMA (17) (Table 1). Walraven and colleagues (13) demonstrated that biofilm architecture was altered in Candida isolates, showing a nucleotide substitution at position S645P of the fks1 gene. The biofilm showed a predominance of cells as well as pseudohyphae and pit-like structures on the surface, suggesting a substantial cell surface defect. We did not find these structures in C. albicans isolates with a nucleotide substitution at position F641S of the fks1 gene. Similarly, Walraven and colleagues did not report the presence of these structures in the isolates showing the F641S substitution (13).
FIG 1.
Biofilm structure of fks mutant strains, wild-type isolates with the same biofilm profile, and intrinsically echinocandin-resistant non-Candida species. Magnification, ×5,000. (a) C. glabrata wild-type, (b) C. glabrata Δ649 fks2, (c) Trichosporon japonicum, (d) C. tropicalis wild-type, (e) C. tropicalis S645F fks1, (f) Rhodotorula mucilaginosa, (g) C. albicans wild-type, (h) C. albicans F641S fks1, (i) Arxula adeninivorans, (j) C. tropicalis wild-type, (k) C. tropicalis F641L fks1, (l) C. tropicalis wild-type, and (m) C. tropicalis R647G fks1 strains were examined.
Intrinsically echinocandin-resistant non-Candida isolates formed biofilms that were mostly LBF (Table 1) with a structure characterized by the absence of hyphae and a low density of cells attached to the plastic surface (Fig. 1). Trichosporon spp. biofilm was characterized by the presence of pseudohyphae and arthrospores, whereas Rhodotorula spp. biofilm was formed predominantly by blastospores with an elongated oval shape.
The three echinocandins and liposomal amphotericin B previously have shown potent in vitro activity against preformed Candida biofilms (5, 6, 22), although data regarding antifungal activity against echinocandin-resistant isolates are very limited. We studied susceptibility to echinocandins and liposomal amphotericin B of preformed biofilms of Candida with acquired resistance to echinocandins, intrinsically echinocandin-resistant non-Candida isolates, and wild-type Candida isolates. To the best of our knowledge, this is the first study in which the activity of liposomal amphotericin B against biofilms formed by echinocandin-resistant clinical yeast isolates has been studied. The effect of the antifungal agents was also studied using SEM.
The antifungal activity of micafungin, anidulafungin, caspofungin, and liposomal amphotericin B against planktonic forms is shown in Table 2. The three echinocandins were barely active against the fks mutant isolates and intrinsically echinocandin-resistant non-Candida isolates. In contrast, liposomal amphotericin B showed high activity against fks mutant Candida and Rhodotorula isolates but not against Trichosporon spp. The antifungal susceptibility of the sessile forms is shown in Table 3. Based on the SMIC50, liposomal amphotericin B (0.29 μg/ml) was the most active drug, followed by micafungin (0.67 μg/ml), anidulafungin (1.15 μg/ml), and caspofungin (1.21 μg/ml) (P < 0.001). However, when a more stringent endpoint (SMIC80) was applied, micafungin (2.63 μg/ml) was the most active drug, followed by anidulafungin (4.49 μg/ml), caspofungin (5.13 μg/ml), and liposomal amphotericin B (7.98 μg/ml) (P < 0.001). We compared the susceptibility of fks mutant Candida isolates, wild-type Candida isolates, and intrinsically echinocandin-resistant non-Candida isolates to the echinocandins and liposomal amphotericin B. Wild-type Candida isolates were the most susceptible to the three echinocandins (P < 0.001), whereas the fks mutant Candida isolates were the most susceptible to liposomal amphotericin B, regardless of the endpoint used (SMIC50 or SMIC80), although the differences were not statistically significant (Table 3).
TABLE 2.
Drug susceptibility of the planktonic form of Candida and non-Candida isolates
| Mutation or in vitro echinocandin resistance status | Species | Planktonic MIC (μg/ml) |
|||
|---|---|---|---|---|---|
| Caspofungin | Micafungin | Anidulafungin | L-amphotericin B | ||
| F641S FKS1 | C. albicans | 0.5 | 1 | 0.25 | 0.062 |
| Δ649 FKS2 | C. glabrata | 4 | 2 | 1 | 0.25 |
| S645F FKS1 | C. tropicalis | 0.5 | 1 | 2 | 0.25 |
| F641L FKS1 | C. tropicalis | 0.125 | 0.125 | 0.062 | 0.25 |
| R647G FKS1 | C. tropicalis | 0.125 | 0.25 | 0.062 | 0.25 |
| Wild type | C. albicans | 0.125 | ≤0.015 | ≤0.015 | 0.125 |
| Wild type | C. albicans | 0.062 | ≤0.015 | ≤0.015 | 0.25 |
| Wild type | C. glabrata | 0.125 | ≤0.015 | ≤0.015 | 0.25 |
| Wild type | C. glabrata | 0.062 | ≤0.015 | ≤0.015 | 0.5 |
| Wild type | C. tropicalis | 0.125 | ≤0.015 | ≤0.015 | 0.25 |
| Wild type | C. tropicalis | 0.125 | 0.031 | ≤0.015 | 0.125 |
| Wild type | C. tropicalis | 0.125 | 0.031 | 0.031 | 0.25 |
| Wild type | C. tropicalis | 0.125 | 0.031 | ≤0.015 | 0.25 |
| Wild type | C. tropicalis | 0.125 | 0.031 | ≤0.015 | 0.25 |
| Wild type | C. tropicalis | 0.125 | ≤0.015 | ≤0.015 | 0.125 |
| Intrinsically resistant | A. adeninivorans | 0.25 | 0.125 | 0.5 | 1 |
| Intrinsically resistant | R. mucilaginosa | 2 | ≥16 | ≥16 | 2 |
| Intrinsically resistant | R. mucilaginosa | ≥16 | ≥16 | ≥16 | 1 |
| Intrinsically resistant | R. mucilaginosa | ≥16 | ≥16 | ≥16 | 0.125 |
| Intrinsically resistant | R. mucilaginosa | ≥16 | ≥16 | ≥16 | 0.25 |
| Intrinsically resistant | R. mucilaginosa | ≥16 | ≥16 | ≥16 | 0.25 |
| Intrinsically resistant | R. mucilaginosa | 1 | ≥16 | ≥16 | 1 |
| Intrinsically resistant | R. mucilaginosa | 1 | ≥16 | ≥16 | 0.25 |
| Intrinsically resistant | T. asahii | ≥16 | ≥16 | ≥16 | ≥16 |
| Intrinsically resistant | T. asahii | ≥16 | ≥16 | ≥16 | ≥16 |
| Intrinsically resistant | T. dermatis | ≥16 | ≥16 | ≥16 | ≥16 |
| Intrinsically resistant | T. japonicum | ≥16 | ≥16 | ≥16 | 8 |
We measured the susceptibility to micafungin, caspofungin, anidulafungin, and liposomal amphotericin B (L-amphotericin B) of the planktonic form of the fks mutant Candida isolates, wild-type Candida isolates, and intrinsically echinocandin-resistant non-Candida species.
TABLE 3.
Antifungal susceptibility to echinocandins and liposomal amphotericin B expressed as SMIC50 and SMIC80 of Candida and non-Candida isolatesa
| Drug and MIC | SMIC50 (in μg/ml) |
SMIC80 (in μg/ml) |
||||
|---|---|---|---|---|---|---|
| fks mutant | Wild type | Intrinsically resistant | fks mutant | Wild type | Intrinsically resistant | |
| Micafungin | ||||||
| GMb | 0.99 | 0.02 | 11.28 | 18.38 | 0.09 | 19.03 |
| MIC50 | 2 | ≤0.015 | ≥32 | ≥32 | ≤0.015 | ≥32 |
| MIC90 | 16 | 0.125 | ≥32 | ≥32 | ≥32 | ≥32 |
| Range | ≤0.015–16 | ≤0.015–0.125 | 0.031–≥32 | 2–≥32 | ≤0.015–≥32 | 0.25–≥32 |
| Anidulafungin | ||||||
| GM | 1.74 | 0.04 | 15.05 | 32 | 0.28 | 20.09 |
| MIC50 | 2 | 0.031 | ≥32 | ≥32 | 0.062 | ≥32 |
| MIC90 | ≥32 | 0.5 | ≥32 | ≥32 | ≥32 | ≥32 |
| Range | 0.062–≥32 | ≤0.015–0.5 | 0.062–≥32 | ≥32–≥32 | 0.031–≥32 | 0.125–≥32 |
| Caspofungin | ||||||
| GM | 1.51 | 0.14 | 6.71 | 12.13 | 0.37 | 32 |
| MIC50 | 2 | 0.125 | ≥32 | ≥32 | 0.25 | ≥32 |
| MIC90 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 |
| Range | 0.062–≥32 | ≤0.015–≥32 | 0.125–≥32 | 0.5–≥32 | 0.031–≥32 | ≥32–≥32 |
| Liposomal amphotericin B | ||||||
| GM | 0.14 | 0.29 | 0.38 | 2.64 | 21.11 | 5.64 |
| MIC50 | 0.062 | 0.25 | 0.25 | 8 | ≥32 | ≥32 |
| MIC90 | 4 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 |
| Range | 0.031–4 | 0.062–≥32 | ≤0.015–≥32 | 0.125–≥32 | 4–≥32 | ≤0.015–≥32 |
Antifungal susceptibility to echinocandins and liposomal amphotericin B was expressed as a 50% and 80% reduction in the metabolic activity of the biofilm (SMIC50 and SMIC80, respectively) of the fks mutant Candida isolates, wild-type Candida isolates, and intrinsically echinocandin-resistant non-Candida isolates.
GM, geometric mean.
We showed that fks mutant Candida isolates, in both the planktonic and the sessile state, were less susceptible to echinocandins than wild-type isolates. Anidulafungin has been reported to be the most active echinocandin against fks mutant Candida isolates when the SMIC80 is applied (13); however, we found that caspofungin was the most active echinocandin drug for this endpoint. Antifungal susceptibility to echinocandins has been reported to vary depending on the fks mutation (23), which can also have an impact on biofilm susceptibility. We aimed to study the anti-biofilm activity of the three echinocandins used in clinical practice. However, in vitro antifungal activity of caspofungin should be interpreted carefully because of the interlaboratory variations reported when using this drug (24).
Liposomal amphotericin B had the highest activity against biofilms formed by isolates with acquired resistance (the P value was not significant) or intrinsic resistance (P < 0.05) to echinocandins; this finding is consistent with other studies, where conventional amphotericin B had high anti-biofilm activity, even against non-Candida isolates (25–29). However, we observed that biofilms formed by C. tropicalis isolates were highly resistant to liposomal amphotericin B, which was unable to achieve an 80% reduction in the metabolic activity of the biofilm. When wild-type C. tropicalis isolates were removed from the analysis, the geometric mean (range) of the liposomal amphotericin B SMIC50 and SMIC80 was 0.015 μg/ml (0.062 to 0.25 μg/ml) and 11.31 μg/ml (4 to 32 μg/ml), respectively. The explanation for this finding is unknown and warrants further investigation.
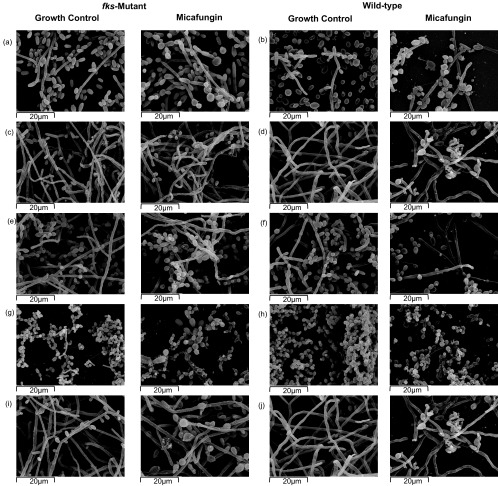
SEM was used to investigate the effect of liposomal amphotericin B and micafungin on the preformed Candida biofilms (Fig. 2 and 3). Liposomal amphotericin B induced subtle changes in C. albicans (both wild-type and fks mutant biofilms) with the presence of shriveled hyphae and a slightly less dense structure. The antifungal effect on C. glabrata biofilms consisted of a prominent decrease in the number of blastospores (in both wild-type and fks mutant isolates). Finally, five C. tropicalis isolates were studied using SEM (three were HBF [R647G and S645F fks1 mutant isolates and the corresponding wild-type isolate], and two were MBF [F641L fks1 mutant isolate and the corresponding wild-type isolate]); liposomal amphotericin B had a limited impact on the structure of HBF biofilms, whereas it led to shriveled hyphae and a less dense structure in MBF biofilms. The impact of micafungin on preformed biofilms differed between Candida wild-type and fks mutant isolates: biofilms formed by wild-type strains were moderately affected, whereas those formed by fks mutant isolates remained unaffected, with the exception of the C. glabrata Δ649 fks2 strain, which showed a slight decrease in biofilm density.
FIG 2.
SEM showing the structure of biofilms formed by fks mutant strains and wild-type controls after treatment with a concentration of liposomal amphotericin equal to the SMIC80 of each isolate. Magnification, ×2,000. (a) C. tropicalis F641L fks1 (growth control, SMIC80 of 16 μg/ml of liposomal amphotericin B), (b) C. tropicalis wild-type (growth control, SMIC80 of 16 μg/ml of liposomal amphotericin B), (c) C. tropicalis R647G fks1 (growth control, SMIC80 of 8 μg/ml of liposomal amphotericin B), (d) C. tropicalis wild-type (growth control, SMIC80 of 16 μg/ml of liposomal amphotericin B), (e) C. albicans F641S fks1 (growth control, SMIC80 of 0.125 μg/ml of liposomal amphotericin B), (f) C. albicans wild-type (growth control, SMIC80 of 16 μg/ml of liposomal amphotericin B), (g) C. glabrata Δ649 fks2 (growth control, SMIC80 of 0.125 μg/ml of liposomal amphotericin B), (h) C. glabrata wild-type (growth control, SMIC80 of 4 μg/ml of liposomal amphotericin B), (i) C. tropicalis S645F fks1 (growth control, SMIC80 of 16 μg/ml of liposomal amphotericin B), and (j) C. tropicalis wild-type (growth control, SMIC80 of 16 μg/ml of liposomal amphotericin B) strains were examined.
FIG 3.
SEM showing the structure of biofilms formed by fks mutant strains and wild-type controls after treatment with a concentration of micafungin equal to the SMIC80 of each isolate. Magnification, ×2,000. (a) C. tropicalis F641L fks1 (growth control, SMIC80 of 16 μg/ml of micafungin), (b) C. tropicalis wild-type (growth control, SMIC80 of 0.0015 μg/ml of micafungin), (c) C. tropicalis R647G fks1 (growth control, SMIC80 of 16 μg/ml of micafungin), (d) C. tropicalis wild-type (growth control, SMIC80 of 0.031 μg/ml of micafungin), (e) C. albicans F641S fks1 (growth control, SMIC80 of 16 μg/ml of micafungin), (f) C. albicans wild-type (growth control, SMIC80 of 8 μg/ml of micafungin), (g) C. glabrata Δ649 fks2 (growth control, SMIC80 of 2 μg/ml of micafungin), (h) C. glabrata wild-type (growth control, SMIC80 of 0.015 μg/ml of micafungin), (i) C. tropicalis S645F fks1 (growth control, SMIC80 of 16 μg/ml of micafungin), and (j) C. tropicalis wild-type (growth control, SMIC80 of 0.031 μg/ml of micafungin) strains were examined.
Our study is subject to two limitations. First, we only included a small set of fks mutant isolates. Second, we did not analyze the mechanism of resistance of C. tropicalis to liposomal amphotericin B, which should be evaluated in future studies.
In conclusion, we showed that the biofilm produced by fks mutant Candida isolates is similar to that produced by wild-type Candida isolates. Liposomal amphotericin B had the highest activity against fks mutant Candida biofilms and intrinsically resistant non-Candida species, with the exception of C. tropicalis.
ACKNOWLEDGMENTS
We thank Thomas O'Boyle for editing the article. Microscopy analysis was performed at the ICTS National Electron Microscopy Center, Universidad Complutense de Madrid.
This study was supported by grant numbers PI11/00167 and PI14/00740 from the Fondo de Investigación Sanitaria (FIS; Instituto de Salud Carlos III; Plan Nacional de I+D+I 2013-2016, Fondo Europeo de Desarrollo Regional FEDER “Una manera de hacer Europa” support) and by a CM-SANTANDER grant (GR3/2014; group 920200). P.E. (CPI15/00115) and J.G. (CPII15/00006) are recipients of a Miguel Servet contract; L.J.M.-Z. (FI12/00265) is supported by FIS.
This study does not present any conflicts of interest for its authors.
REFERENCES
- 1.Ramage G, Martinez JP, Lopez-Ribot JL. 2006. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. 2005. Candida biofilms: an update. Eukaryot Cell 4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathe L, Van Dijck P. 2013. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet 59:251–264. doi: 10.1007/s00294-013-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, Sanguinetti M, Fadda G, Cauda R, Posteraro B. 2012. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 7:e33705. doi: 10.1371/journal.pone.0033705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uppuluri P, Srinivasan A, Ramasubramanian A, Lopez-Ribot JL. 2011. Effects of fluconazole, amphotericin B, and caspofungin on Candida albicans biofilms under conditions of flow and on biofilm dispersion. Antimicrob Agents Chemother 55:3591–3593. doi: 10.1128/AAC.01701-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiori B, Posteraro B, Torelli R, Tumbarello M, Perlin DS, Fadda G, Sanguinetti M. 2011. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob Agents Chemother 55:3031–3035. doi: 10.1128/AAC.01569-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nucci M, Anaissie E, Betts RF, Dupont BF, Wu C, Buell DN, Kovanda L, Lortholary O. 2010. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis 51:295–303. doi: 10.1086/653935. [DOI] [PubMed] [Google Scholar]
- 8.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran-Valle MT, Gago S, Gomez-Lopez A, Cuenca-Estrella M, Jimenez Diez-Canseco L, Gomez-Garces JL, Zaragoza O. 2012. Recurrent episodes of candidemia due to Candida glabrata with a mutation in hot spot 1 of the FKS2 gene developed after prolonged therapy with caspofungin. Antimicrob Agents Chemother 56:3417–3419. doi: 10.1128/AAC.06100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desnos-Ollivier M, Bretagne S, Raoux D, Hoinard D, Dromer F, Dannaoui E. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob Agents Chemother 52:3092–3098. doi: 10.1128/AAC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother 53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vavala E, Colone M, Passariello C, Celestino I, Toccacieli L, Stringaro A, Angiolella L. 2013. Characterization of biofilms in drug-sensitive and drug-resistant strains of Candida albicans. J Chemother 25:87–95. doi: 10.1179/1973947812Y.0000000047. [DOI] [PubMed] [Google Scholar]
- 13.Walraven CJ, Bernardo SM, Wiederhold NP, Lee SA. 2014. Paradoxical antifungal activity and structural observations in biofilms formed by echinocandin-resistant Candida albicans clinical isolates. Med Mycol 52:131–139. doi: 10.1093/mmy/myt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcos-Zambrano LJ, Gómez-Perosanz M, Escribano P, Zaragoza O, Bouza S, Guinea J. 2015. Abstr 7th Cong Trends Med Mycol, poster P001. [Google Scholar]
- 15.Marcos-Zambrano LJ, Escribano P, Rueda C, Zaragoza O, Bouza E, Guinea E. 2013. Comparison between the EUCAST procedure and the Etest for determination of the susceptibility of Candida species isolates to micafungin. Antimicrob Agents Chemother 57:5767–5770. doi: 10.1128/AAC.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinea J, Zaragoza O, Escribano P, Martin-Mazuelos E, Peman J, Sanchez-Reus F, Cuenca-Estrella M. 2014. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain in 2010 and 2011. Antimicrob Agents Chemother 58:1529–1537. doi: 10.1128/AAC.02155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcos-Zambrano LJ, Escribano P, Bouza E, Guinea J. 2014. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int J Med Microbiol 304:1192–1198. doi: 10.1016/j.ijmm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 18.O'Toole GA. 2011. Microtiter dish biofilm formation assay. J Vis Exp 30:2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 21.Marcos-Zambrano LJ, Escribano P, Gonzalez Del Vecchio M, Bouza E, Guinea J. 2014. Micafungin is more active against Candida albicans biofilms with high metabolic activity. J Antimicrob Chemother 69:2984–2987. doi: 10.1093/jac/dku222. [DOI] [PubMed] [Google Scholar]
- 22.Katragkou A, Chatzimoschou A, Simitsopoulou M, Dalakiouridou M, Diza-Mataftsi E, Tsantali C, Roilides E. 2008. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob Agents Chemother 52:357–360. doi: 10.1128/AAC.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, Hope W. 2012. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother 56:2435–2442. doi: 10.1128/AAC.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of Caspofungin MICs for Candida spp. Using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidler M, Salvenmoser S, Muller FM. 2010. Liposomal amphotericin B eradicates Candida albicans biofilm in a continuous catheter flow model. FEMS Yeast Res 10:492–495. doi: 10.1111/j.1567-1364.2010.00618.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramage G, Jose A, Sherry L, Lappin DF, Jones B, Williams C. 2013. Liposomal amphotericin B displays rapid dose-dependent activity against Candida albicans biofilms. Antimicrob Agents Chemother 57:2369–2371. doi: 10.1128/AAC.02344-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes JM, Bizerra FC, Ferreira RC, Colombo AL. 2013. Molecular identification, antifungal susceptibility profile, and biofilm formation of clinical and environmental Rhodotorula species isolates. Antimicrob Agents Chemother 57:382–389. doi: 10.1128/AAC.01647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao Y, Yang S, Cong L, Lu X, Ao J, Yang R. 2014. In vitro activities of antifungal combinations against biofilms and planktonic forms of clinical Trichosporon asahii isolates. Antimicrob Agents Chemother 58:7615–7616. doi: 10.1128/AAC.03817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]