Abstract
Intracellular schizonts of the apicomplexans Theileria annulata and Theileria parva immortalize bovine leukocytes and thereby cause fatal diseases. The hydroxynaphthoquinone buparvaquone is currently the only option for the treatment of theileriosis, and resistance development has been reported. It is therefore tempting to investigate the repurposing of compounds effective against related apicomplexan parasites, such as Plasmodium. Here, we present the results of a screen of 400 compounds included in the open-access Medicines for Malaria Venture (MMV) malaria box on TaC12 cells, a macrophage-derived cell line immortalized by T. annulata schizonts. Using a combination of the classical alamarBlue vitality assay and a recently developed quantitative reverse transcriptase real-time PCR method based on the Theileria TaSP gene, we have identified 5 compounds, characterized their effects on the ultrastructure of TaC12 cells, and investigated whether they easily induce resistance formation. Two compounds, the quinolinols MMV666022 and MMV666054, have 50% inhibitory concentrations (IC50s) of 0.5 and 0.2 μM on TaC12 cells and 5.3 and 5.2 μM on BoMac cells, respectively. Thus, with therapeutic indexes of 11 and 18, they represent promising leads for further development of antitheilerial chemotherapeutics.
INTRODUCTION
Apicomplexan parasites, such as Cryptosporidium, Eimeria, Plasmodium, and Toxoplasma, are of outstanding veterinary and/or human medical importance. Theileria sporozoites are transmitted by ticks, infect cattle, and cause acute and fatal leukoproliferative disease. Theileria parva is found in east, central, and southern Africa and causes East Coast fever, while Theileria annulata occurs in the Mediterranean and Middle East area, northern Africa, India, and the Far East and is the causative agent of tropical theileriosis (1). Theileria-infected cells share several hallmarks of cancer cells, such as resistance to apoptosis, uncontrolled and unlimited proliferation, deregulation of cellular energetics, and acquisition of an invasive and metastatic phenotype (2). Currently, there are different strategies for the prevention and treatment of bovine theileriosis, namely, (i) targeting the tick vector by acaricide treatment (3) or (ii) targeting the parasite itself by vaccination, chemotherapy, or a combination of the two (4, 5). The most efficacious vaccine approach for East Coast fever was developed many years ago and includes infection of cattle with T. parva sporozoites and immediate treatment with a drug that impairs the establishment of infection, such as oxytetracycline (6) or, alternatively, buparvaquone (BPQ), which is currently the only effective compound for the treatment of bovine theileriosis (7–9). BPQ is a hydroxynaphtoquinone related to parvaquone (10, 11). To be effective, the compound must be administered during the early stage of infection, and the drug is not always affordable or available to farmers (12). More recently, the first cases of treatment failures due to resistant parasite populations were reported (13). These treatment failures were shown to be associated with mutations in the Theileria cytochrome b gene encoding the ubiquinone reductase of the respiratory chain (14, 15). Another potential target of BPQ is Theileria prolyl isomerase (TaPIN1), since mutations in the gene were demonstrated in BPQ-resistant cell lines (16). Using a novel assay based on the differential analysis of transcribed RNAs from host and parasite, we have shown that BPQ affects the parasite within a few hours, indicating that, in all likelihood, BPQ acts via rapid metabolic inhibition (17).
The occurrence of BPQ resistance in cattle provides an incentive to search for alternative treatment options. The open-access Medicines for Malaria Venture (MMV) malaria box includes 200 drug-like and 200 probe-like compounds, which represent a subset of the 20,000 in vitro antimalarials identified by the high-throughput screening efforts of St. Jude Children's Research Hospital (Memphis, TN, USA), Novartis, and GlaxoSmithKline (18–20). MMV malaria box compounds have proven to be effective in vitro against the apicomplexans Plasmodium falciparum (blood stage 3D7 and K1 strains) (21), Toxoplasma gondii (22), and Cryptosporidium parvum (23). Apicomplexans share a number of conserved pathways representing potential drug targets (24–26). For instance, the cross-reactivity of inhibitors of calcium-dependent protein kinases (CDPKs) against Plasmodium (27), Toxoplasma (28), and Neospora (29, 30) and the demonstration of profound in vitro and in vivo activity of BPQ against Neospora caninum infection (31) have underscored a potential role for exploiting the possibility of repurposing drugs among apicomplexans.
Here, we report the screening of the MMV malaria box against a macrophage line infected with the schizont stage of T. annulata and the characterization of 5 compounds that could potentially serve as a starting point for the development of novel antitheilerial drugs.
MATERIALS AND METHODS
Cell cultures.
TaC12, a bovine macrophage line infected with T. annulata, was cultured as previously described (17). BoMac, a bovine macrophage line immortalized by the simian virus 40 (SV40) large T antigen, was cultured as described previously (32). Human foreskin fibroblasts (HFF) were cultured as described previously (31).
MMV malaria box compounds.
The MMV malaria box was obtained from the MMV (Geneva, Switzerland). Plate mapping and full data on the malaria box have been made available (http://www.mmv.org/research-development/malaria-box-supporting-information). For more detailed studies, the compounds MMV000760 (compound 1), MMV666022 (compound 2), MMV666023 (compound 3), and MMV666054 (compound 4) were purchased from ChemBridge, and MMV665941 (compound 5) was obtained from AK Scientific, Inc. All the compounds were kept as 10 mM stocks in dimethyl sulfoxide (DMSO) and diluted in the respective culture media for the individual experiments, as indicated.
Cytotoxicity assays.
For the initial screening of the MMV malaria box, TaC12 cells (5 × 103 in 200 μl per well) were seeded into flat-bottom 96-well plates (Greiner Cellstar, Kremsmünster, Austria) and were allowed to adhere for 2 h prior to the addition of 1 μM the malaria box compounds. Some wells received BPQ (0.15 μM; Cross Vet Pharm, Dublin, Ireland) or 0.1% DMSO as positive and solvent controls, respectively. For 50% inhibitory concentration (IC50) determinations, cells were incubated in the presence of 0 to 2 μM.
BoMac cells (2 × 103 cells in 200 μl medium per well) were seeded into 96-well plates and allowed to adhere for 2 h prior to the addition of 0 to 20 μM the respective compounds.
HFF (2 × 103 cells in 200 μl medium per well) were seeded into 96-well plates and allowed to grow to confluent monolayers. Then, the monolayers were exposed to 1 μM selected malaria box compounds.
All cells were cultured for 3 days, and cell viability was measured using the alamarBlue assay as described previously (31).
qRT-PCR.
Quantitative reverse transcriptase real-time PCR (qRT-PCR) assays using primers for bovine actin and for TaSP were performed as described previously (17). Briefly, RNA was isolated with the Qiagen (Hilden, Germany) RNeasy kit, including DNase I digestion, and cDNA was synthesized with 2 μg of RNA using the Qiagen Omniscript kit with random primers according to the manufacturer's instructions. Quantitative PCR was performed with 10 μl of cDNA (diluted 1:50 in water) using the Quanti Tect SYBR green PCR kit (Roche, Basel, Switzerland) in 20-μl standard reaction mixtures containing 0.5 μM forward and reverse primers (MWG Biotech, Ebersberg, Germany). Real-time PCR was performed using a Corbett cycler (Mortlake, Australia), and expression levels are given in arbitrary units relative to the amount of actin RNA, as described previously (17).
TEM.
For transmission electron microscopy (TEM), 106 TaC12 cells were seeded into T25 tissue flasks and cultured for 24 h. Then, the cultures were exposed to 0.5 or 1 μM malaria box compounds, 0.15 μM BPQ, or corresponding amounts of DMSO for 48 h. Fixation, postfixation, and embedding were carried out as previously described (17).
Long-term culture of TaC12 cells in the presence of BPQ and selected malaria box compounds.
For long-term cultures, 106 TaC12 cells were seeded into 25-cm2 culture flasks, and the compounds were added after 2 h of recovery, as indicated. The medium was changed at 3-day intervals. The culture was continued for a maximum of 14 days or until no surviving cells were visible. Then, the cultures were switched to normal medium, and potential regrowth of the parasites was analyzed after 1 week.
Statistics.
For evaluation of the screening of TaC12 cells, compounds were regarded as potential hits when the alamarBlue values were not higher than the mean values of the positive controls (i.e., BPQ) plus 3 times their standard deviation. For evaluation of the screening of HFF, compounds were regarded as cytotoxic when the alamarBlue values were not higher than the mean values of the negative controls (i.e., DMSO) minus 2 times their standard deviation. IC50s were calculated as described previously (33).
RESULTS
Identification and first characterization of five lead compounds.
In a primary assay, TaC12 cells were exposed to 1 μM each malaria box compound and to 150 nM BPQ as a reference compound for a period of 3 days and subjected to the alamarBlue viability assay. As expected, BPQ was highly active against TaC12 cells. In parallel, to eliminate compounds that were highly cytotoxic to mammalian cells, the same screen was performed using confluent HFF. Eleven compounds impaired the viability of TaC12 cells to an extent similar to that with BPQ without impairing the viability of HFF. Subsequently, the IC50s of these 11 compounds were determined. Furthermore, the compounds were subjected to our previously established assay based on the differential expression of host actin and parasite TaSP (17) in order to eliminate compounds that preferentially affected the host compartments of infected cells. For the RT-PCR assay, in control cells, the TaSP expression levels were on the same order of magnitude as the Act levels. The mean values of the relative expression levels were therefore set as 100% in the control cells. Compounds causing a drop in these relative TaSP levels to 50% or less of the control levels were regarded as potentially interesting for further studies. Both assays led to the elimination of six compounds (Table 1 ). Five compounds, referred to here as compounds 1 to 5, inhibited TaC12 at submicromolar concentrations affecting parasite rather than host gene expression. These compounds were retained for subsequent studies. Using the TaSP gene expression assay as described above, we found a clear concentration dependency of relative TaSP mRNA levels with all five compounds, with 3 and 5 having the lowest TaSP levels at 0.5 μM (Fig. 1).
TABLE 1.
Inhibition of TaC12 viability by malaria box compoundsa
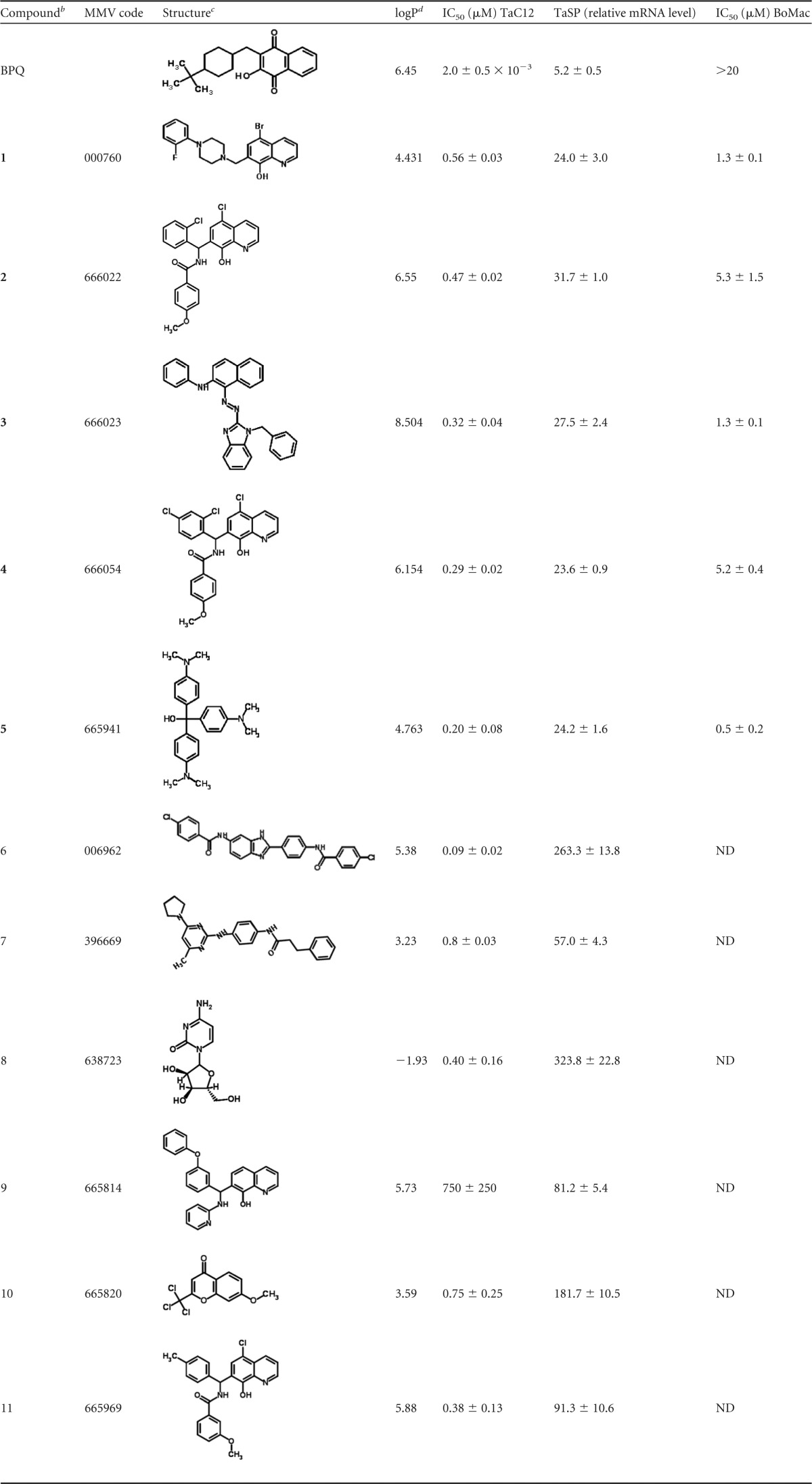
Eleven compounds identified in a previous screening were added to TaC12 cells previously seeded in 96-well plates in concentration series ranging from 0 to 2 μM. DMSO (0.1%) was included as a solvent control and BPQ as a positive control. After 3 days, viability was assayed using the alamarBlue assay. In a second step, in order to see whether the compounds induced a decrease of T. annulata mRNA levels, 107 cells were seeded in the presence of 1 μM the compounds, BPQ (150 nM) as a positive control, or DMSO as a negative control. After 24 h, cells were harvested, RNA was extracted, and mRNA levels of TaSP were quantified by real-time RT-PCR of host cell actin. Mean values ± standard errors (SE) are expressed as percentages of the DMSO control and are given for quadruplicates. For cytotoxicity assays on BoMac, cells were seeded into 96-well plates and treated with concentration series (0 to 20 μM) of compounds 1 to 5 and BPQ. After 3 days, viability was assayed using the alamarBlue assay. All assays were performed in quadruplicate. IC50s with confidence intervals were determined as described previously. ND, not determined.
The five compounds (1 to 5) that were retained for further studies are in boldface.
Structures were obtained by conversion from SMILES annotations via the ChemSpider website (http://www.chemspider.com).
Distribution coefficient octanol/water.
FIG 1.
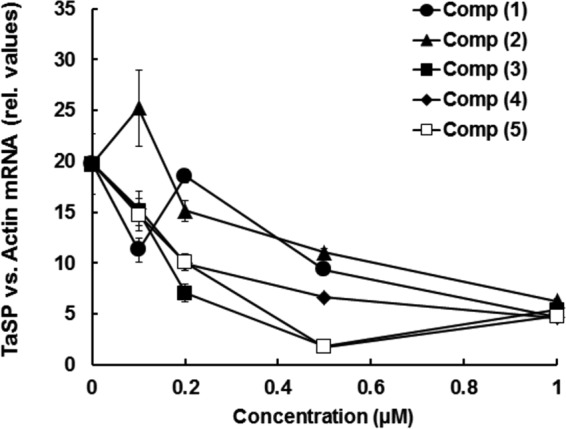
The five most efficient malaria box hits and their dose responses with respect to relative levels of TaSP mRNA in relation to bovine actin transcripts. TaC12 cells were seeded into 25-cm2 culture flasks and treated with 0.1, 0.2, 0.5, and 1 μM each compound (Comp) for 24 h. Relative (rel.) TaSP expression levels (in relation to bovine actin) were assessed by qRT-PCR assay. The experiment was done twice, with essentially identical outcomes. Mean values (± standard errors [SE]) for four biological replicates are shown. Low relative TaSP transcript levels indicated primary action of the drug against T. annulata, and higher relative TaSP levels indicated that compounds could be acting primarily against the host cell.
In order to determine a potential “therapeutic window,” BoMac cells were incubated with concentration series of the five compounds for 3 days and subjected to alamarBlue viability assays. Compound 5 had the highest toxicity for BoMac cells, and compounds 2 and 4 had the lowest and therefore the best therapeutic indexes (34), namely, 11 and 18, of the five compounds (Table 1).
Electron microscopy of TaC12 cultures treated with compounds 1 to 5.
TaC12 cells cultured in the absence of any drugs are shown in Fig. 2A and B. T. annulata schizonts were readily detected in the individual cells. They are located free in the cytoplasm, delineated from the host cell cytoplasm only by the schizont plasma membrane. Typically, 2 to 6 parasite nuclei were visible per section plane. In many instances, electron-dense button-like structures, seemingly acting as membrane connectors and structurally exhibiting some resemblance to tight junctions, were found to be abundantly associated with these schizonts. Host cell mitochondria with normal oval shapes were found near the schizonts. BPQ treatment of TaC12 cells induced clear alterations within the schizont cytoplasm, while the host cell appeared largely unaffected (Fig. 2C and D). Alterations included extensive accumulation of vacuoles filled with electron-dense material of unknown nature and a general disintegration of the cytoplasmic organization of schizonts. Nuclei were no longer discernible, and the button-like structures had disappeared or only residues were still visible. However, the parasite plasma membrane remained morphologically intact and clearly separated the parasite and host cell cytoplasms.
FIG 2.

TEM of untreated and BPQ-treated TaC12 cells. (A and B) Low-magnification view of an untreated control (A), with the boxed area enlarged (B). (C and D) Representative image of a BPQ-treated TaC12 cell (C), with the boxed area shown at higher magnification (D). The arrows indicate the schizont-host cell cytoplasm interface; v, vacuolization within the schizont cytoplasm; ×, button-like structures associated with schizonts; n, parasite nuclei; N, host cell nucleus; mito, host cell mitochondria. Scale bars, 3.4 μm (A), 0.7 μm (B), 1.7 μm (C), and 0.5 μm (D).
In TaC12 cells treated with compound 1, host cell mitochondria lost their typical oval shape and appeared rather swollen, and in some instances, cytoplasmic vacuoles were observed (Fig. 3A). However, the alterations within the schizonts appeared minimal, with only a few small vacuoles and overall intact structural features.
FIG 3.
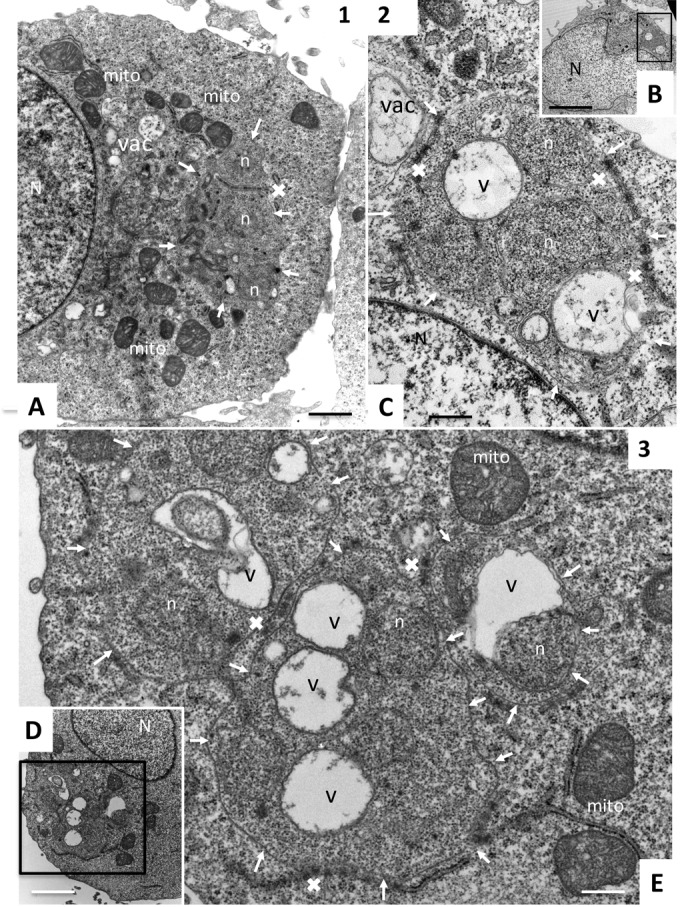
TEM of TaC12 cells treated with compounds 1, 2, and 3. (A) Cell treated with compound 1. (B and C) Low- and high-magnification views, respectively, of compound 2-treated cells. (D and E) Low- and high-magnification images, respectively, of a TaC12 cell treated with compound 3. The arrows indicate the schizont-host cell cytoplasm interfaces; v, vacuolization within the schizont cytoplasm; vac, presence of vacuoles within the host cell cytoplasm; ×, button-like structures or their residues associated with schizonts; n, parasite nuclei; N, host cell nucleus; mito, host cell mitochondria. Scale bars, 0.9 μm (A), 3.4 μm (B), 0.5 μm (C), 3.4 μm (D), and 0.5 μm (E).
Treatment with compound 5 also led to intraschizont vacuole formation, but the overall cytoplasmic organization appeared more intact than that of BPQ-treated cells, and nuclei were still clearly discernible. The button-like structures associated with the schizonts appeared normal. On the host cell side, mitochondrial morphology was clearly impaired, and the organelles appeared less compact and less electron dense than the mitochondria in control cells, indicative of effects on the host cell metabolism (Fig. 3A). Schizonts located in compound 2 (Fig. 3B and C)- and 3 (Fig. 3D and E)-treated TaC12 cells also exhibited extensive cytoplasmic vacuolization, indicating that the schizonts were metabolically impaired. In addition, in both instances, the button-like structures that were evident in untreated TaC12 cells were no longer visible or only residues could be identified. Vacuolization was also observed within the host cell cytoplasm in compound 2-treated cells, but this was less evident in cells treated with compound 3. Most notably, extensive mitochondrial swelling was evident in TaC12 cells treated with compound 3 (Fig. 3).
Schizonts located in compound 4- and 5-treated TaC12 cells (Fig. 4) also exhibited extensive cytoplasmic vacuolization. However, the button-shaped structures in those cells were not affected and appeared rather intact. On the other hand, compound 4 (Fig. 4A and B) induced extensive swelling of host cell mitochondria, similar to what was found for compounds 1 and 3, and treatment with compound 5 (Fig. 4C and D) resulted in mitochondria that were not swollen but rather appeared disintegrated and less electron dense.
FIG 4.

TEM of TaC12 cells exposed to compounds 4 and 5. (A) Low-magnification view of a TaC12 cell exposed to compound 4. (B) Higher-magnification view of the boxed area in panel A. (C) Low-magnification view of a TaC12 cell treated with compound 5. (D) Higher-magnification view of the boxed area in panel C. The arrows indicate the locations of the schizont plasma membranes; v, vacuolization within the schizont cytoplasm; ×, button-like structures or their residues associated with schizonts; n, parasite nucleus; N, host cell nuclei; mito, host cell mitochondria. Scale bars, 2.8 μm (A), 0.8 μm (B), 3.4 μm (C), and 0.5 μm (D).
Long-term treatment of TaC12 cells with BPQ and compounds 1 to 5 revealed the capacity of T. annulata to adapt and/or develop resistance to BPQ, but not malaria box compounds.
In order to determine whether TaC12 cells easily adapt to compounds 1 to 5, cells were treated with the concentration that showed maximum effect on TaSP in the RT-PCR assay, namely, 0.5 μM for compounds 3 and 5 and 1 μM for the others. Moreover, a control with cells treated with 150 nM BPQ was included. We observed an initial dramatic reduction in the number of viable cells within the first 3 to 5 days of BPQ treatment. However, upon extended culture in the presence of BPQ, small colonies of surviving TaC12 cells had formed, which remained viable and resumed proliferation at concentrations up to 2 μM (data not shown). On the other hand, when TaC12 cells were exposed to the five malaria box compounds, no viable cells were visible after 14 days of culture in medium containing the compounds followed by 1 week of culture in drug-free medium, indicating that no adaptation to the compounds took place.
DISCUSSION
Performing a screen with the MMV malaria box, we identified five compounds inhibiting TaC12 cell proliferation preferentially harming the T. annulata schizont. All five compounds are highly lipophilic (logP values above 4). By comparing the IC50s on TaC12 to the IC50s on BoMac cells, we see that compounds 2 and 4 offer convenient therapeutic indexes. One has to keep in mind, however, that both TaC12 and BoMac cells are immortalized, TaC12 by T. annulata and BoMac by the SV40 large T oncogene (32). It is therefore likely that untransformed blood cells react differently to these compounds and could potentially be more resistant. Compounds 2 and 4 are highly similar, differing only by one chlorine group on the quinoline-8-ol scaffold. Other compounds with this scaffold, namely, 9 and 11, also inhibit the proliferation of TaC12 cells but preferentially affect the host cell. This indicates that antitheilerial drug design is a tightrope walk between preferential host cell and parasite toxicities. As indicated by their therapeutic indexes, compounds 2 and 4 are convenient leads for the design of novel drugs as a backup for buparvaquone. Interestingly, both compounds also exhibited profound activity against C. parvum in vitro (23). None of the 5 compounds, however, was active against T. gondii, another intracellular apicomplexan (22). Compound 5 had not only the lowest IC50 on TaC12 cells, but also the highest toxicity for BoMac cells. This compound is the hydrated precursor of crystal violet, a broad-range anti-infective agent used as an antimycotic. It is therefore not surprising that it had also been identified as one of the hits against Cryptosporidium.
Morphologically, all the compounds identified here, except compound 1, induced vacuolization of the schizont cytoplasm, and to different degrees, the treatments resulted in morphological alterations that indicated metabolic impairment of the parasite. However, in all cases, the schizont cytoplasm was still clearly discernible, which indicates that these drugs induce parasite death in a manner that resembles cellular apoptosis rather than necrosis. As shown in a previous study, schizont lobes are connected by button-like structures (17). These structures resemble tight junctions, but their molecular composition and function are unknown. They could be involved in maintaining the structural integrity of the schizont stage, which resides free in the cytoplasm of its host cell. Compounds 2 and 4 affected the integrity of the buttons, whereas treatments with the other compounds did not alter these structures.
The molecular targets of all five compounds are unknown. In a screen for aminopeptidase inhibitors, compound 3 has been identified as one of the hits (35). We have not detected any inhibitory effect of this compound on aminopeptidase activities in crude extracts either from TaC12 cells or from isolated T. annulata schizonts (data not shown). The high lipophilicity of all the compounds not only could be instrumental for the multiple membrane passages that are required to reach the schizont, but also could be indicative of membrane-bound targets. Moreover, these compounds could interact with multiple targets in both the parasite and the host cell, since TaC12 cells were not able to adapt to the compounds during long-term cultures. Affinity chromatography (reference 33 and references therein) would be a tool to identify the proteins that interact with these compounds, especially 2 and 4, two quinolinols that are closely related and that represent the most interesting leads. Both are probe-like compounds and will certainly be modified prior to in vivo applications in a suitable model. Therefore, there are no preclinical ADME (absorption-distribution-metabolism-excretion) data available. Other quinolinols are effective against intracellular tachyzoites, most likely though a mechanism involving generation of reactive oxygen species (36). This could explain the effects on mitochondrial integrity observed in our study.
ACKNOWLEDGMENTS
We thank Kerry Woods and Sven Rottenberg for crucial logistic and moral support and for providing laboratory space for Theileria cell culture. We also gratefully acknowledge Medicines for Malaria Venture in Geneva, Switzerland, for providing the malaria box, and especially Wes Van Voorhis for helpful advice and support.
This work was supported by the Swiss National Science Foundation (grant no. 310030_146162) and the Vetsuisse Faculty of the University of Bern.
We have no conflicts of interest to declare.
Funding Statement
Isabel Hostettler was funded by Vetsuisse Faculty, University of Bern.
REFERENCES
- 1.Mans BJ, Pienaar R, Latif AA. 2015. A review of Theileria diagnostics and epidemiology. Int J Parasitol Parasites Wildl 4:104–118. doi: 10.1016/j.ijppaw.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tretina K, Gotia HT, Mann DJ, Silva JC. 2015. Theileria-transformed bovine leukocytes have cancer hallmarks. Trends Parasitol 31:306–314. doi: 10.1016/j.pt.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Gachohi J, Skilton R, Hansen F, Ngumi P, Kitala P. 2012. Epidemiology of East Coast fever (Theileria parva infection) in Kenya: past, present and the future. Parasit Vectors 5:194. doi: 10.1186/1756-3305-5-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeever DJ. 2007. Live immunisation against Theileria parva: containing or spreading the disease? Trends Parasitol 23:565–568. doi: 10.1016/j.pt.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeever DJ. 2009. Bovine immunity—a driver for diversity in Theileria parasites? Trends Parasitol 25:269–276. doi: 10.1016/j.pt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Spooner PR. 1990. The effects of oxytetracycline on Theileria parva in vitro. Parasitology 100:11–17. doi: 10.1017/S0031182000060066. [DOI] [PubMed] [Google Scholar]
- 7.Brown CG. 1990. Control of tropical theileriosis (Theileria annulata infection) of cattle. Parasitologia 32:23–31. [PubMed] [Google Scholar]
- 8.Mutugi JJ, Young AS, Maritim AC, Linyonyi A, Mbogo SK, Leitch BL. 1988. Immunization of cattle using varying infective doses of Theileria parva lawrencei sporozoites derived from an African buffalo (Syncerus caffer) and treatment with buparvaquone. Parasitology 96:391–402. doi: 10.1017/S0031182000058376. [DOI] [PubMed] [Google Scholar]
- 9.McHardy N, Morgan DW. 1985. Treatment of Theileria annulata infection in calves with parvaquone. Res Vet Sci 39:1–4. [PubMed] [Google Scholar]
- 10.McHardy N, Hudson AT, Morgan DW, Rae DG, Dolan TT. 1983. Activity of 10 naphthoquinones, including parvaquone (993C) and menoctone, in cattle artificially infected with Theileria parva. Res Vet Sci 35:347–352. [PubMed] [Google Scholar]
- 11.Boehm P, Cooper K, Hudson AT, Elphick JP, McHardy N. 1981. In vitro activity of 2-alkyl-3-hydroxy-1,4-naphthoquinones against Theileria parva. J Med Chem 24:295–299. doi: 10.1021/jm00135a011. [DOI] [PubMed] [Google Scholar]
- 12.Morrison WI, McKeever DJ. 2006. Current status of vaccine development against Theileria parasites. Parasitology 133(Suppl):S169–S187. doi: 10.1017/S0031182006001867. [DOI] [PubMed] [Google Scholar]
- 13.Mhadhbi M, Naouach A, Boumiza A, Chaabani MF, BenAbderazzak S, Darghouth MA. 2010. In vivo evidence for the resistance of Theileria annulata to buparvaquone. Vet Parasitol 169:241–247. doi: 10.1016/j.vetpar.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Sharifiyazdi H, Namazi F, Oryan A, Shahriari R, Razavi M. 2012. Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet Parasitol 187:431–435. doi: 10.1016/j.vetpar.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Mhadhbi M, Chaouch M, Ajroud K, Darghouth MA, BenAbderrazak S. 2015. Sequence polymorphism of cytochrome b gene in Theileria annulata Tunisian isolates and its association with buparvaquone treatment failure. PLoS One 10:e0129678. doi: 10.1371/journal.pone.0129678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsolier J, Perichon M, DeBarry JD, Villoutreix BO, Chluba J, Lopez T, Garrido C, Zhou XZ, Lu KP, Fritsch L, Ait-Si-Ali S, Mhadhbi M, Medjkane S, Weitzman JB. 2015. Theileria parasites secrete a prolyl isomerase to maintain host leukocyte transformation. Nature 520:378–382. doi: 10.1038/nature14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostettler I, Müller J, Stephens CE, Haynes R, Hemphill A. 2014. A quantitative reverse-transcriptase PCR assay for the assessment of drug activities against intracellular Theileria annulata schizonts. Int J Parasitol Drugs Drug Resist 4:201–209. doi: 10.1016/j.ijpddr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 19.Meister S, Plouffe DM, Kuhen KL, Bonamy GM, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MC, McNamara CW, Fidock DA, Nagle A, Nam TG, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. 2011. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334:1372–1377. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spangenberg T, Burrows JN, Kowalczyk P, McDonald S, Wells TN, Willis P. 2013. The open access malaria box: a drug discovery catalyst for neglected diseases. PLoS One 8:e62906. doi: 10.1371/journal.pone.0062906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyom FF, Fokou PV, Tchokouaha LR, Spangenberg T, Mfopa AN, Kouipou RM, Mbouna CJ, Donfack VF, Zollo PH. 2014. Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob Agents Chemother 58:5848–5854. doi: 10.1128/AAC.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. 2014. Identification of Cryptosporidium parvum active chemical series by repurposing the open access malaria box. Antimicrob Agents Chemother 58:2731–2739. doi: 10.1128/AAC.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier AT, Dear PH, Konfortov BA, Spriggs HF, Iyer L, Anantharaman V, Aravind L, Kapur V. 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 25.Xu P, Widmer G, Wang Y, Ozaki LS, Alves JM, Serrano MG, Puiu D, Manque P, Akiyoshi D, Mackey AJ, Pearson WR, Dear PH, Bankier AT, Peterson DL, Abrahamsen MS, Kapur V, Tzipori S, Buck GA. 2004. The genome of Cryptosporidium hominis. Nature 431:1107–1112. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- 26.Wernimont AK, Artz JD, Finerty P Jr, Lin YH, Amani M, Allali-Hassani A, Senisterra G, Vedadi M, Tempel W, Mackenzie F, Chau I, Lourido S, Sibley LD, Hui R. 2010. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol 17:596–601. doi: 10.1038/nsmb.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojo KK, Pfander C, Mueller NR, Burstroem C, Larson ET, Bryan CM, Fox AM, Reid MC, Johnson SM, Murphy RC, Kennedy M, Mann H, Leibly DJ, Hewitt SN, Verlinde CL, Kappe S, Merritt EA, Maly DJ, Billker O, Van Voorhis WC. 2012. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest 122:2301–2305. doi: 10.1172/JCI61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, Derocher AE, Inampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, Napuli AJ, Maly DJ, Verlinde CL, Buckner FS, Parsons M, Hol WG, Merritt EA, Van Voorhis WC. 2010. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol 17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojo KK, Reid MC, Kallur Siddaramaiah L, Müller J, Winzer P, Zhang Z, Keyloun KR, Vidadala RS, Merritt EA, Hol WG, Maly DJ, Fan E, Van Voorhis WC, Hemphill A. 2014. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS One 9:e92929. doi: 10.1371/journal.pone.0092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winzer P, Müller J, Aguado-Martínez A, Rahman M, Balmer V, Ortega-Mora L, Ojo KK, Fan E, Maly D, Van Voorhis WC, Hemphill A. 2015. In vitro and in vivo effects of the bumped kinase inhibitor 1294 in the related cyst-forming apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob Agents Chemother 59:6361–6374. doi: 10.1128/AAC.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller J, Aguado-Martinez A, Manser V, Balmer V, Winzer P, Ritler D, Hostettler I, Solís D, Ortega-Mora LM, Hemphill A. 2015. Buparvaquone is active against Neospora caninum in vitro and in experimentally infected mice. Int J Parasitol Drugs Drug Resist 5:16–25. doi: 10.1016/j.ijpddr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stabel JR, Stabel TJ. 1995. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet Immunol Immunopathol 45:211–220. doi: 10.1016/0165-2427(94)05348-V. [DOI] [PubMed] [Google Scholar]
- 33.Müller J, Hemphill A. 2013. New approaches for the identification of drug targets in protozoan parasites. Int Rev Cell Mol Biol 301:359–401. doi: 10.1016/B978-0-12-407704-1.00007-5. [DOI] [PubMed] [Google Scholar]
- 34.Muller PY, Milton MN. 2012. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov 11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 35.Paiardini A, Bamert RS, Kannan-Sivaraman K, Drinkwater N, Mistry SN, Scammells PJ, McGowan S. 2015. Screening the medicines for malaria venture “Malaria Box” against the Plasmodium falciparum aminopeptidases, M1, M17 and M18. PLoS One 10:e0115859. doi: 10.1371/journal.pone.0115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strobl JS, Seibert CW, Li Y, Nagarkatti R, Mitchell SM, Rosypal AC, Rathore D, Lindsay DS. 2009. Inhibition of Toxoplasma gondii and Plasmodium falciparum infections in vitro by NSC3852, a redox active antiproliferative and tumor cell differentiation agent. J Parasitol 95:215–223. doi: 10.1645/GE-1608.1. [DOI] [PubMed] [Google Scholar]


