Abstract
Although the use of probiotics based on Bacillus strains to fight off intestinal pathogens and antibiotic-associated diarrhea is widespread, the mechanisms involved in producing their beneficial effects remain unclear. Here, we studied the ability of compounds secreted by the probiotic Bacillus clausii strain O/C to counteract the cytotoxic effects induced by toxins of two pathogens, Clostridium difficile and Bacillus cereus, by evaluating eukaryotic cell viability and expression of selected genes. Coincubation of C. difficile and B. cereus toxic culture supernatants with the B. clausii supernatant completely prevented the damage induced by toxins in Vero and Caco-2 cells. The hemolytic effect of B. cereus was also avoided by the probiotic supernatant. Moreover, in these cells, the expression of rhoB, encoding a Rho GTPase target for C. difficile toxins, was normalized when C. difficile supernatant was pretreated using the B. clausii supernatant. All of the beneficial effects observed with the probiotic were abolished by the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF). Suspecting the involvement of a secreted protease in this protective effect, a protease was purified from the B. clausii supernatant and identified as a serine protease (M-protease; GenBank accession number Q99405). Experiments on Vero cells demonstrated the antitoxic activity of the purified protease against pathogen supernatants. This is the first report showing the capacity of a protease secreted by probiotic bacteria to inhibit the cytotoxic effects of toxinogenic C. difficile and B. cereus strains. This extracellular compound could be responsible, at least in part, for the protective effects observed for this human probiotic in antibiotic-associated diarrhea.
INTRODUCTION
The gut, one of the biggest surfaces of exchange between the body's internal and external environment, is in permanent contact with antigens. Indeed, the intestinal barrier constitutes an important playground for numerous intestinal pathogen bacteria and toxins.
Among them, Clostridium difficile, an anaerobic Gram-positive bacterium, is a major cause of diarrhea, pseudomembranous colitis, and septicemia, and it can lead to death. Hospital stay and use of antibiotics are major risk factors for contracting this disease (1). Over the past decade, the frequency and severity of C. difficile infections have increased markedly due to the emergence of so-called hypervirulent strains that overproduce toxins (2–4). The C. difficile toxins modify target cell proteins to cause disassembly of the actin cytoskeleton and induce severe inflammation (5).
Expression of several host genes, mainly those involved in cellular processes, cell-cell interactions, apoptosis, and inflammation, is modified during C. difficile infection (6). These include host genes encoding RhoB (regulating actin cytoskeleton and interactions between cells), ZO-1 (tight-junction protein), and ROCK2 (actin cytoskeleton and signaling protein regulator) (7–9).
Equally, many other pathogens, such as Salmonella species, Escherichia coli, Shigella spp., Staphylococcus aureus, and Bacillus cereus, produce several toxins that target intestinal mucosa (10–12). B. cereus, a spore-forming Gram-positive bacterium, is an opportunistic pathogen that is ubiquitous in the environment. Some strains may cause emetic food poisoning (cereulide toxin produced during growth in food) and diarrheal food poisoning when certain enterotoxins (cytotoxin K [CytK], nonhemolytic enterotoxin [Nhe], and hemolysin BL [HBL]) are produced during bacterial growth in the small intestine of the host (13–15). The hemolysin increases vascular permeability, which then causes hemorrhage. The in vitro studies of supernatants produced by B. cereus toxinogenic strains demonstrated the disruption of cell membrane and induction of the apoptotic pathway (16, 17).
Over the last few years, new therapeutic alternatives have been developed to prevent or limit infections, to improve intestinal comfort, and to reinforce antibiotic treatments. One of these alternatives is the use of probiotics against various intestinal pathogen infections and antibiotic-associated diarrhea (18, 19).
Probiotics are “Live microorganisms which when administered in adequate amounts confer a health benefit on the host” (20). Probiotics are known to have a protective role against intestinal pathogens by enhancing intestinal barrier function, interfering with pathogens by competitive exclusion, modulating the host immune system, and stabilizing microbiota (21, 22). Moreover, probiotics can secrete antimicrobial substances, peptides, and proteins which provide protection against pathogens by occasionally inhibiting the action of certain toxins produced by pathogens (19, 23, 24). It has been reported that Saccharomyces boulardii, a probiotic yeast used in the treatment of C. difficile diarrhea and colitis, secretes a protease which digests toxin A and B molecules and its brush border membrane receptor (25, 26). Furthermore, this yeast seems to be able to inhibit in vitro cell adherence of C. difficile (27).
In this study, we focused on Bacillus clausii strain O/C, a sporulating bacterium which constitutes part of the probiotic Enterogermina, one of the oldest commercialized probiotics, which is used for the prevention and treatment of acute diarrhea (28, 29). In vitro and clinical studies have already shown its immune-modulating activity (30, 31). In addition, B. clausii strain O/C produces an antimicrobial substance, clausin, that is active against Gram-positive bacteria like C. difficile and Staphylococcus aureus (31, 32).
In a preliminary study conducted by our laboratory, the enzymatic activity of B. clausii O/C supernatants was studied and a proteolytic activity was noticed. Moreover, secreted proteins were analyzed by SDS-PAGE. About 10 proteins were observed, with a major band at 27 kDa.
The aim of this study was to investigate, using cytotoxicity assays and gene expression analysis, compounds secreted by B. clausii O/C that are involved in the prevention of C. difficile and B. cereus toxicity and to determine if a secreted protease contributes to this effect.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and supernatant production.
B. clausii O/C is a strain which forms part of the commercial probiotic Enterogermina (Sanofi, Milan, Italy). C. difficile VPI 10463 and B. cereus ATCC 14579 were used as toxin producer strains. C. difficile VPI 10463 secretes the two toxins A and B (33). B. cereus ATCC 14579 secretes hemolysin and enterotoxins but not the emetic toxin (34).
B. clausii was cultured aerobically in Mueller-Hinton broth (MH) (Difco Laboratory, Detroit, MI, USA) at 37°C, in a rotary shaker for 3 days, to induce sporulation. To obtain cytotoxic supernatants, C. difficile was grown in brain heart infusion (BHI) medium (BD, Franklin Lakes, NJ, USA) in an anaerobic atmosphere for 3 days at 37°C, whereas B. cereus was grown aerobically overnight in MH at 37°C. Culture supernatants were harvested by centrifugation at 4°C (10,000 × g, 5 min) and filtered onto 0.2-μm cellulose acetate membrane (Merck Millipore, France). C. difficile and B. cereus supernatants were used as the source of toxins.
The proteolytic activity of B. clausii supernatants (OC-SN) was tested preliminarily using agar casein hydrolysis on agar plates containing yeast nitrogen base (YNB) agar medium (Difco Laboratory, Detroit, MI, USA) supplemented with 0.5% casein (35).
Enzyme purification.
Following centrifugation of the culture, the supernatant was filtered through a 0.45-μm-pore-size membrane filter (Millipore Corp., USA). The filtrate was concentrated with an Amicon 8050 UF cell (Amicon Division, W.R. Grace and Co., Beverley, MA) equipped with membrane YM10 (Millipore Corp., USA). The molecular mass cutoff for the membrane was 10,000 Da. The retentate was loaded onto a Superdex 75-10/300 GL column (GE Healthcare, Uppsala, Sweden), equilibrated at a rate of 0.4 ml with Tris-HCl (50 mM, pH 7.5) containing NaCl 0.15 M. Fractions (0.4 ml) were collected and screened for proteolytic activity with N-Suc-(Ala)2-Pro-Phe-pNA assay. Fractions exhibiting protease activity were pooled, desalted on a Micro Bio-Spin 6 column (Life Science Bio-Rad, France), and then dried under vacuum before SDS-PAGE analysis.
Identification of purified enzyme.
The band corresponding to purified protein was excised from a one-dimensional gel stained with Coomassie blue and cut into cubes. After reduction (with 10 mM dithiothreitol [DTT] for 35 min at 56°C) and alkylation (with 55 mM iodoacetamide in the dark for 30 min) of cysteines, dehydrated gel pieces were subjected to trypsin digestion overnight at 37°C. After evaporation, the peptides were solubilized in 20 μl of 2% acetonitrile, 0.05% trifluoroacetic acid, and 2 μl was injected for a 60-min IDA (independent data acquisition) analysis by nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a nanochromatography liquid Ultimate 3000 system (Thermo Fisher) coupled to a TripleTOF 5600+ mass spectrometer (Sciex, Montreal, Canada). The MS/MS data were used to query a B. clausii database extracted from UniProt containing 4,082 proteins using Protein Pilot software (Sciex) with Mascot software (version 2.2; Matrix Science, England).
Proteolytic activity and substrate specificity of B. clausii O/C supernatants and purified protease.
Enzymatic activity of B. clausii O/C vegetative cell (24 h of culture, 0% spores) and spore (3 days of culture, 90% spores) supernatants was tested and compared to that of subtilisin A, produced by Bacillus licheniformis (Sigma-Aldrich, France). The same experiment was conducted with the purified protease secreted by B. clausii O/C.
The substrate specificity of proteolytic compounds was determined using the p-nitroanilide (pNA)-conjugated synthetic peptide substrates N-Suc-Ala-Ala-Ala-pNA, N-Suc-Ala-Ala-Pro-Phe-pNA, and N-Suc-Phe-pNA (Sigma-Aldrich, France). A stock solution (125 mM) of each substrate was prepared in dimethyl sulfoxide (DMSO). Each substrate stock solution was adjusted to 1.25 mM in 50 mM Tris-HCl buffer, pH 8.0, mixed, and preincubated at 37°C.
Eight hundred microliters of this solution was added to samples to test subtilisin A (between 0.1 and 1.0 μg per assay), 50 μl of B. clausii O/C supernatants, 1 μl of the purified protease solution. Each reaction medium was incubated at 37°C. Absorbance was measured at 410 nm for 5 min using Tris-HCl buffer instead of enzymatic solution as a control. One unit of proteolytic activity is defined as the amount of enzyme that released 1 μmol of pNA per min at 37°C and pH 8.
Enzymatic activity assay was also performed with the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) at a 0.5 mM final concentration.
Cell line culture and assay of the cytotoxic supernatants.
Caco-2 cells (a cell lineage obtained from a human colon cancer was used as a model for the human intestine) and Vero cells (a well-established cell line obtained from kidney epithelium of the African green monkey that has been used extensively for testing cytotoxicity, including that of C. difficile strains) were grown in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Sigma-Aldrich, Saint Louis, MO, USA), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Gibco BRL, Grand Island, NY, USA), 0.5% (vol/vol) penicillin and streptomycin (Sigma-Aldrich, Saint Louis, MO, USA), and 1% (vol/vol) nonessential amino acids (Sigma-Aldrich, Saint Louis, MO, USA). Cells were cultivated at 37°C under 5% (vol/vol) CO2 atmosphere until confluence in 25-cm2 tissue culture flasks (Nalge Nunc, Penfield, NY, USA) before being seeded in culture plates. A 105 cellular suspension of cells was seeded into 12-well culture plates containing 2 ml of cell culture medium and then incubated until confluence. The culture medium was renewed every 2 days.
Measurements of supernatant cytotoxic activity.
Different quantities of supernatants of C. difficile (Cd-SN) and B. cereus (Bc-SN) were tested on cells to confirm their cytotoxicity, choose which dose would be used to obtain cytotoxic effect, and evaluate the protective effect of B. clausii O/C supernatant and purified protease (24, 36, 37).
(i) Vero cells.
To test the positive effects of the probiotic supernatant, OC-SN was mixed with Cd-SN in a proportion of 1:3 (vol/vol), whereas it was mixed in a proportion of 1:9 (vol/vol) for Bc-SN. Protective effects of the purified protease were also tested. The purified protease was dissolved in a 50 mM Tris-HCl-NaCl buffer, pH 7.5, so that its enzymatic activity equaled that of OC-SN. Reaction mixtures were incubated and shaken for 2 h at 37°C in the presence or absence of 1 mM PMSF, a serine protease inhibitor, or 1 mM EDTA, a metalloproteinase inhibitor. In order to study the potential effect of coincubation time, we also tested 15 min and 30 min of incubation between OC-SN and toxin supernatant. Final dilutions of 1/25 of treated Cd-SN and 1/2 of treated Bc-SN were placed in wells of Vero cells. As positive controls of toxicity, untreated Cd-SN and Bc-SN were used at the same final dilutions. Untreated OC-SN was also tested under the same conditions to verify the absence of cytotoxicity. Controls of bacterial culture media and/or inhibitors were used.
Additionally, to evaluate the possible effect of OC-SN on the potential cleavage of epithelial cell surface toxin receptors, Vero cells were preincubated with OC-SN for 12 h before treatment with the different cytotoxic supernatant mixtures.
Vero cells were incubated with 2 ml of culture cell media to which the different samples of toxic supernatants were added. The plates were incubated for 24 h with Cd-SN and for 4 h with Bc-SN at 37°C in an atmosphere of 5% (vol/vol) CO2. Every test was performed in triplicate.
(ii) Caco-2 cells.
Cd-SN was mixed with OC-SN in a proportion of 1:1 (vol/vol) and incubated for 2 h, with or without protease inhibitors. A final dilution of 1/20 of Cd-SN was applied on Caco-2 cells for 24 h. Every test was performed in triplicate.
After all treatments, cell viability was evaluated for cell detachment by using crystal violet staining and measuring the residual mitochondrial dehydrogenase activity as described below.
Vero and Caco-2 cell viability analysis. (i) Measurement of cell detachment using crystal violet stain.
The method described by Medrano et al. (37) was used. Shortly, cells were washed with phosphate-buffered saline (PBS) and fixed for 1 min with PBS–2% (vol/vol) formaldehyde, and then the cells were incubated for 20 min with a crystal violet solution (0.13% crystal violet, 5% ethanol, 2% formaldehyde in PBS, wt/vol/vol). After exhaustive washing with PBS, samples were treated for 1 h with 50% (vol/vol) ethanol in PBS. Absorbance was measured at 620 nm using a plate reader (Multiskan FC; Thermo Scientific, Waltham, MA, USA). The percentage of attached cells was calculated as 100 × (A/Ac), where A is the absorbance of treated cells and Ac is the absorbance of untreated control cells.
(ii) Mitochondrial dehydrogenase activity assay (MTT assay).
As previously reported by Medrano et al. (37), cells were washed with PBS and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT; Sigma-Aldrich, Saint Louis, MO, USA) for 4 h at 37°C (final concentration of 0.5 μg/ml in PBS). The MTT assay is based on conversion of MTT to an insoluble purple formazan by mitochondrial dehydrogenase activity, representative of cell viability. Stain was extracted with 0.1 N HCl in isopropanol. After centrifugation, absorbance was measured at 550 nm using a plate reader (Multiskan FC, Thermo Scientific, Waltham, MA, USA). The percentage of remaining activity was calculated as 100 × (A/Ac), where A is the absorbance of treated cells and Ac is the absorbance of untreated control cells.
(iii) Hemolysis assay.
Human blood, pretested for the absence of HIV or hepatitis virus infections and obtained from healthy volunteers (EFS Aquitaine, Bordeaux Blood Bank, Bordeaux, France), was used to evaluate the hemolysis generated by B. cereus 14579. The same treatments used for cell cytotoxicity assays were also used for the hemolysis test. One hundred microliters of human red blood cells (2.5 × 108 cells/ml) and 100 μl of the different samples were placed in a 96-well sterile polystyrene plate. After 1 h of incubation at room temperature, total released hemoglobin was measured at 415 nm (wavelength of hemoglobin absorbance) using a NanoDrop spectrophotometer (NanoDrop 1000; Thermo Scientific, Waltham, MA, USA). The hemoglobin concentration was calculated using 125 as the millimolar extinction coefficient for human hemoglobin (e.g., 128 μg/ml has an optical density of 1.0 at 415 nm) (38, 39). Hemolysis was also checked after 24 h of incubation, with high absorbance showing hemolysis of red blood cells. All analyses were performed on six independent assays.
Gene expression analysis of cells treated with C. difficile supernatants. (i) Cell treatments.
Caco-2 cells were grown as described above, and the same treatments that were used for cell cytotoxicity assays were employed. Cd-SN and cells treated with OC-SN (during 2 h at 37°C) in the presence or absence of 1 mM PMSF were coincubated with Caco-2 cells. After different times of incubation (1 h, 3 h, 6 h, and 20 h), cell morphological changes were observed using microscopy, and gene expression was measured by reverse transcription-quantitative PCR (RT-qPCR). All of the analyses were realized in triplicate.
(ii) RNA isolation and RT-qPCR analysis.
Total RNA from Caco-2 cells was isolated using the RNeasy minikit (Qiagen) according to the manufacturer's recommendations. RNA samples were treated with DNase using the Turbo RNA-free kit (Ambion, Austin, TX, USA). In order to verify RNA integrity, isolated RNA was subjected to 1.2% agarose formaldehyde denaturing gel electrophoresis. The gel was then stained with ethidium bromide and visualized under UV light. Total RNA was quantified using a NanoDrop 1000 (Thermo Scientific) and then normalized to perform the cDNA synthesis, from 1 μg of RNA, by two-step RT-PCR (50°C for 30 min and 85°C for 5 min) with Maxima RT (Fermentas, Vilnius, Lithuania) in an MJ Research PTC-200 PCR thermal cycler (MJ Research, St. Bruno, Canada). qPCR was performed from 1 μl of cDNA with a Maxima Sybr green kit (Fermentas, Vilnius, Lithuania) in 96-well full B/N PCR plates (Bio-Rad, Hercules, USA) on an Opticon Chromo4 (Bio-Rad, Hercules, USA). The thermal cycling consisted of an initial denaturing step at 95°C for 10 min followed by 40 cycles consisting of 95°C for 30 s, 60°C for 30 s, and 72°C for 45 s. Primers and annealing temperatures were used according Janvilisri et al. (6).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software, version 6.0 for Windows (San Diego, CA, USA). Two-tailed Student's t tests were performed for comparisons between the different groups.
RESULTS
Enzymatic activity and substrate specificity of the B. clausii O/C supernatants.
In the preliminary study of supernatants' enzymatic activity, we observed the proteolytic activity of both vegetative cells and spore supernatants. In this study, we characterized the substrate specificity of proteolytic secreted compounds.
The highest specificity was observed for N-Suc-Ala-Ala-Pro-Phe-pNA, a specific substrate for chymotrypsin-like serine proteases (Table 1), such as subtilisin A, produced by Bacillus licheniformis. No hydrolysis was observed for the substrate N-Suc-Phe-pNA, and a relatively low level was observed for N-Suc-Ala-Ala-Ala-pNA. Moreover, the OC-SN enzymatic activity was inhibited by PMSF, a serine protease inhibitor (Table 1).
TABLE 1.
Substrate specificity of B. clausii O/C supernatants toward p-nitroanilide (pNA)-conjugated synthetic peptide substrates
| Supernatant and/or enzyme | Substrate specificity (μmol · min−1 · ml−1) |
||
|---|---|---|---|
| N-Suc-Ala-Ala-Ala-pNA | N-Suc-Ala-Ala-Pro-Phe-pNA | N-Suc-Phe-pNA | |
| B. clausii vegetative cell supernatant | 1.05 × 10−3 | 13 × 10−3 | 0 |
| B. clausii vegetative cell supernatant plus PMSF | 0 | 0 | |
| B. clausii spore supernatant | 5.68 × 10−3 | 54 × 10−3 | 0 |
| B. clausii spore supernatant plus PMSF | 0 | 0 | |
| Subtilisin A | 32.0 × 10−3 | 0 | |
| Subtilisin A plus PMSF | 0 | ||
Vegetative cell supernatant presented approximately 4-fold less enzymatic activity than spore supernatant. Proteolytic compounds seemed to be more secreted during sporulation. Therefore, we decided to work only with the spores' supernatant for the rest of our study.
B. clausii O/C supernatant against the cytotoxic effects induced by C. difficile VPI 10463. (i) Study in Vero cell line.
Vero cells treated with C. difficile supernatant (positive control of toxicity) showed low viability, the percentage of cell attachment was 13.5% ± 0.7%, and the remaining mitochondrial dehydrogenase activity was 16.8% ± 0.9%. That is representative of a high rate of cell death related to C. difficile toxic factors (Fig. 1A and B). Treatment of Cd-SN with the B. clausii supernatant reduced the cytotoxic effects, and neither cell detachment nor loss of mitochondrial activity was observed. This effect depended on Cd-SN/OC-SN coincubation time; maximal protection was achieved when the mix was preincubated for 2 h before incubation with Vero cells. Shorter times of coincubation showed fewer protective effects of OC-SN against toxic Cd-SN, and no protective effects occurred when the mix was preincubated for only 15 or 30 min.
FIG 1.
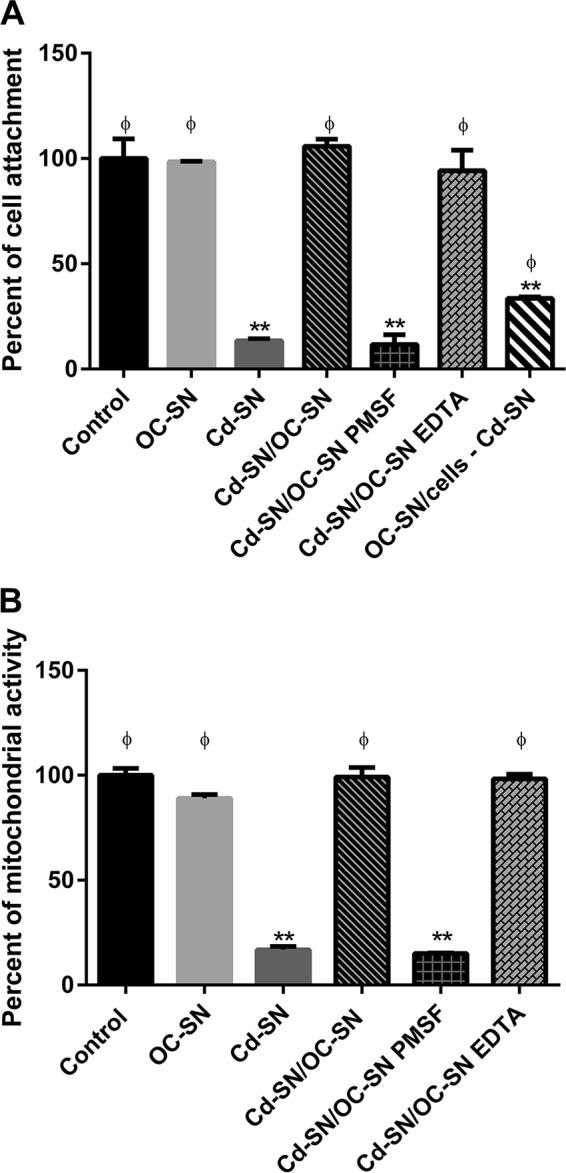
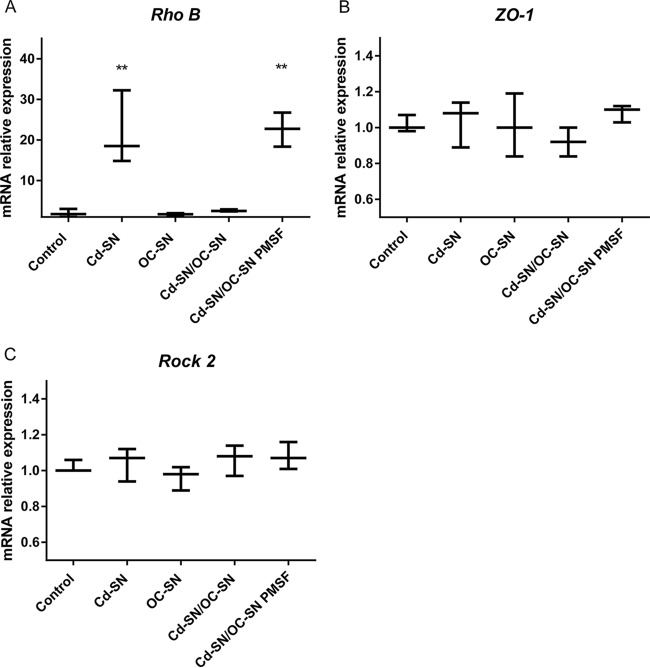
Effect of C. difficile VPI 10463 supernatant (Cd-SN) on Vero cell viability in the presence or absence of B. clausii O/C supernatant (OC-SN). Bars represent the percentage of cell attachment (A) or mitochondrial dehydrogenase activity (B) after incubation of Vero cells with the different treatments assayed (control, Cd-SN, OC-SN, and Cd-SN/OC-SN, with or without the protease inhibitors PMSF and EDTA). Vero cells were also pretreated with OC-SN before incubation with Cd-SN (OC-SN/cells – Cd-SN). The percentage of attachment or remaining mitochondrial dehydrogenase activity was calculated as 100 × (A/Ac), where A is the absorbance of treated cells and Ac is the absorbance of untreated control cells. Results were the averages ± standard deviations (SD) from 3 independent assays. **, significant differences from the corresponding control (P < 0.01); ϕ, significant differences from the Cd-SN condition (P < 0.01).
However, the addition of PMSF during the coincubation abolished the OC-SN beneficial effect (Fig. 1A and B, Cd-SN/OC-SN PMSF). Only 11.7% ± 3.3% of cells remained attached, and a very low level of mitochondrial dehydrogenase activity was observed. The addition of EDTA, a metalloprotease inhibitor (Fig. 1A and B, Cd-SN/OC-SN EDTA), did not affect the OC-SN protective effects.
In order to study if B. clausii supernatant has an effect on cellular toxin receptors, we pretreated Vero cells with OC-SN for 12 h before addition of the cytotoxic Cd-SN. We noticed a cell viability reduction of 33.5% ± 0.6% of cell attachment after 24 h of incubation (Fig. 1A, OC-SN/cells − Cd-SN).
(ii) Study in Caco-2 cell line.
The cytotoxic effects of Cd-SN observed in Caco-2 cells were similar to those observed in Vero cells. The protective effects of OC-SN, as well as the abolition of the protection by PMSF, were also comparable to the results obtained using Vero cells. In addition, microscopy studies showed that Cd-SN altered the morphology of the Caco-2 cell monolayer (cell rounding and cell detachment were observed). These changes were attenuated in the presence of OC-SN (data not shown).
Under our study conditions, the rhoB gene was differentially expressed after 20 h of contact of Caco-2 cells with Cd-SN compared to its expression in nontreated cells (Fig. 2). No significant changes were noticed in the transcription of this gene after 1 h, 3 h, and 6 h of contact (data not shown). C. difficile supernatant induced a 20-fold upregulation of rhoB. In Cd-SN/OC-SN-treated cells, the gene expression level was similar to that of untreated control cells. The addition of PMSF avoided the effect of B. clausii supernatant. No changes in expression were observed for the genes encoding ZO-1 and ROCK2.
FIG 2.
Effect of C. difficile VPI 10463 supernatant on the relative mRNA expression of Caco-2 cells in the presence or absence of B. clausii O/C supernatant. Cd-SN and OC-SN were preincubated (Cd-SN/OC-SN), with or without protease inhibitor (PMSF), before contact with cells. Bars represent the relative expression of genes (in the three independent assays performed) encoding RhoB (A), ZO-1 (B), and ROCK2 (C). **, significant differences from the corresponding control (P < 0.01).
B. clausii O/C supernatant against the cytotoxic effects induced by B. cereus ATCC 14579.
Vero cells treated with B. cereus supernatant (positive control of toxicity) showed low viability, the percentage of cell attachment was 12.3% ± 0.1%, and the remaining mitochondrial dehydrogenase activity was 22.4% ± 0.8% (Fig. 3A and B). Coincubation of Bc-SN with OC-SN avoided the deleterious effect induced by the Bc-SN, as demonstrated by the percentages of cell attachment (Fig. 3A) and mitochondrial activity (Fig. 3B). Furthermore, the time of coincubation of Bc-SN with OC-SN was crucial to counteract toxic effects of Bc-SN. Fifteen minutes of coincubation with the OC-SN was not sufficient to inactivate toxic supernatant, whereas after 30 min, cell attachment was increased to 73.4% ± 3.8% and at 2 h of coincubation to 100% (Fig. 3A). The addition of PMSF during coincubation abolished the OC-SN beneficial effect (Fig. 3A and B, Bc-SN/OC-SN PMSF). However, the addition of EDTA (Fig. 3A and B, Bc-SN/OC-SN EDTA) did not affect the OC-SN protective effects, and the cellular viability remained unaltered.
FIG 3.
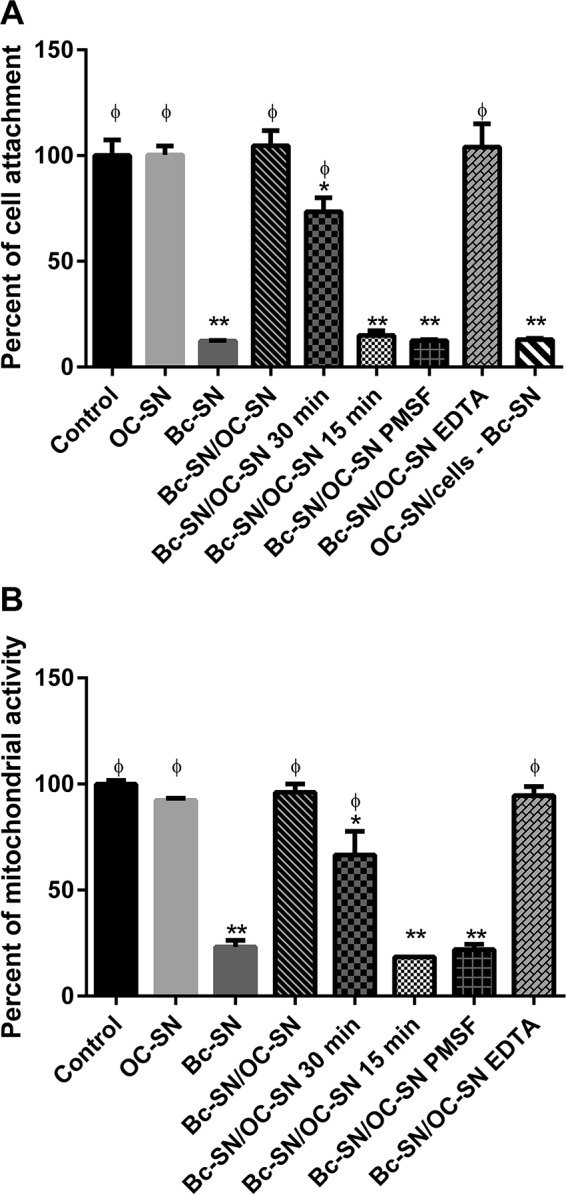
Effect of B. cereus ATCC 14579 supernatant (Bc-SN) on Vero cell viability in the presence or absence of B. clausii O/C supernatant (OC-SN). Different times of coincubation between Bc-SN and OC-SN were tested before incubation with Vero cells (15 min, 30 min, and 2 h). Bars represent the percentage of cell attachment (A) or mitochondrial dehydrogenase activity (B) of cells coincubated with Bc-SN, OC-SN, or Bc-SN/OC-SN with or without protease inhibitors (PMSF and EDTA). Vero cells were also pretreated with OC-SN before incubation with Bc-SN (OC-SN/cells – Bc-SN). The percentage of cell attachment or remaining mitochondrial dehydrogenase activity was calculated as 100 × (A/Ac), where A is the absorbance of treated cells and Ac is the absorbance of untreated control cells. Results were the averages ± SD from 3 independent assays. *, significant differences from the corresponding control (P < 0.05); **, significant differences from the corresponding control (P < 0.01); ϕ, significant differences from the Bc-SN condition (P < 0.01).
Vero cells, pretreated with OC-SN before contact with Bc-SN, presented the same viability percentage as cells directly treated with Bc-SN (Fig. 3A, OC-SN/cells − Bc-SN).
B. clausii O/C supernatant inhibited hemolysis induced by B. cereus extracellular factors.
It was interesting to study the capacity of B. clausii supernatant to counteract another type of toxin, like the hemolysin secreted by B. cereus. Figure 4 shows the strong hemolytic activity of B. cereus extracellular factors on human red blood cells, confirming the presence of a hemolysin. After 1 h of incubation between red blood cells and Bc-SN, the concentration of released hemoglobin was 242 ± 5 μg/ml. This hemolytic effect was totally inhibited when the red blood cells were treated with Bc-SN/OC-SN. In negative controls (OC-SN, MH culture medium, OC-SN PMSF, or OC-SN EDTA), released hemoglobin was not detected.
FIG 4.
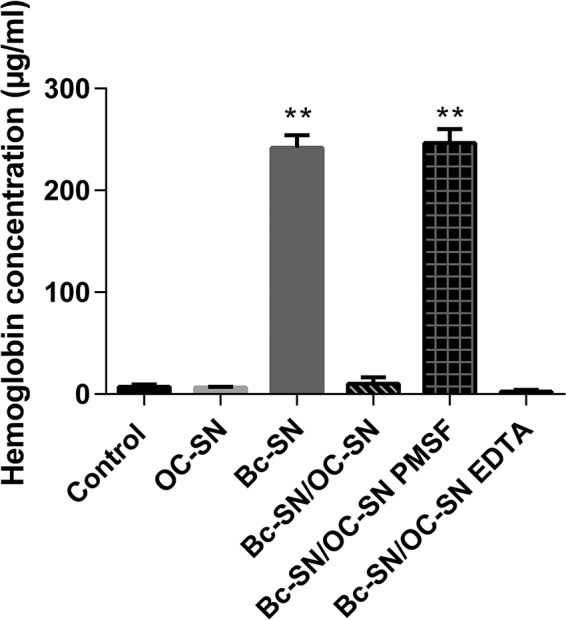
Effect of B. cereus ATCC 14579 supernatant (Bc-SN) on human red blood cells in the presence or absence of B. clausii O/C supernatant (OC-SN). Bars represent the concentration of released hemoglobin when red blood cells were coincubated with Bc-SN, OC-SN, or Bc-SN/OC-SN with or without protease inhibitors (PMSF and EDTA). Results are averages ± SD from 6 values. **, significant differences from the corresponding control (P < 0.01).
Moreover, similar to Vero cell cytotoxicity assays, the protective effect of B. clausii supernatant was counteracted by PMSF but not affected by EDTA.
Characterization of the active secreted compound against C. difficile and B. cereus toxins.
The present results showed a positive effect of B. clausii supernatant against the cytotoxicity of the two pathogens.
The above-described study showed high proteolytic activity of B. clausii O/C supernatants, and we suspected the role of a secreted protease in this beneficial effect. Consequently, proteins secreted by B. clausii were analyzed by SDS-PAGE (Fig. 5), and the major protein, observed at 27 kDa, was identified by MS/MS spectrometry as the M-protease (GenBank accession number Q99405, locus PRTM_BACSK) encoded by the aprE gene with a Mascot score of 1,401, with 17 identified peptides and a coverage of 37.1%. This protein is well described in the UniProt database. This protein of 380 amino acids, for a molecular mass of 38.9 kDa, is processed through signal peptide and propeptide cleavages, leading to the mature protease of 269 amino acids. The resulting active protease has a molecular mass of 26.7 kDa. These data are in good agreement with apparent masses observed in SDS-PAGE. Relating coverage to the active chain leads to a corrected coverage of 52.4% (data not shown).
FIG 5.
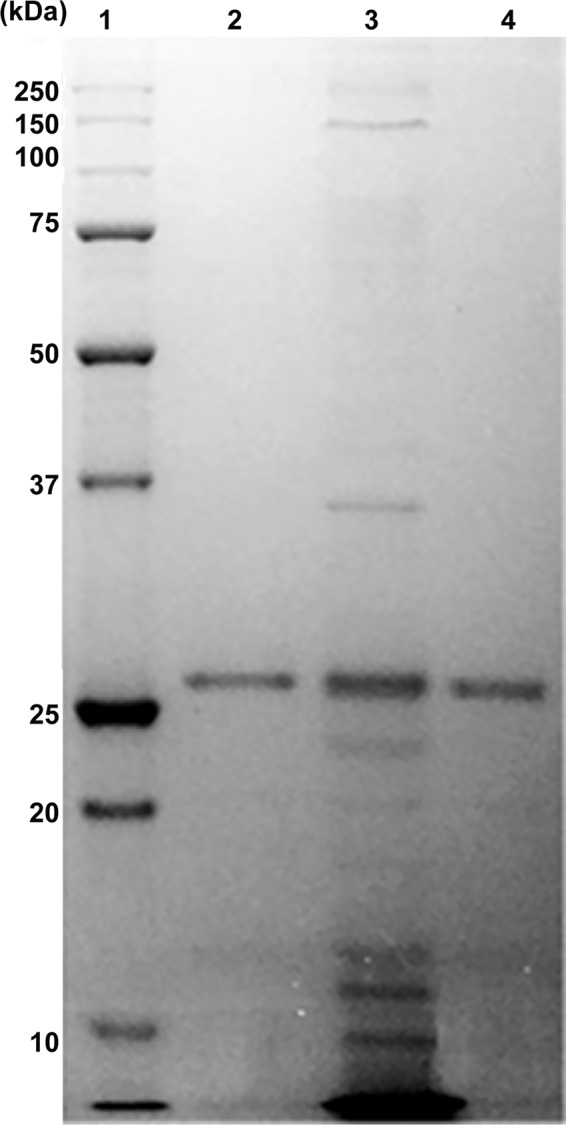
SDS-PAGE of proteins and purified protease secreted by B. clausii O/C. Lane 1, molecular mass marker proteins (Precision Plus Protein standard; Bio-Rad, France); lanes 2 and 4, size exclusion chromatography-purified protease; lane 3, B. clausii O/C spore culture supernatant (crude extract).
We purified this protein to homogeneity by size exclusion chromatography to study its involvement in the observed antitoxic activity. After purification (Table 2 and Fig. 5), the enzymatic specific activity of the purified protease was 13-fold higher than the spore supernatant activity (Table 2).
TABLE 2.
Purification of the 27-kDa protease from B. clausii culture supernatant
| Step | Total amt of proteins (mg) | Total acta (U) | Sp act (U · mg of protein−1) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Culture supernatant | 3.80 | 37.02 | 9.74 | 100 | |
| Ultrafiltration concentrate | 2.85 | 38.20 | 13.40 | 103 | 1.37 |
| Superdex 75 chromatography | 0.175 | 22.67 | 129.54 | 61.2 | 13.30 |
One unit of activity was defined as the amount of enzyme that released 1 μmol of pNA per min at 37°C and pH 8.
B. clausii O/C purified protease against the cytotoxic effects induced by C. difficile VPI 10463 and B. cereus ATCC 14579.
Following these results, cytotoxicity experiments were conducted with the purified M-protease.
Vero cells treated with C. difficile and B. cereus supernatants (positive controls of toxicity) showed low viability, with a percentage of cell attachment of 18.3% ± 4.0% for C. difficile and 13.7% ± 0.9% for B. cereus (Fig. 6 and 7). Coincubation of Cd-SN and Bc-SN with the purified M-protease of B. clausii avoided the cytotoxic effects induced by pathogen supernatants, as demonstrated by the high percentages of cell attachment (Fig. 6 and 7).
FIG 6.
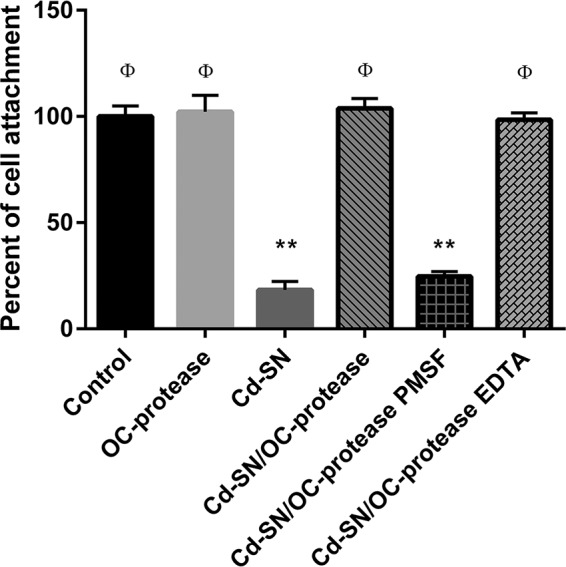
Effect of C. difficile VPI 10463 supernatant (Cd-SN) on Vero cells viability in the presence or absence of B. clausii O/C purified protease (OC-protease). Bars represent the percentage of cell attachment of cells coincubated with Cd-SN, OC-protease, or Cd-SN/OC-protease with or without protease inhibitors (PMSF and EDTA). The percentage of cell attachment was calculated as 100 × (A/Ac), where A is the absorbance of treated cells and Ac is the absorbance of untreated control cells. Results were averages ± SD from 4 independent assays. **, significant differences from the corresponding control (P < 0.01); ϕ, significant differences from the Cd-SN condition (P < 0.01).
FIG 7.
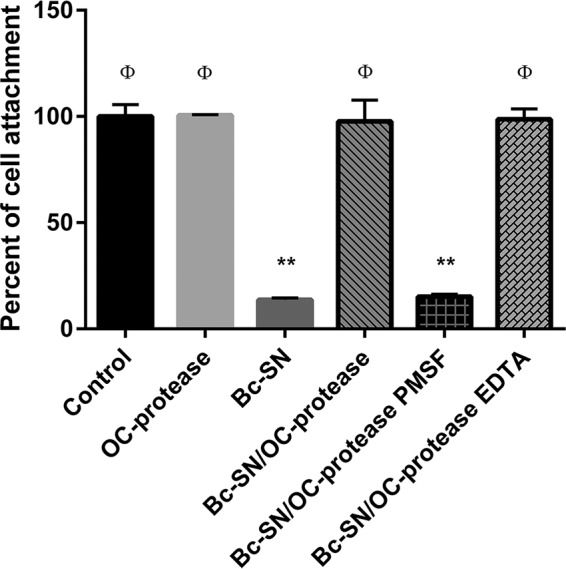
Effect of B. cereus ATCC 14579 supernatant (Bc-SN) on Vero cell viability in the presence or absence of B. clausii O/C purified protease (OC-protease). Bars represent the percentage of cell attachment of cells coincubated with Bc-SN, OC-protease, or Bc-SN/OC-protease with or without protease inhibitors (PMSF and EDTA). The percentage of cell attachment was calculated as 100 × (A/Ac), where A is the absorbance of treated cells and Ac is the absorbance of untreated control cells. Results were averages ± SD from 4 independent assays. **, significant differences from the corresponding control (P < 0.01); ϕ, significant differences from the Bc-SN condition (P < 0.01).
The addition of PMSF during coincubation abolished the protease beneficial effect (Fig. 6 and 7, Cd-SN/OC-protease PMSF and Bc-SN/OC-protease PMSF). On the contrary, the addition of EDTA had no effect (Fig. 6 and 7, Cd-SN/OC-protease EDTA and Bc-SN/OC-protease EDTA) and the cellular viability remained unaltered.
DISCUSSION
Bacillus-based probiotics present an advantage because of the inherent resistance of Bacillus spores (40). Although the Bacillus clausii preparation Enterogermina has been described as an antidiarrheal that also prevents antibiotic-associated diarrhea (28, 29), data describing the functional mechanisms remain very limited. Probiotics may interfere with pathogen invasion by reducing or inhibiting pathogen adherence, producing antimicrobials, or interfering with toxins (41). Indeed, some pathogens, such as C. difficile, B. cereus, Vibrio cholerae, and Escherichia coli, may secrete toxins that are implicated in bacterial virulence (11, 42–44). Proteins secreted and released into the environment might mediate some of the positive effects observed in probiotics (45, 46). In the past, Castagliuolo et al. (25, 26) indicated that a probiotic strain of the yeast Saccharomyces boulardii can excrete a serine protease of 54 kDa that can hydrolyze C. difficile toxin A (which is resistant to trypsin) and toxin B and also can break down the toxin receptor.
In preliminary studies, we looked for functional mechanisms of B. clausii probiotic strains and demonstrated that B. clausii O/C releases an antimicrobial substance(s) active against C. difficile (31). Later, we purified and characterized, from culture supernatant, a bacteriocin, the clausin (47), that inhibits C. difficile strains at potentially useful MICs (0.5 to 1 μg/ml). In the present work, we looked for other mechanisms that could be involved in the probiotic's action against C. difficile and focused our research on the antitoxic activity of the secreted compounds.
In order to experiment on the potential antitoxic effect of compounds secreted by B. clausii O/C, we verified that the OC-SN, supplemented with the protease inhibitors or left unsupplemented, was not cytotoxic and then studied its efficacy against the cytotoxicity of the C. difficile and B. cereus supernatants.
In agreement with the Castagliuolo et al. study on the Saccharomyces boulardii protease (25, 26), secreted compounds of O/C seemed to counteract the cytotoxic effects of C. difficile toxins. However, in our experiment, pretreatment of Vero cells with OC-SN before toxin treatment only slightly abolished the C. difficile cytotoxic effects. Unlike the Saccharomyces boulardii protease (48), the compounds secreted by strain O/C apparently had only a partial effect on the cleavage of the C. difficile toxin receptor sites from the surface of epithelial cells. It may lack a cofactor needed to enhance the secreted compound action, or the time of pretreatment of Vero cells with the OC-SN, before toxin treatment, may not have been appropriate.
Intestinal epithelial cells are targets for enterotoxins. The treatment of Caco-2 and Vero cells with Cd-SN damaged cell integrity and induced cell death. The interaction of enterotoxins with the intestinal mucosa either leads to a direct effect on the cell membrane or has an effect on signal transduction within eukaryotic cells (49). After initial binding with the receptor, C. difficile toxins are internalized and inactivate Rho proteins by glucosylation (50–52). The inactivation of Rho proteins causes the loss of epithelial barrier function, since these small GTPases are critical regulators of tight junction function and are involved in actin polymerization (53).
Cd-SN induced a great increase of rhoB expression. Our results were consistent with previous studies reporting that C. difficile TcdA induces strong upregulation of rhoB in Caco-2 cells (6, 7). When Cd-SN was treated with OC-SN, rhoB expression returned to basal levels. These data indicated that the compounds secreted by B. clausii could damage C. difficile toxins, thus preventing these toxins from binding with cell receptors, being internalized, or glucosylating RhoB.
In addition, Janvilisri et al. (6) found that genes encoding ROCK2 (Rho-associated protein kinase 2, which phosphorylates a large number of important signaling proteins) and ZO-1 (tight-junction protein) were downregulated during C. difficile infection. In our experiment, the expression of these genes was not modified, perhaps because we used a toxic culture supernatant and did not infect the Caco-2 cells directly with C. difficile.
Interestingly, the same protective effect of O/C supernatant was observed against the B. cereus supernatant. The hemolytic activity of the B. cereus strain was also abolished by the compounds secreted by O/C. We noticed the importance of coincubation time between the probiotic supernatant and toxic supernatant to obtain a maximum effect. The action of secreted compounds increased with time until reaching a plateau. Otherwise, preincubation of Vero cells with the probiotic supernatant did not protect cells against B. cereus toxins. In fact, Nhe and HBL enterotoxins are known to form pores in cell membranes. Stenfors Arnesen et al. concluded that the binding is independent of cell membrane receptors (15). More recently, Jeßberger et al. speculated on the existence of a specific enterotoxin receptor for Nhe and probably for HBL, but for now no receptor has been characterized (54).
Since PMSF, a serine protease inhibitor, abolished all antitoxic effects of B. clausii supernatant observed against both C. difficile and B. cereus supernatants, we can assume that a serine protease secreted by the O/C strain is involved in the inactivation of toxins normally cleaving them. No effect of EDTA, a metalloprotease inhibitor, was observed.
After identification of a serine protease in the supernatant, we chose to purify the enzyme to confirm its potential involvement in the supernatant's antitoxic activity.
In this paper, we have demonstrated that the strain secretes a functional protease. This enzyme is a serine protease, the M-protease, mainly produced during the sporulation phase. The enzyme hydrolyzes the peptide bonds on the carboxylic side, after a hydrophobic amino acid, and requires a minimum chain length of ≥3 amino acid residues to express its specificity.
Our results showed a specificity similar to that of subtilisin A produced by Bacillus licheniformis. In addition, Kazan et al. (55) have already characterized a serine alkaline protease from B. clausii GMBAE 42 with activity and specificity very close to those of our protease.
After purification, cytotoxicity experiments on Vero cells were performed with the two pathogen supernatants using the purified protease. The same protective effects against toxins were observed with the purified M-protease. These effects were canceled in the presence of PMSF.
The presented results demonstrated that the protease secreted by B. clausii O/C had a strong protective effect against cytological damages induced by both B. cereus and C. difficile toxic supernatants. All of these data tend to prove the important role of the M-protease in probiotic effects of the O/C strain against toxins. However, we cannot exclude the possibility that other secreted compounds could have an action or a synergistic effect with the protease.
It would be interesting to test the purified protease on other toxins. While we demonstrated an antitoxic activity of this enzyme in vitro, we still have to explore its effect in vivo.
Conclusions.
B. clausii O/C supernatant reduces the cytotoxic effects of C. difficile and B. cereus toxins through the secreted alkaline serine M-protease. Moreover, in the past, we demonstrated that the O/C strain produces an anti-C. difficile substance, the bacteriocin clausin. All of these secreted compounds together become a strong tool to fight against enterotoxigenic pathogens, such as C. difficile, and can at least be partially involved in the clinical effects observed for Enterogermina. Our study opens perspectives for the therapeutic benefits of this probiotic in the treatment of C. difficile-associated diarrhea and should encourage further in vivo studies.
ACKNOWLEDGMENTS
We thank F. Megraud at the Service de Microbiologie, CHU de Bordeaux, for providing C. difficile strain VPI 10463 and Emilie Pinault, responsible of the Service Commun de Recherche et d'Analyses de Biomolécules de Limoges (Université de Limoges), for MS/MS identification of the protease.
Conseil Régional d'Aquitaine and Bordeaux Sciences Agro provided partial grants to Gabrielle Ripert and Silvia M. Racedo.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Kyne L, Hamel MB, Polavaram R, Kelly CP. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 2.Spigaglia P, Barbanti F, Castagnola E, Bandettini R. 2015. Clostridium difficile infection (CDI) in children due to hypervirulent strains PCR-ribotype 027: an emblematic report of two cases. Anaerobe 36:91–93. doi: 10.1016/j.anaerobe.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Yakob L, Riley TV, Paterson DL, Marquess J, Magalhaes RJ, Furuya-Kanamori L, Clements AC. 2015. Mechanisms of hypervirulent Clostridium difficile ribotype 027 displacement of endemic strains: an epidemiological model. Sci Rep 5:12666. doi: 10.1038/srep12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vindigni SM, Surawicz CM. 2015. C. difficile infection: changing epidemiology and management paradigms. Clin Transl Gastroenterol 6:e99. doi: 10.1038/ctg.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen A. 2012. Clostridium difficile toxins: mediators of inflammation. J Innate Immun 4:149–158. doi: 10.1159/000332946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janvilisri T, Scaria J, Chang YF. 2010. Transcriptional profiling of Clostridium difficile and Caco-2 cells during infection. J Infect Dis 202:282–290. doi: 10.1086/653484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhard R, Tatge H, Genth H, Thum T, Borlak J, Fritz G, Just I. 2005. Clostridium difficile toxin A induces expression of the stress-induced early gene product RhoB. J Biol Chem 280:1499–1505. doi: 10.1074/jbc.M406014200. [DOI] [PubMed] [Google Scholar]
- 8.Popoff MR, Geny B. 2009. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta 1788:797–812. doi: 10.1016/j.bbamem.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Popoff MR, Geny B. 2011. Rho/Ras-GTPase-dependent and -independent activity of clostridial glucosylating toxins. J Med Microbiol 60:1057–1069. doi: 10.1099/jmm.0.029314-0. [DOI] [PubMed] [Google Scholar]
- 10.Aschenbach JR, Seidler T, Ahrens F, Schrödl W, Buchholz I, Garz B, Krüger M, Gäbel G. 2003. Luminal Salmonella endotoxin affects epithelial and mast cell function in the proximal colon of pigs. Scand J Gastroenterol 38(7):719–726. doi: 10.1080/00365520310003129. [DOI] [PubMed] [Google Scholar]
- 11.Madhavan TP, Sakellaris H. 2015. Colonization factors of enterotoxigenic Escherichia coli. Adv Appl Microbiol 90:155–197. doi: 10.1016/bs.aambs.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Gray MD, Lacher DW, Leonard SR, Abbott J, Zhao S, Lampel KA, Prothery E, Gouali M, Weill FX, Maurelli AT. 2015. Prevalence of Shiga toxin-producing Shigella species isolated from French travellers returning from the Caribbean: an emerging pathogen with international implications. Clin Microbiol Infect 21(8):765.e9–765.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granum PE, Brynestad S, Kramer JM. 1993. Analysis of enterotoxin production by Bacillus cereus from dairy products, food poisoning incidents and non-gastrointestinal infections. Int J Food Microbiol 17:269–279. doi: 10.1016/0168-1605(93)90197-O. [DOI] [PubMed] [Google Scholar]
- 14.Ehling-Schulz M, Fricker M, Scherer S. 2004. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res 48:479–487. doi: 10.1002/mnfr.200400055. [DOI] [PubMed] [Google Scholar]
- 15.Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606. doi: 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- 16.Beecher DJ, Macmillan JD. 1991. Characterization of the components of hemolysin BL from Bacillus cereus. Infect Immun 59:1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beecher DJ, Schoeni JL, Wong AC. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect Immun 63:4423–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman G. 2012. The role of probiotics in the prevention and treatment of antibiotic-associated diarrhea and Clostridium difficile colitis. Gastroenterol Clin North Am 41:763–779. doi: 10.1016/j.gtc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kolling GL, Wu M, Warren CA, Durmaz E, Klaenhammer TR, Guerrant RL. 2012. Lactic acid production by Streptococcus thermophilus alters Clostridium difficile infection and in vitro toxin A production. Gut Microbes 3:523–529. doi: 10.4161/gmic.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Agriculture Organization of the United Nations (FAO). 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. FAO, Rome, Italy. [Google Scholar]
- 21.Satish Kumar R, Kanmani P, Yuvaraj N, Paari KA, Pattukumar V, Arul V. 2011. Lactobacillus plantarum AS1 binds to cultured human intestinal cell line HT-29 and inhibits cell attachment by enterovirulent bacterium Vibrio parahaemolyticus. Lett Appl Microbiol 53:481–487. doi: 10.1111/j.1472-765X.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 22.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. 2009. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 23.Kakisu E, Abraham AG, Tironi Farinati C, Ibarra C, De Antoni GL. 2012. Lactobacillus plantarum isolated from kefir protects Vero cells from cytotoxicity by type-II Shiga toxin from Escherichia coli O157:H7. J Dairy Res 80:64–71. [DOI] [PubMed] [Google Scholar]
- 24.Carasi P, Trejo FM, Pérez PF, De Antoni GL, de los Serradell MA. 2012. Surface proteins from Lactobacillus kefir antagonize in vitro cytotoxic effect of Clostridium difficile toxins. Anaerobe 18:135–142. doi: 10.1016/j.anaerobe.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. 1996. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun 64:5225–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. 1999. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun 67:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasteyre A, Barc MC, Karjalainen T, Bourlioux P, Collignon A. 2002. Inhibition of in vitro cell adherence of Clostridium difficile by Saccharomyces boulardii. Microb Pathog 32:219–225. doi: 10.1006/mpat.2002.0495. [DOI] [PubMed] [Google Scholar]
- 28.Mazza P. 1994. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm 133:3–18. [PubMed] [Google Scholar]
- 29.Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, Cammarota G, Gasbarrini G, Gasbarrini A. 2004. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther 20:1181–1188. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- 30.Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. 2005. Bacillus clausii exerts immuno-modulatory activity in allergic subjects: a pilot study. Eur Ann Allergy Clin Immunol 37:129–134. [PubMed] [Google Scholar]
- 31.Urdaci MC, Bressollier P, Pinchuk I. 2004. Bacillus clausii probiotic strains: antimicrobial and immunomodulatory activities. J Clin Gastroenterol 38:S86–S90. doi: 10.1097/01.mcg.0000128925.06662.69. [DOI] [PubMed] [Google Scholar]
- 32.Bouhss A, Al-Dabbagh B, Vincent M, Odaert B, Aumont-Nicaise M, Bressolier P, Desmadril M, Mengin-Lecreulx D, Urdaci MC, Gallay J. 2009. Specific interactions of clausin, a new lantibiotic, with lipid precursors of the bacterial cell wall. Biophysics J 97:1390–1397. doi: 10.1016/j.bpj.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson S, Burman LG, Akerlund T. 1999. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology (Reading, Engl) 145(Part 7):1683–1693. doi: 10.1099/13500872-145-7-1683. [DOI] [PubMed] [Google Scholar]
- 34.Ivanova N, Sorokin A, Anderson I, Galleron N, Candelon B, Kapatral V, Bhattacharyya A, Reznik G, Mikhailova N, Lapidus A, Chu L, Mazur M, Goltsman E, Larsen N, D'Souza M, Walunas T, Grechkin Y, Pusch G, Haselkorn R, Fonstein M, Ehrlich SD, Overbeek R, Kyrpides N. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87–91. doi: 10.1038/nature01582. [DOI] [PubMed] [Google Scholar]
- 35.Larsen MD, Kristiansen KR, Hansen TK. 1998. Characterization of the proteolytic activity of starter cultures of Penicillium roqueforti for production of blue veined cheeses. Int J Food Microbiol 43:215–221. doi: 10.1016/S0168-1605(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 36.Minnaard J, Humen M, Pérez PF. 2001. Effect of Bacillus cereus exocellular factors on human intestinal epithelial cells. J Food Prot 64(10):1535–1541. [DOI] [PubMed] [Google Scholar]
- 37.Medrano M, Pérez PF, Abraham AG. 2008. Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int J Food Microbiol 122:1–7. doi: 10.1016/j.ijfoodmicro.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Keller MA, Addya S, Vadigepalli R, Banini B, Delgrosso K, Huang H, Surrey S. 2006. Transcriptional regulatory network analysis of developing human erythroid progenitors reveals patterns of coregulation and potential transcriptional regulators. Physiol Genomics 28:114–128. doi: 10.1152/physiolgenomics.00055.2006. [DOI] [PubMed] [Google Scholar]
- 39.Vinogradov SN, Sharma PK. 1994. Preparation and characterization of invertebrate globin complexes. Methods Enzymol 231:112–124. doi: 10.1016/0076-6879(94)31010-6. [DOI] [PubMed] [Google Scholar]
- 40.Sella SR, Vandenberghe LP, Soccol CR. 2014. Life cycle and spore resistance of spore-forming Bacillus atrophaeus. Microbiol Res 169(12):931–939. doi: 10.1016/j.micres.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Collado MC, Meriluoto J, Salminen S. 2007. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol 45:454–460. doi: 10.1111/j.1472-765X.2007.02212.x. [DOI] [PubMed] [Google Scholar]
- 42.Rai AK, Chattopadhyay K. 2015. Vibrio cholerae cytolysin: structure-function mechanism of an atypical β-barrel pore-forming toxin. Adv Exp Med Biol 842:109–125. doi: 10.1007/978-3-319-11280-0_7. [DOI] [PubMed] [Google Scholar]
- 43.Jeßberger N, Krey VM, Rademacher C, Böhm ME, Mohr AK, Ehling-Schulz M, Scherer S, Märtlbauer E. 2015. From genome to toxicity: a combinatory approach highlights the complexity of enterotoxin production in Bacillus cereus. Front Microbiol 6:560. doi: 10.3389/fmicb.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janoir C. 2016. Virulence factors of Clostridium difficile and their role during infection. Anaerobe 37:13–24. doi: 10.1016/j.anaerobe.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez B, Urdaci MC, Margolles A. 2010. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosal-bacteria interactions. Microbiology 156:3232–3242. doi: 10.1099/mic.0.044057-0. [DOI] [PubMed] [Google Scholar]
- 46.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. 2007. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bressolier P, Brugo MA, Robineau P, Schmitter M, Sofeir M, Urdaci M. April 2007. Peptide compound with biological activity, its preparation and application. WIPO PCT/IB2007/0022003. [Google Scholar]
- 48.Pothoulakis C, Kelly CP, Joshi MA, Gao N, O'Keane CJ, Castagliuolo I, Lamont JT. 1993. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 104(4):1108–1115. [DOI] [PubMed] [Google Scholar]
- 49.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. 2015. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun 83(1):138–145. doi: 10.1128/IAI.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies AH, Roberts AK, Shone CC, Acharya KR. 2011. Super toxins from a super bug: structure and function of Clostridium difficile toxins. Biochem J 436(3):517–526. doi: 10.1042/BJ20110106. [DOI] [PubMed] [Google Scholar]
- 51.Shuyi C, Chunli S, Haiying W, Jufang W. 2015. The Role of Rho GTPases in toxicity of Clostridium difficile toxins. Toxins (Basel) 7(12):5254–5267. doi: 10.3390/toxins7124874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popoff MR, Geny B. 2011. Rho/Ras-GTPase-dependent and -independent activity of clostridial glucosylating toxins. J Med Microbiol 60(Part 8):1057–1069. doi: 10.1099/jmm.0.029314-0. [DOI] [PubMed] [Google Scholar]
- 53.Jank T, Giesemann T, Aktories K. 2007. Rho-glucosylating Clostridium difficile toxins A and B: new insights into structure and function. Glycobiology 17:15R–22R. doi: 10.1093/glycob/cwm004. [DOI] [PubMed] [Google Scholar]
- 54.Jeßberger N, Dietrich R, Bock S, Didier A, Märtlbauer E. 2014. Bacillus cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon 77:49–57. doi: 10.1016/j.toxicon.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 55.Kazan D, Akın Denizci Astring-name>, Kerimak Öner MN, Erarslan A. 2005. Purification and characterization of a serine alkaline protease from Bacillus clausii GMBAE 42. J Ind Microbiol Biotechnol 32:335–344. doi: 10.1007/s10295-005-0260-z. [DOI] [PubMed] [Google Scholar]