Abstract
Eravacycline and comparators were tested against carbapenem- and tigecycline-resistant Enterobacteriaceae and Acinetobacter isolates received at the United Kingdom's national reference laboratory. Eravacycline MICs correlated closely with those of tigecycline but mostly were around 2-fold lower; both molecules retained full activity against isolates with high-level tetracycline and minocycline resistance. MIC90s of eravacycline and tigecycline were raised ca. 2-fold for carbapenem-resistant Enterobacteriaceae compared with carbapenem-susceptible controls, probably reflecting subsets of isolates with increased efflux.
TEXT
Carbapenemase-producing Enterobacteriaceae (CPE) present a growing challenge, as do strains that combine porin loss with AmpC or extended-spectrum β-lactamase (ESBL) activity. Many are susceptible only to tigecycline, colistin, and fosfomycin.
Tigecycline evades the Tet(A) to Tet(E) efflux pumps and ribosome protection mechanisms that cause most tetracycline resistance, but its utility as monotherapy is compromised by (i) disputed breakpoints for Enterobacteriaceae (U.S. Food and Drug Administration [FDA], susceptible [S], ≤2, intermediate [I], 4, and resistant [R], >4 [1]; European Committee on Antimicrobial Susceptibility Testing [EUCAST] [http://www.eucast.org], S, ≤1, I, 2, and R, >2; with no Clinical and Laboratory Standards Institute [CLSI] values), (ii) a lack of breakpoints for Acinetobacter baumannii, (iii) low serum drug peaks, and (iv) an FDA warning of excess mortality (1–5). Despite these concerns, case series suggest that patients with severe CPE infections respond better to colistin-tigecycline combinations than to colistin alone (6, 7).
Eravacycline (TP-434) is a new synthetic “fluorocycline” active against most Gram-negative species (8), again including those with acquired tetracycline efflux pumps and ribosomal protection. It is well tolerated, with simpler pharmacokinetics than tigecycline and higher serum drug levels (9). At 1 mg/kg of body weight intravenous (i.v.) every 12 h (q12h), eravacycline proved noninferior to ertapenem in a phase III trial for complicated intra-abdominal infection (9). A second phase III trial failed to establish eravacycline (1.5 mg/kg i.v. q24h, with step-down to 200 mg oral [p.o.] q12h from day 3) as noninferior to levofloxacin in complicated urinary tract infection, although revised regimens continue to merit study (10).
Against this background, we tested eravacycline in vitro against circulating carbapenem-resistant Enterobacteriaceae and A. baumannii isolates from the United Kingdom and sought to define the interrelationship between eravacycline and tigecycline MICs. The test organisms (n = 369) (Table 1) were recent submissions from United Kingdom clinical diagnostic laboratories to the national reference laboratory. For Enterobacteriaceae, “carbapenem resistant” was defined as resistant at least to ertapenem, as tested by British Society for Antimicrobial Chemotherapy (BSAC) agar dilution methodology (11). Carbapenemase genes were identified by PCR (12). Carbapenem resistance contingent on porin loss plus AmpC or ESBL activity was inferred from the absence of carbapenemase genes together with appropriate cefotaxime-cloxacillin or oxyimino-cephalosporin-clavulanate synergy. Isolates included specifically for tigecycline nonsusceptibility (Tigr) (Table 1) were chosen based on MICs of ≥2 μg/ml by BSAC agar dilution; other organisms were chosen without reference to previous tigecycline MICs. Controls were chosen as carbapenem and tigecycline susceptible and as lacking ESBLs or copious AmpC. MICs were determined by CLSI broth microdilution (13) using plates (Thermofisher, Oakwood Village, OH) containing eravacycline and tigecycline (both 0.06 to 16 μg/ml), minocycline (0.12 to 64 μg/ml), and tetracycline (0.25 to 16 μg/ml). Results were reviewed against EUCAST breakpoints (http://www.eucast.org [values as of the end of 2015]) since EUCAST, unlike CLSI, has values for tigecycline as the major comparator.
TABLE 1.
Relevant phenotypic characteristics of the panel of isolates tested
| Enzyme and/or characteristic | No. of isolates with characteristic |
||||||
|---|---|---|---|---|---|---|---|
| A. baumannii | Citrobacter | E. coli | Enterobacter | Klebsiella | Proteeae | Serratia | |
| KPC | 3 | 10 | 10 | 20a | 2 | ||
| VIM | 4 | 10 | 10 | 20 | 1 | ||
| IMP | 5 | 10 | |||||
| NDM | 5 | 10 | 10 | 20b | 8 | 2 | |
| OXA-48 | 2 | 10 | 10 | 20c | 1 | 2 | |
| Porin loss + AmpCd | 10 | ||||||
| Porin loss + ESBL | 10 | 20 | |||||
| Tigre + carbapenemase | 10 | 20 | |||||
| OXA-23/40/51d/58f | 39 | ||||||
| Tigrg + OXA-23 | 5 | ||||||
| Carbapenem susceptible | 10 | 2 | 10 | 10 | 10 | 5 | 3 |
Ten also with SHV ESBLs.
One also with OXA-48.
Eight also with ESBLs.
Hyperproduced.
Found tigecycline nonsusceptible (MIC of ≥2 μg/ml) by EUCAST criteria on previous BSAC agar dilution testing. Seven isolates had KPC, 9 had NDM, 7 had OXA-48, and 5 had VIM enzymes. All other groups were included without reference to prior tigecycline (or other tetracycline) results.
Nine or ten representatives of each OXA-carbapenemase type listed.
Found tigecycline nonsusceptible (MIC of ≥2 μg/ml) by EUCAST criteria for Enterobacteriaceae on previous BSAC agar dilution testing.
Meropenem (0.03 to 128 μg/ml), amikacin (0.25 to 128 μg/ml), levofloxacin (0.03 to 32 μg/ml), colistin (0.12 to 32 μg/ml), and fosfomycin (8 to 64 μg/ml) were included as additional comparators, and the proportions of the carbapenem-resistant Enterobacteriaceae that were nonsusceptible (intermediate plus resistant) were as follows: amikacin, 27.7%; colistin, 10.6% (excluding Proteeae and Serratia spp.); fosfomycin, 43.7%; levofloxacin, 58.9%; and meropenem, 69.3%. Isolates with NDM carbapenemases were the most multiresistant, with the following proportions nonsusceptible: amikacin, 65.3%; colistin, 7.7% (excluding Proteeae and Serratia spp.); fosfomycin, 36.7%; levofloxacin, 75.5%; and meropenem, 93.9%. Among carbapenem-resistant A. baumannii strains, the proportions nonsusceptible were as follows: amikacin, 66%; colistin, 8%; levofloxacin, 96%; and meropenem, 100%; A. baumannii is inherently resistant to fosfomycin. All of the control Enterobacteriaceae were susceptible to comparators, except (i) Proteeae and Serratia spp. were inherently resistant to colistin, (ii) one Escherichia coli isolate was resistant to colistin at EUCAST's 2-μg/ml breakpoint, and (iii) a few isolates were resistant to fosfomycin. Two of the 10 carbapenem-susceptible A. baumannii controls were nonsusceptible to amikacin, and one was nonsusceptible to levofloxacin.
Eravacycline MICs for the Enterobacteriaceae series (excluding Proteeae, discussed below) were unimodally distributed, as were those of tigecycline (Table 2). Minocycline distributions were unimodal, but with more positive skew (i.e., a wider spread of MICs above than below the mode) than for eravacycline and tigecycline and with a few highly resistant isolates. MIC distributions of tetracycline were bimodal. Although their distributions overlapped considerably, the MICs of eravacycline were mostly 2-fold lower than those of tigecycline, with modes at 0.25 to 0.5 μg/ml, according to the species and resistance group, versus 0.5 to 1 μg/ml. MIC50s of eravacycline (underlined in Table 2) for the carbapenem-resistant Enterobacteriaceae groups mostly were 2-fold higher than those for carbapenem-susceptible control strains, while MIC90s (boldface in Table 2) were 2- or 4-fold higher—a differential also evident for tigecycline MIC90s (not MIC50s). These raised summary MICs partly reflected a larger proportion of Klebsiella versus E. coli isolates among the carbapenem-resistant isolates than the controls (Table 1), coupled with a general trend for Klebsiella to be less susceptible to eravacycline and tigecycline than E. coli (Table 3). Nevertheless, the pattern persisted if only Klebsiella spp. were considered, indicating that a subset of the carbapenem-resistant K. pneumoniae isolates had reduced eravacycline and tigecycline susceptibility.
TABLE 2.
MIC distributions of tetracycline analogues for Enterobacteriaceae, excluding Proteeae, in relation to carbapenem resistance types
| Drug and characteristic(s) (n) | No. of isolates with characteristic(s) at MIC (μg/ml) ofa: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.06 | 0.13 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | |
| Eravacycline | |||||||||||
| KPC (45) | 3 | 13 | 17 | 9 | 2 | 1 | |||||
| VIM (44) | 16 | 18 | 8 | 2 | |||||||
| IMP (15) | 1 | 4 | 4 | 1 | 5 | ||||||
| NDM (42) | 5 | 16 | 9 | 9 | 2 | 1 | |||||
| OXA-48 (44) | 2 | 18 | 15 | 5 | 2 | 2 | |||||
| Porin loss + ESBL/AmpC (40) | 1 | 13 | 10 | 8 | 5 | 3 | |||||
| Susceptible controls (35) | 2 | 9 | 10 | 11 | 3 | ||||||
| Carbapenemase positive chosen as Tigr (30) | 2 | 4 | 4 | 4 | 6 | 9 | 1 | ||||
| Tigecycline | |||||||||||
| KPC (45) | 8 | 14 | 16 | 4 | 3 | ||||||
| VIM (44) | 6 | 16 | 13 | 8 | 1 | ||||||
| IMP (15) | 1 | 7 | 2 | 5 | |||||||
| NDM (42) | 1 | 6 | 18 | 7 | 9 | 1 | |||||
| OXA-48 (44) | 7 | 22 | 10 | 2 | 2 | 1 | |||||
| Porin loss + ESBL/AmpC (40) | 5 | 11 | 12 | 5 | 5 | 2 | |||||
| Susceptible controls (35) | 1 | 10 | 15 | 9 | |||||||
| Carbapenemase-positive chosen as Tigr (30) | 2 | 4 | 6 | 6 | 9 | 3 | |||||
| Minocycline | |||||||||||
| KPC (45) | 1 | 1 | 14 | 11 | 12 | 3 | 3 | ||||
| VIM (44) | 2 | 8 | 13 | 13 | 4 | 3 | 1 | ||||
| IMP (15) | 1 | 5 | 2 | 1 | 3 | 3 | |||||
| NDM (42) | 1 | 8 | 8 | 8 | 9 | 6 | 2 | ||||
| OXA-48 (44) | 2 | 14 | 13 | 7 | 3 | 3 | 2 | ||||
| Porin loss + ESBL/AmpC (40) | 1 | 6 | 13 | 7 | 5 | 6 | 2 | ||||
| Susceptible controls (35) | 2 | 11 | 14 | 6 | 2 | ||||||
| Carbapenemase positive chosen as Tigr (30) | 1 | 2 | 4 | 6 | 4 | 13 | |||||
| Tetracycline | |||||||||||
| KPC (45) | 5 | 8 | 10 | 2 | 2 | 18b | |||||
| VIM (44) | 1 | 8 | 3 | 1 | 31b | ||||||
| IMP (15) | 1 | 3 | 2 | 2 | 1 | 4 | 2b | ||||
| NDM (42) | 4 | 4 | 7 | 1 | 26b | ||||||
| OXA-48 (44) | 8 | 12 | 6 | 3 | 2 | 13b | |||||
| Porin loss + ESBL/AmpC (40) | 1 | 5 | 9 | 5 | 4 | 16b | |||||
| Susceptible controls (35) | 5 | 13 | 12 | 2 | 2 | 1b | |||||
| Carbapenemase positive chosen as Tigr (30) | 1 | 2 | 2 | 3 | 5 | 17b | |||||
MIC50s are underlined, and MIC90s are in boldface; in some cases, these values coincide.
The MIC is greater than or equal to the indicated value.
TABLE 3.
MIC distributions of eravacycline and tigecycline in relation to species and genus, excluding isolates chosen specifically for tigecycline resistance
| Drug and organism (n) | No. of isolates with MIC (μg/ml) ofa: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.13 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | >16 | |
| Eravacycline | ||||||||||
| A. baumannii (55) | 7 | 2 | 6 | 15 | 23 | 2 | ||||
| Citrobacter (11) | 3 | 1 | 5 | 1 | 1 | |||||
| E. coli (60) | 2 | 16 | 35 | 6 | 1 | |||||
| Enterobacter (65) | 9 | 40 | 13 | 3 | ||||||
| Klebsiella (120) | 5 | 43 | 35 | 21 | 13 | 3 | ||||
| Proteeae (15) | 1 | 4 | 3 | 5 | 1 | 1 | ||||
| Serratia (9) | 2 | 3 | 1 | 3 | ||||||
| Tigecycline | ||||||||||
| A. baumannii (55) | 3 | 4 | 6 | 7 | 25 | 10 | ||||
| Citrobacter (11) | 2 | 3 | 4 | 1 | 1 | |||||
| E. coli (60) | 1 | 1 | 33 | 23 | 2 | |||||
| Enterobacter (65) | 1 | 30 | 27 | 4 | 3 | |||||
| Klebsiella (120) | 9 | 48 | 33 | 19 | 9 | 2 | ||||
| Proteeae (15) | 2 | 4 | 4 | 4 | 1 | |||||
| Serratia (9) | 4 | 1 | 4 | |||||||
MIC50s are underlined, and MIC90s are in boldface.
Among the 30 carbapenemase-producing Enterobacteriaceae specifically included as tigecycline nonsusceptible based on prior BSAC agar testing, 18 were confirmed resistant, with MICs of 4 to 16 μg/ml, and another 6 as intermediate, with MICs of 2 μg/ml. MICs of eravacycline remained below those of tigecycline, but with 16 values in the range 4 to 16 μg/ml (Table 2).
The 15 Proteeae isolates (Table 1) comprised 6 Morganella morganii, 5 Providencia rettgeri, and 3 P. stuartii isolates and 1 Proteus mirabilis isolate: 10 isolates had carbapenemases, 8 of which were NDM types. All 15 organisms were resistant to classical tetracyclines. Two were susceptible at tigecycline's EUCAST breakpoint of ≤1 μg/ml, four intermediate (MIC, 2 μg/ml), and nine resistant, with MICs of >2 μg/ml. For eravacycline, 12/15 MICs were from 1 to 4 μg/ml (Table 3), with 10/15 values 2-fold below those for tigecycline.
MICs of eravacycline and tigecycline for the carbapenem-resistant A. baumannii series were unimodally distributed (Table 4), with eravacycline values mostly 2- to 4-fold below tigecycline, clustering at 0.5 to 1 μg/ml versus 1 to 4 μg/ml. MICs of minocycline were widely scattered, with most isolates highly resistant to tetracycline. As with Enterobacteriaceae, eravacycline and tigecycline MIC50s and MIC90s for the carbapenem-resistant groups exceeded those for the susceptible controls. Five A. baumannii isolates, all with OXA-23 carbapenemase, were included based on previously found tigecycline resistance: four “retained” tigecycline MICs of 8 to 16 μg/ml, and MICs of eravacycline for these were 4 to 8 μg/ml.
TABLE 4.
Eravacycline MICs for A. baumannii by carbapenem resistance mechanism
| Drug and characteristic (n) | No. of isolates with MIC (μg/ml) ofa: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.06 | 0.13 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |
| Eravacycline | ||||||||||
| NDM (5) | 1 | 4 | ||||||||
| OXA-23/40/51/58 (39) | 1b | 2 | 12 | 22 | 2 | |||||
| Susceptible controls (10) | 6b | 1 | 2 | 1 | ||||||
| OXA-23, selected as Tigr (5) | 1 | 2 | 2 | |||||||
| Tigecycline | ||||||||||
| NDM (5) | 4 | 1 | ||||||||
| OXA-23/40/51/58 (39) | 1 | 1 | 5 | 22 | 10 | |||||
| Susceptible controls (10) | 3 | 3 | 1 | 1 | 2 | |||||
| OXA-23, selected as Tigr (5) | 1 | 2 | 2 | |||||||
| Minocycline | ||||||||||
| NDM (5) | 1 | 3 | 1 | |||||||
| OXA-23/40/51/58 (39) | 1 | 2 | 2 | 6 | 7 | 7 | 7 | 6 | 1c | |
| Susceptible controls (10) | 7 | 1 | 1 | 1 | ||||||
| OXA-23, selected as Tigr (5) | 1 | 1 | 1 | 2c | ||||||
| Tetracycline | ||||||||||
| NDM (5) | 1 | 1 | 3c | |||||||
| OXA-23/40/51/58 (39) | 4 | 0 | 5 | 30c | ||||||
| Susceptible controls (10) | 6 | 2 | 1 | 1 | ||||||
| OXA-23, selected as Tigr (5) | 5c | |||||||||
MIC50s are underlined, and MIC90s are in boldface.
The MIC is less than or equal to the indicated value.
The MIC is greater than or equal to the indicated value.
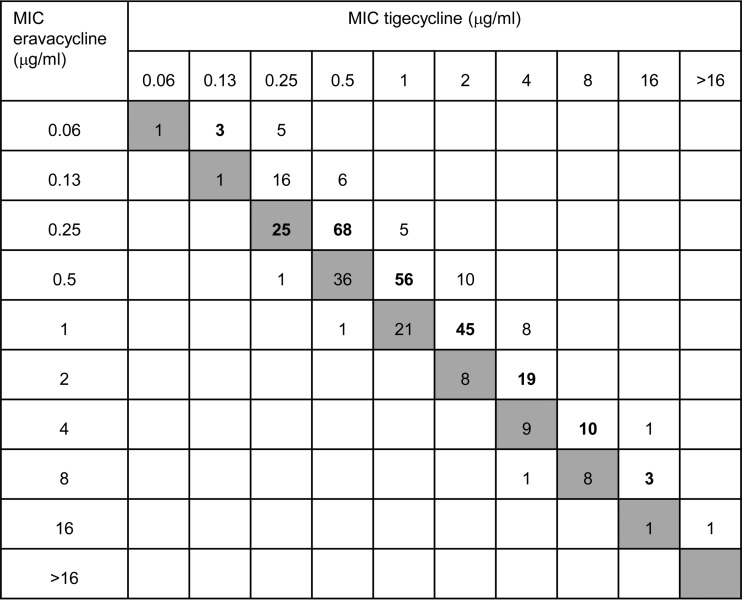
Two key findings emerge. First, eravacycline is 2- to 4-fold more active than tigecycline against carbapenem-resistant Enterobacteriaceae and A. baumannii isolates, but with qualitatively similar behaviors, leading to close correlation between MICs of both molecules (Fig. 1). Second, although (unsurprisingly) little relationship existed between eravacycline MICs and specific carbapenem resistance mechanisms, MIC90s of eravacycline and tigecycline were 2- to 4-fold higher for carbapenem-resistant Enterobacteriaceae and A. baumannii isolates than for the carbapenem-susceptible controls, with a similar MIC50 shift for eravacycline. The likely explanation is that a subset of carbapenem-resistant isolates have upregulated endogenous efflux or reduced permeability, a view supported by a recent Chinese study reporting frequent upregulation of the AcrAB pump in K. pneumoniae isolates with KPC enzymes (14). Upregulation of such pumps is the principal mode of tigecycline resistance in Enterobacteriaceae and A. baumannii (15) and accounts for the intrinsic resistance of Proteeae (16).
FIG 1.
Interrelationship between eravacycline and tigecycline MICs for the full panel of 369 isolates. Gray boxes represent the line of equivalence. Numbers above this line indicate eravacycline is more active, and numbers below indicate tigecycline is more active. Boldface indicates the modal MIC of eravacycline for each tigecycline MIC value.
The small but consistent gains in activity against carbapenem-resistant Enterobacteriaceae and A. baumannii isolates compared with tigecycline, coupled with higher serum drug levels, better tolerability, and more straightforward pharmacokinetics, may translate to an advantage for eravacycline, and clinical investigation is warranted.
ACKNOWLEDGMENTS
This work was funded by Tetraphase.
D.M.L. has served on advisory boards or done ad hoc consultancy for Accelerate, Achaogen, Adenium, Alere, Allecra, Arsanis, AstraZeneca, Auspherix, Basilea, BioVersys, Centauri, Cubist, Cycle, Discuva, Meiji, Nordic, Pfizer, Roche, Shionogi, Tetraphase, VenatoRx, and Wockhardt. In addition, D.M.L. has given paid lectures to AOP Orphan, AstraZeneca, Curetis, Merck, Nordic, Pfizer, and Leo. D.M.L. has relevant shareholdings in Dechra, GSK, Merck, PerkinElmer, and Pfizer amounting to <10% of portfolio value. All other authors have no personal interests to declare. However, P.H.E.'s AMRHAI Reference Unit has received financial support for conference attendance, lectures, research projects, or contracted evaluations from numerous sources, including Achaogen, Allecra Antiinfectives, Amplex, AstraZeneca UK, Becton Dickinson Diagnostics, the BSAC, Cepheid, Check-Points B.V., Cubist Pharmaceuticals, Department of Health, Enigma Diagnostics, Food Standards Agency, GlaxoSmithKline Services, Henry Stewart Talks, IHMA, Merck Sharpe & Dohme, Meiji Seika Kiasya, Momentum Biosciences, Nordic Pharma, Norgine Pharmaceuticals, Rempex Pharmaceuticals, Rokitan, Smith & Nephew UK, Trius Therapeutics, VenatoRx, and Wockhardt.
Funding Statement
The present work was funded by Tetraphase Pharmaceuticals.
REFERENCES
- 1.Food and Drug Administration. 2010. Highlight of prescribing information: Tygacil. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf. [Google Scholar]
- 2.Shen F, Han Q, Xie D, Fang M, Zeng H, Deng Y. 2015. Efficacy and safety of tigecycline for the treatment of severe infectious diseases: an updated meta-analysis of RCTs. Int J Infect Dis 39:25–33. doi: 10.1016/j.ijid.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. 2011. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 11:834–844. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 4.Yahav D, Lador A, Paul M, Leibovici L. 2011. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 66:1963–1971. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 5.Cai Y, Wang R, Liang B, Bai N, Liu Y. 2011. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 55:1162–1172. doi: 10.1128/AAC.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GC, Burgess DS. 2012. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports. Ann Clin Microbiol Antimicrob 11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe JA, O'Brien W, Fyfe C, Grossman TH. 2013. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors KP, Housman ST, Pope JS, Russomanno J, Salerno E, Shore E, Redican S, Nicolau DP. 2014. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother 58:2113–2118. doi: 10.1128/AAC.02036-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhanel GG, Cheung D, Adam H, Zelenitsky S, Golden A, Schweizer F, Gorityala B, Lagacé-Wiens PR, Walkty A, Gin AS, Hoban DJ, Karlowsky JA. 2016. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 76:567–588. doi: 10.1007/s40265-016-0545-8. [DOI] [PubMed] [Google Scholar]
- 11.Anon. 1991. A guide to sensitivity testing. Report of the Working Party on Antibiotic Sensitivity Testing of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 27(Suppl D):1–50. [PubMed] [Google Scholar]
- 12.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—tenth ed. Approved standard M7-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.He F, Fu Y, Chen Q, Ruan Z, Hua X, Zhou H, Yu Y. 2015. Tigecycline susceptibility and the role of efflux pumps in tigecycline resistance in KPC-producing Klebsiella pneumoniae. PLoS One 10:e0119064. doi: 10.1371/journal.pone.0119064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Cai Y, Liu X, Bai N, Liang B, Wang R. 2013. The emergence of clinical resistance to tigecycline. Int J Antimicrob Agents 41:110–116. doi: 10.1016/j.ijantimicag.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Visalli MA, Murphy E, Projan SJ, Bradford PA. 2003. AcrAB multidrug efflux pump is associated with reduced levels of susceptibility to tigecycline (GAR-936) in Proteus mirabilis. Antimicrob Agents Chemother 47:665–669. doi: 10.1128/AAC.47.2.665-669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]