Abstract
ACT-387042 and ACT-292706 are two novel bacterial topoisomerase inhibitors with broad-spectrum activity against Gram-positive and -negative bacteria, including methicillin-resistant Staphylococcus aureus and penicillin- and fluoroquinolone-resistant Streptococcus pneumoniae. We used the neutropenic murine thigh infection model to characterize the pharmacokinetics (PK)/pharmacodynamics (PD) of these investigational compounds against a group of 10 S. aureus and S. pneumoniae isolates with phenotypic resistance to beta-lactams and fluoroquinolones. The in vitro activities of the two compounds were very similar (MIC range, 0.03 to 0.125 mg/liter). Plasma pharmacokinetics were determined for each compound by using four escalating doses administered by the subcutaneous route. In treatment studies, mice had 107.4 to 108 CFU/thigh at the start of therapy with ACT-387042 and 106.7 to 108.3 CFU/thigh at the start of therapy with ACT-292706. A dose-response relationship was observed with all isolates over the dose range. Maximal kill approached 3 to 4 log10 CFU/thigh compared to the burden at the start of therapy for the highest doses examined. There was a strong relationship between the PK/PD index AUC/MIC ratio (area under the concentration-time curve over 24 h in the steady state divided by the MIC) and therapeutic efficacy in the model (R2, 0.63 to 0.82). The 24-h free-drug AUC/MIC ratios associated with net stasis for ACT-387042 against S. aureus and S. pneumoniae were 43 and 10, respectively. The 24-h free-drug AUC/MIC ratios associated with net stasis for ACT-292706 against S. aureus and S. pneumoniae were 69 and 25, respectively. The stasis PD targets were significantly lower for S. pneumoniae (P < 0.05) for both compounds. The 1-log-kill AUC/MIC ratio targets were ∼2- to 4-fold higher than stasis targets. Methicillin, penicillin, or ciprofloxacin resistance did not alter the magnitude of the AUC/MIC ratio required for efficacy. These results should be helpful in the design of clinical trials for topoisomerase inhibitors.
INTRODUCTION
ACT-387042 and ACT-292706 are two novel bacterial topoisomerase inhibitors (NBTIs) with related structures (compounds 32d and 32a, respectively, in reference 1). These compounds belong to a class of antibiotics discovered in the late 1990s (2). Similarly to fluoroquinolones, they bind near the catalytic center of DNA gyrase and topoisomerase IV but are structurally and mechanistically distinct from fluoroquinolones. Due to this differential action, they possess enhanced potency against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) as well as penicillin- and fluoroquinolone-resistant Streptococcus pneumoniae (1, 3–5).
The goal of the present experiments was to assess the in vivo pharmacokinetics (PK)/pharmacodynamics (PD) characteristics of two topoisomerase inhibitors under development in the mouse thigh infection model against two target bacterial species, S. aureus and S. pneumoniae.
(Part of this work was presented at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, September 2015 [21, 22].)
MATERIALS AND METHODS
Organisms, media, and compounds.
Five S. aureus isolates (2 methicillin susceptible and 3 methicillin resistant) and 5 S. pneumoniae isolates (1 penicillin resistant, 2 penicillin intermediate, and 1 fluoroquinolone resistant) were used for these studies (Table 1). All 10 strains were used for in vivo studies with ACT-292706, and 9 of the 10 isolates were similarly studied for ACT-387042. The organisms were chosen to include beta-lactam- and quinolone-susceptible and -resistant isolates. Staphylococcus aureus isolates were grown, subcultured, and quantified by using Mueller-Hinton broth (MHB) and agar (Difco Laboratories, Detroit, MI). Streptococcus pneumoniae isolates were grown, subcultured, and quantified by using sheep blood agar (Remel, Milwaukee, WI). Compounds for in vitro and in vivo studies were supplied by Actelion Pharmaceuticals Ltd. (Allschwil, Switzerland).
TABLE 1.
In vitro susceptibilities of select S. aureus and S. pneumoniae isolates to ACT-387042 and ACT-292706
| Organism | Isolate | ACT-387042 MIC (mg/liter)a | ACT-292706 MIC (mg/liter) | Phenotype |
|---|---|---|---|---|
| S. aureus | ATCC 29213 | 0.06 | 0.06 | Methicillin susceptible |
| ATCC 33591 | 0.06 | 0.03 | Methicillin resistant | |
| 307109 | 0.06 | 0.03 | Methicillin resistant | |
| WIS-1 | 0.03 | 0.03 | Methicillin resistant | |
| ATCC 25923 | 0.06 | 0.03 | Methicillin susceptible | |
| S. pneumoniae | ATCC 49619 | ND | 0.06 | Penicillin intermediate |
| 1396 | 0.125 | 0.125 | Penicillin intermediate | |
| 1293 | 0.125 | 0.125 | Penicillin resistant | |
| MNO-418 | 0.06 | 0.03 | Ciprofloxacin resistant | |
| ATCC 10813 | 0.125 | 0.125 | Penicillin susceptible |
ND, not studied.
In vitro susceptibility studies.
The MICs of each compound and for bacterial strain were determined by using Clinical and Laboratory Standards Institute (CLSI) microdilution methods (6). All MIC assays were performed in duplicate on three separate occasions. The median MICs from replicate assays are reported and were utilized in all PK/PD analyses.
Murine thigh infection model.
The neutropenic murine thigh infection model was used for all in vivo studies. Animals were maintained in accordance with American Association for Accreditation of Laboratory Animal Care (AAALAC) criteria (7). All animal studies were approved by the Animal Research Committees of the William S. Middleton Memorial VA Hospital and the University of Wisconsin. Six-week-old, specific-pathogen-free, female ICR/Swiss mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 23 to 27 g were used. Neutropenia (<100 neutrophils/mm3) was produced by two intraperitoneal injections of cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) at 150 mg/kg of body weight at day −4 and at 100 mg/kg at day −1 from infection.
Organism preparation and infection procedures were as follows. Broth cultures of freshly plated S. aureus bacteria were grown to the logarithmic phase overnight to an absorbance at 580 nm of 0.3 by using a Spectronic 88 spectrophotometer (Bausch and Lomb, Rochester, NY). S. pneumoniae isolates were grown overnight on sheep blood agar. A sterile loop was then used to transfer organisms to sterile saline, and the absorbance was adjusted as described above. After a 1:10 dilution, bacterial counts in the inoculum ranged from 107.4 to 108.0 CFU/ml for ACT-387042 and from 106.7 to 108.3 CFU/ml for ACT-292706. Thigh infections with each of the isolates were produced by injection of 0.1 ml of the inoculum into the thighs of isoflurane-anesthetized mice 2 h before therapy with ACT-387042 or ACT-292706. At the end of therapy (24 h), the organism burden was quantified by CFU determination from whole-organ homogenates. Untreated control animals were similarly assessed at the start- and end-of-therapy time points in all experiments.
Pharmacokinetic studies and analysis.
Single-dose plasma pharmacokinetics studies were performed for each compound following subcutaneous administration. Four dose levels were studied for each compound. The dose levels were chosen to bracket the effective dose range based upon data from previous pilot studies (not shown). The specific doses included 10, 40, 160, and 640 mg/kg for ACT-387042 and 10, 25, 80, and 100 mg/kg for ACT-292706. Plasma from groups of three mice was collected at 6 to 11 time points over the 24-h study period. Plasma drug concentrations of ACT-387042 and ACT-292706 were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) by Actelion Pharmaceuticals Ltd. Based on data fit, an exponential, single, 3-parameter, noncompartmental model was used for pharmacokinetic analysis of ACT-387042 (see Fig. S1 in the supplemental material). In contrast, a linear, noncompartmental model was used for pharmacokinetic analysis of ACT-292706. Relevant pharmacokinetic parameters, including elimination half-life (T1/2), area under the concentration-time curve (AUC), and peak level were determined for each dose in the respective pharmacokinetic studies. Estimation of pharmacokinetic parameters for doses not directly measured for ACT-387042, due to nonlinearity, was performed by using an exponential, single, 3-parameter equation. For ACT-292706, linear interpolation was used to determine pharmacokinetic parameters between doses, and when necessary, linear extrapolation was used to determine the pharmacokinetic parameters above and below the highest and lowest doses. Protein binding was determined by Actelion Pharmaceuticals Ltd. Binding values of 88% and 94% were used for PK/PD analyses of ACT-387042 and ACT-292706, respectively.
Treatment efficacy: pharmacodynamic target determination.
In vivo treatment studies of ACT-387042 and ACT-292706 against each isolate were performed with the thigh model as described above. Dose levels included 0.3125, 1.25, 5, 20, 80, and 320 mg/kg for ACT-387042 and 1.25, 2.5, 5, 10, 20, and 40 mg/kg for ACT-292706. The rationale for the choice of dose levels included an attempt to identify the entire dose-response relationship. Maximal doses were based upon maximally tolerated doses in mice. All dosing regimens were administered by the subcutaneous route every 3 h over the 24-h treatment period. Three mice (6 thigh infections) were used for each dosing regimen. At the end of the treatment period, the organism burden was determined by CFU counts in tissue homogenates. The correlation between efficacy and the PK/PD parameter AUC/MIC ratio (area under the concentration-time curve over 24 h in the steady state divided by the MIC) was determined by nonlinear least-squares multivariate regression (SigmaPlot version 12.3; Systat Software, San Jose, CA). The AUC/MIC ratio was chosen as the predictive pharmacodynamic index as previous studies demonstrated that this index is predictive of efficacy for topoisomerase inhibitors (8–13). While not the focus of the present study, a dose fractionation study was performed to verify the AUC/MIC ratio as the predictive index (see Fig. S2 in the supplemental material). The mathematical model used was derived from the Hill equation, E = (Emax × DN)/(ED50N − DN), where E is the effector, in this case the log change in CFU per thigh between treated mice and untreated controls after the 24-h period of study; Emax is the maximum effect; D is the 24-h total dose; ED50 is the dose required to achieve 50% of the Emax; and N is the slope of the dose-effect curve. The values for the indices Emax, ED50, and N were calculated by using nonlinear least-squares regression. The coefficient of determination (R2) was used to estimate the variance that might be due to regression with the AUC/MIC ratio. The doses required to produce a net static effect compared to the organism burden at the start of therapy (static dose) and 1-log kill (compared to the start of therapy) were calculated for each drug-organism combination. The associated 24-h total and free-drug AUC/MIC ratio targets were calculated. The static and 1-log-kill pharmacodynamic targets for S. aureus and S. pneumoniae were compared by one-way analysis of variance (ANOVA) for each compound for normally distributed data and by Kruskal-Wallis ANOVA on ranks for nonnormally distributed data.
RESULTS
In vitro susceptibility.
The MICs of ACT-387042 and ACT-292706 for the selected S. aureus and S. pneumoniae isolates are listed in Table 1. The MIC ranges of both compounds were relatively similar for S. aureus (range, 0.03 to 0.06 mg/liter) and S. pneumoniae (range, 0.03 to 0.125 mg/liter). Additionally, the MICs are either identical or within a 1-tube dilution when one compares the MICs for both compounds against the same isolate.
Pharmacokinetics.
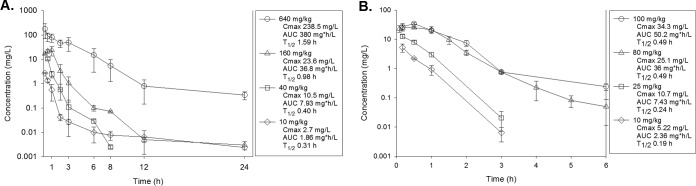
The time course of plasma concentrations of ACT-387042 in mice following subcutaneous doses of 10, 40, 160, and 640 mg/kg are shown in Fig. 1A. Over the dose range studied, the kinetics were nonlinear. The elimination half-life ranged from 0.31 h to 1.59 h. The AUC from 0 h to infinity (AUC0–∞) and peak concentration values for escalating single doses ranged from 1.9 to 380 mg · h/liter and from 2.7 to 239 mg/liter, respectively. An exponential, single, 3-parameter model best fit the PK data over the dose range (see Fig. S1 in the supplemental material). The time course of plasma levels of ACT-292706 in mice following subcutaneous doses of 10, 25, 80, and 100 mg/kg is shown in Fig. 1B. Over the dose range studied, kinetics were linear (maximum concentration of drug in serum [Cmax] and AUC R2 = 0.99). ACT-292706 demonstrated a very short elimination half-life ranging from 0.19 to 0.49 h. The AUC0–∞ and peak concentration values for escalating single doses ranged from 2.4 to 50.2 mg · h/liter and from 4.5 to 34.3 mg/liter, respectively. As noted above, levels of protein binding were previously determined by Actelion Pharmaceuticals Ltd. and were 88% and 94% for ACT-387042 and ACT-292706, respectively. Total and free-drug concentrations were considered in PK/PD calculations for subsequent analyses.
FIG 1.
(A) Plasma ACT-387042 concentrations after subcutaneous administration of single doses of 10, 40, 160, and 640 mg/kg in mice. Each symbol represents the mean ± standard deviation of the levels in the plasma of three mice. Cmax, peak plasma level; AUC, area under the drug concentration-time curve from zero to infinity; T1/2, plasma elimination half-life. (B) Plasma ACT-292706 concentrations after subcutaneous administration of single doses of 10, 25, 80, and 100 mg/kg in mice. Each symbol represents the mean ± standard deviation of the levels in the plasma of three mice.
Treatment efficacy: pharmacodynamic target determination.
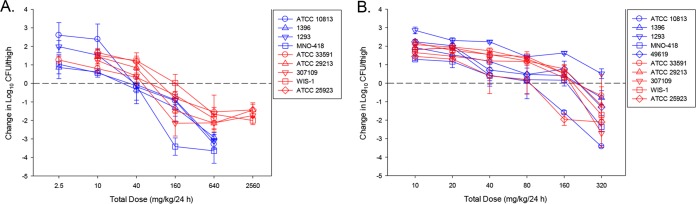
In untreated control mice, the organism burdens increased similarly among the 10 organisms over the 24-h study (S. aureus) mean growth, 2.1 log10 CFU/thigh [range, 1.9 to 2.4 log10 CFU/thigh]; S. pneumoniae mean growth, 2.4 log10 CFU/thigh [range, 1.8 to 3.3 log10 CFU/thigh]. The dose-response curves for ACT-387042 against all isolates are shown in Fig. 2A. The curves are fairly congruent, which is not surprising given the relatively narrow range (2-fold) of MICs. The PK/PD curves demonstrating a treatment effect based on free-drug AUC/MIC exposures for ACT-387042 for S. pneumoniae isolates and S. aureus isolates are shown in Fig. 3A and B, respectively. For both organism groups, the free-drug AUC/MIC ratio was a strong predictor of the treatment effect (R2 values of 0.75 and 0.82, respectively). The total daily doses necessary to achieve net stasis and 1-log kill for ACT-387042 are shown in Table 2. The 24-h static doses ranged from 26 to 53 mg/kg for S. pneumoniae and from 42 to 146 mg/kg for S. aureus. A 1-log-kill endpoint was achieved with all isolates. The 24-h doses necessary to achieve this endpoint ranged from 50 to 128 mg/kg for S. pneumoniae and from 49 to 361 mg/kg for S. aureus. The total doses necessary for each endpoint were not statistically different. The associated free-drug AUC/MIC ratio targets for net stasis and 1-log kill for each isolate are also shown in Table 2. Free-drug AUC/MIC ratio targets of ∼10 and 43 were necessary for net stasis against all S. pneumoniae and S. aureus isolates, respectively. The differences between free-drug AUC/MIC ratio targets for net stasis were statistically different between the two species (P = 0.016). The free-drug AUC/MIC ratio target was ∼2-fold higher for 1-log kill against each group of isolates.
FIG 2.
In vivo dose effects of ACT-387042 (A) and ACT-292706 (B) against select S. pneumoniae (blue lines) and S. aureus (red lines) isolates in a neutropenic mouse thigh model. Each symbol represents the mean and standard deviation from three mice (six thighs). Five or six total drug dose levels were fractionated into an every-3-hour regimen. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. The horizontal line at zero represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth.
FIG 3.
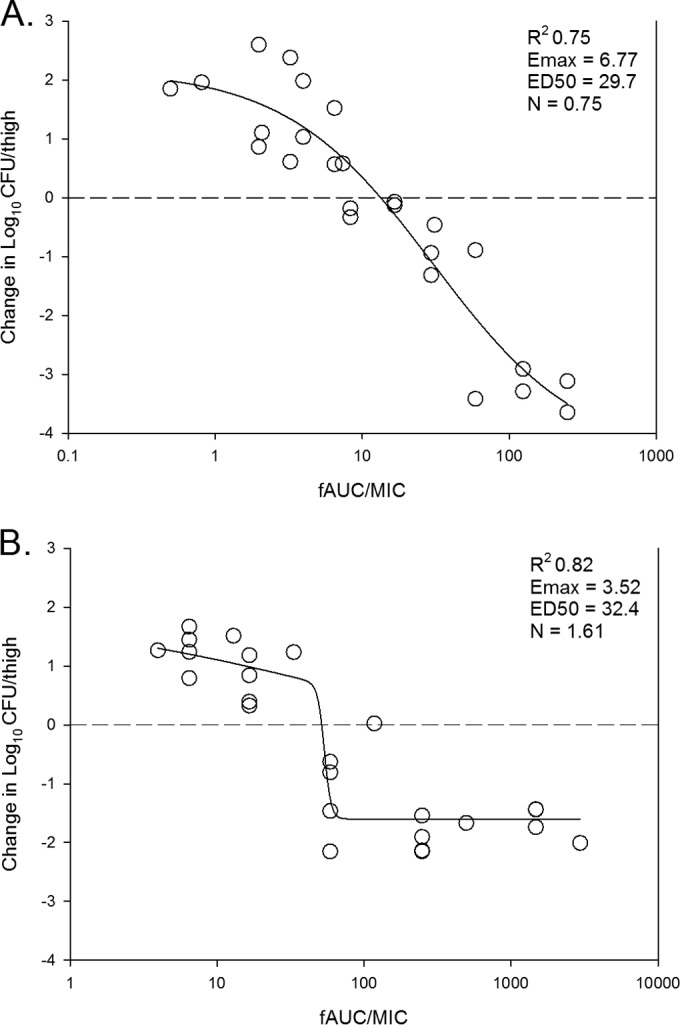
In vivo dose effect of ACT-387042 against select S. pneumoniae (A) and S. aureus (B) isolates in a neutropenic mouse thigh model. Each symbol represents the result of the mean of data for three animals (six thighs). Five or six total drug dose levels were fractionated into an every-3-h regimen. ACT exposure is expressed as the free-drug 24-h AUC/MIC (fAUC/MIC) ratio. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. The horizontal dashed line at zero represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. The R2 value represents the coefficient of determination. The line drawn through the data points is the best-fit line based upon the sigmoid Emax (maximum effect) formula. ED50, 50% maximal effect; N, slope of the best-fit line.
TABLE 2.
Pharmacodynamic targets for ACT-387042 and ACT-292706 against select S. pneumoniae and S. aureus isolatesa
| Drug and organism | Static dose (mg/kg/24 h) | 24-h static-dose AUC/MIC ratio (total drug) | 24-h static-dose AUC/MIC ratio (free drug) | 1-log-kill dose (mg/kg/q24h) | 24-h 1-log-kill AUC/MIC ratio (total drug) | 24-h 1-log-kill AUC/MIC ratio (free drug) |
|---|---|---|---|---|---|---|
| ACT-387042 | ||||||
| SA 29213 | 60.9 | 209.1 | 25.1 | 104.9 | 343.6 | 41.2 |
| SA 33591 | 55 | 191.1 | 22.9 | 208.4 | 669.2 | 80.3 |
| SA 307109 | 42.4 | 153.4 | 18.4 | 48.8 | 172.4 | 20.7 |
| SA WIS-1 | 146.2 | 943.5 | 113.2 | 361.3 | 2,349.8 | 282 |
| SA 25923 | 89.3 | 295.5 | 35.4 | 194.9 | 625.9 | 75.1 |
| Mean | 78.8 | 358.5 | 43* | 183.7 | 832.2 | 99.9 |
| Median | 60.9 | 209.1 | 25.1 | 194.9 | 625.9 | 75.1 |
| SD | 41.4 | 331.2 | 39.7 | 119.1 | 872.7 | 104.7 |
| ACT-387042 | ||||||
| SPN 1396 | 25.7 | 49.5 | 5.9 | 81.8 | 129.4 | 15.5 |
| SPN 1293 | 48.8 | 82.8 | 9.9 | 112.1 | 175.6 | 21.1 |
| SPN MNO-418 | 27.9 | 109.7 | 13.2 | 49.9 | 175.6 | 21.1 |
| SPN 10813 | 53.2 | 89.2 | 10.7 | 128.3 | 199.6 | 24 |
| Mean | 38.9 | 82.8 | 9.9* | 93 | 170.1 | 20.4 |
| Median | 38.35 | 86 | 10.3 | 96.95 | 175.6 | 21.1 |
| SD | 14.1 | 25 | 3 | 34.6 | 29.4 | 3.5 |
| ACT-292706 | ||||||
| SA 29213 | 189 | 782 | 46.9 | NA | ||
| SA 33591 | 186 | 1,540 | 92.4 | NA | ||
| SA 307109 | 158 | 1,307 | 78.4 | 216 | 2,267 | 136 |
| SA WIS-1 | 168 | 1,390 | 83.4 | 254 | 2,919 | 175 |
| SA 25923 | 86 | 695 | 41.7 | 156 | 1,491 | 89 |
| Mean | 157 | 1,143 | 68.6** | 209 | 2,226 | 133 |
| Median | 168 | 1,307 | 78.4 | 216 | 2,267 | 136 |
| SD | 42 | 380 | 22.8 | 49 | 715 | 43 |
| ACT-292706 | ||||||
| SPN 49619 | 136 | 563 | 33.8 | 319 | 2,019 | 121 |
| SPN 1396 | 161 | 318 | 19.1 | NA | ||
| SPN 1293 | NA | NA | ||||
| SPN MNO418 | 76 | 640 | 38.4 | 205 | 2,070 | 124 |
| SPN 10813 | 72 | 136 | 8.2 | 141 | 315 | 19 |
| Mean | 111 | 414 | 24.9** | 222 | 1,468 | 88 |
| Median | 106 | 440.5 | 26.5 | 205 | 2,019 | 121 |
| SD | 44 | 231 | 13.8 | 90 | 999 | 60 |
*, P = 0.016; **, P = 0.012; NA, not achieved; q24h, every 24 h.
The dose-response curves for ACT-292706 against all isolates are shown in Fig. 2B. By visual inspection, there was slightly more variability in the dose-response curves than noted with ACT-387042. The PK/PD exposure-response curves based on free-drug AUC/MIC ratio exposures for ACT-292706 for all S. pneumoniae isolates and all S. aureus isolates are shown in Fig. 4A and B, respectively. The free-drug AUC/MIC ratio was a strong predictor of treatment efficacy (R2 values of 0.63 and 0.72, respectively). The doses necessary to achieve net stasis and 1-log kill for ACT-292706 are shown in Table 2. The 24-h static doses ranged from 72 to 161 mg/kg for S. pneumoniae and from 86 to 189 mg/kg for S. aureus. The 24-h doses necessary to produce a 1-log kill, when achieved, ranged from 141 to 319 mg/kg for S. pneumoniae and from 156 to 216 mg/kg for S. aureus. The total doses necessary for each endpoint were not statistically different. The associated free-drug AUC/MIC ratio targets for net stasis and 1-log kill for each isolate, when achieved, are also shown in Table 2. Free-drug AUC/MIC ratio targets of ∼25 and 69 were necessary for net stasis against all S. pneumoniae and S. aureus isolates, respectively. The differences between free-drug AUC/MIC ratio targets for net stasis were statistically different between the two species (P = 0.012). The free-drug AUC/MIC ratio target was ∼2- to 4-fold higher for 1-log kill against each group of isolates but did not meet statistical significance. However, this evaluation was limited by the slightly smaller number of isolates that achieved the 1-log-kill endpoint.
FIG 4.
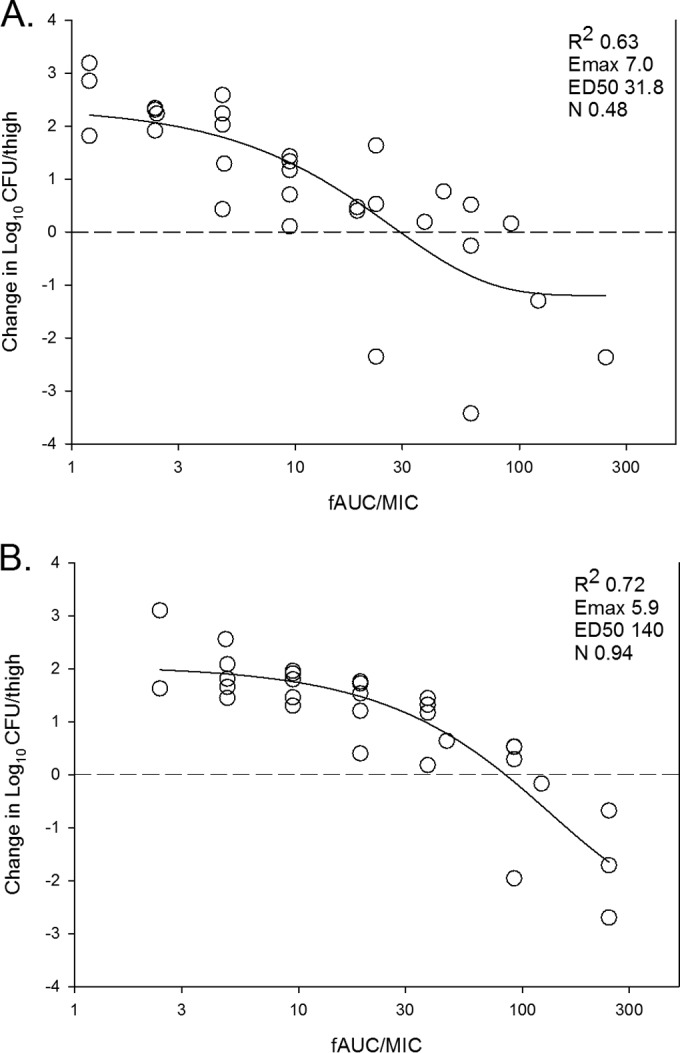
In vivo dose effect of ACT-292706 against select S. pneumoniae (A) and S. aureus (B) isolates in a neutropenic mouse thigh model. Each symbol represents the result of the mean of data for three animals (six thighs). Five or six total drug dose levels were fractionated into an every-3-h regimen. ACT exposure is expressed as the free-drug 24-h AUC/MIC ratio. The burden of organisms was measured at the start and end of therapy. The study period was 24 h. The horizontal dashed line at zero represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. The R2 value represents the coefficient of determination. The line drawn through the data points is the best-fit line based upon the sigmoid Emax (maximum effect) formula. ED50, 50% maximal effect; N, slope of the best-fit line.
DISCUSSION
Pharmacodynamic studies integrate a drug's pharmacokinetic properties, in vitro potency, and antimicrobial activity over time (14). Previous pharmacodynamic studies with fluoroquinolones and NBTIs have examined activity against a variety of pathogens. These studies have consistently shown concentration-dependent action and modest to prolonged postantibiotic effects against Gram-positive and Gram-negative pathogens (9, 10, 12–19). The PK/PD index associated with efficacy given this pattern of activity is typically the AUC/MIC ratio.
In the present studies, we examined the pharmacodynamic activities of two NBTIs in the neutropenic murine thigh model. NBTIs were initially discovered in the late 1990s by researchers at GlaxoSmithKline (GSK) and Aventis. These molecules were non-fluoroquinolone-based topoisomerase inhibitors without cross-resistance to fluoroquinolone-resistant strains. Previously, novel tetrahydropyran-based bacterial topoisomerase inhibitors that act on bacterial type II topoisomerases (DNA gyrase and topoisomerase IV) were discovered by Actelion Pharmaceuticals Ltd. (3). Further chemical modification on the scaffolds shown in those early studies led to two drug candidates, ACT-387042 and ACT-292706 (compounds 32d and 32a, respectively, in reference 1), which were the focus of these PK/PD studies.
We observed a lack of cross-resistance between other antibiotic classes (beta-lactams and fluoroquinolones) and the two NBTIs in these studies, consistent with data from previous work on the NBTI class. In fact, the MIC range was very narrow (2- to 4-fold) using a diverse group of S. aureus and S. pneumoniae isolates with phenotypic resistance to other antibiotic classes. Dose-response curves for each compound demonstrated increasing efficacy with escalating total daily doses, with maximal kill of >3 log10 CFU/thigh. However, among the isolates examined, the outcome appeared consistent with the MIC. Specifically, higher drug doses were required for efficacy against organisms with higher MICs.
In the pharmacodynamic analyses, we chose to examine the PK/PD index AUC/MIC ratio. This was based on data from previous dose fractionation studies with other NBTIs that demonstrated that the AUC/MIC ratio is the PD index linked with efficacy for two candidate NBTI compounds against S. aureus (12, 13) and S. pneumoniae (13) (also see Fig. S2 in the supplemental material). We found that the AUC/MIC ratio is a relatively robust predictor of efficacy against both S. pneumoniae and S. aureus in the murine model (R2 range, 0.63 to 0.82).
Net stasis was observed for ACT-387042 at 24-h free-drug AUC/MIC ratios of 10 against S. pneumoniae and 43 against S. aureus. A single previous study characterized PD targets for an NBTI against Gram-positive pathogens in the murine model (13). In that study, net stasis against a group of S. pneumoniae and S. aureus isolates revealed a 24-h free-drug AUC/MIC ratio target of ∼10. Another study examined the stasis 24-h free-drug AUC/MIC ratio target against two Gram-negative pathogens, Pseudomonas aeruginosa and Escherichia coli, in the murine thigh model, and these targets were 16 and 7, respectively (20). Therefore, the PD targets observed for ACT-387042 in this study were relatively similar to those in the other two reports. The PD targets were numerically higher for ACT-292706 than for ACT-387042, as the stasis 24-h free-drug AUC/MIC ratio targets for this compound were ∼25 and 69 for S. pneumoniae and S. aureus, respectively. The PD target 24-h free-drug AUC/MIC ratios for 1-log-kill endpoints with both NBTIs were 2- to 4-fold higher than those for the stasis endpoints, which has also been observed in the two previous studies of other NBTIs (13, 20).
Compared to fluoroquinolones, the PD targets identified for these NBTIs in the animal model are low. Numerous studies have demonstrated stasis 24-h free-drug AUC/MIC ratio targets that range from 25 to 50 for S. pneumoniae but are higher (range, 50 to 100) for other organisms such as S. aureus (9, 14, 16–18). However, the clinical relevance of this difference between these two drug classes, despite their related mechanisms of action, remains to be determined in clinical trials.
In conclusion, we have demonstrated that two novel NBTIs exhibit potent activity against S. aureus and S. pneumoniae, including isolates with resistance to beta-lactams and fluoroquinolones. The PD index AUC/MIC ratio was a robust predictor of therapeutic efficacy in the murine model for both organism groups. Twenty-four-hour free-drug AUC/MIC ratio targets were lower than those observed for fluoroquinolones and were statistically different between the two species. These findings are an important step forward in the development of NBTIs for clinical use and will be useful to integrate with data from human PK studies to establish dosing regimen design and preliminary susceptibility breakpoints.
Supplementary Material
ACKNOWLEDGMENT
This study was funded by Actelion Pharmaceuticals Ltd.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00363-16.
REFERENCES
- 1.Surivet JP, Zumbrunn C, Rueedi G, Bur D, Bruyere T, Locher H, Ritz D, Seiler P, Kohl C, Ertel EA, Hess P, Gauvin JC, Mirre A, Kaegi V, Dos Santos M, Kraemer S, Gaertner M, Delers J, Enderlin-Paput M, Weiss M, Sube R, Hadana H, Keck W, Hubschwerlen C. 2015. Novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram positive activity and improved safety profile. J Med Chem 58:927–942. doi: 10.1021/jm501590q. [DOI] [PubMed] [Google Scholar]
- 2.Ehmann DE, Lahiri SD. 2014. Novel compounds targeting bacterial DNA topoisomerase/DNA gyrase. Curr Opin Pharmacol 18:76–83. doi: 10.1016/j.coph.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Surivet JP, Zumbrunn C, Rueedi G, Hubschwerlen C, Bur D, Bruyere T, Locher H, Ritz D, Keck W, Seiler P, Kohl C, Gauvin JC, Mirre A, Kaegi V, Dos Santos M, Gaertner M, Delers J, Enderlin-Paput M, Boehme M. 2013. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram-positive activity. J Med Chem 56:7396–7415. doi: 10.1021/jm400963y. [DOI] [PubMed] [Google Scholar]
- 4.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 5.Black MT, Stachyra T, Platel D, Girard AM, Claudon M, Bruneau JM, Miossec C. 2008. Mechanism of action of the antibiotic NXL101, a novel nonfluoroquinolone inhibitor of bacterial type II topoisomerases. Antimicrob Agents Chemother 52:3339–3349. doi: 10.1128/AAC.00496-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 8.Andes D, Craig WA. 1998. Pharmacokinetics and pharmacodynamics of outpatient intravenous antimicrobial therapy. Infect Dis Clin North Am 12:849–860. doi: 10.1016/S0891-5520(05)70024-6. [DOI] [PubMed] [Google Scholar]
- 9.Andes D, Craig WA. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob Agents Chemother 46:1665–1670. doi: 10.1128/AAC.46.6.1665-1670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andes D, Craig WA. 2003. Pharmacodynamics of the new des-f(6)-quinolone garenoxacin in a murine thigh infection model. Antimicrob Agents Chemother 47:3935–3941. doi: 10.1128/AAC.47.12.3935-3941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 37:1073–1081. doi: 10.1128/AAC.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam VH, Chang KT, Yang Z, Newman J, Hu M. 2013. In vitro pharmacodynamics of AZD5206 against Staphylococcus aureus. Antimicrob Agents Chemother 57:1062–1064. doi: 10.1128/AAC.01208-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulik CC, Okusanya OO, Bhavnani SM, Lepak A, Forrest A, Ambrose PG, Hoover JL, Andes DR. 2014. Evaluation of the pharmacokinetics-pharmacodynamics (PK-PD) of GSK2140944 against Staphylococcus aureus and Streptococcus pneumoniae in a murine thigh-infection model, poster A-680. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 14.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10, quiz 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 15.Blaser J, Stone BB, Groner MC, Zinner SH. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother 31:1054–1060. doi: 10.1128/AAC.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig W, Dalhoff A. 1998. Pharmacodynamics of fluoroquinolones in experimental animals, p 208–232. In Kuhlman J, Dalhoff A, Zeiller HJ (ed), Handbook of experimental pharmacology, vol 127 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 17.Lister PD, Sanders CC. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother 43:79–86. doi: 10.1093/jac/43.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Lister PD, Sanders CC. 1999. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 43:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig WA. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis 158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 20.Dougherty TJ, Nayar A, Newman JV, Hopkins S, Stone GG, Johnstone M, Shapiro AB, Cronin M, Reck F, Ehmann DE. 2014. NBTI 5463 is a novel bacterial type II topoisomerase inhibitor with activity against Gram-negative bacteria and in vivo efficacy. Antimicrob Agents Chemother 58:2657–2664. doi: 10.1128/AAC.02778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepak A, Seiler P, Surivet JP, Ritz D, Kohl C, Andes D. 2015. Pharmacokinetic/pharmacodynamic evaluation of a novel topo-isomerase inhibitor, ACT-292706, in a murine thigh and lung infection model against Staphylococcus aureus and Streptococcus pneumoniae, abstr A-047. Abstr 55th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 22.Lepak A, Seiler P, Surivet JP, Ritz D, Kohl C, Andes D. 2015. Pharmacokinetic/pharmacodynamic evaluation of a novel topo-isomerase inhibitor, ACT-387042, in a murine thigh and lung infection model against Staphylococcus aureus (SA) and Streptococcus pneumoniae (SPN) abstr A-046. Abstr 55th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.